ABSTRACT
Although it is known for long time that the peripheral nervous system has the capacity for self-regeneration, the molecular mechanisms by which Schwann cells and extracellular matrix (ECM) guide the injured axons to regrow along their original path, remains a poorly understood process. Due to the importance of ECM molecules during development, constitutive mutant organisms display increased lethality, therefore, conditional or inducible strategies have been used to increase the survival of the organisms and allow the study of the role of ECM proteins. In a recent report published in Neuron, Isaacman-Beck and colleagues (2015) used these pioneering genetic studies on zebrafish combined with in vivo fluorescent imaging, to investigate the micro-environmental conditions required for targeted regeneration of the dorsal motor nerve of zebrafish larvae after laser-transection. A candidate gene approach targeting lh3 basal laminar collagen substrates revealed that the lh3 substrate col4α5 regulates dorsal nerve regeneration by destabilizing misdirected axons. Col4α5 was upregulated in a small population of lh3 expressing Schwann cells located ventrally and ventro-laterally to the injury site and found to co-localize with the molecule slit guidance ligand 1 (slit1a). Capitalizing on the crucial observations of mistargeted regeneration of dorsal nerves in mutant larvae, they put forward a model in which Schwann cells shape an environment that allows and directs axonal regeneration to their original synaptic target. In the light of Isaacman-Beck and colleagues (2015) findings, we will review how their study contributes to the research field, and comment on its potential implications for promoting nerve regeneration after injury.
KEYWORDS: axonal guidance, collagen, dorsal motor nerve, extracellular matrix, lysyl hydroxylase-3, nerve regeneration, Slit1a, Schwann cells
Nerve regeneration in the peripheral nervous system
In the peripheral nervous system (PNS), axonal injury results in the loss of neural functions. Cellular and molecular mechanisms in the periphery contribute to target-specific neuronal regeneration aiming to restore function through proper axonal orientation and targeted innervation.33 After injury, the axon's distal segment undergoes Wallerian degeneration, the inflammatory response of the nervous system to axonal fracture, a process described initially by Augustus Volney Waller in 1850 on frog cranial nerves. This results in fragmentation and disintegration of the distal axonal segment, while the proximal segment is able to regenerate along the original path to the initial target. At the molecular level, both processes are regulated by the expression of apoptotic- or regeneration-associated genes.23 However, it is still under debate whether regenerating axons rely on environmental cues to guide them toward their original target27 and which cues are responsible for this process.
There is a large body of evidence pointing to the regenerative capacity of vertebrate PNS depending on glial cell response. For instance, previous studies demonstrated that after injury, Schwann cells acquire a regenerative phenotype. They proliferate, differentiate, start remyelination and secrete molecular guidance factors,2 generating an extrinsic environment that promotes axonal growth.36 These secreted guidance factors can act as repellents or attractants for regenerating axons by modulating adhesive and protective properties of the extracellular matrix (ECM). The ECM is composed by a mesh of fibrous proteins such as the collagen and glycosaminoglycans (e.g. heparan sulfate); all components are produced intracellularly by neighboring cells and secreted by exocytosis. The pioneering study by Ide and colleagues13 demonstrated that isolated axons from sciatic nerve preparations may regrow on a cell-free substrate, where Schwann cells were completely abolished by repetitive freezing and thawing. These ex vivo experiments pointed to the contribution of an ECM scaffold promoting axon regrowth. Indeed, ECM proteins, like collagens, provide important guiding cues for growing axons.17 After peripheral nerve injury, there is a long-lasting gene expression of collagen at the site of injury, and some collagen types are thought to provide mechanical support for the axonal regeneration, while the presence of others is associated with the axonal re-innervation.15 Before collagen is secreted, it undergoes post-translational modifications depending on its dedicated function. Propyl- and lysyl- hydroxylases (lh) are known to be critical for these early modifications of the precursor molecule of collagen (i.e. pro-collagen). In particular, lysyl hydroxylase 3 (lh3) is involved in the proper secretion, assembly and distribution of certain collagen types.30 Among them, collagen 4α5 (col4α5),11 col18α127 and col19α112 seem to be of specific importance after nerve injuries, since they are glycosylated by lh3 before secretion into the ECM and upregulated at the injury site. Therefore, lh3 contribution in axonal regeneration is evident.
In humans, lh3 is highly expressed in heart and pancreas, while only weak expression has been reported for the brain.34 In mice lh3 is widely expressed during embryogenesis, but its expression during adulthood displays cell- and tissue-specificity. In particular, it is mainly expressed in the heart, lung, liver and testis. In the brain, on the other hand, only a small amount was detectable and was located intracellularly in the pyramidal cells, while its specific role remains unknown.25,26 Regarding zebrafish, apart from lh3 being expressed in the peripheral glia, corresponding temporally with motor axon outgrowth,27 we are not aware of a role in the central nervous system (CNS).
The zebrafish (Danio rerio) larva has been proposed as an appropriate model system for studying vertebrate peripheral nerve regeneration and investigating the role of ECM components involved in this process. The main advantage over other basic model organisms such as the roundworm (Caenorhabditis elegans) or the fruit fly (Drosophila melanogaster) is that the zebrafish, being a vertebrate, shares a high percentage of genetic and functional homology with mammals. Compared to other vertebrate model organisms such as rodents, genetic modifications are much easier (not to mention cheaper and faster) to be introduced into the zebrafish genome, it is possible to control the critical developmental steps, and the external development of transparent embryos allows the real-time visualization. Furthermore, the gestation period is comparably shorter and the number of offspring higher compared with other vertebrate models. In addition to optimization of transgenic technology, the zebrafish's transparent skin at the larva stage facilitates in vivo fluorescent imaging of their relatively simple stereotypic architecture of spinal motor neurons. Pioneering genetic studies on zebrafish gave rise to transgenic fish with targeted mutations in various collagen types.35 However, constitutive mutants of ECM components, which are essential for development, display severe or even lethal phenotypes. To bypass lethality during the critical period of time, conditional or inducible strategies such as Gal4/UAS system10 or the properties of heat-shock proteins, as used by Isaacman-Beck et al.,14 have been used. In addition, technical advances in optical devices and genetic constructs with reporter proteins provided efficient ways for in vivo live-cell imaging to visualize the interaction of transgenically-labeled macrophages with injured peripheral axons.24 Together, the new imaging, genetic and transection techniques provide the tools for investigating the regenerative process of vertebrate peripheral axons in vivo.
How do regenerating neurons navigate to their target?
The aim of the team led by Michael Granato14 was to investigate the environmental conditions required for targeted regeneration. The authors performed laser-transection of the dorsal nerve in zebrafish, harboring the heat-inducible transgene Tg(hsp70l:lh3myc) encoding for a tagged lh3, without damaging its ventral counterpart (Fig. 1A) and followed its regeneration in vivo. They propose that axonal regeneration and migration to their original synaptic targets is guided by components of the ECM through the involvement of Schwann cells.
Figure 1.
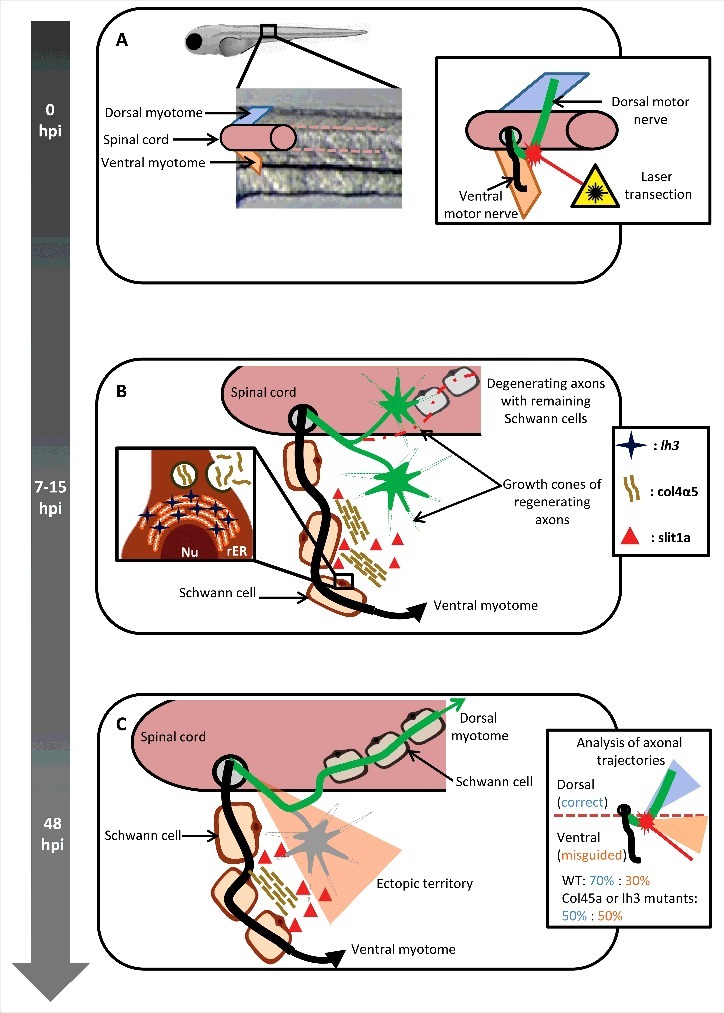
Peripheral glia regulates axonal regeneration. (A) The dorsal motor nerve (green), innervating the dorsal myotome, was laser-transected near the level of the spinal cord exit point, without affecting the integrity of ventral motor nerve (black), innervating the ventral myotome, in 5-day post fertilization zebrafish larvae. 0hpi: 0 hours post injury. (B) 7–15 hours post injury (hpi) the regenerating dorsal motor axons are actively probing the environment in search for their initial trajectories. Schwann cells proximal to the lesion site upregulate the expression of the glycosyltransferase lh3, a transmembrane protein located on the rough endoplasmatic reticulum (rER), resulting in increased secretion of collagen4α5 (col4α5). The repellent molecule slit1a was found to co-localize with the col4a5. Nu: nucleus. (C) 48 hours post injury the misdirected axons (gray) destabilize possibly due to the collagen4α5 dependent accumulation of slit1a, while the rest stabilize and grow toward their original trajectories.
Choosing the right path: Implication of peripheral glia and ECM for proper direction of the regenerating axons
To assess the mechanisms implicated in the regenerative capacity of the PNS, the authors14 relied on selectively fluorescent-labeling of dorsal and ventral motor nerve axons, which allows for in vivo live-cell imaging in transparent zebrafish larvae. Complete laser transection proximal to the diversion site of the dorsal and ventral motor nerve branch (Fig. 1A) revealed that 48 hours post-injury the majority of the regenerating axons grow back toward their initial trajectories. This was confirmed by a modified Sholl analysis19 which uses a circular analysis method, creating a series of concentric circles around the soma of the neuron, their intersection with neurites informs about directionality during axon outgrowth. This analysis revealed that in the first 12 hours sprouting axons and their growth cones acquire a probing behavior seeking for their correct path (Fig. 1B). While most of the extending sprouting axons collapsed, only those that probed dorsally succeeded to stabilize and grow, suggesting the implication of ECM cues at the injury site to promote growth and navigate these axons to their original target. To bypass the critical role of ECM candidates in development, a conditional heat-inducible mutant was used to restrict lh3 expression to early development, allowing testing of how regeneration proceeded in its absence. In this mutant, the dorsal motor axons were not able to grow properly to their original path.
Live-cell imaging of collagen mutant larvae, selected using a candidate gene approach targeting lh3 basal laminar collagen substrates, revealed that the lh3 substrate col4α5, but not col18α1 or col19α1, regulates dorsal nerve regeneration through destabilization of misdirected axons (Fig. 1C). Col4α5 was upregulated in a small population of Schwann cells expressing lh3 that were located ventrally and ventro-laterally to the injury site and found to co-localize with the molecule slit guidance ligand 1 (slit1a). This co-localization is not surprising as slit1a is known to act on robo-1 receptor as a growth cone repellent in vertebrates.3 To see whether lh3 expression could rescue axonal guidance defects in the mutants, Isaacman-Beck and colleagues used an inducible transgene expressing lh3 under the hsp70I heat-shock promoter, and shifted the water temperature from 28.5°C to 37°C, 6 hours after transection, to induce a robust ectopic overexpression of lh3. 48 hours later, this induced overexpression of lh3 had rescued the disoriented re-growth of the dorsal axon in lh3 mutants. Taken together, lh3 expression in peripheral glia is crucial for post-injury peripheral dorsal nerve regeneration, through modulation of col4α5.
A new mechanism uncovered but leaving shadows and burning questions
The authors14 used transgenic zebrafish lines for lh3, col4a5, cola18a1 and col19a1 and in vivo imaging techniques recently developed for application in the zebrafish transparent larva enabling them to put forward a model of target-selective regeneration. In this model, after transection, regenerating dorsal axons extensively probe their environment both ventrally and dorsally. Interestingly, the re-growing axons find their original dorsal trajectories by using ECM cues. They propose that ventrally located lh3-expressing Schwann cells regulate the levels of col4α5, which forms a complex with the repellent molecule slit1a, preventing ventral growth by destabilizing misdirected axons. However, despite their elegant demonstration of a potential mechanism for post-transection dorsal nerve regeneration in zebrafish, many questions remain to be elucidated.
For instance, the study emphasizes the need to invest more the function of col4α5 in comparative research. Indeed, while this protein facilitates nerve regeneration in zebrafish, it is linked with retinal, cochlear and renal function in mice, and col4α5 murine mutants exhibit glomerulonephritis, terminal renal dysfunction and hearing loss (a reminiscent of the Alport syndrome in humans).18 Similarly, although collagen IV subtypes α1 and α2 are ubiquitously distributed in all organs, subtypes α3, α4, α5 and α6 exhibit different spatiotemporal expression patterns and found mainly in renal basal membrane in mice.21 Additionally, in a recent report Chen et al.1 implicate col6α1, another ECM protein, in peripheral nerve regeneration of rodents. They proposed that after nerve injury, upregulation of col6α1 modulates nerve regeneration by recruitment of macrophages at the injury site. Complete col6α1 knockout mice display deficient macrophage migration with a shift to pro-inflammatory, rather than anti-inflammatory phenotype and delayed peripheral nerve regeneration. In zebrafish, col6α1 mutants, exhibit muscle disease attributed to abnormalities in mitochondrial function.31 Taken together, these findings question whether axonal regrowth falls under an evolutionary conserved regulation or is part of species-specific mechanisms.
Furthermore, the reported dorsal nerve specificity of this regenerative mechanism points to an interactive modulation by both external cues and an intrinsic neuronal program. Indeed, previous studies reported that col4α5 is implicated in axonal targeting5 which supports the findings of Isaacman-Beck et al.14 Similarly, regenerating axons are known to express receptors for attractant and repellant molecules as well as for collagens.4 Hence, whether this specificity is due to an innate difference in the molecules expressed on dorsal- and ventral-directed neurons or to the nature of the lesion (full or partial) triggering alternate signaling cascades in both neurons and peripheral glia directing axonal regeneration, remains unknown. Structural and molecular attractive cues secreted from the dorsal compartment might also contribute to target-specific growth. Indeed, Schwann cells of the transected and degenerating axon remain alive, forming a cellular chain (“bands of Bunger”) which provides structural guidance for the regenerating axon within and along the endoneurial tube. In addition, Schwann cells distal from the lesion site proliferate, and produce and secrete neurotrophic factors as well as adhesion molecules that promote axonal regeneration.6 Thus, it is likely that these molecules play a role in the orientation of regenerating axons acting as attractants, increasing system's complexity and precision.
In addition, we need to shed more light onto the contribution, of an organism's innate immune system, acting as a homeostatic regulator, in axonal regeneration. Earlier in vivo studies of Granato's group demonstrated macrophage infiltration into the lesion site.24 After PNS trauma the expected cytokine-mediated inflammatory response at the injury site results in the recruitment of peripheral macrophages. Indicatively, denervated Schwann cells produce high levels of leukemia inhibitory factor, interleukin (IL)-1α, IL-1β and monocyte chemoattractant protein-1, molecules that attract macrophages and augment myelin phagocytosis.28,32 Macrophages display remarkable plasticity and depending on the activating stimulus they adopt pro- or anti-inflammatory phenotypes. A pro-inflammatory phenotype is mainly linked to immune response, whereas an anti-inflammatory phenotype exhibits immunosuppressive properties and facilitates tissue repair and remodeling. At the injury site, macrophages normally secrete immunosuppressive cytokines (i.e., IL-10) and chemokines (i.e., chemokine ligand 17 and ligand 18) that increase phagocytic receptors, upregulate ECM components and growth factors (i.e., neurotrophin -3, 4, 5 and brain-derived neurotrophic factor).22,7 Especially for the ECM molecules, macrophages are able to internalize collagen,20 while IL-4 -one of the first innate signals after tissue injury- stimulates arginase activity in macrophages, an enzyme that promotes the conversion of arginine to ornithine, a precursor of polyamines and collagen, thereby contributing to ECM remodeling16 and possibly assisting to nerve regeneration. Indeed, Goren et al.8 reported that macrophage-depleted mice revealed severely impaired wound morphology and delayed healing. Hence, selective transplantation of macrophages deficient to factors that might contribute to axonal regeneration, at the nerve injury site of macrophage-depleted mice may shed more light on the interaction between the innate immune system and nerve repair.
Furthermore, in the light of Isaacman-Beck et al.'s14 findings, one cannot avoid considering what insight they may provide for CNS regeneration research. Even though CNS nerves are known for their limited ability to regenerate, it has been shown that implanted Schwann cells facilitate rat spinal cord regeneration.36 Taken together this observation and the strategy proposed in Isaacman-Beck et al.'s14 study, might be a further step to study and maybe enhance axonal regrowth, guidance and regeneration in the CNS, by inducing lh3 or similar molecules expression in oligodendrocytes.
Finally, the potential clinical applications of Isaacman-Beck et al.'s14 research are worth commenting on. Surgical approaches following peripheral nerve injury have until recently used autologous nerve grafts to enhance neural repair, however, the risk is high for transplant rejection or malignant growth (for a review see: 9). A new approach may now target the development of artificial grafts which are composed of a physical scaffold comprised by support cells, growth factors and molecules naturally found in the ECM.29 The study of Isaacman-Beck et al.14 provides fundamental and valuable insight into some of the molecular pathways implicated in vertebrate peripheral nerve injury.
Abbreviations
- CNS
central nervous system
- col4α5
collagen4α5
- col18α1
collagen18α1
- col19α1
collagen19α1
- col6α1
collagen6α1
- ECM
extracellular matrix
- lh
lysyl hydroxylases
- PNS
peripheral nervous system
- Robo1/slit1a
slit guidance ligand 1
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgment
We thank Dr. Dominique Bagnard for his guidance and for his thoughtful comments.
Funding
The Joint Master in Neuroscience is supported by NEUREX (www.neurex.org) and by a specific IDEX (Excellence Initiative) pedagogy grant from the University of Strasbourg.
References
- [1].Chen P, Cescon M, Zuccolotto G, Nobbio L, Colombelli C, Filaferro M, Vitale G, Feltri ML, Bonaldo P. Collagen VI regulates peripheral nerve regeneration by modulating macrophage recruitment and polarization. Acta Neuropathol 2015; 129:97-113. [DOI] [PubMed] [Google Scholar]
- [2].Chen ZL, Yu WM, Strickland S. Peripheral regeneration. Annu rev Neurosci 2007; 30:209-33. [DOI] [PubMed] [Google Scholar]
- [3].Devine CA, Key B. Robo-Slit interactions regulate longitudinal axon pathfinding in the embryonic vertebrate brain. Dev Biol 2008; 313(1):371-83; PMID:18061159 [DOI] [PubMed] [Google Scholar]
- [4].Fawcett JW, Keynes RJ. Peripheral nerve regeneration. Annu Rev Neurosci 1990; 13:43-60; PMID:2183684 [DOI] [PubMed] [Google Scholar]
- [5].Fox MA. Novel roles for collagens in wiring the vertebrate nervous system. Curr Opin Cell Biol 2008; 20(5):508-13; PMID:18573651 [DOI] [PubMed] [Google Scholar]
- [6].Fu SY, Gordon T. The cellular and molecular basis of peripheral nerve regeneration. Mol Neurobiol 1997; 14(1-2):67-116; PMID:9170101 [DOI] [PubMed] [Google Scholar]
- [7].Galli SJ, Borregaard N, Wynn TA. Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat Immunol 2011; 12:1035-44; PMID:22012443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Goren I, Allmann N, Yogev N, Schürmann C, Linke A, Holdener M, Waisman A, Pfeilschifter J, Frank S. A transgenic mouse model of inducible macrophage depletion: effects of diphtheria toxin-driven lysozyme M-specific cell lineage ablation on wound inflammatory, angiogenic, and contractive processes. Am J Pathol 2009; 175(1):132-47; PMID:19528348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Grinsell D, Keating CP. Peripheral nerve reconstruction after injury: a review of clinical and experimental therapies. Biomed Res Int 2014; 2014:698256; PMID:25276813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Halpern ME, Rhee J, Goll MG, Akitake CM, Parsons M, Leach SD. Gal4/UAS transgenic tools and their application to zebrafish. Zebrafish 2008; 5(2):97-110; PMID:18554173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Heikkinen J, Risteli M, Wang C, Latvala J, Rossi M, Valtavaara M, Myllylä R. Lysyl hydroxylase 3 is a multifunctional protein possessing collagen glucosyltransferase activity. J Biol Chem 2000; 275(46):36158-63; PMID:10934207 [DOI] [PubMed] [Google Scholar]
- [12].Hilario JD, Wang C, Beattie CE. Collagen XIXa1 is crucial for motor axon navigation at intermediate targets. Development 2010; 137(24):4261-9; PMID:21098567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ide C, Tohyama K, Yokota R, Nitatori T, Onodera S. Schwann cell basal lamina and nerve regeneration. Brain Res 1983; 288(1-2):61-75; PMID:6661636 [DOI] [PubMed] [Google Scholar]
- [14].Isaacman-Beck J, Schneider V, Franzini-Armstrong C, Granato M. The lh3 glycosyltransferase directs target-selective peripheral nerve regeneration. Neuron 2015; 88(4):691-703; PMID:26549330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Koopmans G, Hasse B, Sinis N. The role of collagen in peripheral nerve repair. Int Rev Neurobiol 2009; 87:363-79; PMID:19682648 [DOI] [PubMed] [Google Scholar]
- [16].Kreider T, Anthony RM, Urban JF Jr, Gause WC. Alternatively activated macrophages in helminth infections. Curr Opin Immunol 2007; 19:448-53; PMID:17702561; https://doi.org/ 10.1016/j.coi.2007.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kubo T, Yamashita T, Yamaguchi A, Hosokawa K, Tohyama M. Analysis of genes induced in peripheral nerve after axotomy using cDNA microarrays. J Neurochem 2002; 82:1129-36; PMID:12358760; https://doi.org/ 10.1046/j.1471-4159.2002.01060.x [DOI] [PubMed] [Google Scholar]
- [18].Kuo DS, Labelle-Dumais C, Gould DB. COL4A1 and COL4A2 mutations and disease: insight into pathogenic mechanisms and potential therapeutic targets. Hum Mol Genet 2012; 21:R97-R110; PMID:22914737; https://doi.org/ 10.1093/hmg/dds346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Li GN, Hoffman-Kim D. Evaluation of neurite outgrowth anisotropy using a novel application of circular analysis. J Neurosci Methods 2008; 174(2):202-14; PMID:18674559; https://doi.org/ 10.1016/j.jneumeth.2008.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Madsen DH, Leonard D, Masedunskas A, Moyer A, Jürgensen HJ, Peters DE, Amornphimoltham P, Selvaraj A, Yamada SS, Brenner DA, et al.. M2-like macrophages are responsible for collagen degradation though a mannose receptor-mediated pathway. J Cell Biol 2013; 6:951-66; https://doi.org/ 10.1083/jcb.201301081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Miner JH, Sanes JR. Collagen IV alpha 3, alpha 4, and alpha 5 chains in rodent basal laminae: sequence, distribution, association with laminins, and developmental switches. J Cell Biol 1994; 127:879-91; PMID:7962065; https://doi.org/ 10.1083/jcb.127.3.879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008; 8:958-69; PMID:19029990; https://doi.org/ 10.1038/nri2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Raivich G, Bohatschek M, Da Costa C, Iwata O, Galiano M, Hristova M, Nateri AS, Makwana M, Riera-Sans L, Wolfer DP, et al.. The AP-1 transcription factor c-Jun is required for efficient axonal regeneration. Neuron 2004; 43:57-67; PMID:15233917; https://doi.org/ 10.1016/j.neuron.2004.06.005 [DOI] [PubMed] [Google Scholar]
- [24].Rosenberg AF, Wolman MA, Franzini-Armstrong C, Granato M. In vivo nerve-macrophage interactions following peripheral nerve injury. J Neurosci 2012; 32:3898-909; PMID:22423110; https://doi.org/ 10.1523/JNEUROSCI.5225-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ruotsalainen H, Sipilä L, Kerkelä E, Pospiech H, Myllylä R. Characterization of cDNAs for mouse lysyl hydroxylase 1, 2, and 3, their phylogenetic analysis and tissue-specific expression in the mouse. Matrix Biol 1999; 18:325-9; PMID:10429951; https://doi.org/ 10.1016/S0945-053X(99)00016-5 [DOI] [PubMed] [Google Scholar]
- [26].Salo AM, Sipilä L, Sormunen R, Ruotsalainen H, Vainio S, Myllylä R. The lysyl hydroxylase isoforms are widely expressed during mouse embryogenesis, but obtain tissue- and cell-specific patterns in the adult. Matrix Biol 2006; 25:475-83; PMID:16996725; https://doi.org/ 10.1016/j.matbio.2006.08.260 [DOI] [PubMed] [Google Scholar]
- [27].Schneider VA, Granato M. Genomic structure and embryonic expression of zebrafish lysyl hydroxylase 1 and lysyl hydroxylase 2. Matrix Biol 2006; 26(1):12-9; PMID:17056240; https://doi.org/ 10.1016/j.matbio.2006.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Shamash S, Reichert F, Rotshenker S. The cytokine network of Wallerian degeneration: tumor necrosis factor-alpha, interleukin-1alpha, and interleukin-1beta. J Neurosci 2002; 22:3052-60; PMID:11943808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Shen ZL, Berger A, Hierner R, Allmeling C, Ungewickell E, Walter GF. A Schwann cell-seeded intrinsic framework and its satisfactory biocompatibility for a bioartificial nerve graft. Microsurgery 2001; 21(1):6-11. [DOI] [PubMed] [Google Scholar]
- [30].Sipilä L, Ruotsalainen H, Sormunen R, Baker NL, Lamandé SR, Vapola M, Wang C, Sado Y, Aszodi A, Myllylä R. Secretion and assembly of type IV and VI collagens depend on glycosylation of hydroxylysines. J Biol Chem 2007; 282(46):33381-8; https://doi.org/ 10.1074/jbc.M704198200 [DOI] [PubMed] [Google Scholar]
- [31].Tefler WR, Busta AS, Bonnemann CG, Feldman EL, Dowling JJ. Zebrafish models of collagen VI-related myopathies. Hum Mol Genet 2010; 19:2433-44; https://doi.org/ 10.1093/hmg/ddq126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tofaris GK, Patterson PH, Jessen KR, Mirsky R. Denervated Schwann cells attract macrophages by secretion of leukemia inhibitory factor (LIF) and monocyte chemoattractant protein-1 in a process regulated by interleukin-6 and LIF. J Neurosci 2002; 22:6696-703; PMID:12151548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Valero-Cabré A, Navarro X. Functional impact of axonal misdirection after peripheral nerve injuries followed by graft or tube repair. J Neurotrauma 2002; 19(11):1475-85; PMID:12490012; https://doi.org/ 10.1089/089771502320914705 [DOI] [PubMed] [Google Scholar]
- [34].Valtavaara M, Szpirer C, Szpirer J, Myllylä R. Primary structure, tissue distribution, and chromosomal localization of a novel isoform of lysyl hydroxylase (lysyl hydroxylase 3). J Biol Chem 1998; 273:12881-6; PMID:9582318 [DOI] [PubMed] [Google Scholar]
- [35].Xiao T, Baier H. Lamina specific axonal projections in the zebrafish tectum require the type IV collagen Dragnet. Nat Neurosci 2007; 10:1529-37; PMID:17982451; https://doi.org/ 10.1038/nn2002 [DOI] [PubMed] [Google Scholar]
- [36].Xu XM, Chen A, Guénard V, Kleitman N, Bunge MB. Bridging Schwann cell transplants promote axonal regeneration from both the rostral and caudal stumps of transected adult rat spinal cord. J Neurocytol 1997; 26(1):1-16; PMID:9154524; https://doi.org/ 10.1023/A:1018557923309 [DOI] [PubMed] [Google Scholar]


