ABSTRACT
Strategies to improve retinal progenitor cell (RPC) capacity to yield proliferative and multipotent pools of cells that can efficiently differentiate into retinal neurons, including photoreceptors, could be vital for cell therapy in retinal degenerative diseases. In this study, we found that insulin-like growth factor-1 (IGF-1) plays a role in the regulation of proliferation and differentiation of RPCs. Our results show that IGF-1 promotes RPC proliferation via IGF-1 receptors (IGF-1Rs), stimulating increased phosphorylation in the PI3K/Akt and MAPK/Erk pathways. An inhibitor experiment revealed that IGF-1-induced RPC proliferation was inhibited when the PI3K/Akt and MAPK/Erk pathways were blocked. Furthermore, under the condition of differentiation, IGF-1-pretreated RPCs prefer to differentiate into retinal neurons, including photoreceptors, in vitro, which is crucial for visual formation and visual restoration. These results demonstrate that IGF-1 accelerates the proliferation of RPCs and IGF-1 pretreated RPCs may have shown an increased potential for retinal neuron differentiation, providing a novel strategy for regulating the proliferation and differentiation of retinal progenitors in vitro and shedding light upon the application of RPCs in retinal cell therapy.
KEYWORDS: Retinal progenitor cell (RPC), proliferation, differentiation, insulin-like growth factor-1 (IGF-1), signal pathway
Introduction
Retinal degenerative diseases, including age-related macular degeneration (AMD) and retinitis pigmentosa (RP), are severe blinding lesions and represent serious threats to human health [1]. So far, there is no effective therapy to cure these patients, although stem cell transplantation therapy is a solution that has been proposed in recent years and represents a novel strategy for the treatment of such diseases [2,3].
Retinal progenitor cells (RPCs), which are a type of multi-potential progenitor cell, are isolated from the retina. RPCs not only maintain their ability to self-renew but also maintain multi-directional differentiation potential [4,5]. In the process of early embryonic development, RPCs can differentiate in a specific order to successively produce ganglion cells, amacrine cells, cones cells, horizontal cells, bipolar cells, rod cells and Müller cells [6]. Furthermore, RPCs, a type of seed cell, have been considered for use in cell therapy in retinal degenerative diseases, which has brought hope to patients [2,7]. However, the effective expansion of RPCs and their directional differentiation to retinal neurons, including photoreceptors, have been proven difficult and remain a serious challenge. First, RPCs are relatively difficult to obtain, and they can only be amplified a few generations in vitro, and therefore, the amount of proliferative seed cells and their differentiation capability cannot meet the clinical application needs. Second, RPCs prefer to differentiate into glial cells in vitro rather than retinal neurons, which are more important for visual formation and visual restoration [8]. Many efforts have been dedicated to extending the capacity of proliferation and differentiation toward retinal neurons, such as improvements in RPC isolation methods, changes to the culture media and the application of a culturing carrier [9-13]. It was reported that epidermal growth factor (EGF), a cytokine widely used in the culture medium of RPCs, exerts specific influence on the proliferation and differentiation potential of RPCs to promote gliogenesis [14,15]. Therefore, we questioned that whether there are alternative culture conditions that may better accelerate RPC proliferation and regulate RPC differentiation to generate neurons more effectively. In our efforts to improve this capacity, we observed that IGF-1 may a promising growth factor to refine the proliferation and differentiation potential of RPCs.
IGF-1 is a well-known growth factor composed of 70 single amino acids. It was originally obtained in the human serum by Rinderkencth in 1976 [16]. IGF-1 is a multi-function regulator of cell proliferation. Previous studies have proven that IGF-1 promotes the proliferation of a wide variety of cell types, such as mesenchymal stem cells, embryonic cortical progenitors, and neural stem/progenitor cells, etc. [17–19]. The central nervous system is one of the targets of IGF-1, and the principal effect of IGF-1 in the central nervous system is exerted on the proliferation and differentiation of brain neural progenitors [20]. However, whether IGF-1 has any effects on the proliferation and differentiation of RPCs remains unknown.
In this study, the role of IGF-1 on the proliferation and differentiation potential of RPCs was investigated. Our results showed that IGF-1 is an efficient cytokine that promotes RPC proliferation through IGF-1R, and this effect depends on the phosphorylation of PI3K/Akt and MAPK/Erk signaling cascades. In addition, IGF-1-pretreated RPCs preferentially differentiated into retinal neuronal cells compared to EGF-pretreated RPCs. These results indicate that IGF-1 plays a positive role in governing RPC proliferation and differentiation.
Results
IGF-1 promotes RPC proliferation
As previously described, RPCs were isolated from the fresh retina of postnatal day 1 GFP-transgenic C57BL/6 mice [21]. We identified that more than 80% of cells in the RPC cultures were positive for Nestin (a general marker for retinal progenitor cells) and Vimentin (a marker for retinal progenitor cells) expression (data not shown). These results are consistent with previous reports [21]. Accumulating studies have reported that IGF-1 can promote proliferation of a variety of cells, such as mesenchymal stem cells, embryonic cortical progenitor cells, and neural stem/progenitor cells [22–25], but its effect on RPC proliferation has not been reported. A Cell Counting Kit-8 (CCK-8 assay) was used to evaluate RPC proliferation. When exogenous IGF-1 (20 ng / ml) was added to the cultures, the O.D. value was increased markedly at day 1 and day 3 compared with the cultures without IGF-1 treatment (EGF group), which means that IGF-1 can accelerate the proliferation of RPCs (Fig. 1A). To investigate the effects of different concentrations of IGF-1 on the proliferation of RPCs, cells were cultured in medium containing 20 ng/ml EGF (Control) or 20 ng/ml EGF plus different concentrations of IGF-1 (10 ng/ml, 20 ng/ml, 50 ng/ml). According to the CCK8 assay, the expansion capacity of the RPCs was gradually enhanced in accordance with the concentrations of IGF-1 varying from 10 ng/ml to 50 ng/ml. Nevertheless, when the concentration increased to 50 ng/ml, the promotion effects by IGF-1 on RPC proliferation do not increase significantly compared to the cells treated with 20 ng/ml IGF-1 (Fig. 1B). Hence, a concentration of 20 ng/ml IGF-1 was chosen for further study. In addition, our results also indicate that IGF-1 cannot sustain RPC proliferation without EGF (Fig. 1A, Fig. S1A). Furthermore, the qPCR results show that the mRNA expression levels of Ki-67 in the cells cultured with the IGF-1-supplemented medium was increased compared to the cells in the control group (Fig. 1C). To assess the effect of IGF-1 on the ability of RPCs to form cell spheres, cells were cultured with EGF plus IGF-1 or cultured with EGF as a normal control for 1 day and 3 days respectively. The areas of cell clusters (the green fluorescence) in the IGF-1+ EGF group were larger than the RPC clusters in the EGF group (Fig. 1D), and this result was quantified in Fig. 1E. Moreover, Ki-67 immunocytochemistry and 5-bromodeoxyuridine (BrdU) analysis were also used to evaluate the proliferation of RPCs. The results show that the ratios of Ki-67 (81.3 ± 5.8% and 67.1 ± 6.3% for the IGF-1+EGF group and EGF group, respectively) and BrdU-positive (67.0 ± 5.2% and 45.4 ± 3.8% for the IGF-1+EGF group and EGF group, respectively) cells were increased in the IGF-1+EGF group compared to the EGF group (Fig. 1F-I). Taking together, IGF-1 is capable of promoting RPC proliferation.
Figure 1.
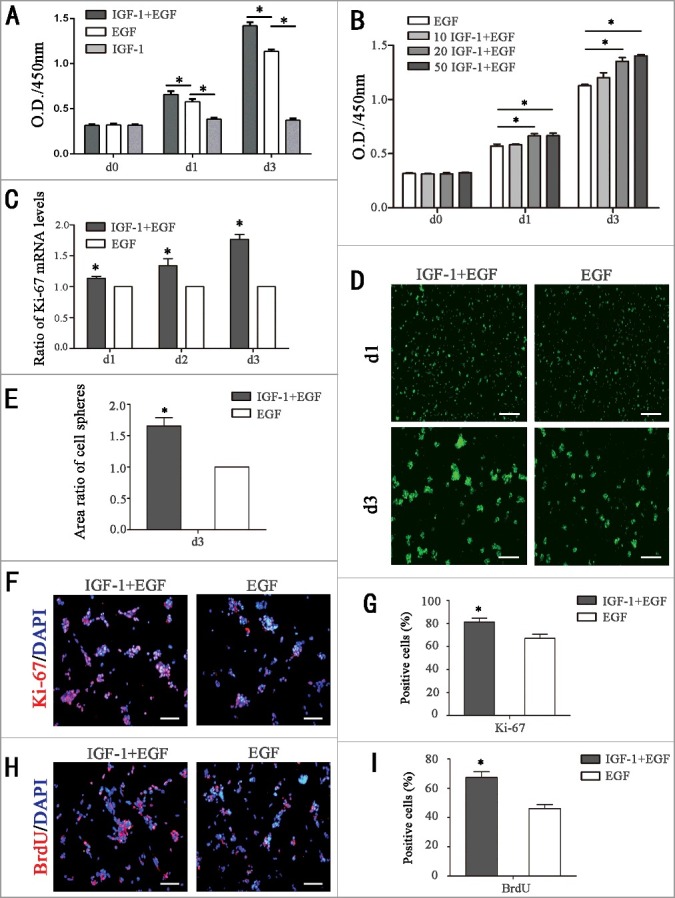
IGF-1 promotes RPC proliferation. (A) The CCK-8 analysis of RPC proliferation in IGF-1 plus EGF group, EGF group and IGF-1 group. The O.D.450 values at day 1 and day 3 were significantly increased in the IGF-1+EGF-treated group compared to the EGF group and only IGF-1 treatment could not maintain the proliferation of RPCs. (B) Detection of the effect of different concentrations of IGF-1 on RPC proliferation by CCK-8 analysis. (C) The qPCR results showed that the mRNA expression level of Ki67 in the IGF-1 plus EGF cultures was higher than that of the EGF group at day1, day2, and day3. (D-E) Fluorescent micrographs of the GFP+ RPCs cultured in IGF-1 plus EGF or EGF for the indicated times. The cell clusters (the green fluorescence) in the IGF-1+EGF cultures were larger than the RPC clusters in the EGF cultures at day 3. (F-I) The immunocytochemistry analysis of the percentages of Ki-67-positive cells and BrdU-positive cells in the RPC cultures treated with IGF-1 plus EGF or EGF showed that the percentages were markedly increased in the RPC cultures treated with IGF-1 plus EGF compared to the EGF group. *P ≤ 0.05 (Student's t-test and ANOVA test). Scale bars: D: 200 μm; F-H: 100 μm.
IGF-1 promotes RPC proliferation by targeting the PI3K/Akt and MAPK/Erk signaling pathways through membrane receptor IGF-1R
To explore whether IGF-1R was involved in the progress of stimulating RPC proliferation, a series of experiments were performed. Our CCK-8 results revealed that the promotion of RPC expansion induced by IGF-1 was weakened, and the O.D. value was backed to EGF group level after blocking the IGF-1Rs by NVP-ADW-742 (NVP) (the chemical inhibitor of IGF-1R) (Fig. 2A). On the other hand, CCK-8 results of EGF group and EGF plus NVP group also indicated that NVP had no influence on the proliferation of RPCs cultured with EGF (Fig. 2A). The qPCR and Ki-67 immunocytochemistry results also indicated that the acceleration of RPC proliferation was attenuated when IGF-1R was blocked by NVP (Fig. 2B-D). The results show the ratios of Ki-67-positive (81.1 ± 6.6%, 66.2 ± 4.8% and 64.2 ± 5.2% for the IGF-1+EGF group, IGF-1 plus NVP group and EGF group, respectively) cells at the time point of day 3 (Fig. 2C-D). These findings indicate that IGF-1 enhances the proliferation of RPCs by activating IGF-1R. Moreover, we also explored the downstream pathways to identify the mechanisms involved the promotion of RPC proliferation by IGF-1. It is well-known that PI3-kinase/Akt and MAPK/Erk cascades are the two main signaling pathways involved in cell proliferation. Thus, Western blot was performed to examine the effects of IGF-1 and EGF on Akt (Ser473) and Erk1/2 phosphorylation in RPC cultures. The RPCs were not treated (No treatment group) or were treated with EGF (EGF group) or IGF-1 plus EGF (EGF+IGF-1 group) for 20 min, and the protein extracts were assessed by Western blot analysis using antibodies against specific phosphorylated residues (p-Akt and p-Erk) or the total protein (Akt and Erk). The results showed that IGF-1+EGF caused stronger phosphorylation of Akt and Erk (Fig. 2E-H). Our results exhibit that EGF induced robust Erk phosphorylation while little Akt phosphorylation in comparison with the no treatment group (Fig. 2E-H). The IGF-1 plus EGF group exhibited prominent induction of both Erk and Akt phosphorylation compared to the no treatment group, and the IGF-1 plus EGF exhibited more remarkable phosphorylation of Akt and Erk compared to the EGF group (Fig. 2E-H). In addition, the effects of EGF plus IGF-1 on promoting Akt and Erk phosphorylation were attenuated when IGF-1R membrane receptors were inhibited by NVP (Fig. 2I-J), indicating that Akt and Erk were downstream of IGF-1R. Moreover, the result also shows that when the IGF-1R transmembrane receptors were inhibited by NVP, the already up-regulated level of Akt and Erk phosphorylation was attenuated back to the levels equivalent to the EGF group (Fig. 2I-J), which suggests that it is IGF-1 that results in stronger phosphorylation of Akt and Erk.
Figure 2.
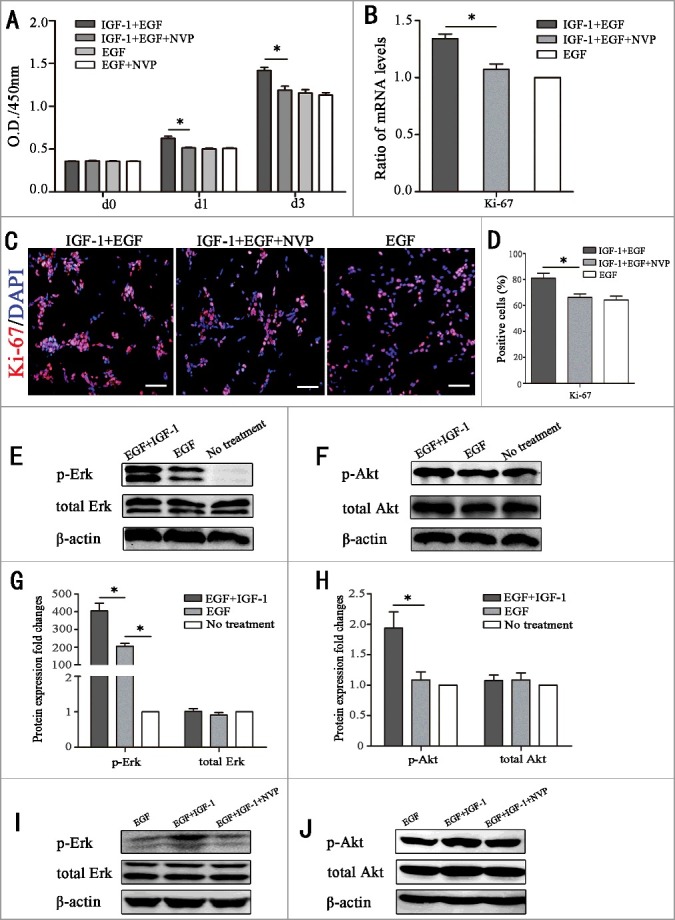
IGF-1 promotes the proliferation of RPCs through the membrane receptor IGF-1R by targeting the AKT and Erk signaling pathways. (A) The CCK-8 analysis of RPC proliferation in EGF+IGF-1 plus NVP-ADW-742 (chemical inhibitors of IGF-1, NVP), EGF+IGF-1, EGF and EGF+NVP. The O.D.450 values on day 1 and day 3 were remarkably decreased in the IGF-1+EGF plus NVP group compared to the IGF-1+EGF group. And the O.D.450 values on day 1 and day 3 had no statistical difference between EGF group and EGF group plus NVP. (B) Ki-67 qPCR expression of RPC proliferation in IGF-1+EGF cultures, IGF-1+EGF cultures with NVP, and EGF cultures. The results showed that the mRNA expression level of Ki67 in the IGF-1+EGF plus NVP cultures was lower than that of the IGF-1+EGF group, but there is no significant difference between IGF-1+EGF plus NVP group and EGF group. (C-D) The immunocytochemistry analysis of the percentages of Ki-67-positive cells in the RPC cultures treated with IGF-1+EGF plus NVP, IGF-1+EGF and EGF. The percentages of positive cells were decreased in the RPC cultures treated with IGF-1+EGF plus NVP compared to the IGF-1+EGF group, but there is no significant difference between IGF-1+EGF plus NVP group and EGF group. (E-H) The RPCs were not treated (no treatment group) or were treated with EGF or IGF-1 plus EGF for 20 min, and the protein extracts were assessed by Western blot analysis using antibodies against specific phosphorylated residues (p-Akt and p-Erk) or the total protein (Akt and Erk). The results showed that IGF-1 caused stronger phosphorylation of Akt and Erk. (I-J) The RPCs were stimulated with IGF-1 (20 ng/mL) in the presence of NVP, and the promotion phosphorylation of Akt and Erk caused by IGF-1 were attenuated by NVP. * P ≤ 0.05 (ANOVA test). Scale bar: 100 μm.
Further, to investigate whether the promoting effects of IGF-1 on RPC proliferation were correlated with phosphorylation of the PI3K/Akt and MAPK/Erk pathways, LY294002 (the chemical inhibitor of PI3K/Akt) and PD98059 (the chemical inhibitor of MAPK/Erk) were add to the IGF-1 plus EGF or EGF cultures, respectively. CCK-8 results revealed that the O.D. value decreased markedly when LY294002 or PD98059 added to cultures with IGF-1 plus EGF, which indicates that both the PI3K/Akt and MAPK/Erk pathways participate in IGF-1 plus EGF induced RPC proliferation (Fig. 3A). However, with regard to the EGF group, the O.D. value had no statistical change when LY294002 was added, while PD98059 can significantly reduce EGF-induced RPC proliferation, which suggests that only MAPK/Erk phosphorylation is correlated with EGF-induced RPC proliferation (Fig. 3B). In combination of the above-mentioned results, we can induce the role of PI3K/Akt and MAPK/Erk phosphorylation on IGF-1-induced RPC proliferation: on one hand, Akt phosphorylation must be correlated with IGF-1-induced proliferation of RPCs because the O.D. value had no statistical change when LY294002 was added to the EGF group, while the O.D. value markedly decreased with the LY294002 in EGF plus IGF-1 group; on the other hand, MAPK/Erk phosphorylation was also correlated with IGF-1-induced RPC proliferation because, although PD98059 had an important effect in weakening RPC proliferation in the EGF group (impairing RPC proliferation to the extent of 20%), PD98059 impaired the proliferation of RPCs to a larger extent in the EGF plus IGF-1 group (impairing RPC proliferation by 40%), which indicates that the part of the IGF-induced proliferation was also partly inhibited by PD98059, and thus, MAPK/Erk phosphorylation must also be correlated with IGF-1-induced RPC proliferation. In addition to the CCK8 analysis, qPCR and immunocytochemistry analyses of the marker Ki-67 were also performed, and the results are consistent with the CCK-8 results. When LY294002 or PD98059 was added to cultures with IGF-1 plus EGF, the Ki-67 mRNA expression level and the ratio of Ki-67 immunocytochemistry positive cells decreased, whereas in EGF cultures, the Ki-67 mRNA expression level and the ratio of Ki-67 immunocytochemistry positive cells decreased only when PD98059 was added (Fig. 3C-H).
Figure 3.
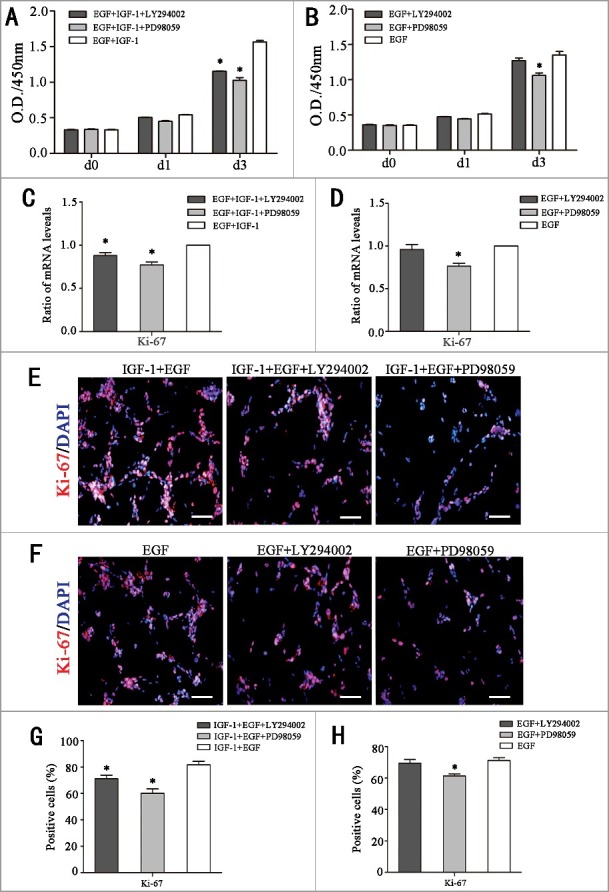
IGF-1 promotes RPC proliferation by targeting the PI3K/Akt and MAPK/Erk signaling pathways. (A-B) RPCs were stimulated with IGF-1 plus EGF or EGF in the presence of LY294002 (chemical inhibitor of Akt) or PD98059 (chemical inhibitor of Erk), and the RPCs’ growth was measured by CCK-8 analysis. Both LY294002 and PD98059 can weaken RPC proliferation, while only PD98059 can reduce EGF-1-induced RPC proliferation instead of LY294002. (C-D) Detection of the mRNA levels of Ki-67 in the RPCs that were treated with IGF-1 plus EGF or EGF in the presence of LY294002 or PD98059. The results showed that IGF-1+EGF-induced mRNA levels of Ki-67 can be reduced by both LY294002 and PD98059 while EGF-induced mRNA levels of Ki-67 only can be reduced by PD98059. (E-H) Ki-67 immunocytochemistry results are in accordance with the mRNA levels of Ki-67: an IGF-1+EGF-induced positive cell ratio of Ki-67 can be reduced by both LY294002 and PD98059, while an EGF-induced positive cell ratio of Ki-67 only can be reduced by PD98059. * P ≤ 0.05 (ANOVA test). Scale bar: 100 μm
From the above results we found that, in comparison to EGF, the addition of IGF-1 caused stronger phosphorylation of Akt and Erk through the IGF-1R membrane receptor, which may account for the reason why the addition of IGF-1 can better promote RPC proliferation.
IGF-1 can keep RPCs undifferentiated state in proliferation condition
As a type of progenitor cells, RPCs maintain their undifferentiated state in the proliferation phase. The qPCR, Western blot and immunocytochemistry were used to check whether IGF-1 changes the undifferentiated state of RPCs. The qPCR (Fig. 4A) showed the mRNA expression levels of Nestin, Vimentin, Pax6 and Chx10 (a cell autonomous regulator of controlling neurogenesis in the primordial retina), and Western blot results (Fig. 4B) showed the protein levels of Nestin, Vimentin, and Pax6, in the RPCs did not markedly change when IGF-1 was added to the cultures. The immunocytochemistry results revealed that the ratios of Nestin-positive (80.6 ± 4.8% and 79.6 ± 5.6% for the IGF-1+EGF and EGF groups, respectively) and Vimentin-positive cells (79.5 ± 6.5% and 81.3 ± 6.1% for the IGF-1+ EGF and EGF groups, respectively) in the IGF-1+ EGF group were comparable to those in the EGF group (Fig. 4C-E). On the other hand, the immunocytochemistry of differentiation makers were also preformed, and the results were negative (Fig. S2). Combined with the above data, these results suggest that IGF-1 not only promotes the proliferation of RPCs but also maintains the undifferentiated state of RPCs.
Figure 4.
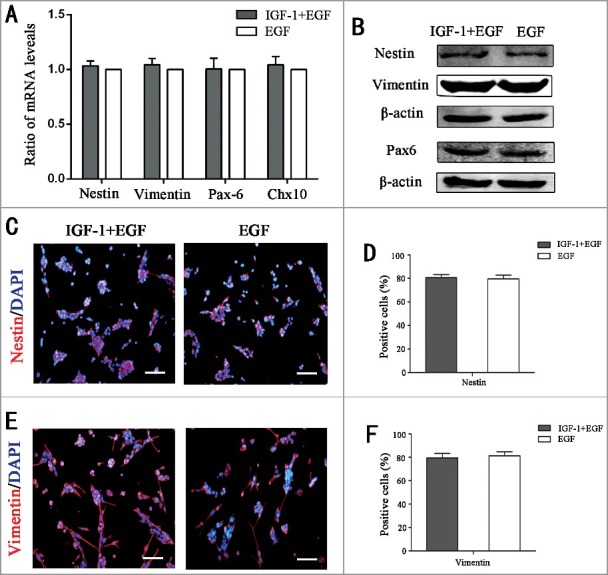
IGF-1 can maintain RPCs in an undifferentiated state in the proliferation condition. (A) The mRNA expression levels of Nestin, Vimentin, Pax-6 and Chx10 in the RPCs cultured with IGF-1 plus EGF or EGF were evaluated by qPCR analysis, and the results showed that there were no obvious differences between the two groups. (B) Western blot analysis of the expression levels of Nestin, Vimentin and Pax6 in the RPCs cultured with IGF-1 plus EGF or EGF. (C-E) Immunocytochemistry analysis of the ratios of Nestin- and Vimentin-positive cells in the RPCs cultured with IGF-1 plus EGF or EGF. The results showed that there were no remarkable differences between the two groups. Scale bar: 100 μm.
The differentiation potential of RPCs toward retinal neurons was enhanced by IGF-1 pretreatment
Previous studies have shown that RPCs cultured in differentiation medium can differentiate into different retinal neuronal and glial cells, including rhodopsin (a marker for rod photoreceptors), recoverin (a marker for rod and corn photoreceptors), β3-tubulin (a marker for neurons), PKC-α (a marker for bipolar cells), Cralbp (a marker for Müller cells), and GFAP (a glial marker) in retinal cells [21]. In the present study, the effect of IGF-1 on the differentiation program of RPCs was investigated. We firstly detected the retinal neuron differentiation gene expression of PRCs under proliferation medium supplemented with IGF, and no significant of these gene expressions were recorded (Fig. S2). Besides, adding IGF-1 directly in RPC differentiation medium attenuated the differentiation ability of RPCs (Fig. S3). Then under differentiation medium, green fluorescence was a label of RPCs, and the results showed that IGF-1+EGF-pretreated RPC cultures (day 1 and day 7) exhibited longer neurites (Fig. 5A-B, Fig. S1B). The processes of pretreatment of RPC cultures were shown in Fig. 5C, which showed the RPCs were cultured in proliferation medium with EGF or proliferation medium with EGF plus IGF-1 for 3 days. Then, all the IGF-1 and EGF was withdrawn, and the culture medium was replaced with differentiation medium containing 10% fetal bovine serum for the following 7 days for differentiation. The IGF-1+EGF-pretreated RPCs were regarded as the experimental group and the EGF-pretreated RPCs were regarded as the control group. The qPCR results showed that the expression levels of β3-tubulin, rhodopsin, recoverin, PKC-α, Cralbp, and GFAP were significantly enhanced in IGF-1+EGF group compared to the EGF group (Fig. 5D). The Western blot analysis and its cartogram showed that the expression levels of β3-tubulin, rhodopsin, PKC-α and GFAP were obviously increased in the IGF-1+EGF group compared to the EGF group, especially for the expression level of rhodopsin, an important protein of the phototransduction cascade that is essential for converting optical stimulation from the outside into electrical signals (Fig. 5E-F). In addition, the immunocytochemistry results showed that the ratios of β3-tubulin (23.5 ± 2.0% and 17.4 ± 2.5% for the IGF-1+EGF group and EGF group, respectively) and ratios of recoverin (24.7 ± 2.3% and 16.6 ± 2.2% for the IGF-1+EGF group and EGF group, respectively) (Fig. 5G-J). The above data show that, compared to the EGF group, the IGF-1+EGF-pretreated RPCs preferentially differentiated into retinal neuronal cells, especially for retinal neurons, including photoreceptors. Although the function of these retinal neurons was relied on further vivo experiments, these data suggest that IGF-1 can improve the differentiation potential of RPCs toward retinal neurons.
Figure 5.
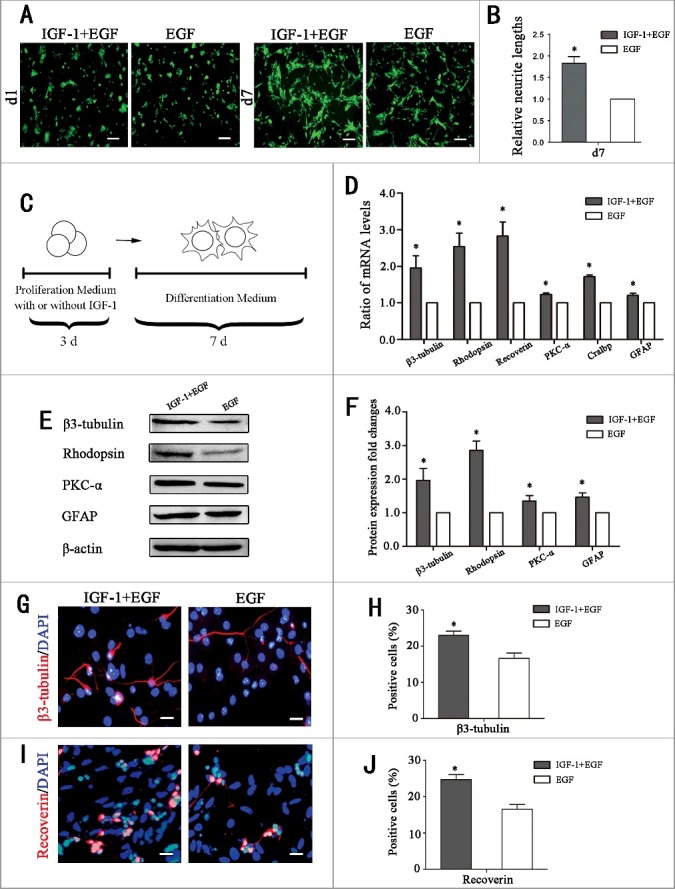
The differentiation potential of RPCs toward retinal neurons was enhanced by IGF-1 pretreatment. (A-B) Fluorescent micrographs of the GFP+ RPCs cultured in differentiation culture for the indicated times. The results showed that the cells in the IGF-1 plus EGF-pretreated RPC cultures exhibited longer neurites than in the EGF group. (C)Schematic diagram of the detection of the influence of IGF-1 on the differentiation of RPCs. RPCs were cultured in proliferation medium with IGF-1+EGF or EGF for 3 days, and then the cells were cultured in differentiation medium for 7 days. (D) The qPCR results showed that the expression levels of β3-tubulin, rhodopsin, recoverin, PKC-α, Cralbp and GFAP in IGF-1+EGF group were obviously increased compared to the EGF group. (E) Western blot analysis showed that the expression levels of β3-tubulin, rhodopsin, PKC-α, and GFAP were enhanced when RPCs pretreated with IGF-1+EGF compared with EGF. (F) Western blot protein expression signal revealed that the IGF-1+EGF pretreated RPCs expressed more β3-tubulin, rhodopsin, PKC-α, and GFAP protein than the EGF group. (G-J) The percentages of β3-tubulin-positive cells and recoverin-positive cells were significantly increased in the IGF-1+EGF group compared to the EGF group. *P ≤ 0.05 (Student's t-test). Scale bars: A: 100 μm, F: 25 μm.
Discussion
Retinal progenitor cells (RPCs) are multipotent cells that maintain the ability to self-renew and generate both retinal neuronal and glial lineages. As seed cells, RPCs have great potential for the cell therapy of retinal degenerative diseases. However, currently, the efficient expansion and differentiation of RPCs into retinal neurons are two of the major concerns of scientists, which ultimately restrict the application of RPCs. In the current study, our data suggest that IGF-1 is an efficient cytokine that promotes RPC proliferation and enhances RPC differentiation toward retinal neurons, which may provide some new insight into the controlled proliferation and differentiation of RPCs.
Thus far, cell therapy using RPCs as seed cells could be one of the most exciting strategies for the treatment of retinal degenerative diseases. However, in the application of this approach, how to generate enough proliferative RPCs is a troublesome issue that researchers currently face. In this study, our data show that IGF-1 plays a role in promoting RPC proliferation. It was reported that IGF-1 can accelerate the proliferation of stem/progenitor cells, such as mesenchymal stem cells, embryonic cortical progenitors and especially neural progenitor cells [22–25]. Our results demonstrate that IGF-1 can be an effective factor to enhance the proliferation capability of RPCs via activating IGF-1R. The data also reveal differences between RPCs and neural stem cells in the downstream signaling pathways of IGF-1R. A previous study demonstrated that IGF-1 promotes the proliferation of neural stem cells via PI3K/Akt signaling pathways [25]. Our data reveal that IGF-1 can promote RPC proliferation through both the MAPK/Erk signaling pathway and the PI3K/Akt signaling pathway, and the MAPK/Erk signaling pathway plays a predominant role in this progress. In addition, EGF is a well-accepted cytokine that maintains survival and promotes the growth of RPCs. And in this study, our results show that EGF promotes RPC proliferation by stimulating marked Erk phosphorylation but little Akt phosphorylation. While IGF-1 plus EGF not only evoked stronger phosphorylation of Erk but also distinctly evoked the phosphorylation of Akt in comparison to EGF, which maybe a possible reason accounting for the better promoting effects of IGF-1 plus EGF rather than EGF on RPC growth. Thus, inhibitor experiments were performed, and the results showed that only when Erk phosphorylation was blocked, the promoting effect of EGF on RPC proliferation was inhibited, while both inhibitors of Erk phosphorylation and Akt phosphorylation work well in reducing the promoting effect of IGF-1 on RPC proliferation, which further confirms the above data in the phosphorylation experiment. As a supplement to traditional culture conditions, IGF-1 can enhance the proliferation capacity of RPCs. This study may pave the way for improving the proliferation capacity of RPCs and sheds light upon further clinical applications of RPCs for retinal degenerative diseases.
The gradual development progress from an embryo to any system or organ involves the coordination of the proliferation and differentiation of stem or progenitor cells. In neurogenesis, only cells exit the cell cycle, and they can go through an integrated program incorporating the expression of many genes and the generation of different proteins [26,27]. Previous evidence suggests that this procedure relies on the action of multiple secreted cytokines [25]. IGF-1 is one of the important cytokines that regulates both cell proliferation and neuronal differentiation. Previous studies have found that exogenously added IGF-1 accelerates the differentiation of multipotent neural progenitor cells [25,28,29]. Most important of all, it was reported that the IGF-1 supplemented culture system enhances the ability to produce photoreceptors from human embryonic stem cells [28]. In the current study, our data reveal that IGF-1+EGF pretreated RPCs can generate more neurons, including the most interesting photoreceptors, which are essential for converting optical stimulation from the outside into electrical signals [23,30] that always undergo irreversible degeneration in retinal degenerative diseases [4,31,32]. Although IGF-1 promotion of RPCs differentiation requires further study, our results offer a promising strategy for generating a pool of optimized seed cells with enhanced neuronal differentiation potential. In summary, our results demonstrate that IGF-1 plays an efficient role in enhancing the proliferation of RPC in vitro via IGF-1R, and this process relies on the phosphorylation of the MAPK/Erk signaling pathways and the phosphorylation of the PI3K/Akt signaling pathways. IGF-1+EGF-pretreated RPCs may prefer to differentiate into retinal neurons, including photoreceptors, in vitro. These data suggest that IGF-1 can promote RPC expansion and enhance RPC differentiation toward retinal neurons, which are crucial features for the future treatment of retinal degenerative diseases, but the effect of IGF-1 on RPCs in vivo requires further research.
Materials and methods
Isolation and culture of murine retinal progenitor cells (RPCs)
The RPCs were isolated from fresh neural retina tissue of postnatal day 1 C57BL/6 mice, and they were genetically modified with GFP (a kind gift from Dr. Masaru Okabe, University of Osaka, Japan). Afterwards, the RPCs were plated in T25 flasks in fresh proliferation medium that consisted of advanced DMEM/F12 (Invitrogen, Carlsbad, CA, USA), 1% N2 neural supplement (Invitrogen), 2 mM L-glutamine (Invitrogen), and 20 ng/ml recombinant human epidermal growth factor (EGF, Invitrogen) or insulin-like growth factor-1 (IGF-1; Sigma-Aldrich, Saint Louis, MO, USA). The culture medium was changed at regular intervals of 2 days, and the cells were passaged every 3 to 4 days. For differentiation of RPCs, the cells were cultured with differentiation medium that contained DMEM/F12 (Invitrogen), 1% N2 neural supplement (Invitrogen), 2 mM L-glutamine (Invitrogen), and 10% fetal bovine serum (FBS) (Invitrogen) without EGF. The differentiation media were changed every 2 days. To detect the differentiation
potential influence of IGF-1 on RPCs, the cells were first cultured in proliferation medium containing 20 ng / ml of IGF-1+EGF or 20 ng / ml EGF for 3 days, then, withdraw IGF-1 and EGF, add 10% FBS to further culture for 7 days. All animals used in this study were prudently handled according to the ARVO animal usage standards, and animal procedures received approval from the Animal Care and Use Committee of the Schepens Eye Research Institute, where the original derivation of the cells was performed.
Cell counting and morphology detection in RPC culture
RPC proliferation capacity was evaluated using a cell counting kit (CCK8; Dojindo, Japan). The RPCs were seeded in 96-well plates and incubated at 37°C with proliferation medium containing EGF (20 ng/ml), EGF (20 ng/ml) plus IGF-1 (10, 20, or 50 ng/ml), EGF (20 ng/ml) plus IGF-1(20 ng/ml) plus NVP-ADW742 (Selleck, Houston, TX, USA), EGF (20 ng/ml) plus IGF-1(20 ng/ml) plus LY294002 (Sigma-Aldrich), or EGF (20 ng/ml) plus IGF-1 (20 ng/ml) plus PD98059 (Sigma-Aldrich). The absorbance of each well was measured at 450 nm using a microplate reader (ELX800, BioTek, USA) at 0 d, 1 d, and 3 d of cell culture. The working concentrations of NVP, LY294002, and PD98059 were 0.17 μM, 1.4 μM, and 10μM.
To assess the effect of IGF-1 on RPC proliferation morphology, the same concentration of RPCs was seeded in proliferation medium containing IGF-1+EGF and EGF, respectively. To determine the size of cell spheres, images of 6 random fields of view / well at day1 and day3 after cell seeding were recorded by a fluorescence microscope (Olympus BX51, Olympus, Japan) and analyzed by Image J software (National Institutes of Health (NIH), Bethesda, MD, USA). The area ratio of cell spheres was calculated via dividing cell sphere area in IGF-1+EGF group by the cell sphere area in EGF group [33]. To detect the differentiation potential influence of IGF-1 on RPCs, cells were treated as previous described and average lengths of the neurites were obtained by ImageJ software (NIH) through semiautomatically tracing individual neurites of cells in 3 separate images taken from IGF-1+EGF or EGF group (about 100 cells per image). The relative neurite lengths were expressed via dividing average neurite lengths of IGF-1+EGF group by average neurite lengths of EGF group [34,35].
RNA isolation, reverse transcription and quantitative polymerase chain reaction (qPCR)
To detect the expression levels of stem cell markers (Nestin, Vimentin, Pax-6 and Chx10), the RPCs are cultured under proliferation medium with IGF-1+EGF or EGF for 3 days. To detect the expression levels differentiation markers (β3-tubulin, Rhodopsin, Recoverin, PKC-α, Cralbp and GFAP), the cells were first cultured in proliferation medium with IGF-1+EGF or EGF for 3 days, then, withdraw IGF-1 and EGF, add 10% FBS to further culture for 7 days. Total RNA was extracted from each sample of cultured cells with Trizol (Invitrogen) in accordance with the manufacturer's instructions. DNase I was used to avoid genomic DNA contamination. The concentration and purity of the total RNA were determined spectrophotometrically at OD260/280 nm ratios between 1.9 and 2.1 using spectrophotometer and Nanodrop 2000 software. Then, 1000 ng of qualified RNA was reverse transcribed using a PrimeScript™ RT reagent kit (Perfect Real Time; TaKaRa, Dalian, China). The qPCR was carried out in a 10-µl total volume including 5 µl of 2 × Power SYBR Green PCR Master Mix (Applied Biosystems), 1 µl of diluted cDNA, and 150 nM of gene-specific primers, and the rest was replenished by nuclease-free water (Invitrogen). The PCR efficiency of the reaction was detected with primers using serial dilutions of the cDNA (1:1, 1:5, 1:25, 1:125, 1:625, and 1:3,125). The primer sequences are shown in Table 1. The samples were amplified using a 7500 Real-Time PCR Detection System (Applied Biosystems, Irvine, CA), and after amplification for 40 cycles, the relative mRNA was analyzed using the Pfaffl method [36]. The cycle threshold (Ct) value represents the number of PCR cycles at which an increase in fluorescence signal (and therefore cDNA) can be detected above background and the increase is exponential for the particular gene. The results are expressed as fold-change relative to untreated controls after normalizing to the expression of β-actin of each sample.
Table 1.
Primers used for qPCR.
| Genes | Accession no. | Forward(5'-3') | Reverse(5'-3') | Annealing temperature (°C) | Product size(base pairs) |
|---|---|---|---|---|---|
| Ki-67 | X82786 | cagtactcggaatgcagcaa | cagtcttcaggggctctgtc | 60 | 170 |
| Nestin | NM_016701 | aactggcacctcaagatgt | tcaagggtattaggcaagggg | 60 | 235 |
| Vimentin | NM_011701 | tggttgacacccactcaaaa | gcttttggggtgtcagttgt | 60 | 269 |
| Pax-6 | NM_013627 | agtgaatgggcggagttatg | acttggacgggaactgacac | 60 | 132 |
| Chx10 | NM_238086 | caatgctgtggcttgcttta | cttgagagccactgggctac | 60 | 157 |
| β3-tubulin | NM_023279 | cgagacctactgcatcgaca | cattgagctgaccagggaat | 60 | 152 |
| Rhodopsin | NM_145383 | tcaccaccaccctctacaca | tgatccaggtgaagaccaca | 60 | 216 |
| Recoverin | NM_009038 | atggggaatagcaagagcgg | gagtccgggaaaaacttggaata | 60 | 179 |
| Pkc-α | NM_011101 | cccattccagaaggagatga | ttcctgtcagcaagcatcac | 60 | 212 |
| Cralbp | NM_020599 | agggtctttgttcacggagat | tgccactagagcgttcctaaa | 60 | 297 |
| GFAP | NM_010277 | agaaaaccgcatcaccattc | tcacatcaccacgtccttgt | 60 | 184 |
| β-actin | NM_007393 | agccatgtacgtagccatcc | ctctcagctgtggtggtgaa | 60 | 152 |
Western blot analysis
The cells were harvested at the indicated time points after the RPCs in each group were incubated with different media, the total proteins were extracted, and their concentrations were detected using a BCA Kit (Pierce, Rockford, IL, USA) according to the manufacturer's protocol. Proteins were separated by sodium dodecyl sulfate–polyacrylamide gel (SDS-PAGE) electrophoresis, and then transferred to 0.22 mm polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). After blocking with 5% BSA, the membranes were incubated with mouse monoclonal anti-Nestin (BD, San Jose, CA, USA 1:500), rabbit monoclonal anti-Vimentin (Millipore, 1:1000), rabbit monoclonal anti-Pax-6 (Biolegend, San Diego, CA, USA), mouse monoclonal anti-Rhodopsin (Millipore, 1:200), mouse monoclonal anti-PKC-α (Millipore, 1:200), mouse monoclonal anti-β3-tubulin (Millipore, 1:1000), and mouse monoclonal anti-GFAP (Millipore, 1:1000) antibodies overnight at 4°C, followed by incubation with horseradish peroxidase-conjugated secondary antibodies (Sigma-Aldrich, 1:5000). For the phosphorylation experiment, the RPCs were washed with phosphate-buffered saline (PBS), starved for 1 h in DMEM/F12 medium, and incubated with 20 ng/ml EGF or 20 ng/ml EGF + 20 ng/ml IGF-1 or proliferation medium without EGF and IGF-1 for 20 min. Then, the total cellular proteins were harvested and a BCA kit (Pierce) was used to analyze the protein concentrations. Proteins were separated by sodium dodecyl sulfate–polyacrylamide gel (SDS-PAGE) electrophoresis and then transferred to 0.22 mm polyvinylidene fluoride membranes (Millipore). After blocking with 5% BSA, the membranes were incubated with rabbit monoclonal anti-Akt (Cell Signaling Technology (CST), Danvers, MA, USA, 1:1000), rabbit monoclonal anti-P-Akt (CST, 1:1000), rabbit monoclonal anti-Erk (CST,1:1000), rabbit monoclonal anti-P-Erk (CST, 1:1000) and mouse anti-β-actin (Sigma-Aldrich, 1:5000) antibodies overnight at 4°C, followed by incubation with horseradish peroxidase-conjugated secondary antibodies (1:5000, Sigma-Aldrich). Protein expression images were visualized using the Odyssey V 3.0 image scanning (LI-COR).
Immunocytochemistry
RPCs were seeded on glass coverslips (VWR, West Chester, PA, USA) coated with laminin (Sigma-Aldrich) in 12-well plates. Following incubation in the proliferation medium for 3 days or in the differentiation medium for 7 days, the cells were subsequently fixed with 4% paraformaldehyde (Sigma-Aldrich) for 20 min, permeabilized with 0.3% Triton X-100 (Sigma-Aldrich) and blocked with 10% normal goat serum (Invitrogen) for 1 h. Then, the cells were subjected to immunofluorescent staining using mouse monoclonal anti-Ki-67 (1:200, BD), mouse monoclonal anti-Nestin (1:200, Millipore), mouse monoclonal anti-Vimentin (1:200, Millipore), mouse monoclonal anti-β3-tubulin (1:100, Millipore), or rabbit polyclonal anti-Recoverin (Millipore, 1:200) antibodies overnight at 4°C. Fluorescent-labeled secondary antibodies (Alexa Fluor546-goat anti-mouse/rabbit, BD, 1:800) were used after washing with PBS 3 times, and the cell nuclei were then counterstained with 4',6-diamidino-2-phenylindole (DAPI, Invitrogen). Negative control samples were processed in parallel but without the primary antibody. The immunoreactive cells were visualized and imaged with a fluorescence microscope (Olympus BX51). The percentage of positive cells was determined by dividing the number of immunopositive cells by the number of nuclei stained with DAPI. For 5-bromo-2-deoxyuridine (BrdU) incorporation, RPCs were cultured in proliferation medium for 3 days, and then 10 mM BrdU (Sigma-Aldrich) was used to treat cells for 10 hours. The cells were detected using a monoclonal anti-BrdU antibody (CST) after incubation in proliferation medium for 12 hours.
Statistical analyses
All experiments in our study were performed in triplicate except where otherwise specified. The experimental statistics are expressed as the mean ± the standard derivation (SD). Statistical analyses were performed using Student's t-test and ANOVA test, and the difference was considered significant for p ≤ 0.05.
Supplementary Material
Funding Statement
This research was supported by the National Natural Science Foundations of China (81570883, 31500835), Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant Support (20161316), Natural Science Foundation of Ningbo Science and Technology Department (2010A610033) and The Science and Technology Commission of Shanghai (17DZ2260100)
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors are grateful to Dr. Henry Klassen and Dr. Michael J. Young for the original mouse RPCs. This research was supported by the National Natural Science Foundations of China (81570883, 31500835), Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant Support (20161316), Natural Science Foundation of Ningbo Science and Technology Department (2010A610033) and The Science and Technology Commission of Shanghai (17DZ2260100).
References
- [1].Margalit E, Sadda SR. Retinal and optic nerve diseases. Artif Organs. 2003;27(11):963–974. PMID:14616515. doi: 10.1046/j.1525-1594.2003.07304.x. [DOI] [PubMed] [Google Scholar]
- [2].Jeon S, Oh IH. Regeneration of the retina: toward stem cell therapy for degenerative retinal diseases. BMB Rep. 2015;48(4):193–199. PMID:25560700. doi: 10.5483/BMBRep.2015.48.4.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Klassen H, Sakaguchi DS, Young MJ. Stem cells and retinal repair. Prog Retin Eye Res. 2004;23(2): 149–181. PMID:15094129. doi: 10.1016/j.preteyeres.2004.01.002. [DOI] [PubMed] [Google Scholar]
- [4].Steedman MR, Tao SL, Klassen H, et al. Enhanced differentiation of retinal progenitor cells using microfabricated topographical cues. Biomed Microdevices. 2010;12(3):363–369. PMID:20077017. doi: 10.1007/s10544-009-9392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gu P, Harwood LJ, Zhang X, et al. Isolation of retinal progenitor and stem cells from the porcine eye. Mol Vis. 2007;13:1045–1057. PMID:17653049. [PMC free article] [PubMed] [Google Scholar]
- [6].Usui A, Mochizuki Y, Iida A, et al. The early retinal progenitor-expressed gene Sox11 regulates the timing of the differentiation of retinal cells. Development. 2013;140(4):740–750. PMID:23318640. doi: 10.1242/dev.090274. [DOI] [PubMed] [Google Scholar]
- [7].Klassen H. Stem cells in clinical trials for treatment of retinal degeneration. Expert Opin Biol Ther. 2016;16(1):7–14. PMID:26414165. doi: 10.1517/14712598.2016.1093110. [DOI] [PubMed] [Google Scholar]
- [8].Zhang D, Ni N, Chen J, et al. Electrospun SF/PLCL nanofibrous membrane: a potential scaffold for retinal progenitor cell proliferation and differentiation. Sci Rep. 2015;5:14326. PMID:26395224. doi: 10.1038/srep14326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hu Y, Luo M, Ni N, et al. Reciprocal actions of microRNA-9 and TLX in the proliferation and differentiation of retinal progenitor cells. Stem Cells Dev. 2014;23(22): 2771–2781. PMID:24901604. doi: 10.1089/scd.2014.0021. [DOI] [PubMed] [Google Scholar]
- [10].Xia X, Ahmad I. let-7 microRNA regulates neurogliogenesis in the mammalian retina through Hmga2. Dev Biol. 2016;410(1):70–85. PMID:26698218. doi: 10.1016/j.ydbio.2015.12.010. [DOI] [PubMed] [Google Scholar]
- [11].Xia J, Luo M, Ni N, et al. Bone marrow mesenchymal stem cells stimulate proliferation and neuronal differentiation of retinal progenitor cells. PLoS One. 2013;8(9): e76157. PMID:24098776. doi: 10.1371/journal.pone.0076157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ni N, Zhang D, Xie Q, et al. Effects of let-7b and TLX on the proliferation and differentiation of retinal progenitor cells in vitro. Sci Rep. 2014;4:6671. PMID:25327364. doi: 10.1038/srep06671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zahir T, Klassen H, Young MJ. Effects of ciliary neurotrophic factor on differentiation of late retinal progenitor cells. Stem Cells. 2005;23(3):424–432. PMID:15749937. doi: 10.1634/stemcells.2004-0199. [DOI] [PubMed] [Google Scholar]
- [14].Angenieux B, Schorderet DF, Arsenijevic Y. Epidermal growth factor is a neuronal differentiation factor for retinal stem cells in vitro. Stem Cells. 2006;24(3): 696–706. PMID:16179425. doi: 10.1634/stemcells.2005-0190. [DOI] [PubMed] [Google Scholar]
- [15].Deleyrolle L, Marchal-Victorion S, Dromard C, et al. Exogenous and fibroblast growth factor 2/epidermal growth factor-regulated endogenous cytokines regulate neural precursor cell growth and differentiation. Stem Cells. 2006;24(3):748–762. PMID:16166253. doi: 10.1634/stemcells.2005-0138. [DOI] [PubMed] [Google Scholar]
- [16].de Pablo F, de la Rosa EJ.. The developing CNS: a scenario for the action of proinsulin, insulin and insulin-like growth factors. Trends Neurosci. 1995;18(3):143–150. PMID:7754526 [DOI] [PubMed] [Google Scholar]
- [17].Zhao P, Deng Y, Gu P, et al. Insulin-like growth factor 1 promotes the proliferation and adipogenesis of orbital adipose-derived stromal cells in thyroid-associated ophthalmopathy. Exp Eye Res. 2013;107:65–73. PMID:23219871. doi: 10.1016/j.exer.2012.11.014. [DOI] [PubMed] [Google Scholar]
- [18].Hodge RD, D'Ercole AJ, O'Kusky JR. Insulin-like growth factor-I accelerates the cell cycle by decreasing G1 phase length and increases cell cycle reentry in the embryonic cerebral cortex. J Neurosci. 2004;24(45):10201–10210. PMID:15537892. doi: 10.1523/jneurosci.3246-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kouroupi G, Lavdas AA, Gaitanou M, et al. Lentivirus-mediated expression of insulin-like growth factor-I promotes neural stem/precursor cell proliferation and enhances their potential to generate neurons. J Neurochem. 2010;115(2): 460–474. PMID:20681949. doi: 10.1111/j.1471-4159.2010.06939.x. [DOI] [PubMed] [Google Scholar]
- [20].Gorecki DC, Beresewicz M, Zablocka B. Neuroprotective effects of short peptides derived from the Insulin-like growth factor 1. Neurochem Int. 2007;51(8):451–458. PMID:17582656. doi: 10.1016/j.neuint.2007.04.030. [DOI] [PubMed] [Google Scholar]
- [21].Zhang Y, Shen B, Zhang D, et al. miR-29a regulates the proliferation and differentiation of retinal progenitors by targeting Rbm8a. Oncotarget. 2017;8(19):31993–32008. PMID:28404883. doi: 10.18632/oncotarget.16669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ahmad I, Dooley CM, Thoreson WB, et al. In vitro analysis of a mammalian retinal progenitor that gives rise to neurons and glia. Brain Res. 1999;831(1-2):1–10. PMID:10411978. doi: 10.1016/S0006-8993(99)01376-1. [DOI] [PubMed] [Google Scholar]
- [23].Li T, Lewallen M, Chen S, et al. Multipotent stem cells isolated from the adult mouse retina are capable of producing functional photoreceptor cells. Cell Res. 2013;23(6):788–802. PMID:23567557. doi: 10.1038/cr.2013.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ye P, D'Ercole AJ. Insulin-like growth factor actions during development of neural stem cells and progenitors in the central nervous system. J Neurosci Res. 2006;83(1):1–6. PMID:16294334. doi: 10.1002/jnr.20688. [DOI] [PubMed] [Google Scholar]
- [25].McMorris FA, Mozell RL, Carson MJ, et al. Regulation of oligodendrocyte development and central nervous system myelination by insulin-like growth factors. Ann N Y Acad Sci. 1993;692:321–334. PMID:8215042. doi: 10.1111/j.1749-6632.1993.tb26247.x. [DOI] [PubMed] [Google Scholar]
- [26].de Pablo F, de la Rosa EJ. The developing CNS: a scenario for the action of proinsulin, insulin and insulin-like growth factors. Trends Neurosci. 1995;18(3):143–150. PMID:7754526. doi: 10.1016/0166-2236(95)93892-2. [DOI] [PubMed] [Google Scholar]
- [27].Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132(4):598–611. PMID:18295578. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mellough CB, Collin J, Khazim M, et al. IGF-1 Signaling Plays an Important Role in the Formation of Three-Dimensional Laminated Neural Retina and Other Ocular Structures From Human Embryonic Stem Cells. Stem Cells. 2015;33(8):2416–2430. PMID:25827910. doi: 10.1002/stem.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ma J, Guo C, Guo C, et al. Transplantation of Human Neural Progenitor Cells Expressing IGF-1 Enhances Retinal Ganglion Cell Survival. PLoS One. 2015;10(4):e0125695. PMID:25923430. doi: 10.1371/journal.pone.0125695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].MacLaren RE, Pearson RA, MacNeil A, et al. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444(7116):203–207. PMID:17093405. doi: 10.1038/nature05161. [DOI] [PubMed] [Google Scholar]
- [31].Alves CH, Pellissier LP, Vos RM, et al. Targeted ablation of Crb2 in photoreceptor cells induces retinitis pigmentosa. Hum Mol Genet. 2014;23(13):3384–3401. PMID:24493795. doi: 10.1093/hmg/ddu048. [DOI] [PubMed] [Google Scholar]
- [32].Bhutto I, Lutty G. Understanding age-related macular degeneration (AMD): relationships between the photoreceptor/retinal pigment epithelium/Bruch's membrane/choriocapillaris complex. Mol Aspects Med. 2012;33(4):295–317. PMID:22542780. doi: 10.1016/j.mam.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chung DJ, Wong A, Hayashi K, et al. Effect of hypoxia on generation of neurospheres from adipose tissue-derived canine mesenchymal stromal cells. Vet J. 2014;199(1):123–130. PMID:24252224. doi: 10.1016/j.tvjl.2013.10.020. [DOI] [PubMed] [Google Scholar]
- [34].Havlicek S, Kohl Z, Mishra HK, et al. Gene dosage-dependent rescue of HSP neurite defects in SPG4 patients' neurons. Hum Mol Genet. 2014;23(10):2527–2541. PMID:24381312. doi: 10.1093/hmg/ddt644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bica L, Liddell JR, Donnelly PS, et al. Neuroprotective copper bis(thiosemicarbazonato) complexes promote neurite elongation. PLoS One. 2014;9(2):e90070. PMID:24587210. doi: 10.1371/journal.pone.0090070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. PMID:11328886. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


