ABSTRACT
Drought stress provokes jasmonic acid (JA) signaling, which mediates plant stress responses; moreover, growing numbers of studies suggest that JA is involved in the modulation of root development under drought stress. Recently, we showed that JA promotes differentiation of xylem from procambial cells in Arabidopsis roots. Further molecular and genetic approaches revealed that the effect of JA on xylem development is caused by suppression of cytokinin responses, suggesting that JA antagonistically interacts with cytokinin to modulate xylem development. Here, we showed that, similar to JA, drought stress promotes xylem development. This suggests that the antagonistic interaction between JA and cytokinin is involved in drought-mediated xylem development, a hypothesis supported by the observation that drought stress increases JA responses and decreases cytokinin responses. Based on these findings, we propose that drought stress promotes xylem development, and the antagonistic interaction between JA and cytokinin is deeply involved in this process.
KEYWORDS: Cytokinin, drought, hormonal interaction, jasmonic acid, xylem
Plants dynamically coordinate their development and defenses in response to environmental stresses such as drought, and modulation of growth may help plants survive environmental stresses.1-4 Drought stress affects root development and growth and our recent study revealed that drought stress promotes the differentiation of xylem from meristematic procambial cells in root vascular tissues.5 (Fig. 1) In Arabidopsis roots, xylem cells develop in a single axis.6 Wild-type plants grown in polyethylene glycol (PEG)-containing medium designed to simulate drought stress conditions formed extra xylem adjacent to the xylem axis, in contrast to wild-type plants grown in normal conditions. Quantification of the number of xylem cells showed that roots of wild-type plants grown in normal conditions developed approximately 4 xylem cells but wild-type plants grown on PEG-containing medium developed approximately 5 xylem cells, suggesting that the PEG-mediated stress promotes xylem development.
Figure 1.
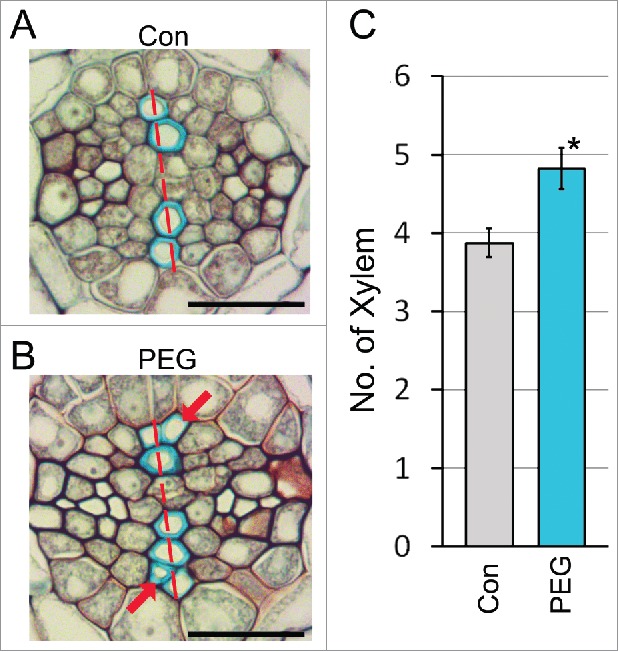
PEG-mediated drought stress promotes xylem development in roots. Xylem development of wild-type (Col-0) roots grown in PEG-untreated (A) and -treated (B) conditions. Four-day-old seedlings germinated on normal Murashige and Skoog (MS) media were transferred to the control media (1/2 MS medium, water potential = −0.25 MPa) or PEG-containing media (water potential = −0.70 MPa), respectively. After 8 days, the roots collected from these plants were sectioned transversly and stained with toluidine blue. Root sections were taken from the root maturation zone 2 mm above the transition zone, which is located between the meristem and basal differentiation region. The red arrows and dotted lines indicate extra xylem cells and the xylem axis. (C) Quantification of the number of xylem cells formed in these roots (n>15). Error bars represent S.E. and asterisks indicate statistically significant differences between the corresponding samples and their control (p < 0.01, Student's t-test). Scale bar = 20 μm.
The interaction between hormones, especially between the hormones that govern stress responses and development, regulates developmental flexibility under stress conditions.3,4,7-11 Cytokinin is an essential hormone controlling cell proliferation and root xylem development.6,12,13,14 For example, wooden leg (wol) mutants with severe defects in cytokinin signaling display all-xylem phenotypes in their vascular tissues, and the mutant plants that lack expression of Type-B ARABIDOPSIS RESPONSE REGULATORs (ARRs) such as ARR1 and ARR12 produce more xylem.6,13,14 These observations indicate that cytokinin is a key negative regulator inhibiting xylem development in root vascular tissues. The finding that exogenous cytokinin treatment suppresses xylem formation supports this hypothesis (Fig. 2A). Together with previous findings that expression of the genes responsible for cytokinin responses tended to be downregulated by environmental stresses,15-17 these findings suggest that modulation of the cytokinin response is deeply involved in xylem development under PEG-mediated drought stress conditions.
Figure 2.
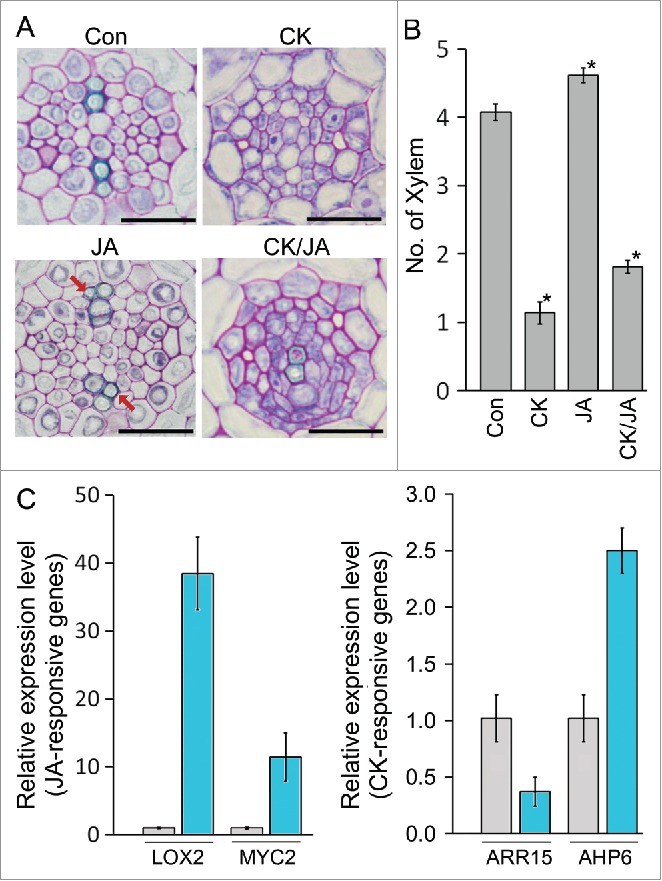
JA and cytokinin responses in PEG-treated roots. (A) Xylem development of Col-0 roots grown in the indicated conditions for 7 days (cytokinin [CK], 50 nM BAP; JA, 10 μM MeJA; CK/JA, 10 μM MeJA and 50 nM BAP). The red arrows indicate extra xylem. (B) Quantification of the number of xylem cells formed in these roots (n>21). Error bars represent S.E. and asterisks indicate statistically significant differences between the corresponding samples and the control (p < 0.01, Student's t-test). (C) Expression of JA-responsive and cytokinin-responsive genes in the roots grown in the PEG-untreated (grey) and -treated (blue) conditions. Total RNAs were extracted from 8-day-old Col-0 roots grown in PEG-untreated (water potential = −0.25 MPa) and -treated (water potential = −0.70 MPa) conditions. Error bars represent S.D. Scale bar = 20 μm.
In contrast to cytokinin, JA and its related stresses such as drought and oxidative stress promote xylem differentiation,5,18 in agreement with our finding that JA promotes formation of extra xylem (Fig. 2A). This indicates that cytokinin and JA have opposite functions in xylem development and suggests that cytokinin and JA antagonistically interact in root xylem development. When the number of xylem cells was quantified, the plants co-treated with cytokinin and JA had more xylem cells compared with the plants treated with cytokinin alone, but fewer xylem cells compared with the plants treated with JA alone (Fig. 2B). These results support the idea that cytokinin and JA antagonistically interact in root xylem development. The results also suggest that PEG-mediated drought stress promotes xylem development by modulating the JA and cytokinin responses. To explore this, we analyzed JA and cytokinin responses in roots grown on PEG-containing medium by quantifying expression levels of JA-induced genes such as LOX2 and MYC2 and cytokinin-induced genes such as ARR15 (Fig. 2C). Transcript levels of JA-induced genes such as LOX2 and MYC2 increased while transcript levels of cytokinin-induced genes such as ARR15 decreased in response to the stress. Unlike ARR15, expression of AHP6 whose expression is downregulated by cytokinin6 was upregulated by the stress. These findings indicated that the PEG-mediated drought stress activates JA responses and suppresses cytokinin responses, suggesting that the PEG-mediated drought stress affects xylem development by modulating the JA and cytokinin responses. The previous result showing that JA-responsive transcription factor MYC2 promotes xylem differentiation by activating expression of AHP6, a negative regulator of cytokinin response5 partially supports this. Collectively, our findings suggest that the antagonistic interaction between cytokinin and JA mediates xylem differentiation under drought stress.
Growing numbers of studies have proposed that JA antagonistically interacts with cytokinin in various aspects of plant physiology and development. For example, JA strongly inhibits cytokinin-induced callus growth19 and suppresses the effect of cytokinin on chloroplast development and the plant defense system.20,21 A recent study of circadian stress responses supports the antagonistic interaction between JA and cytokinin.22 These suggest that the JA–cytokinin interaction is largely involved in the modulation of plant physiology and development in response to environmental stresses such as drought. However, the molecular mechanisms controlling the JA–cytokinin interaction are largely unknown. Further studies will expand our understanding of the molecular mechanisms underlying this process.
Funding Statement
Rural Development Administration PJ01364301 and PJ01323901; National Research Foundation of Korea [NRF-2014R1A1A2054261].
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was carried out with the support of “Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ01364301 and PJ01323901)” Rural Development Administration, Republic of Korea and the National Research Foundation of Korea grant funded by the Korean Government (MOE) [NRF-2014R1A1A2054261].
References
- 1.Dinneny JR, Long TA, Wang JY, Jung JW, Mace D, Pointer S, Barron C, Brady SM, Schiefelbein J, Benfey PN. Cell identity mediates the response of Arabidopsis roots to abiotic stress. Science. 2008;320:942–5. doi: 10.1126/science.1153795. [DOI] [PubMed] [Google Scholar]
- 2.Fahad S, Bajwa AA, Nazir U, Anjum SA, Farooq A, Zohaib A, Sadia S, Nasim W, Adkins S, Saud S. Crop production under drought and heat stress: Plant responses and management options. Front Plant Sci. 2017;8:1147. doi: 10.3389/fpls.2017.01147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huot B, Yao J, Montgomery BL, He SY. Growth–defense tradeoffs in plants: A balancing act to optimize fitness. Mol Plant. 2014;7:1267–87. doi: 10.1093/mp/ssu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang D-L, Yao J, Mei C-S, Tong X-H, Zeng L-J, Li Q, Xiao LT, Sun TP, Li J, Deng XW. Plant hormone jasmonate prioritizes defense over growth by interfering with gibberellin signaling cascade. Proc Nat Acad Sci. 2012;109:E1192–E200. doi: 10.1073/pnas.1201616109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jang G, Chang SH, Um TY, Lee S, Kim J-K, Do Choi Y. Antagonistic interaction between jasmonic acid and cytokinin in xylem development. Sci Rep. 2017;7:10212. doi: 10.1038/s41598-017-10634-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bishopp A, El-Showk S, Weijers D, Scheres B, Friml J, Benková E, Benková E, Mähönen AP, Helariutta Y. A mutually inhibitory interaction between auxin and cytokinin specifies vascular pattern in roots. Curr Biol. 2011;21:917–26. doi: 10.1016/j.cub.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 7.Pieterse CM, Zamioudis C, Berendsen RL, Weller DM, Van Wees SC, Bakker PA. Induced systemic resistance by beneficial microbes. Annu Rev Phytopathol. 2014;52:347–75. doi: 10.1146/annurev-phyto-082712-102340. [DOI] [PubMed] [Google Scholar]
- 8.Hou X, Lee LYC, Xia K, Yan Y, Yu H. DELLAs modulate jasmonate signaling via competitive binding to JAZs. Dev Cell. 2010;19:884–94. doi: 10.1016/j.devcel.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 9.Kazan K, Manners JM. JAZ repressors and the orchestration of phytohormone crosstalk. Trends Plant Sci. 2012;17:22–31. doi: 10.1016/j.tplants.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Yoon S, Lee D-K, Yu IJ, Kim YS, Do Choi Y, Kim J-K. Overexpression of the OsbZIP66 transcription factor enhances drought tolerance of rice plants. Plant Biotechnol Rep. 2017;11:53–62. doi: 10.1007/s11816-017-0430-2. [DOI] [Google Scholar]
- 11.Zhong T, Zhang L, Sun S, Zeng H, Han L. Effect of localized reduction of gibberellins in different tobacco organs on drought stress tolerance and recovery. Plant Biotechnol Rep. 2014;8:399–408. doi: 10.1007/s11816-014-0330-7. [DOI] [Google Scholar]
- 12.Schaller GE, Street IH, Kieber JJ. Cytokinin and the cell cycle. Curr Opin Plant Biol. 2014;21:7–15. doi: 10.1016/j.pbi.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 13.Mähönen AP, Bonke M, Kauppinen L, Riikonen M, Benfey PN, Helariutta Y. A novel two-component hybrid molecule regulates vascular morphogenesis of the Arabidopsis root. Genes & Dev. 2000;14:2938–43. doi: 10.1101/gad.189200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yokoyama A, Yamashino T, Amano Y-I, Tajima Y, Imamura A, Sakakibara H, Mizuno T. Type-B ARR transcription factors, ARR10 and ARR12, are implicated in cytokinin-mediated regulation of protoxylem differentiation in roots of Arabidopsis thaliana. Plant and Cell Physiol. 2007;48:84–96. doi: 10.1093/pcp/pcl040. [DOI] [PubMed] [Google Scholar]
- 15.Hare P, Cress W, Van Staden J.. The involvement of cytokinins in plant responses to environmental stress. Plant Growth Regul. 1997;23:79–103. doi: 10.1023/A:1005954525087. [DOI] [Google Scholar]
- 16.Howe GA, Schilmiller AL. Oxylipin metabolism in response to stress. Curr Opin Plant Biol. 2002;5:230–6. doi: 10.1016/S1369-5266(02)00250-9. [DOI] [PubMed] [Google Scholar]
- 17.Argueso CT, Ferreira FJ, Kieber JJ. Environmental perception avenues: The interaction of cytokinin and environmental response pathways. Plant, Cell & Env. 2009;32:1147–60. doi: 10.1111/j.1365-3040.2009.01940.x. [DOI] [PubMed] [Google Scholar]
- 18.Ghuge SA, Carucci A, Rodrigues-Pousada RA, Tisi A, Franchi S, Tavladoraki P, Angelini R, Cona A. The apoplastic copper AMINE OXIDASE1 mediates jasmonic acid-induced protoxylem differentiation in Arabidopsis roots. Plant Physiol. 2015;168:690–707. doi: 10.1104/pp.15.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ueda J, Kato J. Inhibition of cytokinin‐induced plant growth by jasmonic acid and its methyl ester. Physiol Plant. 1982;54:249–52. doi: 10.1111/j.1399-3054.1982.tb00255.x. [DOI] [Google Scholar]
- 20.Naseem M, Kaltdorf M, Hussain A, Dandekar T. The impact of cytokinin on jasmonate-salicylate antagonism in Arabidopsis immunity against infection with Pst DC3000. Plant Signaling & Behavior 2013;8:e26791. doi: 10.4161/psb.26791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu L, Li H, Zeng H, Cai Q, Zhou X, Yin C. Exogenous jasmonic acid and cytokinin antagonistically regulate rice flag leaf senescence by mediating chlorophyll degradation, membrane deterioration, and senescence-associated genes expression. J Plant Growth Regul. 2016;35:366–76. doi: 10.1007/s00344-015-9539-0. [DOI] [Google Scholar]
- 22.Nitschke S, Cortleven A, Iven T, Feussner I, Havaux M, Riefler M, Schmülling T. Circadian stress regimes affect the circadian clock and cause jasmonic acid-dependent cell death in cytokinin-deficient arabidopsis plants. Plant Cell. 2016;28:1616–39. [DOI] [PMC free article] [PubMed] [Google Scholar]


