ABSTRACT
Introduction: Soft tissue Sarcomas (STS) are rare malignances, with high mortality rates. Half of patients develop metastasis. The presence of isolated Circulating Tumor Cells (CTCs) and Circulating Tumor Microemboli (CTM) in the blood may be early markers of tumor invasion. Epidermal Growth Factor (EGF) family receptors can also influence this process.
Objectives: to quantify CTCs and identify CTM as well as the EGF Receptor (EGFR) protein expression in these cells and correlate with clinical outcome in metastatic STS.
Materials and methods: Approximately 8mL of blood was prospectively collected from patients with different types of high-grade STS, before the beginning of chemotherapy. The samples were processed and filtered by ISET (Rarecells, France) for the isolation and quantification of CTCs and CTMs. EGFR expression was analyzed by immunocytochemistry (ICC) on CTCs/ CTMs.
Results: We analyzed 18 patients with median age of 49 years (18-77 y). The positivity for EGFR protein expression in CTCs was observed in 93.75% of the patients. This result shows that targeting EGFR positive CTCs from STS origen can be translated in clinical benefit for some patients. In addition, if target therapy is chosen, the EGFR expression in CTCs can be used in follow-up to measure treatment effectiveness.
Conclusions: This is the first study to demonstrate the expression of EGFR protein in CTCs from sarcoma patients. It may open an area for future investigations. The next step is to characterize CTCs in a larger cohort of patients to better understand the role of EGFR in sustaining tumor metastasis in sarcomas.
KEYWORDS: circulating tumor cells, circulating tumor microemboli, EGFR, soft-tissue-sarcomas
Introduction
Soft Tissue Sarcomas (STS) are a heterogenous group of neoplasms. Originated from mesenchymal tissue, these tumors have different morphological patterns.1 STS represent 1% of all cancers in adults and occur in fat, nerves, blood vessels, muscles and deep skin tissues.2 Despite the low incidence of these tumors, occurrence is more common in adolescents and young adults in comparison to other cancers, as a consequence, STS can harm individuals in their most productive period of life. STS are mainly treated and cured by surgery. Radiation therapy decreases the local recurrence chance. Adjuvant chemotherapy offers modest improvement in overall survival. Dissemination of STS occurs predominantly by angiovascular pathway. Lymph node metastases are rare. The most frequent site of distant relapse is the lung.
Current methods to detect recurrence or metastasis in STS patients are radiological exams. Computed Tomography (CT) scan, Magnetic Resonance Imaging (MRI) and Positron Emission Tomography (PET) are the most used technologies and, in general, can detect disease in advanced stages or macrometastasis. However, these methods are limited by the tumor size, and are able to detect the presence of disease when the metastasis reaches 1 cm3 or more.3
The development of new methods to early detection of recurrence and metastasis could change the manner STS are managed. Early detection of presence of tumor cells could impact the dosage and even the type of systemic therapy for each individual patient.
The presence of isolated Circulating Tumor Cells (CTCs) and/or Circulating Tumor Microemboli (CTM) in the blood of patients may be early markers of tumor invasion and dissemination. It has been demonstrated that these cells circulate in the blood for months or year(s) before the development of macrometastases.4,3,5 In contrast to carcinomas, few studies have examined the detection of CTCs and CTMs in sarcomas,6 due to the mesenchymal features of these tumors and the fact that the methods for detecting cells in the blood without epithelial markers are scarce. Most methods for detection of CTCs were initially developed and validated to detect carcinomas, by the use of epithelial markers to distinguish CTC from leucocytes. The “isolation by size of epithelial tumor cells” (ISET) technique, which consists on polycarbonate filters with circular pores of 8 μm diameter for CTC enrichment and cytological detection from blood samples, has being widely used. Some authors have addressed the high sensitivity and specificity of the method, and others have been comparing the ISET with antibody-based methodologies, showing ISET's higher sensitivity and specificity.7,8
Some clinical trials that included patients with carcinomas have demonstrated that the presence of malignant cells in the peripheral blood is associated with poor prognosis.9 However, only a few studies have shown the prognostic role of CTM in solid tumors (colon, prostate, kidney, non-small cell lung cancer and head and neck).10-16
CTMs are clusters of three or more CTCs that can play an important role in metastatic process.17 CTMs provide a cell-cell adhesion advantage against shear stress in the blood stream and activate signaling for anti-apoptosis and protection from anoikis.14
In addition to CTCs and CTMs, other factors influence the process of metastases such as the overexpression of EGFR family receptors, found in various cell types including those of epithelial, mesenchymal and neuronal origin. This family of receptors includes Her1 (EGFR, ErbB1), Her2 (Neu,ErbB2), Her3 (ErbB3), and Her4 (ErbB4).18 EGFR/ErbB signaling has been involved in cell proliferation, migration, motility and invasion of malignant cells.19 EGFR is overexpressed in many cancers, including HNSSC (Head and Neck Squamous Cell Carcinoma) and lung cancer, as well as in gastrointestinal tumors. Several studies evaluating the association between EGFR overexpression and survival rate have been reported. The increase in the tumoral EGFR protein expression is associated with reduced survival in solid tumors.20-23 However, there are no studies about the expression of this protein in CTCs from sarcoma patients.
Therefore, the aim of this study was to evaluate the prognostic impact of CTCs and CTMs in sarcomas patients, and also to analyse EGFR protein expression in these cells and to correlate with clinical outcome.
Materials and methods
Patients and samples
For the analysis of CTCs by the ISET® (Rarecells Diagnostics, Paris, France) peripheral blood samples were obtained from patients with metastatic STS, before the beginning of chemotherapy. All patients were treated at the A.C. Camargo Cancer Center, São Paulo, Brazil and were included in this study between August 2015 and December 2016. Written informed consent was obtained from the patients prior to any test. This study was approved by the local Research Ethics Committee (CEP protocol 2081/15). Inclusion criteria were: age > 18 y old; one of the four high grade histology subtypes (synovial, pleomorphic, leyomisosarcoma and liposarcoma); first or second line of palliative chemotherapy; presence of metastatic disease detected by conventional imaging methods; performance status < 2; candidates to receive active chemotherapy regimens (antracyclin and non-antracyclin containing protocol). After accrual, patients were classified according to the line of treatment: first or second line. Blood sample collection was performed prior to the initiation of palliative chemotherapy.
The CTC and CTM were correlated with Progression-Free Survival (PFS). Conventional response was assessed by Response Evaluation Criteria In Solid Tumors (RECIST) criteria.
ISET assay
Blood samples were drawn in EDTA tubes (BD Vacutainer®) with immediate gentle agitation after blood collection. If samples were not processed immediately after blood withdrawal, the tubes were left on a blood homogenizer at room temperature until processing within 4 hours after blood collection.
The ISET assay was performed as described previously.24 The samples were processed on platform as manufacturer's instructions. Eight mL of whole blood was diluted up to 80 mL with buffer containing 0.02% formaldehyde incubated for 10 min at room temperature and filtered through a membrane having 8 µm pore size. To preserve cell integrity, the filtration pressure was optimized to −10 kPa. The membrane was then washed once with phosphate-buffered saline (PBS). After processing, filters were dried, wrapped in an aluminum sheet and stored frozen at -20 °C until use.
Immunocytochemistry
The spots membranes were submitted to dual color immunocytochemistry (ICC) (DAB+/Permanent Red; DakoTM) on 24 wells plate. Antigen retrieval was then performed using Antigen Retrieval Solution (DakoTM). Cells were hydrated with tris-buffered saline (TBS) 1X for 20 min and permeabilized with TBS + Triton X-100 for 5 min and endogenous peroxides were blocked with 3% hydrogen peroxide in the dark for 15 min. The spots were incubated with antibodies diluted on TBS 10% fetal calf serum. To amplify the antibody signal, the spots were incubated with Envision G/2 Doublestain System, Rabbit/Mouse (DakoTM) followed by 10 min of incubation with DAB+/Permanent Red; (DakoTM). The spots were then washed with PBS between the steps. Cells were stained with hematoxylin and analyzed by light microscope (BX61-Olympus). To distinguish CTCs and CTMs from white blood cells, it was used anti-CD45 antibody (1:100 – CusaBio, Polyclonal antibody, Lot: G0227Y). CTCs were characterized based on the following criteria: negative staining for CD45, nucleus size ≥ 12 µm, hyperchromatic and irregular nucleus, visible presence of cytoplasm, and a high nucleus–cytoplasm ratio (80%).15 Cell clusters were considered as CTM if they contained three or more CTCs.25 EGFR antibody (1:100 – CusaBio Polyclonal antibody, Lot: C041A) was used to search for EGFR expression on CTCs and CTMs. Negative and positive controls were performed for each ICC staining. For negative controls, cell line A549 spiked in healthy blood was used as follow: by omitting the primary antibody, to ensure the exclusion of cross-reactivity; and by including the primary antibody, to guarantee the specificity of the antibody, as it is known that A549 do not express EGFR. For positive control we used FaDu cell line, which accordingly to The Human Protein Atlas (http://www.proteinatlas.org/) expresses EGFR protein. Both cell lines were acquired from ATCC® HTB-43™.
For EGFR expression analysis, cells were classified according to staining. No staining was considered negative and any staining was classified as positive.
Statistical analysis
For statistical analysis, a description of each group was performed according to clinical and pathologic characteristics. The determination of two groups of observations with respect to a cut-off was estimated using the maximum of the standardized log-rank statistic proposed by Lausen & Schumacher.26 The positivity ratio of EGFR was calculated using the number of EGFR-positive CTCs present on ISET membrane spot divided by the total CTCs present in the same spot. PFS was defined as the beginning of palliative chemotherapy and first detection of progression of the disease. It was obtained using the Kaplan-Meier method and the difference between curves was compared using the log-rank method. The PFS was correlated to EGFR staining and line of treatment. The Statistical analysis was performed using SPSS software for Windows, version 15. The p value was considered significant if ≤ 0.05.
Results
A total of 18 patients were included in this study. Clinical and pathological characteristics were obtained from medical records and are summarized in Table 1. There were 10 male and 8 female patients, with median age of 49.3 y (18–77 y). There were included 7 synovial sarcoma (38.8%), 5 pleomorphic sarcoma (22.2%), 4 leiomyosarcoma (27.7%), one liposarcoma (5.5%). One patient initially diagnosed as leiomyosarcoma was, in fact, better classified as rhabdomyosarcoma after pathologic review.
Table 1.
Sarcoma patients' clinic-pathological characteristics.
| Variable | Nº. | % |
|---|---|---|
| Total number of patients | 18 | 100 |
| Age at entry study, y | ||
| Median (range) | 49.33 (18 – 77) | |
| Gender | ||
| Male | 10 | 55.55 |
| Female | 8 | 44.44 |
| Histological subtype | ||
| Synovial Sarcoma | 7 | 38.88 |
| Leiomyosarcoma | 5 | 27.77 |
| Pleomorphic Sarcoma | 4 | 22.22 |
| Liposarcoma | 1 | 5.55 |
| Rhabdomyosarcoma | 1 | 5.55 |
| Progression of disease after CTC collection | ||
| No | 9 | 50 |
| Yes | 9 | 50 |
| Median CTC/mL number (range) | 2.0 (0 –11.0) | |
| CTM baseline | ||
| No | 13 | 72.22 |
| Yes | 5 | 27.77 |
Regarding systemic treatment, 10 patients received first line chemotherapy to treat metastatic disease and 8 patients received second or third line chemotherapy. The combination of Antracyclin (Doxorubicin or Epirubicin) plus Ifosfomide was used in three patients in first line and none in second and third line. Gemcitabine + Docetaxel were used as second line in two patients. Regimens and lines of treatment are summarized in Table 2.
Table 2.
Patients' treatment, outcome and CTCs counting and characterization.
| Patient ID | Histological subtype | Positivity ratio EGFR | Number of EGFR positive CTCs in 1ml of blood | Number of EGFR negative CTCs in 1ml of blood | CTC/1ml | CTM | Collection before the first-line treatment | Treatment received after CTC collection | Line of treatment |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Pleomorphic Sarcoma | 1 | 4 | 0 | 1.25 | – | Yes | Gemcitabine + Docetaxel | 1st |
| 2 | Rhabdomyosarcoma | 1 | 3 | 0 | 1 | – | Yes | Ifosfamide + Doxorubicin | 1st |
| 3 | Liposarcoma | 1 | 5 | 0 | 11.25 | + | Yes | Doxorubicin Monotherapy | 1st |
| 4 | Synovial Sarcoma | 0.7 | 5 | 1 | 4 | + | Yes | Ifosfamide + Etoposide | 1st |
| 5 | Pleomorphic Sarcoma | 1 | 2 | 0 | 4.5 | + | Yes | Epirubicin + Ifosfamide | 1st |
| 6* | Leiomyosarcoma | 1 | 1 | 0 | 0.75 | – | No | Ifosfamide + Etoposide | 2nd |
| 7* | Pleomorphic Sarcoma | 0.1 | 2 | 12 | 6.25 | + | No | Dacarbazine + Gemcitabine | 3rd |
| 8 | Leiomyosarcoma | 0.8 | 5 | 1 | 2 | – | Yes | Ifosfamide + Doxorubicin | 1st |
| 9* | Leiomyosarcoma | 0.3 | 1 | 2 | 0.75 | – | No | Doxorubicin + Dacarbazine | 2nd |
| 10 | Synovial Sarcoma | 0 | 0 | 7 | 2 | – | No | Ifosfamide | 2nd |
| 11 | Synovial Sarcoma | 1 | 2 | 0 | 1 | – | Yes | Epirubicin + Ifosfamide | 1st |
| 12 | Leiomyosarcoma | 1 | 1 | 0 | 0.93 | – | Yes | Dacarbazine Monotherapy | 1st |
| 13 | Synovial Sarcoma | – | – | – | 0 | – | Yes | Ifosfamide + Doxorubicin | 1st |
| 14 | Synovial Sarcoma | 0.5 | 1 | 1 | 1.87 | – | No | Ifosfamide | 2nd |
| 15 | Leiomyosarcoma | 0.5 | 2 | 2 | 9.6 | – | No | Epirubicin + Ifosfamide | 2nd |
| 16 | Synovial Sarcoma | – | – | – | 0 | – | No | Ifosfamide | 3rd |
| 17 | Synovial Sarcoma | 0.5 | 2 | 2 | 5 | + | Yes | Epirubicin + Ifosfamide | 1st |
| 18 | Pleomorphic Sarcoma | 0.5 | 2 | 2 | 5.66 | – | No | Doxorubicin | 2nd |
Positivity Ratio: The positivity ratio of EGFR was calculated as the number of EGFR-positive CTCs present on ISET membrane spot divided by the total CTCs present in the same spot.
CTC count per mL of blood: CTCs were counted in four spots of the membrane, which corresponds to 4 mL blood. After counting we calculated the mean of these four spots to obtain the amount of CTCs per1 mL of blood, according to Krebs et al. (2012).15
patients that underwent resection of primary tumor and metastasis in first line treatment were excluded from the progression-free survival analysis.
CTCs were detected in 17 patients (94.4%). The median number of CTCs detected by ISET® in these patients was 2.0 CTC/mL (0–11 CTCs/mL). CTMs were found in 5 patients (27.7%). CTCs and CTMs are shown in Fig. 1. Patients treated in first line had a median count of 1.93 CTC/ml and 1.62 CTC/ml in second or third line chemotherapy.
Figure 1.
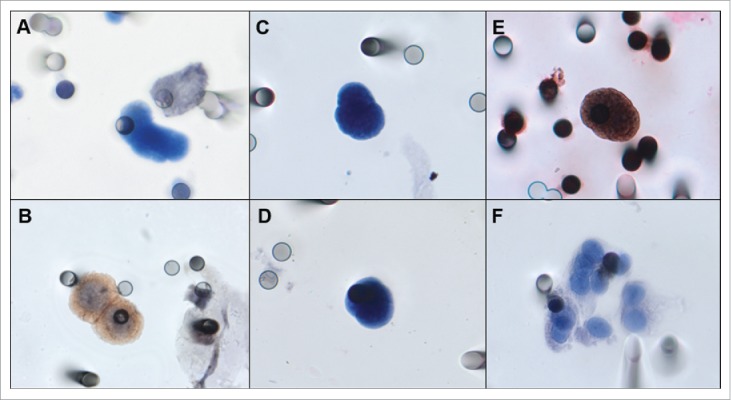
A) Negative control, A-549 cell line “spiked” in healthy blood and negative for EGFR. B) Positive control, FaDu cell line “spiked” in healthy blood and stained for EGFR. C, D) Examples of an isolated CTC of sarcoma patient with cytomorphological features (negative staining for CD45, nucleus size ≥ 12 µm, hyperchromatic and irregular nucleus, visible presence of cytoplasm, and a high nucleus–cytoplasm ratio (Krebs, et al., 2012)15. E) Immunocytochemistry of CTC with anti-EGFR antibody and counterstaining with DAB. F) One CTM from STS patient observed in the blood filtered using the ISET.
Three patients that underwent resection of primary tumor and metastasis were excluded from the statistical analysis of PFS. The median PFS of the remaining 15 patients was 7.7 months (0–11.0 months).
We classified patients as positive and negative for CTM. Although patients with positive CTM in the blood had inferior median PFS time (5.0 months versus not reached; p = 0.724) in relation to those without CTM, it was not statistically significant (Table 2).
We hypothesized that patients in first line had low CTCs count. Based on this, we analyzed the first-line patients separately and established the cut off level of 1 CTC per ml for positivity and absence of CTC as negative. We observed that patients with presence of CTCs had inferior median PFS, when compared to those with absence of these cells, but without statistical significance (p = 0.317).
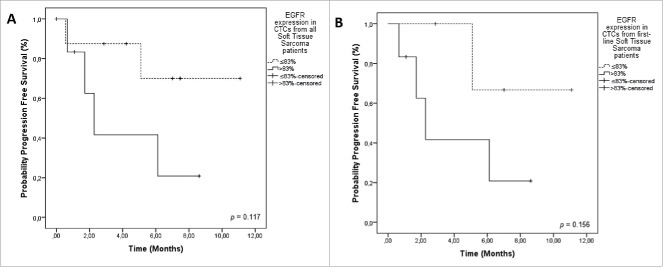
Among the 18 patients evaluated for EGFR expression, two did not have any CTC at the spot analysed. From the 16 patients that had CTCs, 15 were positive for EGFR expression (93.75%). EGFR was negative in 3 patients with synovial sarcoma subtype. We established a cut-off for EGFR expression in CTCs and classified patients as positive and negative for its expression using the maximum of the standardized log-rank statistic proposed by Lausen and Schumacher (1992) (> 83% of expression = positive; ≤ 83% of expression = negative). We calculated PFS only for the 15 patients not submitted to surgery. The PFS of patients with positive EGFR compared to negative ones was 2.2 months x NR (not reached) (p = 0.117). We also analyzed the expression of EGFR in first-line patients. The PFS of first line patients with EGFR + CTCs was 2.2 months versus NR, for EGFR- CTCs (p = 0.156). Although without reaching statistical significance, the curves are clearly separated, indicating a possible correlation of EGFR expression in CTCs with poor prognosis (Table 2 and Fig. 2). No CTM presented staining for EGFR.
Figure 2.
Progression-Free Survival (PFS) in relation to EGFR staining on CTCs from sarcoma patients. A) PFS of all patients included. EGFR expression in STS patients (> 83% = positive EGFR staining on CTCs 2.2 months); ≤ 83% = negative EGFR staining on CTCs (NR) (p = 0.117). B) PFS including only patients treated in first line. EGFR + CTCs was 2.2 months versus NR, for EGFR- CTCs (p = 0.156). Notes: Dotted line: patients without expression of EGFR. Continuous line: patients with expression of EGFR. NR = not reached.
Discussion
Sarcomas are considered relatively rare neoplasms. Despite the poor prognosis of these neoplasms, there are few studies evaluating the role of liquid biopsy and correlating with outcome. Recently, Nicolazzo & Gradilone reported the difficulty found in the detection of CTC and also mentioned the importance of these cells for STS patients, opening a new scenario for research and improvement in the management of patients with sarcomas.27 Previously, we have reported that isolation, detection, and characterization of CTCs from the blood of patients with STS is feasible using a size-based/cytoplathological approach instead of polymerase chain reaction (PCR)-based molecular tests.24 Moreover, in this study, our group also showed the sensitivity and specificity of ISET, by counting the cells of culture lineage before filtering and testing blood from healthy patients.
In the present study, we used ISET to search for CTCs from patients with metastatic sarcoma, to detect the presence of CTM and the expression of EGFR protein in CTCs/CTM. The high detection of CTCs in metastatic STS patients (94.4%) found here are promising, considering the difficulty in the follow up of patients with this disease.
The presence of CTCs and CTM and their relation to tumor progression have been observed in some studies, such as small-cell lung cancer and metastatic melanoma, showing their relation to poor prognosis.14,28 We observed CTM in 5 patients with a detection rate of 27,7%.
The detection of CTMs were correlated to poor outcome in metastatic breast cancer and metastatic castration-naïve prostate cancer and by using another method, it was shown that CTM represents around 2 to 5% of CTCs.29,30
Using ISET, Hou et al. demonstrated that 26% of patients with small cell lung cancer have CTM, a percentage similar to our results with STS (27.7%).14 Although CTM have not the same magnitude in prognosis such as CTC, these authors found that the cell clusters were correlated to poor PFS. Patients with CTM had median PFS of 4.6 months, compared to those with no CTMs with 8.2 months. Our findings are also in agreement with a study by Long et al., with metastatic melanoma patients.28 CTCs were detected in 85% of their patients, 34% with 2 to 6 CTMs. Overall Survival (OS) was significantly worse in patients with CTMs, independently of the therapeutic strategy (p < 0.001 for dacarbazine-treated patients and p = 0.0064, for dacarbazine plus vemurafenib -treated patients).
Our study is the first to explore the presence of EGFR protein in CTCs from patients with STS. The positivity for EGFR protein in CTCs was observed in 93.75% of the patients (15 of 16).
EGFR expression is a strong prognostic feature in multiple solid tumor types. Targeting EGFR is a strong therapeutic option for the treatment of many tumors currently.31 However, the role of EGFR in sarcoma is still unclear. In a study conducted by Sato et al., the authors analyzed the expression of epidermal growth factor receptor (EGFR) in formalin-fixed primary tumor of 281 patients with STS and observed positive staining in 168 of 281 (60%) patients.32 The overexpression of EGFR was significantly associated with high histological grade (p = 0.001).32 Yang et al., found promising results in STS tissues, as EGFR was expressed in 36/46 of STS samples distributed among different histological subtypes.33
In a recent study, Sannino, et al., suggested that certain sarcoma subtypes reside in a “metastable” state characterized by the expression of both epithelial and mesenchymal features. They proposed that certain sarcoma subtypes can suffer EMT/MET-related processes and that the activation of EMT/MET-related programs can lead to reversible phenotypic changes with specific stimuli.34 Moreover, they described that “metastable” phenotype may allow individual tumor cells to acquire the characteristics of more differentiated epithelial or mesenchymal cells, and the molecular heterogeneity could lead to highly aggressive clinical behavior in sarcomas patients, because the entire tumor will take advantage of both the EMT- and MET related biological features.
The epithelial-to-mesenchymal transition (EMT) is a reversible process, where epithelial cells reduce their intercellular adhesions and proliferative capacity while gaining a mesenchymal phenotype with migratory and invasive properties.34 The potential existence of EMT-related process in sarcomas allow them to be mesenchymal or epithelial under specific conditions, with important clinical implications. EGFR is a key factor in epithelial malignancies, and its activity enhances tumor growth, invasion, and metastasis. We believe that our findings with the EGFR protein reinforce the idea of EMT process in CTCs from sarcoma patients and its interference on the metastatization process. As the expression of EGFR by tumors typically confers a more aggressive phenotype, we believe that the same process occurs in CTCs from sarcoma, since these cells undergo numerous transformations along their trajectory.
The absence of EGFR expression on CTMs needs to be deeply explored. We believe that the protection conferred by the microembolus structure inhibits the initiation of the EGFR expression. Within the CTM structure, CTCs are no longer exposed to external factors, such as cytokines (which may be involved in EGFR expression) while being protected by host cells, such as endothelial cells, neutrophils, monocytes, and platelets, which can add protection against external agents and another immune system-related cells into blood circulation.
Considering that the main treatment to STS is surgical removal, and that these tumors are difficult to treat with chemotherapy, our results are encouraging and point new target for sarcoma treatment. We hypothesized that EGFR expression by CTCs of non-epithelial tumors can explain the process of invasion and dissemination. Maybe, in the future, targeting EGFR positive CTC from STS origen could translate in clinical benefit for some patients.
CTCs, CTM and EGFR expression in these cells can be used as tools to measure the effectiveness of treatment and also better select patients for clinical intervention. Studies with a larger cohort of patients, with well-defined treatment and follow up are necessary to confirm our data and to evaluate the role of CTC, CTM and EGFR expression for STS patients, as well as the clinical impact of our findings.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank National Institute for Science and Technology in Oncogenomics and Therapeutic Innovation (INCT FAPESP/CNPq 2014/50943-1) and Coordination for the Improvement of Higher Education Personnel (CAPES 1548678) for the financial support.
References
- 1.Singer S, Demetri GD, Baldini EH, Fletcher CD. Management of soft-tissue sarcomas: an overview and update. Lancet Oncol. 2000;1:75–85. doi: 10.1016/S1470-2045(00)00016-4. PMID:11905672 [DOI] [PubMed] [Google Scholar]
- 2.Mackall CL, Meltzer PS, Helman LJ. Focus on sarcomas. Cancer Cell. 2002;2(3):175–8. doi: 10.1016/S1535-6108(02)00132-0. PMID:12242149 [DOI] [PubMed] [Google Scholar]
- 3.Klein CA. Parallel progression of primary tumours and metastases. Nat Rev Cancer. 2009;9(4):302–12. doi: 10.1038/nrc2627. PMID:19308069 [DOI] [PubMed] [Google Scholar]
- 4.Paterlini-Brechot P, Benali NL. Circulating tumor cells (CTC) detection: clinical impact and future directions. Cancer Lett. 2007;253(2):180–204. doi: 10.1016/j.canlet.2006.12.014. PMID:17314005 [DOI] [PubMed] [Google Scholar]
- 5.Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK, Vonderheide RH, et al.. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148(1–2):349–61. doi: 10.1016/j.cell.2011.11.025. PMID:22265420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang L, Asatrian G, Dry SM, James AW. Circulating tumor cells in sarcomas: a brief review. Med Oncol Northwood Lond Engl. 2015;32(1):430. doi: 10.1007/s12032-014-0430-9. [DOI] [PubMed] [Google Scholar]
- 7.Vona G, Sabile A, Louha M, Sitruk V, Romana S, Schütze K, Capron F, Franco D, Pazzagli M, Vekemans M, et al.. Isolation by size of epithelial tumor cells. Am J Pathol. 2000;156(1):57–63. doi: 10.1016/S0002-9440(10)64706-2. PMID:10623654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khoja L, Backen A, Sloane R, Menasce L, Ryder D, Krebs M, Board R, Clack G, Hughes A, Blackhall F, et al.. A pilot study to explore circulating tumour cells in pancreatic cancer as a novel biomarker. Br J Cancer. 2012;106(3):508–16. doi: 10.1038/bjc.2011.545. PMID:22187035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lucci A, Hall CS, Lodhi AK, Bhattacharyya A, Anderson AE, Xiao L, Bedrosian I, Kuerer HM, Krishnamurthy S. Circulating tumour cells in non-metastatic breast cancer: a prospective study. Lancet Oncol. 2012;13(7):688–95. doi: 10.1016/S1470-2045(12)70209-7. PMID:22677156 [DOI] [PubMed] [Google Scholar]
- 10.Knisely WH, Mahaley MS. Relationship between size and distribution of spontaneous metastases and three sizes of intravenously injected particles of VX2 carcinoma. Cancer Res. 1958;18(8 Part 1):900–5. PMID:13573362 [PubMed] [Google Scholar]
- 11.Brandt B, Junker R, Griwatz C, Heidl S, Brinkmann O, Semjonow A, Assmann G, Zänker KS. Isolation of prostate-derived single cells and cell clusters from human peripheral blood. Cancer Res. 1996;56(20):4556–61. PMID:8840959 [PubMed] [Google Scholar]
- 12.Molnar B, Ladanyi A, Tanko L, Sréter L, Tulassay Z. Circulating tumor cell clusters in the peripheral blood of colorectal cancer patients. Clin Cancer Res Off J Am Assoc Cancer Res. 2001;7(12):4080–5. [PubMed] [Google Scholar]
- 13.Kats-Ugurlu G, Roodink I, de Weijert M, Tiemessen D, Maass C, Verrijp K, van der Laak J, de Waal R, Mulders P, Oosterwijk E, et al.. Circulating tumour tissue fragments in patients with pulmonary metastasis of clear cell renal cell carcinoma. J Pathol. 2009;219(3):287–93. doi: 10.1002/path.2613. PMID:19731255 [DOI] [PubMed] [Google Scholar]
- 14.Hou J-M, Krebs MG, Lancashire L, Sloane R, Backen A, Swain RK, et al.. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2012;30(5):525–32. doi: 10.1200/JCO.2010.33.3716. [DOI] [PubMed] [Google Scholar]
- 15.Krebs MG, Hou J-M, Sloane R, Lancashire L, Priest L, Nonaka D, Ward TH, Backen A, Clack G, Hughes A, et al.. Analysis of circulating tumor cells in patients with non-small cell lung cancer using epithelial marker-dependent and -independent approaches. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2012;7(2):306–15. [DOI] [PubMed] [Google Scholar]
- 16.Fanelli MF, Oliveira TB, Braun AC, Corassa M, Abdallah EA, Nicolau UR, da Silva Alves V, Garcia D, Calsavara VF, Kowalski LP, et al.. Evaluation of incidence, significance, and prognostic role of circulating tumor microemboli and transforming growth factor-β receptor I in head and neck cancer. Head Neck. 2017;39(11):2283–2292 doi: 10.1002/hed.24899. PMID:28815787 [DOI] [PubMed] [Google Scholar]
- 17.Chen J-Y, Tsai W-S, Shao H-J, Wu J-C, Lai J-M, Lu S-H, Hung TF, Yang CT, Wu LC, Chen JS, et al.. Sensitive and specific biomimetic lipid coated microfluidics to isolate viable circulating tumor cells and microemboli for cancer detection. PloS One. 2016;11(3):e0149633. doi: 10.1371/journal.pone.0149633. PMID:26938471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141(7):1117–34. doi: 10.1016/j.cell.2010.06.011. PMID:20602996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Egloff AM, Grandis JR. Targeting epidermal growth factor receptor and SRC pathways in head and neck cancer. Semin Oncol. 2008;35(3):286–97. doi: 10.1053/j.seminoncol.2008.03.008. PMID:18544443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee CS, Redshaw A, Boag G. Epidermal growth factor receptor immunoreactivity in human laryngeal squamous cell carcinoma. Pathology (Phila). 1997;29(3):251–4. [DOI] [PubMed] [Google Scholar]
- 21.Grandis JR, Chakraborty A, Zeng Q, Melhem MF, Tweardy DJ. Downmodulation of TGF-alpha protein expression with antisense oligonucleotides inhibits proliferation of head and neck squamous carcinoma but not normal mucosal epithelial cells. J Cell Biochem. 1998;69(1):55–62. doi: 10.1002/(SICI)1097-4644(19980401)69:1%3c55::AID-JCB6%3e3.0.CO;2-U. PMID:9513046 [DOI] [PubMed] [Google Scholar]
- 22.Adjei AA, Rowinsky EK. Novel anticancer agents in clinical development. Cancer Biol Ther. 2003;2(4 Suppl 1):S5–15. PMID:14508076 [PubMed] [Google Scholar]
- 23.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5(5):341–54. doi: 10.1038/nrc1609. PMID:15864276 [DOI] [PubMed] [Google Scholar]
- 24.Chinen LTD, Mello CAL, Abdallah EA, Ocea LM, Buim ME, Breve NM, et al.. Isolation, detection, and immunomorphological characterization of circulating tumor cells (CTCs) from patients with different types of sarcoma using isolation by size of tumor cells: a window on sarcoma-cell invasion. OncoTargets Ther. 2014;7:1609–17. doi: 10.2147/OTT.S62349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khoja L, Shenjere P, Hodgson C, Hodgetts J, Clack G, Hughes A, Lorigan P, Dive C. Prevalence and heterogeneity of circulating tumour cells in metastatic cutaneous melanoma. Melanoma Res. 2014;24(1):40–6. doi: 10.1097/CMR.0000000000000025. PMID:24201293 [DOI] [PubMed] [Google Scholar]
- 26.Lausen B, Schumacher M. Maximally Selected Rank Statistics. Biometrics. 1992;48(1):73. doi: 10.2307/2532740. [DOI] [Google Scholar]
- 27.Nicolazzo C, Gradilone A. Significance of circulating tumor cells in soft tissue sarcoma. Anal Cell Pathol Amst. 2015;2015:697395. PMID:26167450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Long E, Ilie M, Bence C, Butori C, Selva E, Lalvée S, Bonnetaud C, Poissonnet G, Lacour JP, Bahadoran P, et al.. High expression of TRF2, SOX10, and CD10 in circulating tumor microemboli detected in metastatic melanoma patients. A potential impact for the assessment of disease aggressiveness. Cancer Med. 2016;5(6):1022–30. doi: 10.1002/cam4.661. PMID:26945789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, Spencer JA, Yu M, Pely A, Engstrom A, Zhu H, et al.. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158(5):1110–22. doi: 10.1016/j.cell.2014.07.013. PMID:25171411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carlsson A, Nair VS, Luttgen MS, Keu KV, Horng G, Vasanawala M, et al.. Circulating tumor microemboli diagnostics for patients with non-small-cell lung cancer. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2014;9(8):1111–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicholson RI, Gee JM, Harper ME. EGFR and cancer prognosis. Eur J Cancer Oxf Engl 1990. 2001;37 Suppl 4:S9–15. [DOI] [PubMed] [Google Scholar]
- 32.Sato O, Wada T, Kawai A, Yamaguchi U, Makimoto A, Kokai Y, Yamashita T, Chuman H, Beppu Y, Tani Y, et al.. Expression of epidermal growth factor receptor, ERBB2 and KIT in adult soft tissue sarcomas: a clinicopathologic study of 281 cases. Cancer. 2005;103(9):1881–90. doi: 10.1002/cncr.20986. PMID:15772959 [DOI] [PubMed] [Google Scholar]
- 33.Yang J-L, Hannan MT, Russell PJ, Crowe PJ. Expression of HER1/EGFR protein in human soft tissue sarcomas. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2006;32(4)466–8. [DOI] [PubMed] [Google Scholar]
- 34.Sannino G, Marchetto A, Kirchner T, Grünewald TGP. Epithelial-to-mesenchymal and mesenchymal-to-epithelial transition in mesenchymal tumors: A paradox in sarcomas? Cancer Res. 2017;77(17):4556–61. doi: 10.1158/0008-5472.CAN-17-0032. PMID:28811330 [DOI] [PubMed] [Google Scholar]