ABSTRACT
Epithelial-mesenchymal transition (EMT) is potentially involved in increasing metastasis of oral squamous cell carcinoma (OSCC). Periodontal pathogens are well-known for their ability to induce intense immune responses and here we investigated whether they are involved in inducing EMT. Cultures of OSCC cell line (H400) were treated separately with heat-killed periodontal pathogens F. nucleatum, or P. gingivalis or E. coli LPS for 8 d. EMT-associated features were assayed using sq-PCR and PCR-arrays, for EMT-related markers, and ELISAs for TGF-β1, TNF-α, and EGF. The migratory ability of cells was investigated using scratch and transwell migration assays. E-cadherin and vimentin expression was assessed using immunofluorescence while Snail activation was detected with immunocytochemistry. In addition, the integrity of the cultured epithelial layer was investigated using transepithelial electrical resistance (TEER). PCR data showed significant upregulation after 1, 5, and 8 d in transcription of mesenchymal markers and downregulation of epithelial ones compared with unstimulated controls, which were confirmed by immunofluorescence. Periodontal pathogens also caused a significant increase in level of all cytokines investigated which could be involved in EMT-induction and Snail activation. Exposure of cells to the bacteria increased migration and the rate of wound closure. Downregulation of epithelial markers also resulted in a significant decrease in impedance resistance of cell monolayers to passage of electrical current. These results suggested that EMT was likely induced in OSCC cells in response to stimulation by periodontal pathogens.
KEYWORDS: cadherins, cytokines, invasiveness, migration, mesenchymal cells, neoplasm metastasis, oral keratinocytes
Introduction
Oral cancer is a life-threatening condition if it is not diagnosed and treated in its early stages.1 Epidemiological data indicates that oral cancer is the nineteenth most common cancer causing mortality in the UK, with oral squamous cell carcinoma (OSCC) being the predominant form accounting for over 90% of oral malignancy cases.2,3 OSCC has around 40–50% 5-year survival rates due to recurrence and metastasis.4 The invasiveness of cancer cells, has been attributed, in part, to a process called epithelial-mesenchymal transition (EMT).5-7 Accordingly, the expression of certain epithelial structural and adhesion molecules is lost while the expression of mesenchymal cell molecules is increased, consequently giving epithelial cells a mesenchymal-like phenotype, at least to some extent.6,8,9
Persistent chronic inflammation due to microbial challenge may favor EMT-induction in various organs including lungs, liver, and intestine10 and recent data indicates that infection by the periodontal pathogens P. gingivalis and F. nucleatum may drive the inflammatory host response and also increase OSCC invasiveness.11,12 Oral epithelium responds to the presence of periodontal pathogens by secreting chemokines and cytokines such as transforming growth factor-β1 (TGF-β1)13 epidermal growth factor14 and tumor necrosis factor-α (TNF-α)15-17 which have been suggested to trigger the onset of EMT either independently or synergistically.13,18,19 EGF appears to induce EMT by increasing expression of the transcriptional factor Twist which regulates cell differentiation and lineage definition.20 EMT has been suggested to be responsible for the upregulation of vimentin, a mesenchymal intermediate filament protein, as well as downregulation of the epithelial attachment protein E-cadherin which, in turn could facilitate cell motility and compromise epithelial integrity.21 A similar mechanism of Twist upregulation is reportedly induced by TNF-α to trigger EMT22 and furthermore, cross talk between Twist-Snail signaling appears to be critical in inducing EMT-like features, increasing anoikis resistance, facilitating cell migration and consequently metastasis.23-25 EMT-related features are usually investigated by using a range of assays including PCR, immunocytochemistry, scratch-wound and transwell migration assays.26-29
Recent data from a case-control study has claimed that periodontitis could represent a risk factor for OSCC independent of other risk factors.30 Periodontal pathogens, particularly Gram negative bacteria and their products are well known for their ability to elicit intense chronic inflammatory and immune responses which could trigger EMT. The aim of this work therefore was to examine the potential of F. nucleatum and P. gingivalis in driving EMT in OSCC in vitro.
Materials and methods
Cell culture
The OSCC cell line (H400),31 passage numbers 14–35, was cultured in Dulbecco's modified Eagle's medium (DMEM) (SAFC Biosciences, UK) supplemented with 10% fetal calf serum (FCS) at 37°C in 5% CO2 Cultures were treated individually with media-only (DMEM and 10% FCS, negative control) or the heat-killed periodontal pathogens, F. nucleatum and P. gingivalis, at a ratio of 100 bacteria per cell,16,32 and 20 μg/ml E. coli LPS (EMT-positive control). Culture supernatants collected from days 1, 5, and 8 were used for further analysis.
Semi-quantitative reverse transcriptase-polymerase chain reaction (sq-RT-PCR) and PCR-array analysis
Total RNA was extracted from cultures using RT lysis buffer (Qiagen, UK) and quantified spectrophotometrically (BioPhotometer plus, Eppendorf, Germany) and visualized in a 1% agarose gel with SYBR Gold. Single stranded cDNA was synthesized from 1 μg of RNA using the Tetro kit (Bioline, UK) or the RT2 First strand kit (Qiagen, UK).
For sq-RT-PCR cDNA templates were amplified using a thermal cycler (Mastercycler, Eppendorf, Germany) using selected primers (Table 1). PCR products were visualized following separation in 1.5% agarose gels supplemented with ethidium bromide (10 mg/ml) (Sigma, UK). Images were captured using GeneSnap software (Syngene, USA), and analyzed using GeneTools software (Syngene, USA). Relative levels of PCR products were calculated and normalized against the housekeeping gene, GAPDH. All analyses were performed twice and in duplicate for each experiment.
Table 1.
Details of genes studied, primer sequences and semi-quantitative RT-PCR conditions. Accession numbers were obtained from GenBank (Tm: Melting temperature).
| wGene name | Gene symbol | Accession number | Sequence (5′ → 3′) | Tm (°C) | Product (bp) | Number of cycles |
|---|---|---|---|---|---|---|
| Tumor necrosis factor-α | TNF-α | NM_000594.2 | F-AAG AAT TCA AAC TGG GGC CT | 60 | 402 | 38 |
| R-GGC TAC ATG GGA ACA GCC TA | ||||||
| Epidermal growth factor | EGF | NM_001963 | F-CTTGGGAGCCTGAGCAGAAA | 60 | 521 | 32 |
| R-TGGTTGTGGTCCTGAAGCTG | ||||||
| E-cadherin | CDH1 | NM_004360 | F-CAAGTGCCTGCTTTTGATGA | 60 | 339 | 34 |
| R-GCTTGAACTGCCGAAAAATC | ||||||
| Vimentin | VIM | NM_003380.3 | F-CGGGAGAAATTGCAGGAGGA | 60 | 588 | 31 |
| R-ACGAAGGTGACGAGCCATTT | ||||||
| Snail-1 | SNAl1 | NM_005985.3 | F-CGGACCCACACTGGCGAGAAG | 60 | 433 | 30 |
| R-CAGCTGCCCTCCCTCCACAGA | ||||||
| Twist family bHLH transcription factor 1 | TWIST1 | NM_000474 | F- AGACCTAGATGTCATTGTTTCCAGA | 60 | 454 | 28 |
| R-CAGGCCAGTTTGATCCCAGT |
For PCR array analysis, the cDNA template was combined with RT2 SYBER Green Mastermix (Qiagen, UK) before dispensing into the PCR-array plate (96-well format) (Qiagen, UK). Plates were loaded into a thermal cycler (ROCHE, Switzerland). Cycling conditions were 1 cycle at 95°C for 10 min, followed by 45 cycles at 95°C for 15 sec, then terminated by incubation at 60°C for 1 min. Data were collected by LightCycler 480 software and analyzed using the Qiagen online tool (www.SABiosciences.com/pcrarraydataanalysis.php).
TGF-β1, TNF-α and EGF ELISA
Levels of TGF-β1, TNF-α and EGF in culture supernatants were assayed 3 times, in triplicate for each time point, using ELISA kits (Quantikine ELISA Kit, R&D Systems, USA) following the manufacturer's instructions.
Scratch wound assay
H400 cells were cultured to confluence in 6-well plates and either treated with media only or bacterial components for 8 d. The epithelial sheet was disrupted to produce a scratch-wound using a sterile disposable plastic pipette tip of 10 μm diameter (Fig 3A) and rinsed with phosphate buffered saline (PBS) before media replenishment.33 Images were captured immediately after scratching (0 hr) using phase contrast microscopy (Primo Vert, Zeiss, Germany), and at 12 and 24 hr. Images were analyzed using Java-based ImageJ34 and the distance measured at 10 points, using a superimposed grid, between the wound edges. Three images were taken at each time-point and data were averaged. The experiments were repeated 3 times and performed in triplicate for each time point.
Figure 3.
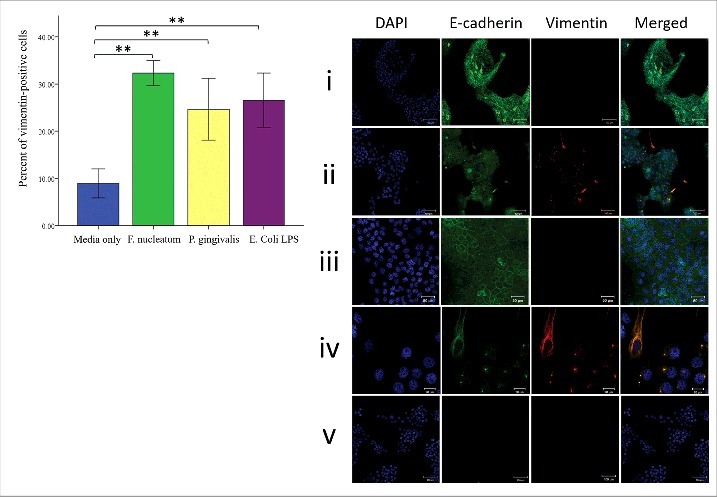
Periodontal bacteria modulate vimentin and E-cadherin expression in H400 cells after 8-days culture. (A) Cells with vimentin positive staining were counted and expressed as a percentage. Analysis indicated a significant increase between cells stimulated with bacteria and unstimulated control in terms of vimentin expression. (B) Immunofluorescence staining indicates that F. nucleatum and P. gingivalis induce expression of vimentin (red) (ii) when compared with the control group treated with media only (i). Scale bars = 100 μm. Higher magnification showing that unstimulated control (iii) maintained normal E-cadherin distribution and negatively expressed vimentin. Scale bar = 50 μm. while stimulated cultures (iv) showed presence of vimentin-positive cells which either exhibit mesenchymal-like morphology and retained some characteristics of their parental origin by expressing E-cadherin or cluster of epithelial cells simultaneously expressing vimentin with downregulation of E-cadherin from periphery of cells. Scale bars = 20 μm. Negative controls (cultures treated with secondary antibodies only) were included to exclude possibility of unspecific staining (v). (** = P< 0.001).
Transwell migration assay
Cells (5 × 104) were treated with media only or bacterial components for up to 8 d. Cultures were trypsinized and seeded in 200 μl FCS-free media on the upper compartment of tissue culture insert (Greiner Bio-One, Germany) assembled in the 24-well plate. Media supplemented with FCS (700 μl) were added to the bottom chamber of each well. Cultures were incubated at 37°C and 5% CO2 for 12 hr. Non-migrated cells in the upper compartment of the membrane were removed using a cotton swab; cells on the lower surface of the membrane were fixed with 4% paraformaldehyde for 15 min followed by Giemsa staining (Sigma, UK).35 Cell counts were performed on 4 random fields of view using phase contrast microscopy (Primo Vert, Zeiss, Germany). All experiments were performed 3 times and in triplicate.
Immunofluorescence and immunohistochemistry analysis
H400 cells were cultured on sterilized glass coverslips (22 × 22 mm) for 8 d. Cultures were stimulated separately, for 8 days, either with heat-killed F. nucleatum, P. gingivalis or E. coli LPS (20 μg/ml), the control comprised media alone. Cultures were prepared for immunofluorescence and immunohistochemical staining by fixing in 4% paraformaldehyde for 15 min. Cells were permeabilized with 0.25% Triton X-100 in PBS (10 min). Non-specific binding of antibodies was blocked by incubating samples for 30 min with 5% bovine serum albumin (BSA) in phosphate-buffered saline (PBS), followed by incubation with primary antibodies (1:100) for Anti-E-cadherin (rabbit polyclonal IgG), Anti-vimentin (mouse monoclonal IgG) (Santa Cruz, USA), and Anti-Snail (rabbit polyclonal IgG) (Abcam, UK) overnight at 4°C. For immunofluorescence, secondary antibodies were applied for 1 hr at room temperature Antibodies used were FITC-conjugated (bovine anti-rabbit) and TRITC-conjugated (goat anti-mouse) for immunofluorescence, both antibodies were from Santa Cruz (USA). Cell nuclei were stained with DAPI (Sigma, UK) and images captured using a confocal microscope (Zeiss, Germany). Analysis was performed on 5 random fields, clusters of cells expressing vimentin were selected, in 3 slides for each group to determine the number of vimentin positive cells compared with total cell number.
For Snail staining, biotin-conjugated (mouse anti-rabbit) (Abcam, UK) was used and counterstained with Mayer's hematoxylin. Slides were viewed by using an inverted microscope (Primo Vert, Zeiss, Germany). A total of 4 random fields from each sample were used to calculate the percent of Snail-positive cells in the fields examined. Both assays were repeated 3 times and performed in triplicate.
Transepithelial electrical resistance (TEER)
This assay was developed to examine integrity of epithelial monolayers in vitro by measuring their resistance to the passage of an electric current. Normal barrier function is characterized by increased electrical impedance and the opposite is applied to compromised epithelial layers. Tissue culture inserts with 0.4 μm pore membrane (Greiner bio-one, Germany) were inserted in 24-well plates and seeded with H400 cells (7 × 104) in the upper chamber.36 Two-hundred microliters of media was applied in the upper chamber and 500 μl in the lower compartment. Cultures were exposed separately to media only (control), heat-killed F. nucleatum and P. gingivalis or E. coli LPS (20 μg/ml). Measuring transepithelial electrical resistance (TEER) started at a point where stable recordings were obtained from the culture using an AC-based ohmmeter (EVOM2, USA). The experiment was repeated 3 times and performed in triplicate, TEER measurements were recorded in (Ω) after subtracting the background resistance of the membrane only out of each read out to obtain the resistant of the epithelial layer.
Statistical analysis
Results are expressed as mean ± one standard deviation. Analyses were performed using one-way ANOVA between multiple groups and post-hoc test to compare differences. Statistically significant results were considered for P values < 0.05.
Results
Analysis of EMT-associated gene and protein expression in H400 cultures exposed to bacterial components
Analysis using sq-RT-PCR demonstrated that exposure of H400 cells to heat-killed periodontal pathogens and E. coli LPS resulted in gene expression profiles reportedly representative of EMT. Eight days of culture bacterial stimulation resulted in upregulation of vimentin, Snail, and Twist (∼6-fold upregulation, P< 0.001, Fig. 1). F. nucleatum exposure stimulated the highest increase in gene expression of these molecules compared with the other 2 bacterial stimulants. Transcript levels of the epithelial adhesion molecule, E-cadherin, was downregulated following exposure to bacterial stimulation by up to 6-fold (P< 0.001) relative to controls. Significant upregulation of several cytokines and growth factors was also detected. EGF transcription levels were increased up to 6-fold (P< 0.001), while TNF-α and TGF-β1 showed a ∼2-fold upregulation at all time-points compared with controls (Fig 2D, E, F). The highest changes detected were following 8-days of stimulation. Further analysis of samples for this time-point using PCR-arrays demonstrated a comparable pattern of expression for these genes. Moreover; PCR-array analysis indicated changes had occurred to several other EMT-related indicators and hence supported the induction of this phenotype. These included genes expression changes in mesenchymal, epithelial, transcriptional factors, cytokines, signaling pathways and enzymes (Table 2).
Figure 1.

Exposure of H400 cells to heat-killed F. nucleatum, P. gingivalis, and 20 μg/ml E. coli LPS produced changes in EMT-related indicators. Gel images and densitometric analysis showing fold changes relative to highest or lowest levels as determined for selected EMT-related genes using semi-quantitative RT-PCR analysis. F. nucleatum and P. gingivalis produced significant changes in (A) E-cadherin level after 24 hr cell stimulation and continued till day 8 (∼5-fold). Other EMT-related genes [(B) vimentin, (C) Twist, and (D) Snail-1] were upregulated up to 5-fold compared with unstimulated control in response to periodontal pathogens from days 1–8 (* = P< 0.05, ** = P< 0.001).
Figure 2.

H400 cells were treated with media only (DMEM supplemented with 10% FCS), or heat inactivated periodontal pathogens F. nucleatum and P. gingivalis (100 bacteria per cell), and 20 μg/ml E. coli LPS over 8 d. Supernatant collected from each group after 1, 5, and 8 d and assayed using ELISA for (A) TGF-β1 which significantly increased with F. nucleatum and E. coli LPS (∼2 folds) at day 1, while P. gingivalis showed no difference compared with the control. Day 5 and 8 data showing significant upregulation for all test groups (∼6-folds). (B) EGF and (C) TNF-α, results showing similar pattern of stimulation to that of TGF-β1 in which P. gingivalis did not produce any statistical difference in the amount of EGF and TNF-α released in comparison with the control. At day 5 and 8 all bacterial groups significantly upregulated the level of both cytokines in the supernatant. The same picture is applied to the increase in gene transcription for the same cytokines (D, E, F), the only exception is the significant increased transcription of TNF-α associated with P. gingivalis after 24 h (* = P< 0.05, ** = P< 0.001).
Table 2.
EMT PCR-array fold changes in transcription of EMT-related genes following exposure to bacterial components. Table was generated by using Qiagen online analysis tool (www.SABiosciences.com/pcrarraydataanalysis.php).
| Fold changes relative to control |
||||
|---|---|---|---|---|
| Gene name | Gene Symbol | F. nucleatum | P. gingivalis | E. Coli LPS |
| Transforming growth factor β1 | TGF-β1 | 2.8 | 2.2 | 1.6 |
| Bone Morphogenic protein 7 | BMP-7 | 2.5 | 4.7 | 6 |
| Glycogen synthase kinase 3 β | GSK3β | 1.6 | 4.6 | 2.7 |
| Jagged 1 | JAG1 | 4.5 | 2 | 1.3 |
| Notch 1 | NOTCH1 | 6.9 | 2.1 | 1.9 |
| Snail-1 | SNAl1 | 4.3 | 6.3 | 1.9 |
| Slug | SNAl2 | 1.5 | 3.1 | 1.6 |
| Zinc finger E-box binding homeobox 1 | ZEB1 | 3.2 | 7.8 | 2.9 |
| E-cadherin | CDH1 | −5.4 | −4.3 | −3.5 |
| β-Catenin | CTNNB1 | −2 | −3.1 | −1 |
| Cytokeratin 14 | KRT14 | −2.1 | −2.5 | −1.1 |
| N-cadherin | CDH2 | 6.3 | 2.4 | 5.7 |
| Vimentin | VIM | 5.5 | 4.8 | 2.6 |
| Matrix metalloproteinases-2 | MMP2 | 2.4 | 2 | 3.1 |
| Matrix metalloproteinases-9 | MMP9 | 6.5 | 2.1 | 2.4 |
| Smad family member 1 | SMAD1 | 2.4 | 1.8 | 2.4 |
| Twist family bHLH transcription factor 1 | TWIST1 | 2 | 4 | 6.6 |
Levels of TGF-β1, EGF and TNF-α (Fig. 2) in the supernatant of H400 cultures stimulated with bacteria increased significantly (P< 0.05) over the 8-day culture period. At day 1, only P. gingivalis exposure did not produce a significant increase in levels of these cytokines secreted by the H400 cultures. In general, periodontal pathogens caused 1–10-fold increases, relative to controls, in the levels of TGF-β1, EGF and TNF-α over the experimental period.
Analysis of immunofluorescence images (Fig. 3A) showed an increase in the percentage of vimentin positive stained H400 cells following bacterial exposure (P< 0.05) at 8-days culture. This change was also accompanied by a decrease of E-cadherin expression on the cell membrane of stimulated cells when compared with controls and was associated with co-existence of vimentin positive staining (Fig 4A). Consistent with these findings, immunohistochemistry analysis (Fig. 4B) indicated a significant increase (P< 0.05) in the proportion of Snail-positive cells, following the same period of stimulation as compared with the control.
Figure 4.

Stimulation of H400 cells with periodontal bacteria resulted in significant increase in Snail-positive cells. (A) Immunocytochemical staining of H400 cell, growing on 22 × 22 mm coverslips, showing increased Snail expression. Activated cells manifested by dark brown discoloration of the nucleaus and/or cytoplasm (green arrow), while cells treated with media only mostly exhibit dark blue nucleaus (red arrow). Scale bars are(50 μm) (B) Analysis of percent of Snail-positive cells showing that P. gingivalis and F. nucleatum elicited the higher response (up to 70%) when compare with unstimulated control. (C) H400 cells were cultured on a 0.4 μm pore membrane insert and stimulated with periodontal pathogens. Resistance levels were stabilized in all groups after 4 days, from this point TEER measurements were recorded up to day 8. Analysis of data showed that resistance in response to bacterial stimulation was significantly decreased from day 4 to 8 in comparison with the media control group. Following 5 d of exposure to periodontal pathogens, cells in stimulated cultures exhibited fibroblast-like morphology while unstimulated control maintained normal epithelial sheet architecture (D). Scale bars = 50 μm. (* = P< 0.05, ** = P< 0.001).
Epithelial integrity and migratory responses in H400 cultures exposed to bacteria
As EMT is associated with a decrease of expression of molecules involved in cellular attachment, it is suggested that this is a contributor to cell dispersion. in vitro TEER was performed to assess H400 culture integrity. Figure 4C shows that resistance in H400 monolayers (cultured in the presence of bacterial components) was significantly lower than controls (P< 0.05) at day 4 following exposure to F. nucleatum, P. gingivalis, and E. coli LPS. All bacterial exposures from day 4 till 8 exhibited significantly reduced TEER, indicating that integrity of epithelial sheets in the cultures was compromised after exposure to periodontal pathogens. Consistent with these findings, culture images (Fig 4D) showed that cells treated with bacteria showed scattering associated with morphological changes resembling that of fibroblasts, while unstimulated cultures maintained a classical epithelial cobble-stone appearance.
Increased cell locomotion is regarded as an indicator of acquisition of a mesenchymal phenotype. H400 cells previously exposed to periodontal pathogens over 8 d were used in the transwell migration assay. Data (Fig. 5A) indicated that periodontal pathogens increased migration rates in H400 cells (P< 0.001) as compared with media-only treated cells. To explore further the migratory ability, a scratch-wound assay was undertaken on H400 confluent monolayers exposed to the periodontal pathogens (8 days). Wounding was repeated many times on several samples, stimulated and control, and carefully selected to ensure that all of them had the same initial gap width (500 μm). This approach was used to eliminate any variation in closure rate due to difference in distance between wounded edges. Measurements at 12 hr did not show any significant difference in gap closures between test and control groups. However, at 24 hr, cultures exposed to bacteria exhibited greater gap closures (P< 0.05) when compared with controls (Fig. 5B, C).
Figure 5.

(A) Analysis of transwell migration assay for H400 cells stimulated over 8 d with periodontal pathogens. Data demonstrated that a higher number of cells had migrated through the membrane in comparison with control. Confluent monolayers of H400 cells were disrupted by scratching with a 10 μm pipette tip following 8 d of exposure to heat-inactivated F. nucleatum and P. gingivalis and 20 μg/ml E. coli LPS. (B) Images were captured immediately following scratching and then after 12 and 24 hr culture. Scale bars are 100 μm (C) Measurements of the wound width at 12 hr indicated no significant difference, however at 24 hr scratch closure was significantly increased in all cultures exposed to bacterial components in comparison with unstimulated control. Data is shown as mean + SD. Experiments were performed in triplicate. (* = P< 0.05, ** = P< 0.001).
Discussion
The poor prognosis of OSCC represents a significant health challenge despite advances in diagnostic and therapeutic measures mainly due to the high recurrence and metastasis rates.3 The oral tissues are exposed to a relatively large population of potentially pathogenic bacteria, in particular, Gram negative anaerobes.37 Recent research has highlighted a potential involvement of periodontal pathogens, F. nucleatum and P. gingivalis, in increased aggressiveness of OSCC.11,12 Samples from highly malignant gingival squamous cell carcinoma showed an abundant presence of P. gingivalis,38 however their role and reason for the observed association remain unclear.
Recent studies on EMT indicated that this process provides a potential mechanism to explain some of the required steps leading to metastasis.5,7 In the current work, we investigated the possible effect of periodontal pathogens in triggering EMT in OSCCs in vitro. The transition of cell phenotype by EMT requires activation of certain inducer molecules of which Snail and Twist appear to be relevant candidates.20,23 Snail is a zinc-finger transcription factor that increases in activity during inflammation and is a well characterized regulator of E-cadherin expression.39 In addition, increased Snail expression has been reported in recurrent and metastatic OSCCs, in comparison with cancer cells from the primary tumor.24 PCR data showed that both transcriptional factors were increased <6-fold over 8 d of culture following bacterial stimulation. Notably the activation of these transcriptional factors requires activation of signaling pathways by a range of cytokines. Consistent with this was data from the EMT PCR-array which indicated increased transcription of essential EMT-signaling pathways GSK3β, JAG1, and NOTCH. However, the changes in signaling pathways were not equally significant among bacterial stimuli investigated (Table 2). This may provide insight into the differential activation of certain pathways by different bacteria. While PCR-array data was consistent with our sq-RT-PCR findings it also identified further changes: upregulation of MMP-2, -3 and -9 compared with controls. These molecules are involved in the breakdown of the basement membrane, which could facilitate tumor metastasis.8,9
Previous work by Milward and coworkers16 showed that presence of non-viable periodontopathogens in the culture of epithelial cells increase transcription TNF-α, IL-1β, and IL-8. In this study, TGF-β1, EGF and TNF-α were initially selected for investigation as they share a common EMT-signaling pathway which results in stabilization and activation of Snail.39,40 We originally hypothesized that these factors may drive the EMT phenotype chronically due to local inflammatory events. Assaying levels of TGF-β1, EGF and TNF-α in the supernatant from H400 cultures showed that bacterial components increased production of these cytokines although this was not evident for P. gingivalis 24 hr post-exposure. Notably these findings were consistent with previous reports indicating that P. gingivalis had a weaker effect, compared with E. coli LPS, in stimulating TNF-α over a 24 hr period both in vivo and in vitro.41
Evidence for EMT triggering requires detection of certain changes which have been described previously as major criteria including downregulation of adhesion molecules, increased migratory ability, and expression of new mesenchymal markers.42 Transcription of E-cadherin, the central protein of adherens junctions involved in cell attachment, is reportedly decreased significantly following increased transcription of Snail,43 and Twist.25 E-cadherin downregulation was also accompanied by upregulation of N-cadherin (Table 2) and supports the switching from epithelial into mesenchymal cadherins which is considered one of the main features of EMT.8,9 Snail activity, and E-cadherin suppression, stimulated by periodontal pathogens, was confirmed here by immunohistochemical staining. Snail activation after 8 d of bacterial stimulation showed that 70% of cells were activated by F. nucleatum and P. gingivalis. Immunofluorescence staining and PCR data showed a simultaneous upregulation of vimentin expression after stimulation with bacterial products, further supporting the process of EMT induction in the H400 OSCC line in vitro.44 Up-regulation of vimentin has been reported to be induced by EGF, which is a key regulator of proliferation and migration of cells.45 Transitioned cells co-expressing vimentin and E-cadherin cells were elongated associated with E-cadherin internalization. Other cells still forming epithelial clusters with E-cadherin distributed around the cell membrane; however, vimentin could be detected in their cytoplasm. (Fig. 3B).
Exposure to periodontal pathogens resulted in a higher rate of closure of the scratch-wounds inflicted on H400 monolayer cultures. In the present study, the migratory ability of the cells was investigated by using simple Boyden or transwell-migration assays which can provide thorough information about cell migration through a barrier in response to a chemo-attractant agent.46 In addition, the wound-healing assay which is simple, cheap and well-developed method to measure cell migration in vitro47 was used to further confirm the increased motility of epithelial cells in response to stimuli. Our results indicated that wound closure was consistent with an increase in numbers of migrated cells as supported by the transwell migration assay (Fig. 5A). The same pattern of migration rates was detected in both assays for F. nucleatum and P. gingivalis. These findings are also consistent with the PCR and immunofluorescence data demonstrating downregulation of E-cadherin as well as the TEER results showing decreased epithelial integrity which would potentially enable cell motility. The decrease in electric resistance of the cultures to the passage of a current correlated with the loss of cellular attachment and changes in cellular morphology as observed with light microscopy (Fig 4D). The increased vimentin expression was a potential indicator for the presence of cell populations with high migratory capabilities as well as populations usually associated with cancers.48 These data were in agreement with previous results21 which demonstrated increased cell motility was correlated with vimentin expression and associated with E-cadherin downregulation. In addition, compromised epithelial integrity may suggest onset of EMT due to decrease in cellular junctions.6 Notably this occurs because of E-cadherin and other epithelial adherence protein downregulation. This feature was also supported by the decreased electrical impedance following exposure to bacteria.
In conclusion, this study suggested that the periodontal pathogens elicit changes in H400 cells, OSCC cell line, at the molecular, structural and behavioral levels which are potentially indicative of EMT. Further investigation on the potential of periodontal pathogens to induce EMT in different cell lines and primary cells are likely to complement our findings and provide additional information on the clinical importance of EMT induction, and their relation to compromised integrity of epithelial tissue, invasion and metastasis.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This study was sponsored by Iraqi Ministry of Higher Education and Scientific Research. GL acknowledges support from EPSRC grant EP/M023869/1.
References
- [1].Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: A Cancer J Clinicians 2011; 61:69-90; PMID:21296855 [DOI] [PubMed] [Google Scholar]
- [2].Choi S, Myers JN. Molecular pathogenesis of oral squamous cell carcinoma: implications for therapy. J Dental Res 2008; 87:14-32; PMID:18096889; https://doi.org/ 10.1177/154405910808700104 [DOI] [PubMed] [Google Scholar]
- [3].Feller L, Lemmer J. Oral squamous cell carcinoma: epidemiology, clinical presentation and treatment. J Cancer Therapy 2012; 3:263-8; https://doi.org/ 10.4236/jct.2012.34037 [DOI] [Google Scholar]
- [4].Marsh D, Suchak K, Moutasim KA, Vallath S, Hopper C, Jerjes W, Upile T, Kalavrezos N, Violette SM, Weinreb PH, et al.. Stromal features are predictive of disease mortality in oral cancer patients. J Pathol 2011; 223:470-81; PMID:21294121; https://doi.org/ 10.1002/path.2830 [DOI] [PubMed] [Google Scholar]
- [5].Jechlinger M, Grunert S, Beug H. Mechanisms in epithelial plasticity and metastasis: insights from 3D cultures and expression profiling. J Mammary Gland Biol Neoplasia 2002; 7:415-32; PMID:12882526; https://doi.org/ 10.1023/A:1024090116451 [DOI] [PubMed] [Google Scholar]
- [6].Radisky DC. Epithelial-mesenchymal transition. J Cell Sci 2005; 118:4325-6; PMID:16179603; https://doi.org/ 10.1242/jcs.02552 [DOI] [PubMed] [Google Scholar]
- [7].Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial–mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol 2006; 172:973-81; PMID:16567498; https://doi.org/ 10.1083/jcb.200601018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest 2009; 119:1420-8; PMID:19487818; https://doi.org/ 10.1172/JCI39104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial–mesenchymal transition. Nat Rev Mol Cell Biol 2014; 15:178-96; PMID:24556840; https://doi.org/ 10.1038/nrm3758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hofman P, Vouret-Craviari V. Microbes-induced EMT at the crossroad of inflammation and cancer. Gut Microbes 2012; 3:176-85; PMID:22572828; https://doi.org/ 10.4161/gmic.20288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Binder Gallimidi A, Fischman S, Revach B, Bulvik R, Maliutina A, Rubinstein AM, Nussbaum G, Elkin M. Periodontal pathogens Porphyromonas gingivalis and Fusobacterium nucleatum promote tumor progression in an oral-specific chemical carcinogenesis model. Oncotarget 2015; 6:22613-23; PMID:26158901; https://doi.org/ 10.18632/oncotarget.4209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ha NH, Woo BH, Kim DJ, Ha ES, Choi JI, Kim SJ, Park BS, Lee JH, Park HR. Prolonged and repetitive exposure to Porphyromonas gingivalis increases aggressiveness of oral cancer cells by promoting acquisition of cancer stem cell properties. Tumour Biol 2015; 36:9947-60; PMID:26178482; https://doi.org/ 10.1007/s13277-015-3764-9 [DOI] [PubMed] [Google Scholar]
- [13].Wendt MK, Smith JA, Schiemann WP. Transforming Growth Factor-β-Induced Epithelial-Mesenchymal Transition Facilitates Epidermal Growth Factor-Dependent Breast Cancer Progression. Oncogene 2010; 29:6485-98; PMID:20802523; https://doi.org/ 10.1038/onc.2010.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Schröder NWJ, Morath S, Alexander C, Hamann L, Hartung T, Zähringer U, Göbel UB, Weber JR, Schumann RR. Lipoteichoic Acid (LTA) of Streptococcus pneumoniaeand Staphylococcus aureus Activates Immune Cells via Toll-like Receptor (TLR)-2, Lipopolysaccharide-binding Protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J Biol Chem 2003; 278:15587-94; PMID:12594207; https://doi.org/ 10.1074/jbc.M212829200 [DOI] [PubMed] [Google Scholar]
- [15].Socransky SS, Haffajee AD. Microbial mechanisms in the pathogenesis of destructive periodontal diseases: a critical assessment. J Periodontal Res 1991; 26:195-212; PMID:1831843; https://doi.org/ 10.1111/j.1600-0765.1991.tb01646.x [DOI] [PubMed] [Google Scholar]
- [16].Milward MR, Chapple IL, Wright HJ, Millard JL, Matthews JB, Cooper PR. Differential activation of NF-κB and gene expression in oral epithelial cells by periodontal pathogens. Clin Exp Immunol 2007; 148:307-24; PMID:17355248; https://doi.org/ 10.1111/j.1365-2249.2007.03342.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sahingur SE, Yeudall WA. Chemokine Function in Periodontal Disease and Oral Cavity Cancer. Frontiers Immunol 2015; 6:214; PMID:25999952; https://doi.org/ 10.3389/fimmu.2015.00214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ackland ML, Newgreen DF, Fridman M, Waltham MC, Arvanitis A, Minichiello J, Price JT, Thompson EW. Epidermal growth factor-induced epithelio-mesenchymal transition in human breast carcinoma cells. Lab Invest J Technical Methods Pathol 2003; 83:435-48; PMID:12649344; https://doi.org/ 10.1097/01.LAB.0000059927.97515.FD [DOI] [PubMed] [Google Scholar]
- [19].Zhang S, Wang X, Iqbal S, Wang Y, Osunkoya AO, Chen Z, Chen Z, Shin DM, Yuan H, Wang YA, et al.. Epidermal Growth Factor Promotes Protein Degradation of Epithelial Protein Lost in Neoplasm (EPLIN), a putative metastasis suppressor, during epithelial-mesenchymal transition. J Biol Chem 2013; 288:1469-79; PMID:23188829; https://doi.org/ 10.1074/jbc.M112.438341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lo HW, Hsu SC, Xia W, Cao X, Shih JY, Wei Y, Abbruzzese JL, Hortobagyi GN, Hung MC. Epidermal Growth Factor Receptor Cooperates with Signal Transducer and Activator of Transcription 3 to Induce Epithelial-Mesenchymal Transition in Cancer Cells via Up-regulation of TWIST Gene Expression. Cancer Res 2007; 67:9066-76; PMID:17909010; https://doi.org/ 10.1158/0008-5472.CAN-07-0575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Misra A, Pandey C, Sze SK, Thanabalu T. Hypoxia Activated EGFR Signaling Induces Epithelial to Mesenchymal Transition (EMT). PLoS One 2012; 7:e49766; PMID:23185433; https://doi.org/ 10.1371/journal.pone.0049766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Li CW, et al.. Epithelial-Mesenchyme transition induced by TNF-α requires NF-κB-mediated transcriptional upregulation of Twist1. Cancer Res 2012; 72:1290-300; PMID:22253230; https://doi.org/ 10.1158/0008-5472.CAN-11-3123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tepass U, Truong K, Godt D, Ikura M, Peifer M. Cadherins in embryonic and neural morphogenesis. Nat Rev Mol Cell Biol 2000; 1:91-100; PMID:11253370; https://doi.org/ 10.1038/35040042 [DOI] [PubMed] [Google Scholar]
- [24].Takkunen M, Grenman R, Hukkanen M, Korhonen M, García de Herreros A, Virtanen I. Snail-dependent and -independent epithelial-mesenchymal transition in oral squamous carcinoma cells. J Histochem Cytochem 2006; 54:1263-75; PMID:16899764; https://doi.org/ 10.1369/jhc.6A6958.2006 [DOI] [PubMed] [Google Scholar]
- [25].Smit MA, Geiger TR, Song JY, Gitelman I, Peeper DS. A Twist-Snail Axis Critical for TrkB-Induced Epithelial-Mesenchymal Transition-Like Transformation, Anoikis Resistance, and Metastasis. Mol Cell Biol 2009; 29:3722-37; PMID:19414595; https://doi.org/ 10.1128/MCB.01164-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Qiao B, Johnson N, Gao J. Epithelial-mesenchymal transition in oral squamous cell carcinoma triggered by transforming growth factor-b1 is Snail family-dependent and correlates with matrix metalloproteinase-2 and-9 expressions. Int J Oncol 2010; 37:663-8; PMID:20664935 [DOI] [PubMed] [Google Scholar]
- [27].Tang Y, Herr G, Johnson W, Resnik E, Aho J. Induction and analysis of epithelial to mesenchymal transition. JoVE (J Visual Exp) 2013; 78:e50478; https://doi.org/ 10.3791/50478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wang Y, Liu J, Ying X, Lin PC, Zhou BP. Twist-mediated Epithelial-mesenchymal Transition Promotes Breast Tumor Cell Invasion via Inhibition of Hippo Pathway. Scientific Reports 2016; 6:24606; PMID:27920433; https://doi.org/ 10.1038/srep24606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Doerner AM, Zuraw BL. TGF-β 1 induced epithelial to mesenchymal transition (EMT) in human bronchial epithelial cells is enhanced by IL-1β but not abrogated by corticosteroids. Respiratory Res 2009; 10:100; PMID:19857272; https://doi.org/ 10.1186/1465-9921-10-100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Tezal M, Sullivan MA, Reid ME, Marshall JR, Hyland A, Loree T, Lillis C, Hauck L, Wactawski-Wende J, Scannapieco FA. CHronic periodontitis and the risk of tongue cancer. Arch Otolaryngol Head Neck Surg 2007; 133:450-4; https://doi.org/ 10.1001/archotol.133.5.450 [DOI] [PubMed] [Google Scholar]
- [31].Prime SS, Nixon SV, Crane IJ, Stone A, Matthews JB, Maitland NJ, Remnant L, Powell SK, Game SM, Scully C. The behaviour of human oral squamous cell carcinoma in cell culture. J Pathol 1990; 160:259-69; PMID:1692339; https://doi.org/ 10.1002/path.1711600313 [DOI] [PubMed] [Google Scholar]
- [32].Dierickx K, Pauwels M, Van Eldere J, Cassiman JJ, Van Steenberghe D, Quirynen M. Viability of cultured periodontal pocket epithelium cells and Porphyromonas gingivalis association. J Clin Periodontol 2002; 29:987-96; PMID:12472991; https://doi.org/ 10.1034/j.1600-051X.2002.291103.x [DOI] [PubMed] [Google Scholar]
- [33].Kim J, Jeong H, Lee Y, Kim C, Kim H, Kim A. HRG-β1-driven ErbB3 signaling induces epithelial–mesenchymal transition in breast cancer cells. BMC Cancer 2013; 13:383-3; PMID:23937725; https://doi.org/ 10.1186/1471-2407-13-383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Schneider CA, Rasband WS, Eliceiri KW. NIH image to ImageJ: 25 years of image analysis. Nature Methods 2012; 9(7):671–5; https://doi.org/ 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Schaeffer D, Somarelli JA, Hanna G, Palmer GM, Garcia-Blanco MA. Cellular migration and invasion uncoupled: increased migration is not an inexorable consequence of epithelial-to-mesenchymal transition. Mol Cell Biol 2014; 34:3486-99; PMID:25002532; https://doi.org/ 10.1128/MCB.00694-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sume SS, Kantarci A, Lee A, Hasturk H, Trackman PC. Epithelial to mesenchymal transition in gingival overgrowth. Am J Pathol 2010; 177:208-218; PMID:20489142; https://doi.org/ 10.2353/ajpath.2010.090952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Paster BJ, Boches SK, Galvin JL, Ericson RE, Lau CN, Levanos VA, Sahasrabudhe A, Dewhirst FE. Bacterial diversity in human subgingival plaque. J Bacteriol 2001; 183:3770-83; PMID:11371542; https://doi.org/ 10.1128/JB.183.12.3770-3783.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Katz J, Onate MD, Pauley KM, Bhattacharyya I, Cha S. Presence of Porphyromonas gingivalis in gingival squamous cell carcinoma. Int J Oral Sci 2011; 3:209-15; PMID:22010579; https://doi.org/ 10.4248/IJOS11075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wu Y, Zhou BP. Snail: More than EMT. Cell Adhesion Migration 2010; 4:199-203; https://doi.org/ 10.4161/cam.4.2.10943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wang H, Wang HS, Zhou BH, Li CL, Zhang F, Wang XF, Zhang G, Bu XZ, Cai SH, Du J. Epithelial–Mesenchymal Transition (EMT) Induced by TNF-α Requires AKT/GSK-3β-mediated stabilization of snail in colorectal cancer. PLoS One 2013; 8:e56664; PMID:23431386; https://doi.org/ 10.1371/journal.pone.0056664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Liu R, Desta T, Raptis M, Darveau RP, Graves DT. Porphyromonas gingivalis and E. coli lipopolysaccharide exhibit different systemic but similar local induction of inflammatory markers. J Periodontol 2008; 79:1241-7; PMID:18597607; https://doi.org/ 10.1902/jop.2008.070575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest 2009; 119:1429-37; PMID:19487819; https://doi.org/ 10.1172/JCI36183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Batlle E, Sancho E, Francí C, Domínguez D, Monfar M, Baulida J, García De Herreros A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol 2000; 2:84-9; PMID:10655587; https://doi.org/ 10.1038/35000034 [DOI] [PubMed] [Google Scholar]
- [44].Scanlon CS, Van Tubergen EA, Inglehart RC, D'Silva NJ. Biomarkers of Epithelial-Mesenchymal transition in squamous cell Carcinoma. J Res 2013; 92:114-21; PMID:23128109; https://doi.org/ 10.1177/0022034512467352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Paccione RJ, Miyazaki H, Patel V, Waseem A, Gutkind JS, Zehner ZE, Yeudall WA. Keratin down-regulation in vimentin-positive cancer cells is reversible by vimentin RNA interference, which inhibits growth and motility. Am Association Cancer Res 2008; 7:2894-2903 [DOI] [PubMed] [Google Scholar]
- [46].Justus CR, Leffler N, Ruiz-Echevarria M, Yang LV. In vitro Cell Migration and Invasion Assays. J Vis Exp 2014; PMID:24962652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protocols 2007; 2:329-33; PMID:17406593; https://doi.org/ 10.1038/nprot.2007.30 [DOI] [PubMed] [Google Scholar]
- [48].Yoon Y, Liang Z, Zhang X, Choe M, Zhu A, Cho HT, Shin DM, Goodman MM, Chen ZG, Shim H. CXC Chemokine Receptor-4 Antagonist Blocks Both Growth of Primary Tumor and Metastasis of Head and Neck Cancer in Xenograft mouse models. Cancer Res 2007; 67:7518-24; PMID:17671223; https://doi.org/ 10.1158/0008-5472.CAN-06-2263 [DOI] [PubMed] [Google Scholar]


