ABSTRACT
Parasitic plants establish vascular-conducting cells in an intrusive organ called haustorium. In haustoria of a stem parasitic plant, Cuscuta japonica, the presence of cells expressing cell-type-specific genes of phloem companion cell, phloem sieve element, procambial cell and xylem vessel has recently been demonstrated. Differentiation of these vascular cells is regulated in a manner similar to that in conventional vascular tissues. However, the initiation of procambial cells occurs concomitantly with the differentiation of vascular-conducting cells. The differentiation process of phloem also differed from that of conventional vascular tissues because enucleation of sieve elements appeared to be impeded. These results collectively imply that the vascular differentiation process in haustoria of parasitic plants may be different from that in conventional vascular tissues.
KEYWORDS: Cuscuta, differentiation, dodder, haustorium, parasitic plants, phloem, procambium
Parasitic plants establish vascular-conducting cells in an intrusive organ called haustorium. A serial array of haustorial vascular-conducting cells serves as a bridge between the vascular systems of host and parasite plants and facilitates the transport of water and nutrients from host to parasite plants. Experiments using various tracer dyes demonstrated that haustorial vascular-conducting cells function in the transport of substances from the host to the parasite.1-3 However, it remains unclear whether the differentiation of vascular-conducting cells in haustoria occur in the same manner as in conventional vascular tissues.4-7
We have recently revealed that cell type-specific genes are expressed in haustoria of a stem parasitic dodder, Cuscuta japonica (Cj).8 Expression of CjAPL, CjSEOR1, CjWOX4 and CjTED7 was confirmed in Cj haustoria, demonstrating that phloem companion cells,9 phloem sieve elements,10 procambial cells11 and developing xylem vessels12 are present in haustoria, respectively. In addition to these cell type-specific genes, we confirmed the expression of genes that regulate vascular cell differentiation,13 including CjCLE41, CjGSK3 and CjBES1 in haustoria.8 The expression level of CjCLE41, which encodes a peptide called the tracheary element differentiation inhibitory factor (TDIF) that maintains the activity of the stem cell,11 started to increase at 72 hour after attachment (h.a.a.), which is just before the differentiation of xylem vessels in haustoria, and it showed the maximum level at 96 h.a.a. when xylem vessels started to differentiate. Expression of CjGSK3 continuously decreased, while that of CjBES1 continuously increased from 72 to 120 h.a.a., which coincided with the formation of continuous xylem vessel. These results suggest that the TDIF-TDR-GSK3 signaling pathway controls the differentiation of xylem in haustoria in a manner similar to that in roots and stems. In situ hybridization demonstrated that the expression of CjWOX4 and CjCLE41 was localized in the adjacent cells, but was not overlapped with the haustorial xylem vessel, suggesting that distinct phloem/procambial- and xylem domains were established in haustoria.8
In haustoria, the expression of CjWOX4 and CjCLE41, both of which are involved in the maintenance of procambial cells, and CjAPL, CjSEOR1 and CjTED7, which are cell type-specific genes, showed a remarkable increase from 72 to 96 h.a.a.8 These results suggest that differentiation of procambial- and vascular-conducting cells started concomitantly, and the latter continued until the later stages of haustorial development (Fig. 1). However, it remains unclear how procambial cells are initiated in this differentiation scheme (Fig. 1). Haustorial cells of Cj are initiated in the cortex of stem by forming a meristem-like region called prehaustorium. The expression level of CjWOX4 was much lower in prehaustoria at 48 h.a.a. than in haustoria at 72 h.a.a.8 We did not successfully identify cells expressing CjWOX4 using in situ hybridization in prehaustoria at 48 h.a.a. At 72 h.a.a., cells expressing CjWOX4 were localized in the central part of the haustorium, suggesting that specification of procambial cells was already completed before the onset of xylem vessel formation.8 These results suggest two possible hypotheses. Procambial cells might be initiated in haustoria (not in prehaustoria) when they were elongated (Fig. 1, right). Alternatively, an identity of procambial cells might have been established in prehaustoria (Fig. 1, left) and expression level of CjWOX4 increased during the elongation of haustorial cells because elongating cells increase their ploidy, as observed in dark-grown hypocotyls.14 To test these hypotheses, a methodology enabling higher-sensitivity detection of gene expression, such as transformation using promoter-reporter constructs, needs to be developed for Cuscuta spp.
Figure 1.
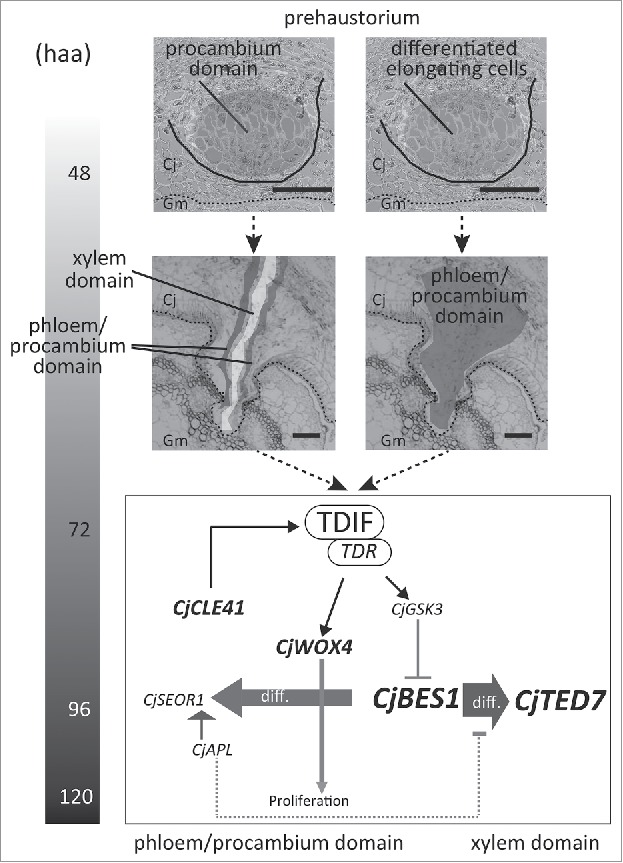
Differentiation of vascular-conducting cells in haustoria of Cuscuta japonica. Upper: In the prehaustorium, cells started elongating. CjWOX4, a procambium-specific gene, was not detected either by RT-PCR or in situ hybridization.8 Thus, it is unclear whether procambial cells are established (grey, left) or not (right) in the prehaustorium. Middle: between 48 and 72 h.a.a., before the onset of xylem vessel formation (white, left), procambial cells expressing CjWOX4 (grey, left) were established. This does not exclude the possibility that phloem and xylem has been differentiated. Lower: after 72 h.a.a., haustorial procambial cells differentiated into distinct phloem/procambial- and xylem domains. Solid line indicates the boundary of the prehaustorium. Dotted line indicates the boundary of the parasite, Cuscita japonica (Cj) and the host, Glycine max (Gm). Scale bar, 50 μm.
Differentiation of phloem-conducting cells in haustoria also appears to be different from the conventional phloem. Although phloem-conducting cells are arranged in a linear conductive array, some of the cells contain nuclei,8 suggesting that they are not mature sieve elements (SE). The presence of nucleated SEs has been reported in several parasitic plants including Cuscuta gronovii,15 Alectra vogelii16 and Phelipanche ramosa.16 Recently, the presence of nucleated phloem-conducting cells has also been reported in haustoria of Phelipanche aegyptiaca.17 In haustoria of P. aegyptiaca, a homolog of NAC-DOMAIN CONTAINING TRANSCRIPTION FACTOR 45/86 (NAC45/86), PaNAC45, and homologs of NAC45/86-DEPENDENT EXONUCLEASE-DOMAIN PROTEIN 1 (NEN1) and NEN4, PaNEN1 and PaNEN4, were expressed in higher levels than in the protrusion organs of the tubercle in which enucleated SEs are differentiated.17 NAC45/86 encodes a transcription factor protein that regulates the downstream processes of SE maturation, and NEN1 and NEN4 encode exonucleases responsible for the degradation of nuclei in SE.18 In Cj, the expression of CjNEN4 in haustorium was confirmed and it increased from 72 to 96 h.a.a. (Fig. 2A). The contradiction between the retention of nuclei and expression of an exonuclease gene implied a mechanism repressing the nuclear degradation. Establishment of SE is controlled by the OCTOPUS–BRASSINOSTEROID INSENSITIVE 2-BRI1-EMS-SUPPRESSOR 1 (OPS-BIN2-BES1) regulatory pathway that controls SE differentiation and the ALTERED PHLOEM DEVELOPMENT (APL)-NAC45/86-NEN regulatory pathway that controls SE enucleation (Fig. 2B).19 Nucleated SEs are frequently observed in the ops loss-of-function mutant of Arabidopsis,20,21 which implies that the expression of OPS may be lower in haustorial phloem-conducting cells than in the conventional phloem. The occurrence of a crosstalk between OPS-BIN2-BES1 and APL-NAC45/86-NEN regulatory pathways needs to be clarified (Fig. 2B).
Figure 2.
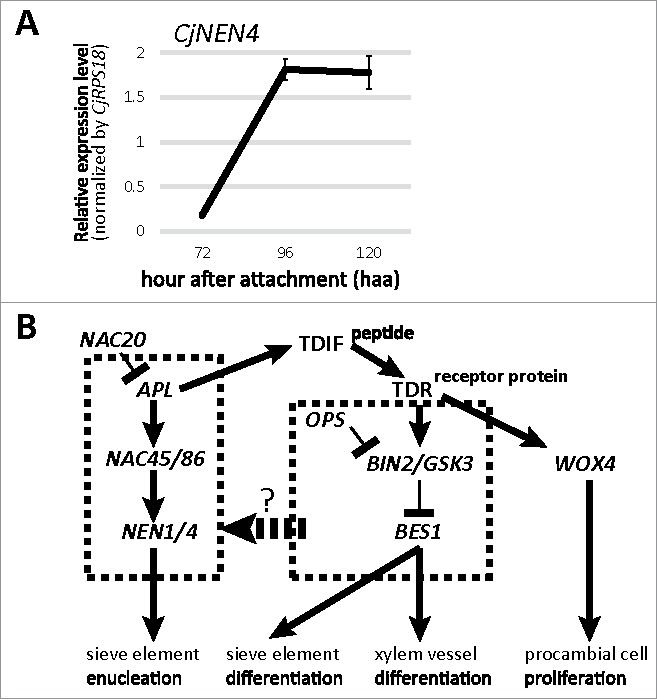
Regulation of phloem-conducting cell differentiation in haustoria of Cuscuta japonica. (A) Expression profile of CjNEN4 in haustoria. Expression level increased from 72 to 96 h.a.a., which is inconsistent with the retention of nuclei. Expression levels were normalized by that of C. japonica ribosome protein S18 gene (CjRPS18). Mean and standard error of three biological replicates are indicated. (B) Hypothesis for the regulation of differentiation and enucleation of phloem-conducting cells. Main hypothesis is that OPS-BIN2-BES1 regulatory pathway may promote the enucleation by controlling APL-NAC45/86-NEN regulatory pathway, although the point of action has not been identified (dotted arrow). In Cj haustoria, expression of OPS, BIN2, or BES1 may be lower than conventional vascular tissues, which results in retention of nuclei in phloem-conducting cells.
In conclusion, the presence of cells that have attributes of vascular elements was confirmed in Cj haustoria. Differentiation of these elements was regulated by TDIF-TDR-WOX4- and TDIF-TDR-GSK3 regulatory pathways in a manner similar to that in conventional vascular tissues. However, the initiation of procambial cells may differ. The differentiation process of SE also appears to differ from that in conventional vascular tissues because enucleation of SE appears to be impeded. This implies a possible crosstalk between the OPS-BIN2-BES1 and APL-NAC45/86-NEN regulatory pathways.
Funding Statement
This work was supported by the Scientific Research in Innovative Areas project “The Plant Cell Wall as an Information Processing System” (MEXT, Japan) to KA (grant no. 15H01237), and by the Grant-in-Aid for Scientific Research (B) to KA (grant no. 16H04875).
Abbreviations
- APL
ALTERED PHLOEM DEVELOPMENT
- BES
BRI1-EMS-SUPPRESSOR
- BIN
BRASSINOSTEROID-INSENSITIVE
- Cj
Cuscuta japonica
- CLE
CLAVATA3/EMBRYO SURROUNDING REGION-RELATED
- GSK
glycogen synthase kinase
- haa
hours after attachment
- NEN
NAC45/86-DEPENDENT EXONUCLEASE-DOMAIN PROTEIN
- OPS
OCTOPUS
- SE
sieve element
- SEOR
SIEVE ELEMENT OCCLUSION-RELATED
- TDIF
tracheary element differentiation inhibitory factor
- TDR
TDIF-receptor
- TED
TRACHEARY ELEMENT DIFFERENTIATION-RELATED
- WOX
WUSCHEL-RELATED HOMEOBOX
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Haupt S, Oparka KJ, Sauer N, Neumann S. Macromolecular trafficking between Nicotiana tabacum and the holoparasite Cuscuta reflexa. J Exp Bot. 2001;52:173–7. doi: 10.1093/jexbot/52.354.173. [DOI] [PubMed] [Google Scholar]
- 2.Birschwilks M, Haupt S, Hofius D, Neumann S. Transfer of phloem-mobile substances from the host plants to the holoparasite Cuscuta sp. J Exp Bot. 2006;57:911–21. doi: 10.1093/jxb/erj076. [DOI] [PubMed] [Google Scholar]
- 3.Birschwilks M, Sauer N, Scheel D, Neumann S. Arabidopsis thaliana is a susceptible host plant for the holoparasite Cuscuta spec. Planta. 2007;226:1231–41. doi: 10.1007/s00425-007-0571-6. [DOI] [PubMed] [Google Scholar]
- 4.Fukuda H. Signals that control plant vascular cell differentiation. Nat Rev Mol Cell Biol. 2004;5:379–91. doi: 10.1038/nrm1364. [DOI] [PubMed] [Google Scholar]
- 5.Hirakawa Y, Kondo Y, Fukuda H. Regulation of vascular development by CLE peptide-receptor systems. J Integr Plant Biol. 2010;52:8–16. doi: 10.1111/j.1744-7909.2010.00904.x. [DOI] [PubMed] [Google Scholar]
- 6.Ohashi-Ito K, Fukuda H. Functional mechanism of bHLH complexes during early vascular development. Curr Opin Plant Biol. 2016;33:42–47. doi: 10.1016/j.pbi.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Campbell L, Turner S. Regulation of vascular cell division. J Exp Bot. 2017;68:27–43. doi: 10.1093/jxb/erw448. [DOI] [PubMed] [Google Scholar]
- 8.Shimizu K, Hozumi A, Aoki K. Organization of vascular cells in the haustorium of the parasitic flowering plant Cuscuta japonica. Plant Cell Physiol. 2017. December 11. doi: 10.1093/pcp/pcx197. [DOI] [PubMed] [Google Scholar]
- 9.Bonke M, Thitamadee S, Mähönen AP, Hauser MT, Helariutta Y. APL regulates vascular tissue identity in Arabidopsis. Nature. 2003;426:181–6. doi: 10.1038/nature02100. [DOI] [PubMed] [Google Scholar]
- 10.Rüping B, Ernst AM, Jekat SB, Nordzieke S, Reineke AR, Müller B, Bornberg-Bauer E, Prüfer D, Noll GA. Molecular and phylogenetic characterization of the sieve element occlusion gene family in Fabaceae and non-Fabaceae plants. BMC Plant Biol. 2010;10:219. doi: 10.1186/1471-2229-10-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirakawa Y, Kondo Y, Fukuda H. TDIF peptide signaling regulates vascular stem cell proliferation via the WOX4 homeobox gene in Arabidopsis. Plant Cell. 2010;22:2618–29. doi: 10.1105/tpc.110.076083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Endo S, Pesquet E, Yamaguchi M, Tashiro G, Sato M, Toyooka K, Nishikubo N, Udagawa-Motose M, Kubo M, Fukuda H, et al.. Identifying new components participating in the secondary cell wall formation of vessel elements in zinnia and Arabidopsis. Plant Cell. 2009;21:1155–65. doi: 10.1105/tpc.108.059154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kondo Y, Fukuda H. The TDIF signaling network. Curr Opin Plant Biol. 2015;28:106–10. doi: 10.1016/j.pbi.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Narukawa H, Yokoyama R, Komaki S, Sugimoto K, Nishitani K. Stimulation of cell elongation by tetraploidy in hypocotyls of dark-grown arabidopsis seedlings. PLoS One. 2015;10:e0134547. doi: 10.1371/journal.pone.0134547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Truscott FH. On the regeneration of new shoots from isolated dodder haustoria. Am J Bot. 1958;45:169–77. doi: 10.1002/j.1537-2197.1958.tb12205.x. [DOI] [Google Scholar]
- 16.Dörr I. Sieve elements in haustoria of parasitic angiosperms In: Behnke H-D, Sjolund RD editors. Sieve elements: Comparative structure, induction and development. Berlin, Germany: Springer-Verlag; 1990. p. 239–56. [Google Scholar]
- 17.Ekawa M, Aoki K. Phloem-conducting cells in haustoria of the root-parasitic plant Phelipanche aegyptiaca retain nuclei and are not mature sieve elements. Plants (Basel). 2017;5:E60. doi: 10.3390/plants6040060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furuta KM, Yadav SR, Lehesranta S, Belevich I, Miyashima S, Heo JO, Vatén A, Lindgren O, De Rybel B Van Isterdael G, et al.. Plant development. Arabidopsis NAC45/86 direct sieve element morphogenesis culminating in enucleation. Science. 2014;345:933–7. doi: 10.1126/science.1253736. [DOI] [PubMed] [Google Scholar]
- 19.Heo JO, Blob B, Helariutta Y. Differentiation of conductive cells: a matter of life and death. Curr Opin Plant Biol. 2017;35:23–29. doi: 10.1016/j.pbi.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Anne P, Azzopardi M, Gissot L, Beaubiat S, Hématy K, Palauqui JC. OCTOPUS negatively regulates BIN2 to control phloem differentiation in Arabidopsis thaliana. Curr Biol. 2015;25:2584–90. doi: 10.1016/j.cub.2015.08.033. [DOI] [PubMed] [Google Scholar]
- 21.Truernit E, Bauby H, Belcram K, Barthélémy J, Palauqui JC. OCTOPUS, a polarly localised membrane-associated protein, regulates phloem differentiation entry in Arabidopsis thaliana. Development. 2012;139:1306–15. doi: 10.1242/dev.072629. [DOI] [PubMed] [Google Scholar]


