ABSTRACT
Nicotinic acetylcholine receptors (nAChRs) have vital functions in processes of neurotransmission that underpin key behaviors. These pentameric ligand-gated ion channels have been used as targets for insecticides that constitutively activate them, causing the death of insect pests. In examining a knockout of the Dα1 nAChR subunit gene, our study linked this one subunit with multiple traits. We were able to confirm previous work that had identified Dα1 as a target of the neonicotinoid class of insecticides. Further, we uncovered roles for the gene in influencing mating behavior and patterns of sleep. The knockout mutant was also observed to have a significant reduction in longevity. This study highlighted the severe fitness costs that appear to be associated with the loss of function of this gene in natural populations in the absence of insecticides targeting the Dα1 subunit. Such a fitness cost could explain why target site resistances to neonicotinoids in pest insect populations have been associated specific amino acid replacement mutations in nAChR subunits, rather than loss of function. That mutant phenotypes were observed for the two behaviors examined indicates that the functions of Dα1, and other nAChR subunits, need to be explored more broadly. It also remains to be established whether these phenotypes were due to loss of the Dα1 receptor and/or to compensatory changes in the expression levels of other nAChR subunits.
KEYWORDS: behavior, courtship, ends-out gene targeting, nicotinic acetylcholine receptor, insecticide resistance, longevity
Introduction
Nicotinic acetylcholine receptors (nAChRs) are ligand-gated ion channels that facilitate fast-synaptic signalling in the central nervous system (CNS) in insects.1 The subunits form pentamers that, when present at the synaptic membrane, are able to bind their native ligand, acetylcholine (ACh). nAChR subunits are classified as being α or non-α. Both heteromeric and homomeric receptor subtypes have been identified in vertebrates. In D. melanogaster 10 genes encode receptor subunits (7 α and 3 β – the non-α subunits in insects).2 Considering all of the possible homomeric and heteromeric combinations, the potential exists for thousands of receptor subtypes to be produced.2,3 Binding of ACh is co-ordinated by six recognised domains (or loops) in the N-terminal, extracellular domain of neighboring subunits. Three loops, A, B and C, contribute to the α subunit primary interface when a “YXCC” motif is present at Loop C. β subunits lack this motif. Either another α or a β subunit contribute loops D, E and F to form the complementary subunit interface.4 Each nAChR protein subunit has 4 transmembrane domains (TM1->TM4). The binding of the ligand instigates a conformational change in the receptor that leads to an influx of cations through the receptor ion pore, a structure lined by the TM2 domains of the five nAChR subunits. The various combinations of receptor subunits that have been tested using electrophysiology in cell lines or Xenopus oocytes display different pharmacological responses to agonists, including Ach, and antagonists, while also differing in their channel gating kinetics.5 The roles of the insect nAChRs and how different subunits contribute to behavior remains a fertile ground for research.
Our study focussed on the phenotypic characterization of a loss of function mutation for the Dα1 nAChR subunit gene.6 Multiple phenotypes were identified. The Dα1 receptor subunit had previously been implicated in insecticide resistance phenotypes, specifically to several members of the neonicotinoid class of insecticides but, in addition, the deletion of Dα1 was shown to perturb sleep and courtship behavioral circuitry in ways that would be likely to impact fitness in a competitive field environment. In this Extra Views article we present additional data on this Dα1 knockout and discuss their implications in relation to the fitness of insects carrying resistance mutations in this gene.
Receptors and resistance
One of the first reported phenotypes associated with insect nAChRs was found in a field collected population of the Brown Planthopper, Nilaparvata lugens, that was then selected in the laboratory for 35 generations, yielding a 250 fold increase in resistance to the neonicotinoid insecticide imidacloprid. Mutations in two nAChR subunit genes, Nlα1 and Nlα3, both with the same Y151S amino acid replacement.7 This mutation would disrupt the loop B region involved in binding of ACh. Although the Nlα1Y151S allele reached fixation under lab selection, and despite the high level of resistance conferred, neither of these mutations has been detected in the field. 7 Several other nAChR subunit genes have since been associated with insecticide resistance.
In D. melanogaster, chemical mutagenesis and insecticide selection led to the isolation of mutant alleles of the Dα1 and Dβ2 subunit genes that conferred resistance to many neonicotinoids.8,9 A mutation in the β1 subunit that conferred resistance to imidacloprid was isolated from field populations of the green peach aphid, Myzus persicae. 10 This mutation produced an amino acid replacement (R81T) that would impact the loop D region of the agonist binding pocket of the receptor. While multiple resistance mechanisms were operating in these field resistant aphids, experiments partitioning out the impact of the R81T replacement mutation suggested that it had a ∼234-fold increase in imidacloprid resistance, compared to a susceptible lab aphid clone. The discovery of the spread in field populations of the R81T resistance mutation suggest that target site mutations pose a threat to the effectiveness of neonicotinoid insecticides in controlling insect pests. The majority of cases of field resistance to neonicotinoids have been associated with increased activity of drug metabolising enzymes that typically yield lower levels of resistance than is observed with mutations in nAChR targets.11 It is possible that the incidence of target site resistance, and the spectrum of mutations that confer it, are constrained by associated fitness costs. It is therefore essential that the range of functions of the nAChR subunits targeted by insecticides be examined in detail to assess the potential for such costs. Further, there has been great concern about the impact of neonicotinoid insecticides on non-target insect species, particularly behavioral changes in honeybees, so there is a need to determine how perturbations of receptor function impacts insect behavior.12,13
Creation and characterization of a Dα1 gene knockout
Ends out gene targeting was used to delete the entire 57 kb gene region of Dα1, replacing it with a white+ selectable marker cassette.6 A line containing this deletion was then crossed to a Cre recombinase line to remove the white+ cassette to establish the Dα1KO line. The first phenotypic test performed was for resistance to neonicotinoids. Results for imidacloprid (25 fold resistance), were similar to those reported for the Dα1 allele, Dα1EMS1, a frameshift mutation disrupting the TM4 domain with an additional 72 missense amino acids.9 Resistance to nitenpyram (47 fold resistant) was higher than that observed for Dα1EMS1 (13 fold resistance). These resistance ratios were calculated relative to each allele's respective susceptible genetic background strain. None of the resistance ratios are as high as those attributed to the receptor subunits mutations in N. lugens strain (both Nlα1 and Nlα3 Y151S mutations) or to the M. persicae clone carrying the R81T mutation in the β1 subunit. In the N. lugens case, this may not be surprising given that there are mutations in two genes that contribute to the overall level of resistance observed in the strain. The higher resistance conferred by the R81T mutation in β1 subunit of M. persicae, compared to the more modest effect of the Dα1KO in Drosophila might be due to larger contribution of the β1 as opposed to the α1 subunit in multimeric receptor complexes. However, it must be acknowledged that the actual level of resistance conferred by the R81T mutation is not known. In contrast to the situation here, where the only difference between Dα1KO and the control line is the deletion at the Dα1 locus, the aphid R81T resistant line and the susceptible control used for comparison did not share the same genetic background. Therefore, variation outside the Mpβ1 locus could contribute to the level of resistance observed. Indeed, studies with the cytochrome P450 inhibitor, PBO, suggest that one or more P450s may contribute to the level of resistance observed for both imidacloprid and thiamethoxam.10 The notion that resistance could be controlled by variation at multiple loci in field populations of pests is not surprising given the strong selective pressures applied with insecticides. A recent study by Zimmer et al (2016) further underlines the challenges of accurately determining the contribution of variation in single genes to resistance in situations where the genetic background is not known.14 In recapitulating the G275E mutation in the α6 gene found in field populations of F. occidentalis in D. melanogaster, it was found that the level of resistance associated with the mutation was 66-fold, compared to the >300,000 fold reported in the pest.15
In addition to nitenpyram and imidacloprid, we have examined the level of resistance to another neonicotinoid insecticide, dinotefuran. The Dα1KO mutant exhibited dinotefuran resistance, with significantly higher survival of the mutant strain compared to controls, at all doses tested (Fig. 1). There is a significant difference in the survival of both homozygotes and heterozygotes on the 0.2 ppm dose; However, the phenotype appears to be incompletely recessive, similar to other neonicotinoids with the 0.4 ppm dose clearly discriminating between the hetero and homozygous state (Fig. 1). We note that the levels of resistance to dinotefuran were far lower than those observed with imidacloprid, perhaps because dinotefuran might bind to multiple nAChR subunits with similar affinity or have additional targets that imidacloprid does not have. It should be noted that the Dα1EMS1 mutants did not exhibit resistance to dinotefuran,8 which could be explained if this mutant, in contrast to Dα1KO, possibly because the EMS induced allele is not a complete loss of function mutation.
Figure 1.

Larval insecticide bioassay of Dα1KO on the neonicotinoid insecticide, dinotefuran. Standard semolina flyfood media was dosed with appropriate volumes of 0.1% w/v dinotefuran/acetone solution. Five replicates of 50 1st instar larvae were placed into vials and were stored in the dark until adult eclosion, when they were scored. Mortality data were corrected for control mortality using Abbott's formula and plotted with 95% confidence intervals.37
Are “rescue” phenotypes using the GAL4-UAS expression system for nAChRs biologically relevant?
With the expression of a UAS-Dα1 cDNA driven in the CNS with by the pan-neuronal elav-GAL4 driver, we observed an increase in the susceptibility of the Dα1KO flies to both imidacloprid and nitenpyram (16, Fig S2). This partial rescue of the wild type (susceptibility) phenotype occurs likely because the target for these insecticides is being restored in the knockout mutant. Having validated this rescue system for insecticide resistance, we next used it to assess whether the behavioral phenotypes observed in the Dα1KO mutant were due to Dα1 loss of function.
We first used the Wiggle Index, which measures the behavioral response to insecticide exposure over time in terms of the relative movement (thrashing) of larvae, before and after addition of a insecticide (Fig. 2).17 Significant differences in the relative movement are detectable within 15 minutes of exposure to the compound (Fig. 2). In the analysis of the Dα1KO background control, larval movement is greatly reduced over time when exposed to imidacloprid, whereas a smaller reduction in movement was observed in the knockout. Relative movement is rescued to background control levels when UAS-Dα1 is driven by elav-GAL4 (Fig. 2B). Concordant with other neonicotinoid resistant Dα1 alleles, the reporter only knockout was still affected by imidacloprid demonstrated by the small, but significant reduction in relative movement relative to its movement pre-exposure. This could suggest that other neonicotinoid sensitive receptor subtypes lacking Dα1 subunits are expressed, either endogenously or as a result of the Dα1 knockout. The latter would involve the compensatory expression of other receptor subunits and the corresponding change in the pharmacology of the receptors as the cause of the phenotype. This possibility can be investigated using dominant negative subunit alleles or by constraining the receptor subtypes produced through the use of concatenated constructs with receptor subunits restricted to a specific partner, thereby only allowed to form a specific extracellular ligand binding interface, as has been performed for vertebrate subunits.18,19
Figure 2.
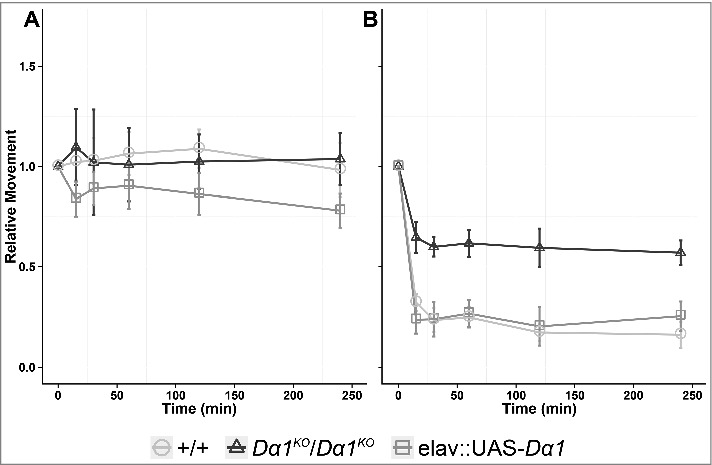
Relative movement ratio analysis of the Dα1 GAL4-UAS rescue system. Following the protocol of Denecke, et al., 2015, 3rd instar larvae were bathed in (A) 5% sucrose or (B) a 5% sucrose:25 ppm imidacloprid solution, and their movement recorded over time.17 The differences in movement relative to their starting activity is presented in the graph as a relative movement ratio. No difference is observed between the three genotypes in 5% sucrose solution across the duration of the experiment. At 25 ppm imidacloprid all three genotypes show a rapid and sustained reduction in movement. Both the background control and rescue larvae have a much greater reduction in relative movement when compared the reporter-only knockout.
Driving the expression of any gene using GAL4-UAS, whether using a ubiquitous, tissue specific or even a “native” GAL4 driver, raises the question as to whether the observed results reflect the replacement of the wild type protein, or are due to other effects, such as too much or mis-expressed protein. The results from rescue experiments for Dα1 appear to be valid, particularly given the phenotype of rescue towards a wild type phenotype.
Previous rescue studies on other nAChR subunit genes have been performed for the Dα7 subunit which is involved in the D. melanogaster escape response 20 and the Dα6 subunit, loss of which is associated with resistance to the spinosyn class of insecticides.21 In the case of Dα7, complete rescue of a loss of the long latency response in the TTM (jump muscle) was performed using a giant fibre specific c17-GAL4 line. For Dα6, the rescue of susceptibility to the insecticide spinosad was observed with both the pan-neuronal elav-GAL4 driver and a Dα6 enhancer. In the case of Dα6, the reported levels of response were similar to, but not exactly the same as in wild type. Further, the more widely and strongly expressed elav-Gal4 lead to a stronger rescue. This could indicate the enhancer region fragment from Dα6 does not include all neuronal cell types that Dα6 is normally expressed in or the level of expression is not high enough. There is also the possibility that the random insertion of the GAL4 driver into the genome impacted the expression pattern and, hence, the level of rescue. Rescue experiments need to be conservatively interpreted; However there is no current evidence that use of the GAL4-UAS expression system carries any systemic flaws that would preclude its use in investigating nAChR function.
Dα1KO flies show defects in sleep
The expression patterns of nAChRs and the distribution of cholinergic neurons in fly adult brains have been broadly examined at both the RNA and protein level.1,22–24 Expression of Dα1 was detected using immunohistochemistry in the neuropiles of the optic lobes, protocerebrum, deutocerebrum, as well as the thoracic ganglion of adults,25 localization that support a role of these receptors in important centres in the brain that control behavior. The phenotypes reported for nAChR mutants prior to our study were consistent with this, with impacts found on sleep and escape behavior. Studies on the Dα7gfA1 allele as well as on the Dα7 knockout strain found that this subunit was involved in the escape response behavior of D. melanogaster.20 Other studies associated the reduction of Dα3 expression to specific sleep neurons (using GAL4/UAS mediated RNA interference) in a short sleep mutant background (the sleepless, sssP1 allele) with an increase in sleep to wild type levels.26 Further, Dα4 protein levels were reported to cycle, dependent on sleep homeostasis rather than under the control of the circadian clock, with wild type flies having protein level peaks during afternoon and night-time sleep.27 Higher levels of Dα4 protein expression are also found in mutant backgrounds of several genes that have short sleep phenotypes, including the fumin, insomniac and sssP1 alleles. Our study used previously published methods 28 to measure sleep activity with DAM2, the Drosophila Activity Monitor (Trikinetics Inc.). The amount of night sleep of Dα1KO flies was shown to be significantly reduced due to both fewer bouts of sleep and a shorter duration of these bouts. Day sleep of the Dα1KO flies was largely unaffected. When we examined the sleep bout length for every hour of the day, it suggested mutant flies have trouble maintaining a sleep bout more than 15–20 minutes long, regardless of time of day (Fig. 3A). As reported by Faville et. al., (2015), wild type flies generally enter the deepest stage of sleep 20–40 minutes after the initiation of a bout of sleep.29 Flies are more easily aroused to an active wake state when sleep extends beyond this time. Does this mean that Da1KO flies are unable to sleep deeply? To address this question, we used the Drosophila ARousal Tracking (DART) system to measure the level of responsiveness of sleeping flies to mechanical stimuli. Overall, the knockout flies were less reactive to stimuli compared to control flies, especially during daytime (Fig. 3B). Based on these data, it appears that the loss of Dα1 function did not affect sleep intensity, but rather sleep quantity by shortening sleep bouts at night. This loss could possibly be compensated for by increasing day sleep amount or intensity. There seems to be a trend towards an increased number of sleep bouts during the day (Fig. 3C), while on the other hand, sleep was more intense than in the background control during the day for Dα1KO flies.
Figure 3.
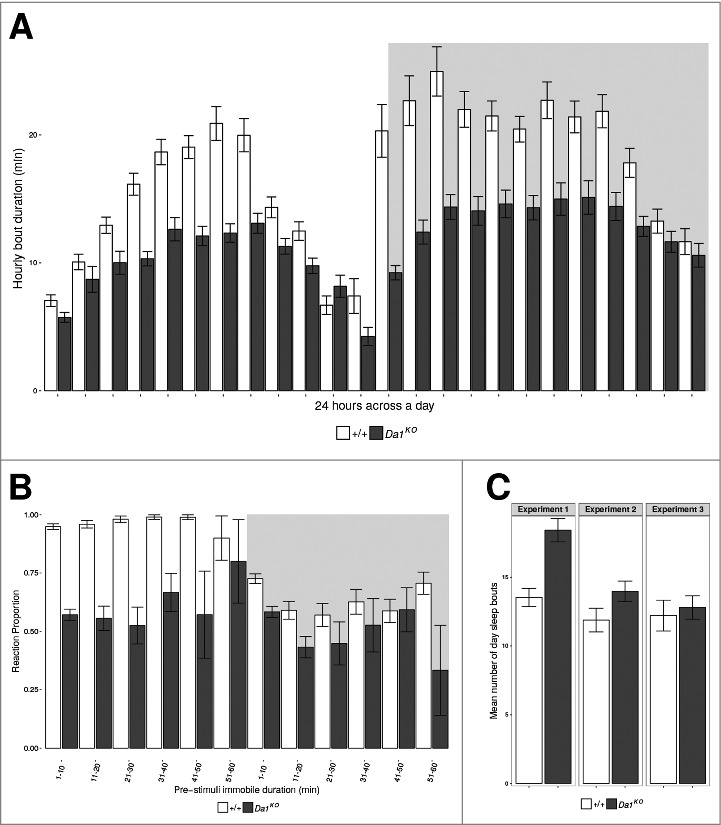
Sleep intensity of Dα1 knock-out. (A) Mean duration of sleep bouts for every hour of a day. White background: day-time, gray background: night-time. (B) Proportion of population reactive to stimuli based on pre-stimuli immobility time. White background: day-time, gray background: night-time. The longer flies had been immobile, the lower the proportion of flies that would react due to being in deeper sleep state. Wild-type flies generally have lighter sleep ie. more reactive during day-time than night-time. (C) Mean number of sleep bouts during day time across different experiments. All error bars are standard errors of the means. For (A) and (B), control n = 44, knockout n = 38. For (C), experiment 1: control n = 30, knockout n = 32, experiment 1: control n = 32, knockout n = 31, experiment 3: control n = 32, knockout n = 32.
Mating ability of Dα1KO flies is impaired
For the first time a mutation in an nAChR subunit gene was associated with changes in courtship behavior. In comparison to the 100% of wild type males that were able to initiate courtship in trials, only 65% of knockout males initiated courtship with wild type females and 79% with knockout females. Looking more closely at the time taken to initiate copulation (copulation latency), only 15% of mutant males had mated with wild type females within 10 minutes, compared to 90% for the wild type control males. When paired with knockout females, the relative percentages were 3% for knockout males and 56% for wild type control males. Thus copulation latency depended on both sexes, but the genotype of the male was the major contributing factor to this phenotype.
Dα1KO flies have significantly shorter life spans
In a standard longevity assay, adult lifespan was reduced to a 34 day median survivorship compared to 52 d for controls. It remains to be seen whether this reduction is from the cumulative impact of Dα1 loss of function across many traits or a product of only one or a few traits. Determining this will require greater understanding of other Dα1 subunit's other roles, but it is likely that the reduction in sleep is a significant contributing factor.30,31
Conclusions and future directions
Our study has demonstrated several roles of the Dα1 nAChR subunit and we also validated a GAL4-UAS rescue system that can be used in future work. We envisage future studies focussing on using this system to examining receptor expression in specific neurons for Dα1 and orthologues from other species, to dissect the impact of subunits in different neurons on behavior. Similarly, rescue systems for other nAChR subunits could assist in dissection of independent receptor roles in the insect brain and also identify the neurons critical for insect responses to insecticides. Expression of resistance alleles discovered in the field could help determine fitness costs and allow more accurate assessment of the likely persistence of the allele in the absence of the selection pressure of insecticide use.
Partial or complete loss of function α6 alleles have been shown to confer high levels of spinosad resistance in D. melanogaster and several pest species,32–34 with little or no other phenotypes observed. This is in marked contrast with the multiple mutant phenotypes observed in flies lacking the Dα1 subunit. Under field conditions, the reduction in fitness is likely to be even greater than that observed under optimal laboratory conditions. While alleles with a small reduction in α1 function may arise to confer neonicotinoid resistance, the evolution and spread of complete loss of function alleles of α1 would seem unlikely. Anecdotal evidence for this is that the N. lugens Y151S in the Nlα1 and Nlα3 subunits have not been detected in the field. Indeed, the only resistance mutation that has been reported is the R81T amino acid replacement in the β1 subunit of Myzus persicae that is likely to only impact imidacloprid binding, while retaining the capacity of the receptor to respond to ACh.7,10 This does not exclude the possibility of fitness costs associated with the M. persicae β1 allele.
There is already a large amount of literature suggesting that exposure to compounds that target the nAChRs lead to behavioral changes and it is likely that the loss or perturbed function of these receptors will affect many other behaviors that are modulated by nAChRs.35 The widespread distribution of cholinergic neurons suggests many behavioral roles. Analysis of more phenotypes, particularly testing learning and memory paradigms, are likely to provide further insights. In a recent study, Barnstedt et al., demonstrated olfactory memories are stored as plasticity of cholinergic Kenyon cell – mushroom body output neurons – and they identified roles for the Dα1, Dα4, Dα5, and Dα6 nAChR subunits in these neurons.36 More specifically, they found that RNAi knockdown of the Dα1 subunit led to a change in odour-evoked behavioral response, switching behavior of flies from avoidance to an approach behavior.
Insects have not been extensively analysed after knockout of nAChR subunits for any changes in protein levels and/or localization in other subunits. Understanding the mechanisms controlling the compensation and regulation of receptors and how this translates to different pharmacological subtypes is an important research direction to be pursued. One of the more difficult tasks is to determine whether the phenotypes observed are due to the loss of function of a single subunit, such as Dα1, or due to compensatory changes in the expression of other nAChR subunits. One approach that may assist with this is an analysis of a wider range of alleles. This could encompass resistance alleles identified in the field, together with targeted changes such as the P146S dominant negative Dα6 allele.16 Such manipulation would allow comparison of a complete knockout to a situation where the subunit has impaired function, but is still assembled into the native receptor pentamers, possibly getting around the complicating issue of compensation by other nAChR subunits. Use of knockouts as well as replacement/rescue with different receptors, or predicted orthologues from other insect species, may assist in understanding the subtle differences in receptor pharmacology that underlie changes in behavioral responses. Work along these lines will also help determine which receptors are most important for the complex behaviors of beneficial insects and could guide a more informed debate on the use of compounds that disrupt them.
Abbreviations
- nAChR
nicotinic acetylcholine receptor
- ACh
Acetylcholine
- CNS
Central Nervous System
- DART
Drosophila ARousal Tracking
Acknowledgements
The authors would like to acknowledge Australian Research Council Discovery Grant (DP120100788 – PB) and Australian Postgraduate Award (JS) and Dowd Foundation Scholarship (JS) as sources of funding for this project. We would also like to thank the editor and reviewers for their constructive input on this manuscript.
Funding
Department of Industry, Innovation, Science, Research and Tertiary Education, Australian Government | Australian Research Council (ARC)
References
- 1.Chintapalli VR, Wang J, Dow JA. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat Genet. 2007;39(6):715–20. doi: 10.1038/ng2049. [DOI] [PubMed] [Google Scholar]
- 2.Sattelle DB, Jones AK, Sattelle BM, Matsuda K, Reenan R, Biggin PC. Edit, cut and paste in the nicotinic acetylcholine receptor gene family of Drosophila melanogaster. Bioessays. 2005;27(4):366–76. doi: 10.1002/bies.20207. [DOI] [PubMed] [Google Scholar]
- 3.Schulz R, Bertrand S, Chamaon K, Smalla KH, Gundelfinger ED, Bertrand D. Neuronal nicotinic acetylcholine receptors from Drosophila: two different types of alpha subunits coassemble within the same receptor complex. J Neurochem. 2000;74(6):2537–46. [DOI] [PubMed] [Google Scholar]
- 4.Corringer PJ, Le Novere N, Changeux JP. Nicotinic receptors at the amino acid level. Annu Rev Pharmacol Toxicol. 2000;40:431–58. doi: 10.1146/annurev.pharmtox.40.1.431. [DOI] [PubMed] [Google Scholar]
- 5.Lansdell SJ, Millar NS. The influence of nicotinic receptor subunit composition upon agonist, alpha-bungarotoxin and insecticide (imidacloprid) binding affinity. Neuropharmacology. 2000;39(4):671–9. doi: 10.1016/S0028-3908(99)00170-7. [DOI] [PubMed] [Google Scholar]
- 6.Somers J, Luong HN, Mitchell J, Batterham P, Perry T. Pleiotropic Effects of Loss of the Dalpha1 Subunit in Drosophila melanogaster: Implications for Insecticide Resistance. Genetics. 2017;205(1):263–271. doi: 10.1534/genetics.116.195750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Z, Williamson MS, Lansdell SJ, Denholm I, Han Z, Millar NS. A nicotinic acetylcholine receptor mutation conferring target-site resistance to imidacloprid in Nilaparvata lugens (brown planthopper). Proc Natl Acad Sci U S A. 2005;102(24):8420–5. doi: 10.1073/pnas.0502901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perry T, Chan JQ, Batterham P, Watson GB, Geng C, Sparks TC.. Effects of mutations in Drosophila nicotinic acetylcholine receptor subunits on sensitivity to insecticides targeting nicotinic acetylcholine receptors. Pesticide Biochemistry and Physiology. 2012;102(1):56–60. doi: 10.1016/j.pestbp.2011.10.010. [DOI] [Google Scholar]
- 9.Perry T, Heckel DG, McKenzie JA, Batterham P. Mutations in Dalpha1 or Dbeta2 nicotinic acetylcholine receptor subunits can confer resistance to neonicotinoids in Drosophila melanogaster. Insect Biochem Mol Biol. 2008;38(5):520–8. doi: 10.1016/j.ibmb.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Bass C, Puinean AM, Andrews M, Cutler P, Daniels M, Elias J, Paul VL, Crossthwaite AJ, Denholm I, Field LM et al.. Mutation of a nicotinic acetylcholine receptor beta subunit is associated with resistance to neonicotinoid insecticides in the aphid Myzus persicae. BMC Neurosci. 2011;12:51. doi: 10.1186/1471-2202-12-51. PMID:21627790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bass C, Denholm I, Williamson MS, Nauen R. The global status of insect resistance to neonicotinoid insecticides. Pestic Biochem Physiol. 2015;121:78–87. doi: 10.1016/j.pestbp.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Gill RJ, Ramos-Rodriguez O, Raine NE. Combined pesticide exposure severely affects individual- and colony-level traits in bees. Nature. 2012;491(7422):105–8. doi: 10.1038/nature11585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams GR, Troxler A, Retschnig G, Roth K, Yañez O, Shutler D, Neumann P, Gauthier L. Neonicotinoid pesticides severely affect honey bee queens. Sci Rep. 2015;5:14621. doi: 10.1038/srep14621. PMID:26459072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zimmer CT, Garrood WT, Puinean AM, Eckel-Zimmer M, Williamson MS, Davies TG, Bass C. A CRISPR/Cas9 mediated point mutation in the alpha 6 subunit of the nicotinic acetylcholine receptor confers resistance to spinosad in Drosophila melanogaster. Insect Biochem Mol Biol. 2016;73:62–9. doi: 10.1016/j.ibmb.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puinean AM, Lansdell SJ, Collins T, Bielza P, Millar NS. A nicotinic acetylcholine receptor transmembrane point mutation (G275E) associated with resistance to spinosad in Frankliniella occidentalis. J Neurochem. 2013;124(5):590–601. doi: 10.1111/jnc.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Somers J, Nguyen J, Lumb C, Batterham P, Perry T. In vivo functional analysis of the Drosophila melanogaster nicotinic acetylcholine receptor Dα6 using the insecticide Spinosad. Insect Biochem Mole Biol. 2015. doi: 10.1016/j.ibmb.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 17.Denecke S, Nowell CJ, Fournier-Level A, Perry T, Batterham P. The Wiggle Index: An Open Source Bioassay to Assess Sub-Lethal Insecticide Response in Drosophila melanogaster. PLoS One. 2015;10(12):e0145051. doi: 10.1371/journal.pone.0145051. PMID:26684454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carbone AL, Moroni M, Groot-Kormelink PJ, Bermudez I. Pentameric concatenated (alpha4)(2)(beta2)(3) and (alpha4)(3)(beta2)(2) nicotinic acetylcholine receptors: subunit arrangement determines functional expression. Br J Pharmacol. 2009;156(6):970–81. doi: 10.1111/j.1476-5381.2008.00104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groot-Kormelink PJ, Broadbent S, Beato M, Sivilotti LG. Constraining the expression of nicotinic acetylcholine receptors by using pentameric constructs. Mol Pharmacol. 2006;69(2):558–63. [DOI] [PubMed] [Google Scholar]
- 20.Fayyazuddin A, Zaheer MA, Hiesinger PR, Bellen HJ. The nicotinic acetylcholine receptor Dalpha7 is required for an escape behavior in Drosophila. PLoS Biol. 2006;4(3):e63. doi: 10.1371/journal.pbio.0040063. PMID:16494528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perry T, Somers J, Yang YT, Batterham P. Expression of insect α6-like nicotinic acetylcholine receptors in Drosophila melanogaster highlights a high level of conservation of the receptor:spinosyn interaction. Insect Biochem Mole Biol. 2015;64:106–15. doi: 10.1016/j.ibmb.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 22.Salvaterra PM, Kitamoto T. Drosophila cholinergic neurons and processes visualized with Gal4/UAS-GFP. Brain Res Gene Expr Patterns. 2001;1(1):73–82. doi: 10.1016/S1567-133X(01)00011-4. [DOI] [PubMed] [Google Scholar]
- 23.Schloss P, Betz H, Schröder C, Gundelfinger ED. Neuronal nicotinic acetylcholine receptors in Drosophila: antibodies against an alpha-like and a non-alpha-subunit recognize the same high-affinity alpha-bungarotoxin binding complex. J Neurochemis. 1991;57(5):1556–62. doi: 10.1111/j.1471-4159.1991.tb06351.x. [DOI] [PubMed] [Google Scholar]
- 24.Schulz R, Sawruk E, Mülhardt C, Bertrand S, Baumann A, Phannavong B, Betz H, Bertrand D, Gundelfinger ED, Schmitt B. D alpha3, a new functional alpha subunit of nicotinic acetylcholine receptors from Drosophila. J Neurochem. 1998;71(2):853–62. [DOI] [PubMed] [Google Scholar]
- 25.Schuster R, Phannavong B, Schröder C, Gundelfinger ED. Immunohistochemical localization of a ligand-binding and a structural subunit of nicotinic acetylcholine receptors in the central nervous system of Drosophila melanogaster. J Comp Neurol. 1993;335(2):149–62. doi: 10.1002/cne.903350202. [DOI] [PubMed] [Google Scholar]
- 26.Wu M, Robinson JE, Joiner WJ. SLEEPLESS is a bifunctional regulator of excitability and cholinergic synaptic transmission. Curr Biol. 2014;24(6):621–9. doi: 10.1016/j.cub.2014.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi M, Yue Z, Kuryatov A, Lindstrom JM, Sehgal A. Identification of Redeye, a new sleep-regulating protein whose expression is modulated by sleep amount. Elife. 2014;3:e01473. doi: 10.7554/eLife.01473. PMID:24497543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilestro GF, Cirelli C. pySolo: a complete suite for sleep analysis in Drosophila. Bioinformatics. 2009;25(11):1466–7. doi: 10.1093/bioinformatics/btp237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faville R, Kottler B, Goodhill GJ, Shaw PJ, van Swinderen B. How deeply does your mutant sleep? Probing arousal to better understand sleep defects in Drosophila. Sci Rep. 2015;5:8454. doi: 10.1038/srep08454. PMID:25677943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koh K, Evans JM, Hendricks JC, Sehgal A. A Drosophila model for age-associated changes in sleep:wake cycles. Proc Natl Acad Sci U S A. 2006;103(37):13843–7. doi: 10.1073/pnas.0605903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaw PJ, Tononi G, Greenspan RJ, Robinson DF. Stress response genes protect against lethal effects of sleep deprivation in Drosophila. Nature. 2002;417(6886):287–91. doi: 10.1038/417287a. [DOI] [PubMed] [Google Scholar]
- 32.Baxter SW, Chen M, Dawson A, Zhao JZ, Vogel H, Shelton AM, Heckel DG, Jiggins CD. Mis-spliced transcripts of nicotinic acetylcholine receptor alpha6 are associated with field evolved spinosad resistance in Plutella xylostella (L.). PLoS Genet. 2010;6(1):e1000802. doi: 10.1371/journal.pgen.1000802. PMID:20062520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsu JC, Feng HT, Wu WJ, Geib SM, Mao CH, Vontas J. Truncated transcripts of nicotinic acetylcholine subunit gene Bdalpha6 are associated with spinosad resistance in Bactrocera dorsalis. Insect Biochem Mol Biol. 2012;42(10):806–15. doi: 10.1016/j.ibmb.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 34.Perry T, McKenzie JA, Batterham P. A Dalpha6 knockout strain of Drosophila melanogaster confers a high level of resistance to spinosad. Insect Biochem Mol Biol. 2007;37(2):184–8. doi: 10.1016/j.ibmb.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 35.Williamson SM, Wright GA. Exposure to multiple cholinergic pesticides impairs olfactory learning and memory in honeybees. J Exp Biol. 2013;216(Pt 10):1799–807. doi: 10.1242/jeb.083931. PMID:23393272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barnstedt O, Owald D, Felsenberg J, Brain R, Moszynski JP, Talbot CB, Perrat PN, Waddell S. Memory-Relevant Mushroom Body Output Synapses Are Cholinergic. Neuron. 2016;89(6):1237–47. doi: 10.1016/j.neuron.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenheim JA, Hoy MA. Confidence Intervals for the Abbott's Formula Correction of Bioassay Data for Control Response. Journal of Economic Entomology. 1989;82(2):331–335. doi: 10.1093/jee/82.2.331. [DOI] [Google Scholar]


