ABSTRACT
The thymus supports differentiation of T cell precursors. This process requires relocation of developing thymocytes throughout multiple microenvironments of the organ, mainly with thymic epithelial cells (TEC), which control intrathymic T cell differentiation influencing the formation and maintenance of the immunological synapse. In addition to the proteins of the major histocompatibility complex (MHC), this structure is supported by several adhesion molecules. During the process of thymopoiesis, we previously showed that laminin-mediated interactions are involved in the entrance of T-cell precursors into the thymus, as well as migration of differentiating thymocytes within the organ. Using small interference RNA strategy, we knocked-down the ITGA6 gene (which encodes the CD49f integrin α-chain) in cultured human TEC, generating a decrease in the expression of the corresponding CD49f subunit, in addition to modulation in several other genes related to cell adhesion and migration. Thymocyte adhesion to TEC was significantly impaired, comprising both immature and mature thymocyte subsets. Moreover, we found a modulation of the MHC, with a decrease in membrane expression of HLA-ABC, in contrast with increase in the expression of HLA-DR. Interestingly, the knockdown of the B2M gene (encoding the β-2 microglobulin of the HLA-ABC complex) increased CD49f expression levels, thus unraveling the existence of a cross-talk event in the reciprocal control of CD49f and HLA-ABC. Our data suggest that the expression levels of CD49f may be relevant in the general control of MHC expression by TEC and consequently the corresponding synapse with developing thymocytes mediated by the T-cell receptor.
KEYWORDS: extracellular matrix, HLA-ABC, HLA-DR, integrin α6, RNA interference, thymic epithelial cells
Introduction
The thymic microenvironment is crucial for intrathymic T cell migration and maturation by providing inductive signals. This tissue is essentially composed by thymic epithelial cells (TEC), which play a vital role in T cell development and induction of self-tolerance for adaptive immunity. TEC are responsible for the production of the majority of the inductive signals, including cytokines, chemokines and components of extracellular matrix (ECM).1-4
The thymus is microanatomically structured in discrete cortical and medullary regions that contain phenotypically and functionally distinct TECs, as well as thymocytes at defined stages of maturation.5-7 Thymocyte development takes place during a stepwise progression and requires tuned migration through the distinct thymic regions, where maturing thymocytes interact with microenvironmental cells. Those interactions are mediated by a series of ligand/receptor molecular associations, as for example those in the immunological synapse involving major histocompatibility complex (MHC) and the T-cell receptor (TCR), respectively expressed by TEC and developing thymocytes, as well as cell adhesion/cell migration related TEC/thymocyte interactions.1,7,8 Although in real life the thymic epithelium is a tridimensional (3D) network, adhesion of developing thymocytes with cultured TEC can be seen in 2D conditions, with formation of typical synaptic structures, as revealed by confocal microscopy.9 Moreover, we can measure changes in the human TEC-thymocyte adhesion degree by experimentally modulating cell adhesion-related molecules such as ECM ligands and receptors, and semaphorins, among others.9-11
During the course of classical immunological synapse formation between T-cells antigen-presenting cells (APCs), the adhesive proprieties of immune cells are crucial for the dynamic assembly of signaling complexes. Integrins give support to those interactions and, in mature immunological synapses, there is an external integrin ring, formed by lymphocyte function-associated antigen 1 (LFA-1), which surrounds central TCR clusters in the contact zone.12,13 Moreover, different microdomains containing immunoreceptors (MHC proteins, pathogen recognition receptors, integrins, among others) have been reported in APCs, being important for efficient pathogen recognition, the formation of the immunological synapse, and subsequent T cell activation.14
The thymic synapse is the first T cell-specific synapse, although the relevance of signaling complex formation to TCR signaling during thymus development is much less clear. The first thymic synapse investigated was the one inducing negative selection of thymocytes, which have rearranged their TCR and express both CD4 and CD8 accessory molecules on their membranes.15 It was shown that positively selecting synapses, studied in the mouse model, are established during multiple short encounters with microenvironmental cells.15 Very few papers actually deal with the intrathymic T cell/microenvironmental cell synapse and also virtually nothing is known concerning the human thymus. Interestingly however, it has been reported that α-distroglycan, a subunit of the distroglycan complex (which binds the ECM molecule laminin), plays a role in the intrathymic immunological synapse, since blocking this molecule through different strategies resulted in altered intrathymic T-cell development, as revealed in fetal mouse thymus organ cultures.16
Components of ECM are critically involved in the formation of thymic microenvironments and T cell development, by establishing molecular bridges between thymocytes and microenvironmental cells. The major membrane receptors for adhesion to ECM proteins in metazoa are integrins, transmembrane heterodimers composed of α and β subunits, comprising 18 α and 8 β members, so far known to assemble into 24 distinct dimers.17 ECM-integrin interactions play a critical role in the regulation of signaling pathways that coordinate several biologic processes, including cell adhesion, migration proliferation and survival.18,19
In a large series of studies, we showed that TEC constitutively produce various ECM molecules and express the corresponding integrin-type receptors.8,20 In particular, others and we have demonstrated the expression of laminin isoforms in human and murine thymus, as well as the expression of functional laminin receptors by TEC and developing thymocytes, which play a role in the adhesion of thymocytes to TEC in normal murine and human thymuses.21
CD49f is an ECM cell adhesive protein, α6 integrin chain, encoded by the ITGA6 gene, which can associate with β1 or β4 sub-unities to form laminin receptors.22 In normal epithelial tissues, α6β4 has been implicated in forming and stabilizing of hemi-desmosomes, which contribute to the organization and maintenance of epithelial structure. Accordingly, the loss of ITGA6 or ITGB4 gene function, in humans, is associated with some forms of epidermolysis bullosa.23
Particularly in the thymus, CD49f participates in the homing of T cell progenitors into this organ and may function as costimulatory molecule for thymocyte development.24-27 Accordingly, thymocyte adhesion on microenvironmental cells, as well as thymocyte migration, is dependent on laminin-mediated interaction, through integrin-type receptors, such as VLA-6 (α6β1 or CD49f/CD29).21,28 Recently, we showed that siRNA targeting ITGA6 in TEC could modulate cell migration-related genes, including those coding for ECM as well as chemokine ligands and receptors, promoting a significant decrease in cell adhesion to laminin.28
In a second vein, the integrin β4 subunit is associated with proliferation of endothelial cells through a molecular association with HLA-ABC, which corresponds to class I molecules of the major histocompatibility complex.29,30
Using the small interference RNA strategy, we examined here the engagement of ITGA6 and its corresponding protein CD49f, in human TEC, looking for its putative role on the TEC-related immunological synapse. We observed modulation of immunological synapse-related genes, including MHC class I and class II, as well as cell adhesion molecules and chemokine receptors. These changes were associated with other morphological and functional alterations, including TEC shape shrinkage and decrease in capacity to adhere to laminin, as well as decrease in proliferation of TEC in vitro. Our data thus unravels the role of the CD49f integrin subunit expressed by the human thymic epithelium upon the TEC expression of MHC molecules and other cell adhesion proteins, crucial for the formation and maintenance of the immunological synapse between TEC and developing thymocytes. It is thus conceivable that the maintenance of the immunological synapse involving human TEC is much more complex than what has been so far reported for the immunological synapse in the periphery of the immune system.
Materials and methods
Cell culture conditions and transient transfection
The human TEC line was obtained from an infant thymus by explant technique and limiting dilution cloning, being derived from explants of a postnatal organ.31 It has been kindly provided by Dr. Maria Luiza Toríbio (Universidad Autonoma de Madrid, Madrid, Spain). These cells express constitutively MHC class I and class II molecules (HLA-ABC and HLA-DR, respectively) as well as several cell adhesion proteins, including ICAM-1, VCAM-1 and various integrin-type ECM receptors including VLA-3, VLA-4, VLA-5 and VLA-6;10,31 being able to interact with thymocytes.10,32 The expression of those key TEC molecules was confirmed in present work. Cells were cultured in 10% fetal bovine serum-supplemented RPMI 1640 medium at 37°C in a 5% CO2 atmosphere. The experimental protocol for expression of siRNA via transient transfection was applied using 3 ITGA6 specific siRNAs (sc-43129A: 5′- CCAUCACAGUAACUCCUAAtt-3′, 5′-UUAGGAGUUACUGUGAUGGtt-3′; sc-43129B: 5′-GGAUAUGCCUCCAGGUUAAtt-3′, 5′-UUAACCUGGAGGCAUAUCCtt-3′; sc-43129C: 5′-CCAAACUGAUCCAGUAUAAtt-3′, 5′-UUAUACUGGAUCAGUUUGGtt-3′), or the negative control siRNA (scrambled sequence); all synthesized by Santa Cruz Biotechnology (Santa Cruz Co., catalog sc-43129). Cells were seeded in 6-well plates 1 day before transfection at 2 × 105 cells per well, and grown in media supplemented with 10% fetal bovine serum (FBS) and antibiotics (penicillin/streptomycin) until 60 to 80% confluence. ITGA6 specific and Scramble siRNAs (5nM) were complexed with Lipofectamine 2000 (Invitrogen, catalog 11668019) according to the manufacturer's instructions and applied to each well in a total volume of 500 µl OptiMEM (Gibco, catalog 31985070). Cells were incubated 6 hours at 37°C in 5% CO2 atmosphere and transfection medium was removed and replaced with complete medium after 6 h. Experiments were conducted 48 hours after transfection.
Quantitation of mRNA by real-time reverse transcriptase-polymerase chain reaction (RT-PCR) and PCR array
RNA extraction was performed using RNeasy Mini Kit (QIAGEN, 74104). Total RNA was quantified using ND8000 spectrophotometer (Thermo Scientific NanoDrop Products, DE, EUA) and RNA integrity was tested using Agilent 2100 Bioanalyzer (Agilent Technologies, CA, USA). The extraction was carried with TEC after 48 hours after transfection, following the manufacturer's protocol. cDNA (cDNA) synthesis was performed using Superscript II Reverse Transcriptase with oligo-dT primers (Invitrogen, catalog 18064014), in accordance with the manufacturer's instructions, using 2.0 μg of extracted RNA per sample. Amplification conditions for each cycle were as follows: 5 min at 65°C; 2 min at 42°C, 50 min at 42°C and 15 min at 60°C. The final cDNA products were diluted 10-fold and amplified using FAST SYBR Green Master Mix (Applied Biosystems, catalog 4385612) in a 25 μL reaction mixture that was pipetted into each well of a 96-well optical plate. All standard dilutions were run in triplicate.
Real-time PCR was performed using a 2-step cycling program involving an initial single cycle of 95°C for 10 min, followed by 40 cycles of 95°C for 15 seconds, then 60°C for 1 min in the StepOnePlus™ Real-Time PCR System (Applied Biosystems, NY, USA) with Sequence Detector System software 1.6.3. A first derivative dissociation curve was performed (95°C for 1 min, 65°C for 2 min, then ramped from 65°C to 95°C at a rate of 2°C/min). The formation of a single peak at temperatures higher than 80°C confirmed the presence of a single PCR product in the reaction mixture.
The RNA extracts from both knocked down and control TEC, in 5 independent experiments, were used in Human Extracellular matrix and adhesion molecules e Chemokines and chemokine receptors RT2 Profiler PCR Arrays (QIAGEN, respectively catalogs PAHS-013Z and PAHS-022Z) in 96-well plates, designed for 84 focused genes and 5 housekeeping genes, following the manufacturer's protocol. The first-strand cDNA was synthetized using the RT2 First Strand Kit in accordance with the manufacturer's instructions, using 1.0 μg of extracted RNA per sample. The array plates, RT2 First Strand Kit and RT2 SYBR Green Mastermix were purchased from QIAGEN.
The fluorescence accumulation data of real-time RT-PCR reaction of each sample were used to fit 4 parameters sigmoid curves to represent each amplification curve using the library qpcR33 for the R statistical package version 2.14.1.34 Endogenous controls used in the normalization between the different amplified samples were selected among ACTB, B2M, GAPDH, HPRT1 e RPL13A human genes by the method geNorm.35
Immunohistochemistry
Transfected TEC were grown in Lab-Tek chambers, washed in PBS and ethanol fixed at room temperature for 10 min. Samples were incubated with PBS/BSA 1% to block non-specific fluorochrome binding; being then subjected to the anti-human CD49f goat polyclonal antibody (Santa Cruz Co., catalog sc-10730) for 45 min at room temperature. After 3 gentle PBS washings, the Alexa 546-coupled anti-goat Ig secondary antibody (Invitrogen, A-21085) was applied for one hour followed by PBS and nucleus staining with DAPI (Invitrogen, CA, USA). Fluorochrome-labeled Abs was detected using a Zeiss Axio Imager A2 microscope (Carl Zeiss, Oberkochen, Germany). Images were acquired with a CCD camera (Hamamatsu Orca, Shizuoka, Japan). As negative controls, primary antibodies were replaced by an unrelated anti-human goat immunoglobulin followed by Alexa 546-coupled anti-goat Ig secondary antibody. This condition did not generate any significant labeling.
Flow cytometry
We also performed cytofluorometric analyses for evaluating the membrane density of the following integrin α or β chains: CD49b, CD49c, CD49d, CD49f, CD29 and CD104 (respectively α2, α3, α4, α6, β1 and β4 integrin chains). TEC were immunostained with corresponding specific fluorochrome-labeled antibodies, or unrelated Ig isotype-matched negative controls. Briefly, after 48h of gene silencing procedures, the cells were detached of the tissue culture plates using EDTA and centrifuged before being transferred to wells within a round-bottom 96-well plate (Nunc) and blocked in suspension with normal human serum for 15 min at 4°C. The cells were then treated with the fluorochrome-labeled primary mouse anti-human antibody or unrelated control (1:10 dilution) for 30 min in dark chamber; washed in PBS and re-suspended in the fixing solution, 2% formaldehyde in PBS. All antibodies were purchased from BD Bioscience.
Cell cycle status was determined by flow cytometry using Propidium Iodide (PI), in combination with KI-67 staining. The analysis was carried with TEC after 48 hours after transfection. Cells were detached of the tissue culture plates using EDTA and re-suspended in 10% fetal bovine serum-supplemented RPMI 1640 medium. Cells were washed twice with permeabilization buffer with saponin (Perm buffer, eBioscience, catalog 00–8333–56) and re-suspended in the same buffer. Cells were stained with the Alexa 488 coupled anti-KI-67 antibody (1:20 dilution) for 30 minutes at 4°C in the dark followed by wash Perm buffer and fixed (paraformaldehyde and saponin) for 30 minutes at 4°C (Fix/Perm buffer, eBioscience). The cells were then washed with ice-cold PBS, re-suspended in 0,1% Triton X-100 solution (Sigma-Aldrich, catalog 9002–93–1) and incubated with ribonuclease (0,2 mg/mL) and propidium iodide (20 μg/mL) for 30 minutes at room temperature. The anti-Ki67 polyclonal antibody was purchased from Abcam (catalog ab15580).
To verify the ability of siRNA to affect cell viability, one million of human TEC cells transfected with ITGA6 specific or Scramble siRNAs as control were stained using Annexin-V/propidium iodide (PtdIns) kit (BD Bioscience, 556547). The externalization of phosphatidylserine in apoptotic cells was assessed using Annexin-V conjugated to green-fluorescent FITC dye while PI was included to characterize dead cells. Thymocytes incubated with dexamethasone (Sigma-Aldrich, catalog 50–02–2) during 8 hours at 37°C, in a CO2 incubator were used as positive control for cell death. In all cases, cells were analyzed by flow cytometry, and dot plots were generated for the simultaneous Annexin V and PI labeling.
In all experiments, the fluorescence intensity of labeled TEC was determined with a FACSCanto II flow cytometer (BD Biosciences, San Diego, CA, USA), with at least 10,000 events recorded for each sample derived from at least 3 independent assays. The analyses were performed using the BD FACSDiva 6.1.3 or the Summit softwares (respectively from BD Biosciences, CA, and Dako, Carpinteria, USA).
Immunoblotting
Protein expression was also determined by immunoblotting. Cells were washed with phosphate-buffered saline (PBS) buffer, lysed in protease inhibitor (Sigma-Aldrich, catalog P8340) passing through a 27 gauge needle. Lysates were cleared by centrifugation at 12, 000 g for 5 min, and the concentration of protein was determined with Qubit® 2.0 Fluorometer (Invitrogen, CA, USA). Equal amounts (10μg) of protein were fractionated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE; 10% polyacrylamide) before transfer to nitrocellulose membranes (Invitrogen, catalog LC2006). The protein transfer was performed using the iBlot® 7-Minute Blotting System (Invitrogen, CA, USA). Membranes were blocked with 5% milk in Tris-tween buffer (25 mM Tris–HCl, pH 7.4, 150 mM NaCl, 2.7 mM KCl, 0.1% Tween 20) for 2 h at room temperature. After being washed thoroughly 3 times during 10 min with the same buffer, the membranes were incubated with the anti-CD49f rabbit antibody (Cell Signaling, catalog 3750S), washed and subjected to the peroxidase-coupled goat anti-rabbit IgG (Abcam, catalog ab6721) (1:1500 dilution) for 2 h at 37 °C, then washed 3 times again. Secondary antibody was used according to the manufacturer's instructions, and its detection was improved by applying the enhanced chemiluminescence kit (GE Healthcare, catalog RPN2232).
Thymocyte-Thymic epithelial cell adhesion assay
Human TEC cultures with 48 h of transfection were used to verify the ability of siRNA ITGA6 transfected TEC to bind thymocytes. To test this, it was applied 2 distinct techniques. One approach was the co-culture in the 24 well plates for phenotyping analysis by flow cytometry of those thymocytes that remained adhered on the TEC monolayer. We exposed TEC monolayer to 50 thymocytes per TEC for one hour at 37°C in a 5% CO2 atmosphere, nonadherent cells were gently washed out, and adhered thymocytes were harvested and phenotyped. Cells were washed and subsequently submitted to 3-color immunofluorescence staining as described previously.10,32 A second approach to evaluate thymocyte/TEC adhesion was by direct counting, under the microscope, the numbers of thymocytes which had adhered to TEC cultures. TECs were trypsinized, replated to 8-well Labtek chambers (4 × 103 cells/0.5 ml in each chamber) for 12–18 h, exposed to 50 thymocytes per TEC for one hour at 37°C in a 5% CO2 atmosphere, non-adherent thymocytes were gently washed out, and cover glasses were fixed in cold absolute ethanol for 5 minutes and stained with Giemsa or used for immunoflurescence. Countings were integrated in the form of an association index (AI) and calculated using the following formula, previously validated for this type of analysis.10,32
At least 300 thymic epithelial cells with or without adhered thymocytes, were counted per well. These experiments were repeated at least 3 times, with 2 separate observers performed counting blind. We did not consider the growth rates; however, the evaluation by 2 different observers after transfections did not suggest any modifications in growth rates when compared no treated with treated cells.
Human thymuses were obtained as a by-product of cardiac surgery performed on children at Necker Hospital. The study was approved by the Necker Hospital Ethical Committees for human research and was performed according to the European Union guidelines and the declaration of Helsinki.
In silico molecular docking
The 3-dimensional (3D) model of the CD49f sub-unit was generated by comparative modeling method using the program Modeler 9v11.36 The crystal structure of integrin CD49e (PDB code: 1JV2) was identified as the best template after PSI-BLAST (http://nar.oxfordjournals.org/content/25/17/3389.short) analysis against the Protein Data Bank (PDB).37 The docking calculations were performed with HADDOCK easy interface (http://haddock.science.uu.nl/services/HADDOCK/haddockserver-easy.html) using the standard protocols and automatically determining values.38 This interface requires only starting structures and lists of interacting residues. The generated 3D model of CD49f was used as a starting structure, as well as the crystallographic structure of β-2-microglobulin (B2M) available at PDB. The interaction residues were based on the interactions derived from the X-ray crystallographic structure of MHC class II histocompatibility antigen interaction with B2M (PDB code: 3JTS). The best 10 solutions were clustered and sorted according to the intermolecular energy (sum of van der Waals, electrostatic and ambiguous interaction restrains energy terms) and their average buried surface area.
Statistical analyses
The comparison of means of normalized gene expression values of PCR arrays between the 2 groups (unpaired) was performed using Student's t tests. Results were represented in graphs displaying the expression levels mean ± standard error of mean of each group relative to mean of the control group (fold-change), defined by the arbitrary value of 1. Also, the statistical analysis of the cytofluorometry data was performed using Student's t tests to compare differences between the 2 groups (unpaired). In this case, results were presented as mean ± standard error of the mean fluorescence intensity. Two-tailed levels of significance less than or equal to 0.01 and 0.05 were considered as “highly significant” and “significant,” respectively.
Functional protein association networks, including direct (physical) as well as indirect (functional) associations, were inferred using the platform freely available in the framework of the STRING database (http://string-db.org), developed by STRING CONSORTIUM 2016 which includes SIB - Swiss Institute of Bioinformatics, CPR - NNF Center for Protein Research and EMBL - European Molecular Biology Laboratory.
Results
Anti-ITGA6 siRNA oligonucleotides impair the expression of CD49f and other integrin subunits by human TEC
To assess the consequences of CD49f (α6 integrin) expression loss, human TEC were transfected with oligonucleotides (siRNA) that target the ITGA6 mRNA (siITGA6). We did confirm our recent results28 showing a significant knockdown of ITGA6 gene expression, as ascertained by real time PCR with primers located in the regions of siRNAs degradation. The measurement of mRNA levels was assessed using the carefully designed RT-qPCR primers for each one of the 3 individual siRNAs duplexes (Supplementary Information, Fig. 1). We found 70–80% reduction of ITGA6 mRNA in TEC transfected with ITGA6 siRNA as compared with the scramble oligonucleotide. As expected, ITGA6 gene knockdown was also reflected at the CD49f protein expression levels, as seen by immunoblotting and flow cytometry analyses (Supplementary Information, Fig. 1). The immunoblotting analysis suggests virtual complete loss of the CD49f protein, which is at variance with the significant amounts detected by flow cytometry. This apparent discrepancy is likely due to the fact that the protein extraction for the immunoblotting experiments did not preserve the entire membrane fraction, and the soluble fraction may be more affected by the action of gene knockdown process.
Figure 1.
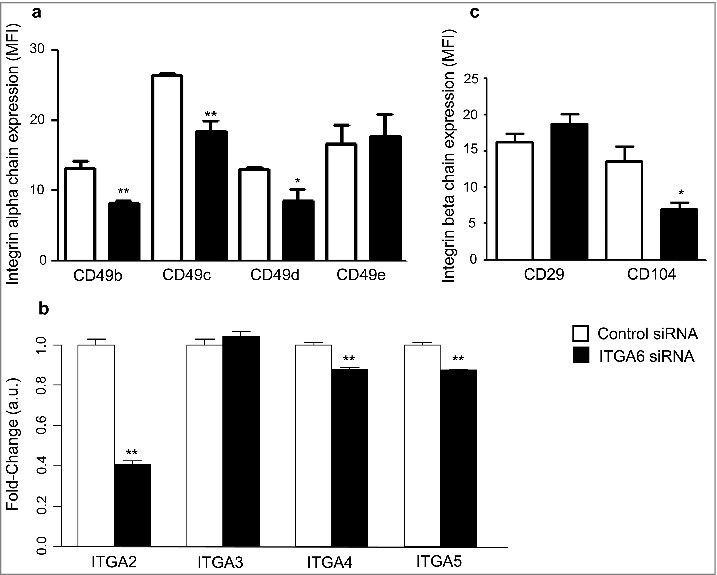
ITGA6 knockdown affects the expression of various integrin chains by human thymic epithelial cells. Cultured TEC were transfected with a siRNA negative control duplex or a siRNA duplex against human ITGA6 (Control siRNA and ITGA6 siRNA, respectively). In panels a and b, the membrane expression of α and β chains were analyzed by flow cytometry using monoclonal antibodies specific for each human integrin chain. Data were expressed as mean fluorescence intensities. Effects of ITGA6 knockdown on expression levels of some corresponding genes were ascertained by RT-qPCR, as seen in panel c. These results shown are representative of 5 independent experiments and error bars indicate standard error. Data are shown as relative expression values, normalized by housekeeping genes expression. Significant differences were identified by Student's t test (*p < 0.05; **p < 0.01).
The surface expression of other α-integrin subunits, CD49b, CD49c, and CD49d, was also decreased following the ITGA6 gene knockdown, whereas CD49e expression was not modified on the cell surface (Fig. 1a). Furthermore, by using RT-qPCR we found that the expression of ITGA2, ITGA4 and ITGA5 was down-modulated in ITGA6 knocked-down cells, averaging 59%, 12% and 12% (Fig. 1b). Interestingly, in contrast to ITGA2, ITGA4 and ITGA5 mRNA, the mRNA levels of ITGA3 were not affected in the cells. This is consistent with previous published data in which α6β4 integrin regulates the transcription of α2 and the translation of α3 integrin subunits.39
To determine whether expression of α6β4 (CD49f/CD104) and α6β1 (CD49f/CD29) integrins were suppressed on the TEC surface following transfection with siITGA6, cells were analyzed by flow cytometry. Considering that the β4 subunit forms heterodimer specifically with the α6 subunit, the knockdown of this subunit resulted in depletion of the 44% in surface expression of β4 integrin (Fig. 1c). Nevertheless, the expression of β1 integrin was not affected in protein level on the membrane of TEC (Fig. 1b). Like β1, α5 integrin subunit levels were not significantly decreased in the α6 knocked-down TEC (Fig. 1a). These findings indicate that, in addition to the decrease of α6β1, cell surface expression of α6β4 is diminished in siITGA6 transfected human TEC.
Effects of down-regulating ITGA6 on thymocyte adhesion as well as adhesion molecules expressed on TEC
Considering our previous data showing that antibodies to the laminin receptor could significantly inhibit adhesion of developing thymocytes to TEC, in both mice and humans,40-42 we evaluated whether ITGA6 siRNA could also promote similar effects, when compared with controls. We found a dramatic decrease in thymocyte adhesion on ITGA6 siRNA-treated TEC, as ascertained by the association index, which is calculated based on number of TEC with thymocytes as well as number of thymocytes bound to TEC (Fig. 2a–b).
Figure 2.
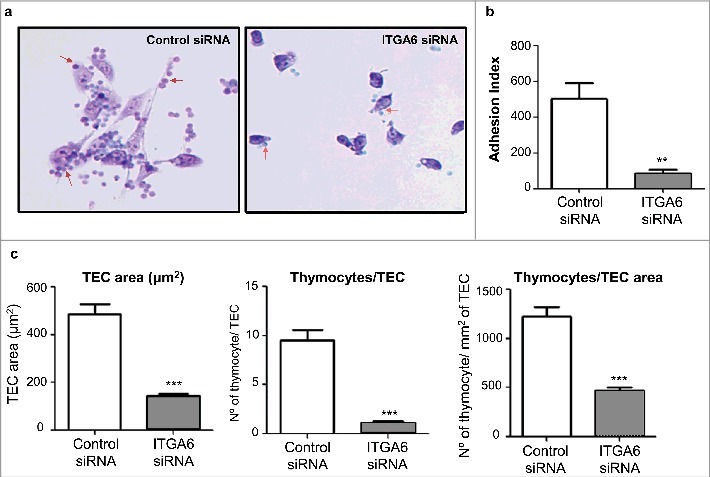
Decrease of thymocyte adhesion to human thymic epithelial cells following knockdown of ITGA6. TEC transfected with control siRNA or siRNA specific for the gene ITGA6 with 48 hours received thymocytes for 1 hour of adhesion. The cells were fixed and stained with Giemsa. Panel a illustrates representative microscopic fields of both TEC groups. Red arrows in each of the photomicrographs indicate thymocytes adhered to the TEC. Original magnification X200. The adhesion index (panel b) represents the quantification of differences in the numbers of adhered thymocytes to TEC, in which values correspond to mean ± standard error (n = 4). Since ITGA6 knockdown also diminished TEC area on the culture substrate (seen in panel c on the left), we also quantified the numbers of thymocytes per TEC and per TEC mm2 (panel c, middle and right side, respectively), and in all case there was a significant reduction when ITGA6 was knocked down. Significant differences were ascertained by Student's t test (**p < 0.01; ***p < 0.001). Error bars indicate standard deviations (n = 5)
Since we also observed a considerable diminution of the average surface of TEC after ITGA6 siRNA treatment, we also calculated the numbers of thymocytes adhered per TEC, as well as the numbers of adhered thymocytes per TEC surface (expressed in mm2). As seen in Fig. 2c, in both cases the diminution of adhered thymocytes was highly significant.
The interference upon cell adhesion following ITGA6 siRNA treatment on TEC could be explained, not only by the decrease in CD49d/CD29-mediated interaction, but also by the modulation in extracellular matrix and other adhesion genes observed after the ITGA6 knockdown. Among the genes analyzed by PCR Array, we found 42 genes who modulation of expression was statistically significant, being either down- or upregulated (Fig. 3a). Fifteen genes were up-modulated as compared the expression levels in ITGA6 siRNA versus control siRNA treated TEC, whereas the expression of 27 genes was downregulated and the expression of 2 genes was completely abolished in ITGA6 knocked treated TEC, SELE and TIMP3 (Supplementary Information, Table 1).
Figure 3.
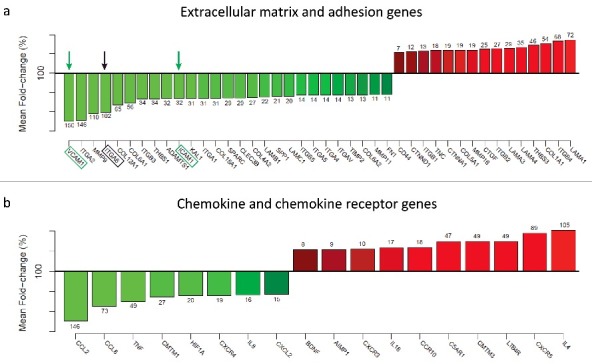
ITGA6 knockdown affects the expression of extracellular matrix and chemokine genes by human thymic epithelial cells. Cultured TEC were transfected with a siRNA negative control duplex or a siRNA duplex against human ITGA6 (Control siRNA and ITGA6 siRNA, respectively). Quantitative RT-PCR was conducted in quintuplicate and the relative mRNA expression level were normalized to geometric mean levels of expression of reference, namely B2M, HPRT1, RPL13A, GAPDH and ACTB. In graphics a and b, the effects of ITGA6 knockdown on expression levels of several genes and respective percentage of modulation, as ascertained by PCR Array, where the mean values of the control group correspond to 100%. The arrows indicate the ITGA6, as well as 2 adhesion molecules known to be part of the immunological synapse, namely ICAM-1 and VCAM-1. Results shown in the panels are representative of 5 independent experiments and significant differences were identified by Student's t tests (p < 0.05).
We next asked whether other important group of genes, the chemokine receptors and ligands, would be affected by knockingdown ITGA6. Chemokine receptors and ligands have been implicated in signal transmission at the immunological synapse between T lymphocytes and their cellular partners,43 being involved with membrane compartmentalization and regulating local T-cell adhesiveness through lymphocyte function-associated antigen (LFA)-1 activation.44-47 The knockdown of ITGA6 promoted huge modulation in several chemokines genes (Fig. 3b). Of the 84 genes studied in this group, 79 genes were expressed in TEC, among which 18 presented a significant modulation in the expression, where 8 were down modulated and 10 presented up modulation when ITGA6 was abrogated. These results suggest that knockdown of ITGA6 suppresses cell spreading and adhesion of thymic epithelial cell and that α6 integrin plays a key role in the expression of cell adhesion genes.
We then evaluated whether the adhesion blockade targeted preferentially a given CD4/CD8-defined thymocyte subset (Fig. 4). This was not the case, since all immature and mature subsets were similarly affected after adhesion in ITGA6 siRNA-treated TEC, when compared with control TEC, strongly suggesting that there is no preferential reduction of a subpopulation of thymocytes due to decreased the density of expression of CD49f in TEC.
Figure 4.
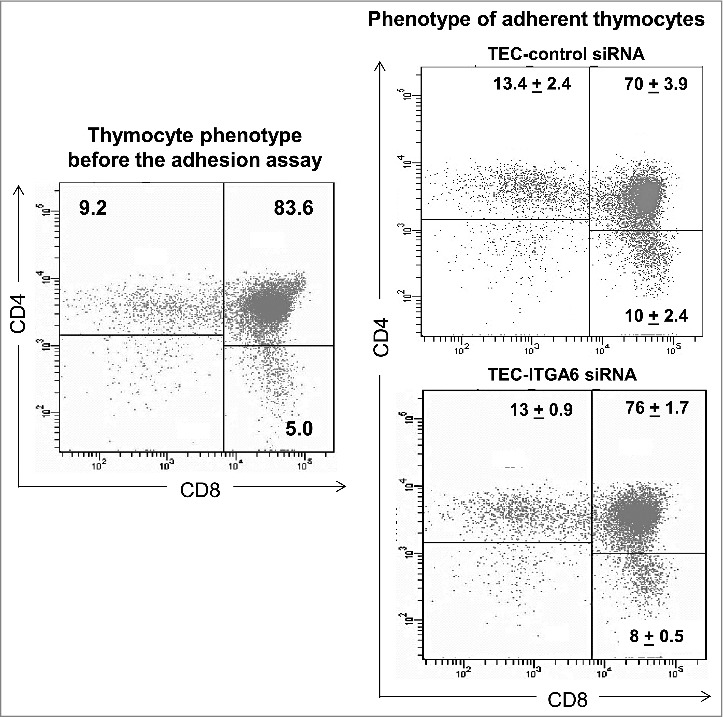
Decrease of thymocyte adhesion to human thymic epithelial cells following knockdown of ITGA6 affects both mature and immature thymocytes. Normal thymocytes were led to adhere onto growing TEC, treated either with control or ITGA6 siRNA. After washing the co-cultured, adherent thymocytes were harvested and submitted with cytofluorometry for simultaneous detection of CD4 and CD8 molecules. For comparison, we also included the typical CD4/CD8 profile of human thymocytes freshly-isolated, thus before co-culture (left panel). The numbers inside each dot plot indicate the percentages ± standard deviation of cells inside the rectangles. The right panels clearly show that, although knockdown ITGA6 largely decrease the total numbers of thymocytes, such decrease is not specific for a given stage of differentiation since their proportions are similar in the 2 conditions (Control siRNA and ITGA6 siRNA).
Since siITGA6 TEC showed a decreased in number of cells, we evaluated cell death in these preparations using the Annexin V/PI double-labeling. We found that the ITGA6 knockdown did not influence cell viability, with both Control-siRNA and ITGA6-siRNA treated cells were primarily Annexin V-FITC and PI negative, indicating that they were viable and not undergoing apoptosis (Supplementary, Fig. 2). Taken together, these data indicate that ITGA6-knockdown induced arrest in proliferation of TEC, without affecting apoptosis.
ITGA6 knockdown in human TEC modulates immunological synapse related genes
The impact of ITGA6 siRNA (applied on TEC) upon T cell adhesion might have consequences in T cell development and selection, since interactions of these cells with TEC have a crucial role in the regulation of these processes, particularly in the formation and maintenance of immunological synapse. Therefore, we investigated other synapse related molecules in the human thymic epithelium. As shown in Fig. 3a, gene expression of 2 important adhesion molecules relevant in the immunological synapse and known to be potent co-stimulatory molecules,48,49 namely vascular cell adhesion molecule (VCAM-1) and the intercellular cell adhesion molecule (ICAM-1) was down modulated after the knockdown of ITGA6, decreasing 60% and 24%, respectively. In this condition, we also found significant down regulation in the expression of ITGAL and ITGA4 genes that encode α chains of other integrin-type surface receptors LFA-1 and VLA-4 (CD49d/CD29), which also play a significant role in both the formation of the supramolecular activation cluster in the immunological synapse and in cellular crosstalk50 (Fig. 3a).
A further immunologic synapse related molecule is the chemokine receptor CXCR4, whose activation is related to synapse maintenance,51 and mutations of the CXCR4 gene, seen in the WHIM syndrome, impair the stability of T-cell synapse.52,53 Targeting ITGA6 gene by siRNA in cultured TEC did induce a decrease in the expression of the CXCR4 gene, as revealed by qRT-PCR (Fig. 3a).
We then searched whether or not the expression levels of class I and class II major histocompatibility complex, HLA-ABC and HLA-DR, was affected by the knock-down of ITGA6 in human TEC. Interestingly, as compared with controls, this treatment promoted a consistent decrease in membrane expression of HLA-ABC, whereas the expression of HLA-DR was increased, as determined in 5 independent experiments (Fig. 5).
Figure 5.
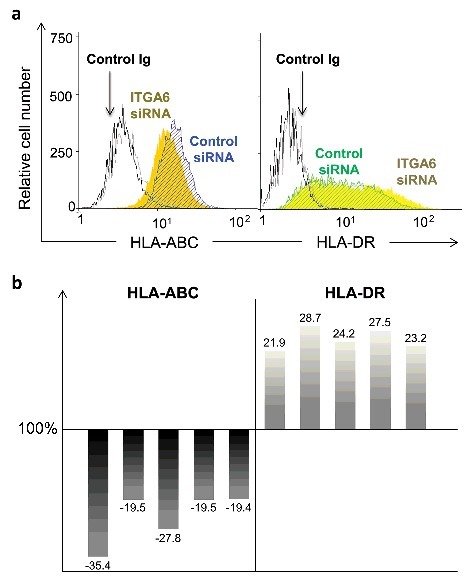
ITGA6 knockdown in human thymic epithelial cells affects the expression of molecules of the major histocompatibility complex. The HLA class I and class II molecules were evaluated on the surface of TEC by cytofluorometry after ITGA6 gene knockdown experiments. Panel a depicts cytofluorometric profiles for detection of HLA-ABC and HLA-DR. In this panel, light and dark gray histograms represent isotype control staining for negative control and ITGA6siRNA-treated cells respectively. Filled yellow histogram depicts the cells transfected with ITGA6 siRNA and the hachured are the cells with control siRNA. Differences in the expression levels of HLA-ABC and HLA-DR expression in ITGA6 knockdown TEC (also verified by flow cytometry), are seen in 5 independent experiments, with siRNA control being set as 100%. The percentages of change in the expression of HLA-ABC (left) and HLA-DR (right) in ITGA6siRNA treated cells are represented by each bar. Significant differences were identified in each independent experiment by Student's t tests paired (p < 0.003).
Previous reports showed that HLA-ABC can interact with the β4 integrin subunit in endothelial cells.29,30 To explore the possibility of the α6 integrin subunit, which pairs with β4 integrin, also interacting with HLA-ABC in the human thymic epithelium, we applied a transfection protocol using β2-microglobulin (B2M) siRNA in cultured TEC. Transfection efficiency was determined by flow cytometry analysis and RT-qPCR at 48h after introduction of the B2M siRNA. The protein levels of HLA-ABC complex, which contains the β2-microglobulin, decreased 50% on the membrane of TEC, when compared with control cells, and the mRNA level of B2M was downregulated around 73% (Fig. 6a and 6b).
Figure 6.
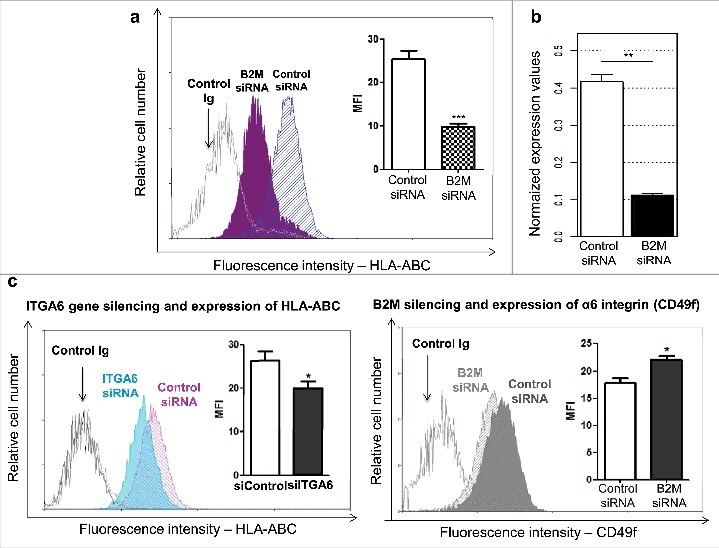
Bidirectional modulation of α6 integrin and HLA-ABC in human thymic epithelial cells. Cultured TEC were transfected either with siRNA oligonucleotides targeting the B2M gene (B2M siRNA) or with a scramble siRNA oligonucleotide sequence (Control siRNA). Panel a depicts cytofluorometric profiles showing the effects of B2M gene knockdown, in terms of the corresponding protein membrane expression level: as compared with control siRNA, β2-microglobulin expression on TEC is significantly reduced after using B2M-siRNA. Insert shows the mean fluorescence intensity, also revealing such a reduction, and quantified in 4 independent experiments. B2M Gene expression impairment was further confirmed by real time RT-PCR, seen in panel b, after normalization to geometric mean levels of expression of reference genes HPRT, TFRC and RPL13A. Panel c reveals that siRNA ITGA6 knockdown reduces protein expression levels of HLA-ABC, as seen by the flow cytometry profiles and mean fluorescence intensity (MFI). Conversely, when cells were transfected with B2M siRNA the expression of integrin α6 subunit was increased as seen in panel d. In all flow cytometry profiles aspecific fluorescence levels were recorded by using an unrelated control Igs. Light and dark gray histograms represent isotype negative control staining for control and ITGA6siRNA-treated cells respectively. In the various experiments data are expressed as mean ± standard error (n = 4). Significant differences were identified by 2-tailed unpaired Student's t tests (*p < 0.05; **p < 0.01; ***p < 0.001).
We then evaluated cytofluorometric analyses of the membrane expression levels of CD49f and B2M, in conditions of reciprocal knocking down of ITGA6 and B2M genes. As compared with controls, in 4 independent experiments the ITGA6 knockdown resulted in decrease in B2M protein contents in TEC, whereas the B2M gene knockdown increased CD49f expression levels. This was ascertained by analyzing the relative cell numbers and means of fluorescence intensity of each protein (Fig. 6c). Such experiments clearly show the existence of a cross-talk event in the reciprocal control of CD49f and HLA-ABC.
Hypothetically, this cross-talk might be through direct physical interaction between the 2 molcules. We then approached this possibility by performing in silico docking experiments. We identified that 2 residues in the β-propeller region, present in the extracellular portion of the α6 integrin chain, interact with 2 residues on the N-terminal region of the β2-microglobulin. The residues likely involved in this interaction are Lys58 and Leu289 in α6 integrin chain and Gln8 and Tyr10 present in β2-microglobulin. These results are summarized in Fig. 7.
Figure 7.
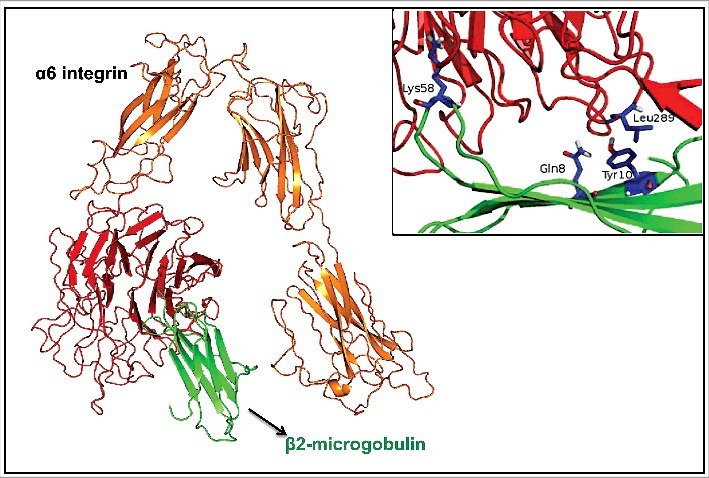
Three-dimensional structural prediction of α6 integrin and β2-microglobulin direct interaction. In orange and red is the α6 integrin and in green is β2-microglobulin. The arrows are representing the α-helices and loops; sticks represent aromatic residues likely involved in protein-protein interaction. The insert highlights in blue the residues likely involved in the interaction.
Discussion
Intrathymic laminin/integrin mediated interactions are known to be relevant in interactions between developing thymocytes with epithelial and nonepithelial components of the thymic microenvironment.40-42,54 Recently we showed that targeting the ITGA6 gene in human TEC [using the siRNA strategy, with consequent inhibition of CD49f, the α chain that forms the heterodimers α6β1 (CD49f/CD29) and α6β4 (CD49f/CD104)] resulted in a complex alteration in cell migration-related gene networks in thymus epithelium, including several chemokines and ECM ligands.28 In this same study, we further revealed that ITGA6 gene targeting also decreased TEC proliferation and changed the morphology of the growing cells, without affecting cell viability. This was confirmed herein with the experiments designed to detect cell death through double labeling for Annexin V/PI in both control and ITGA6 targeted cells.
We also showed that abrogation of ITGA6 gene by transient siRNA specific gene targeting in human TEC down-regulates the membrane expression of other integrin chains, such as α2, α3, α4 and β4, consequently diminishing, various laminin receptors (α6β1, α6β4, α2β1, α3β1) and the fibronectin receptor α4β1, which also binds the adhesion molecule VCAM-1. Interestingly enough, VCAM-1 gene expression was also decreased after applying ITGA6 siRNA in cultured human TEC.
Considering the above data, it was plausible to hypothesize that the same treatment on growing TEC would impair adhesion of developing thymocytes. This was actually the case. Such a decrease comprised all CD4/CD8-defined thymocyte subsets, a finding that is further supported by the decreasing in the TEC/thymocyte adhesion, previously showed in TEC treated by anti-α6 integrin.40,42
Importantly, we also found that the surface expression of HLA-ABC and HLA-DR were modulated by the knockdown of the integrin α6 subunit in TEC.
Although the flow cytometry plots of HLA-ABC and CD49f may convey the impression of a minimal difference, the quantitative data concerning the mean fluorescence intensity revealed statistically significant reduction of HLA-ABC after ITGA knockdown, and significant increase of CD49f in siB2M treated cells. One possible explanation is that if that the presence of CD49d is necessary to maintain physiological levels of HLA-ABC on TEC membranes. Accordingly, the decrease of CD49f results in diminution of membrane HLA-ABC. Once the membrane levels of HLA-ABC diminish, there may be a sort of homeostatic feedback loop (which is β2-microglobulin dependent) that ultimately enhances CD49f, with consequent resetting HLA-ABC levels to physiological conditions.
Furthermore, we detected a bidirectional modulation between integrin α6 and HLA-ABC molecule, suggesting a direct mutual regulation in the expression of these molecules on TEC membranes. This is further supported by the in silico experiments showing direct points of interactions. In this context, it is noteworthy that proliferation of endothelial cells is stimulated through a mutual dependency between HLA-ABC and integrin β4.29
Although we do not provide biochemical/biophysical evidence the in silico findings do open the possibility of a direct protein-protein interaction involving CD49f and β2-microglobulin, which might explain why a modulation of one molecule could result in the modulation of the other. In any case, we clearly demonstrate herein that ITGA6 gene targeting in the human thymic epithelium differentially regulates the expression of immunological synapse-related genes, with important consequences upon the ability of developing thymocytes to adhere onto CD49f-deficient TEC. Moreover, the fact that ITGA6 gene knockdown inhibits MHC class I and enhances MHC class II molecules on TEC membranes suggests that the expression levels of CD49f (forming α6β1 or α6β4 integrins) may be relevant in the general control of MHC expression by TEC and consequently the corresponding TCR-mediated interactions with developing thymocytes, so that CD49f deficiency might rather skewing the intrathymic generation of T-cells toward CD4+ thymocytes.
Lastly, the fact that large numbers of genes involved in cell adhesion and migration together with MHC genes raises the hypothesis that a much broader molecular network is involved in the regulation of the immunological synapse, on the TEC side. Searching for potential protein networks, we did find in the literature points of connection grouping together MHC and cell adhesion related networks, as summarized in Fig. 8. Conceptually, the data summarized in this figure brings the hypothesis that the maintenance of the immunological synapse involving human TEC is much more complex than what has been so far reported for the more studied immunological synapse in the periphery of the immune system. Such a concept precludes that the intrathymic selection of the T-cell repertoire, through MHC/TCR interactions, may be much more complex regulation protein networks.
Figure 8.
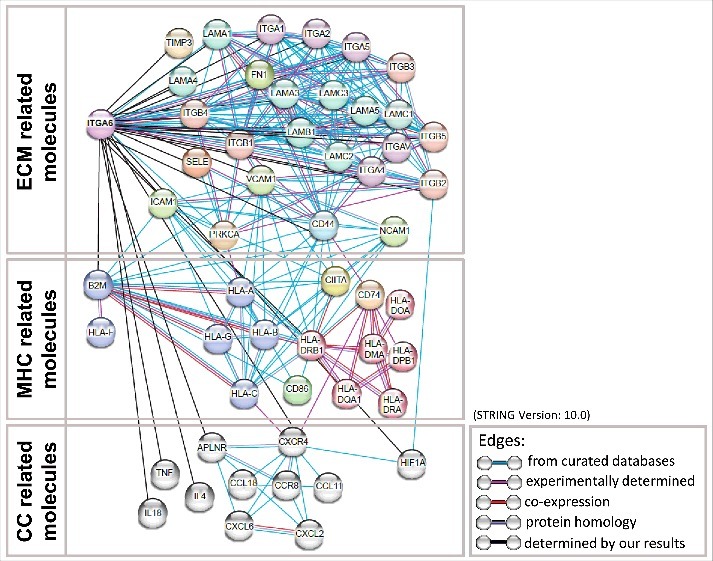
Direct and indirect protein-protein associations related with Integrin α6 and HLA-ABC and HLA-DR. Altered proteins identified in the present study and some curated key proteins provided by STRING tool were subjected to global protein network analysis using STRING tool (version 10) (http://string.embl.de/). The proteins were grouped according to molecules related with components of extracellular matrix (ECM) (top panel), major histocompatibility complex (MHC) (middle panel) and chemokines (CC) (bottom panel). The connecting lines between protein nodes indicate protein-protein interactions, specifically related with information available in curated database (blue lines), experimentally determined (pink lines), co-expressed protein (red lines), proteins whose present homology (violet lines) and possible relationship determined by our present study (black lines). The protein-protein interactions network demonstrated that Protein Kinase C α (PRKCA), CD44 molecule (CD44), Integrin β2 (ITGB2), Intercellular Adhesion Molecule 1 (ICAM1), C-X-C Motif Chemokine Receptor 4 (CXCR4) and Hypoxia Inducible Factor 1α Subunit (HIF1A), beyond Integrin α6, were key nodes of such protein-protein interactions, possibly implicated of changes induced by this single gene knock-down in the human thymic epithelium. As seen within the figure, protein-protein interactions were categorized as those from: curated databases; experimentally determined; co-expression; protein homology; determined by our results.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Prof. Mireille Dardenne and the Necker Children's Hospital for providing the thymus samples.
Funding
This work was funded by the Oswaldo Cruz Foundation (Fiocruz); Brazilian National Council for Scientific and Technological Development (CNPq); Carlos Chagas Filho Foundation for Funding Research in the State of Rio de Janeiro (Faperj); Brazilian National Coordination for Advancement of University Personnel (CAPES) and Mercosur Fund for Structural Convergence (FOCEM, Mercosur).
Author contributions
DCF.G., A.T.R.V. and W.S. conceived the experiments; M.R.A., M.M.B.F., G.L., V.C.A and A.T.R.V. performed data analysis; DCF.G., E.S.V.V., A.L., and D.A.M.C. performed experiments and analyzed data, DCF.G., D.A.M.C., V.C.A., A.T.R.V. and W.S. wrote the manuscript, W.S. coordinated the research.
References
- [1].Petrie HT, Zúñiga-Pflücker JC. Zoned out: functional mapping of stromal signaling microenvironments in the thymus. Annu Rev Immunol 2007; 25:649-79; PMID:17291187; https://doi.org/ 10.1146/annurev.immunol.23.021704.115715 [DOI] [PubMed] [Google Scholar]
- [2].Anderson G, Takahama Y. Thymic epithelial cells: Working class heroes for T cell development and repertoire selection. Trends Immunol [Internet] 2012; 33:256-63. Available from: https://doi.org/10.1016/j.it.2012.03.005; PMID:22591984; https://doi.org/ 10.1016/j.it.2012.03.005 [DOI] [PubMed] [Google Scholar]
- [3].Shah DK, Zúñiga-Pflücker JC. An overview of the intrathymic intricacies of T cell development. J Immunol [Internet] 2014; 192:4017-23. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24748636; PMID:24748636; https://doi.org/ 10.4049/jimmunol.1302259 [DOI] [PubMed] [Google Scholar]
- [4].Halkias J, Melichar HJ, Taylor KT, Robey EA, Halkias J, Melichar HJ, Taylor KT, Robey EA. Tracking migration during human T cell development. Cell Mol Life Sci [Internet] 2014. [cited 2016 Sep 5]; 71:3101-17. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24682469; PMID:24682469; https://doi.org/ 10.1007/s00018-014-1607-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Miller JFAP. The golden anniversary of the thymus. Nat Rev Immunol [Internet] 2011. [cited 2016 Sep 5]; 11:489-95. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21617694; PMID:21617694; https://doi.org/ 10.1038/nri299310.1038/nri3010 [DOI] [PubMed] [Google Scholar]
- [6].Miller JFAP. The discovery of thymus function and of thymus-derived lymphocytes. Immunol Rev [Internet] 2002. [cited 2016 Sep 8]; 185:7-14. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12190917; PMID:12190917; https://doi.org/ 10.1034/j.1600-065X.2002.18502.x [DOI] [PubMed] [Google Scholar]
- [7].Ciofani M, Zúñiga-Pflücker JC. The thymus as an inductive site for T lymphopoiesis. Annu Rev Cell Dev Biol 2007; 23:463-93; PMID:17506693; https://doi.org/ 10.1146/annurev.cellbio.23.090506.123547 [DOI] [PubMed] [Google Scholar]
- [8].Savino W, Mendes-Da-Cruz DA, Silva JS, Dardenne M, Cotta-De-Almeida V. Intrathymic T-cell migration: A combinatorial interplay of extracellular matrix and chemokines? Trends Immunol 2002; 23:305-13; PMID:12072370; https://doi.org/ 10.1016/S1471-4906(02)02224-X [DOI] [PubMed] [Google Scholar]
- [9].Lepelletier Y, Smaniotto S, Hadj-Slimane R, Villa-Verde DMS, Nogueira AC, Dardenne M, Hermine O, Savino W. Control of human thymocyte migration by Neuropilin-1/Semaphorin-3A-mediated interactions. Proc Natl Acad Sci U S A [Internet] 2007. [cited 2015 Aug 5]; 104:5545-50. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1838472&tool=pmcentrez&rendertype=abstract; PMID:17369353; https://doi.org/ 10.1073/pnas.0700705104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ribeiro-Carvalho MM, Farias-de-Oliveira DA, Villa-Verde DMS, Savino W. Triiodothyronine modulates extracellular matrix-mediated interactions between thymocytes and thymic microenvironmental cells. Neuroimmunomodulation 2002; 10:142-52; https://doi.org/ 10.1159/000067175 [DOI] [PubMed] [Google Scholar]
- [11].Linhares-Lacerda L, Ribeiro-Alves M, Nogueira ACM, de A, Mendes-da-Cruz DA, Magalhães DA, Dardenne M, Passos GA, Savino W. RNA interference-mediated knockdown of CD49e (α5 integrin chain) in human thymic epithelial cells modulates the expression of multiple genes and decreases thymocyte adhesion. BMC Genomics [Internet] 2010. [cited 2016 Sep 8]; 11(Suppl 5):S2 Available from: http://www.ncbi.nlm.nih.gov/pubmed/21210968; https://doi.org/ 10.1186/1471-2164-11-S5-S2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Dustin ML, Colman DR. Neural and immunological synaptic relations. Science [Internet] 2002. [cited 2016 Sep 8]; 298:785-9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12399580; PMID:12399580; https://doi.org/ 10.1126/science.1076386 [DOI] [PubMed] [Google Scholar]
- [13].Dustin ML, Chakraborty AK, Shaw AS. Understanding the structure and function of the immunological synapse. Cold Spring Harb Perspect Biol [Internet] 2010. [cited 2016 Sep 8]; 2:a002311. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20843980; PMID:20843980; https://doi.org/ 10.1101/cshperspect.a002311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zuidscherwoude M, de Winde CM, Cambi A, van Spriel AB. Microdomains in the membrane landscape shape antigen-presenting cell function. J Leukoc Biol [Internet] 2014. [cited 2016 Sep 8]; 95:251-63. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24168856; PMID:24168856; https://doi.org/ 10.1189/jlb.0813440 [DOI] [PubMed] [Google Scholar]
- [15].Richie LI, Ebert PJR, Wu LC, Krummel MF, Owen JJT, Davis MM. Imaging synapse formation during thymocyte selection: inability of CD3zeta to form a stable central accumulation during negative selection. Immunity [Internet] 2002. [cited 2016 Sep 8]; 16:595-606. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11970882; PMID:11970882; https://doi.org/ 10.1016/S1074-7613(02)00299-6 [DOI] [PubMed] [Google Scholar]
- [16].Gong Y, Zhang R, Zhang J, Xu L, Zhang F, Xu W, Wang Y, Chu Y, Xiong S. Alpha-dystroglycan is involved in positive selection of thymocytes by participating in immunological synapse formation. FASEB J [Internet] 2008. [cited 2016 Sep 8]; 22:1426-39. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18171694; PMID:18171694; https://doi.org/ 10.1096/fj.07-08792410.1096/fj.07-9264com [DOI] [PubMed] [Google Scholar]
- [17].Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res 2010; 339:269-80; PMID:19693543; https://doi.org/ 10.1007/s00441-009-0834-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wolfenson H, Lavelin I, Geiger B. Dynamic regulation of the structure and functions of integrin adhesions. Dev Cell [Internet] 2013. [cited 2016 Sep 8]; 24:447-58. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23484852; PMID:23484852; https://doi.org/ 10.1016/j.devcel.2013.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Schwartz MA, Assoian RK. Integrins and cell proliferation: regulation of cyclin-dependent kinases via cytoplasmic signaling pathways. J Cell Sci [Internet] 2001. [cited 2016 Sep 8]; 114:2553-60. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11683383; PMID:11683383 [DOI] [PubMed] [Google Scholar]
- [20].Savino W, Mendes-Da-Cruz DA, Smaniotto S, Silva-Monteiro E, Villa-Verde DMS. Molecular mechanisms governing thymocyte migration: combined role of chemokines and extracellular matrix. J Leukoc Biol 2004; 75:951-61; PMID:15020651; https://doi.org/ 10.1189/jlb.1003455 [DOI] [PubMed] [Google Scholar]
- [21].Savino W, Mendes-da-Cruz DA, Golbert DCF, Riederer I, Cotta-de-Almeida V. Laminin-Mediated Interactions in Thymocyte Migration and Development. Front Immunol [Internet] 2015. [cited 2016 Sep 8]; 6:579. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26635793; PMID:26635793; https://doi.org/ 10.3389/fimmu.2015.00579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sonnenberg A, Linders CJ, Daams JH, Kennel SJ. The alpha 6 beta 1 (VLA-6) and alpha 6 beta 4 protein complexes: tissue distribution and biochemical properties. J Cell Sci [Internet] 1990. [cited 2016 Sep 8]; 96(Pt 2):207-17. Available from: http://www.ncbi.nlm.nih.gov/pubmed/1698797; PMID:1698797 [DOI] [PubMed] [Google Scholar]
- [23].Pulkkinen L, Uitto J. Mutation analysis and molecular genetics of epidermolysis bullosa. Matrix Biol [Internet] 1999. [cited 2016 Sep 8]; 18:29-42. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10367729; PMID:10367729; https://doi.org/ 10.1016/S0945-053X(98)00005-5 [DOI] [PubMed] [Google Scholar]
- [24].Ruiz P, Wiles M V, Imhof BA. Alpha 6 integrins participate in pro-T cell homing to the thymus. Eur J Immunol [Internet] 1995. [cited 2015 Aug 5]; 25:2034-41. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7621877; PMID:7621877; https://doi.org/ 10.1002/eji.1830250735 [DOI] [PubMed] [Google Scholar]
- [25].Chang AC, Salomon DR, Wadsworth S, Hong MJ, Mojcik CF, Otto S, Shevach EM, Coligan JE. Alpha 3 beta 1 and alpha 6 beta 1 integrins mediate laminin/merosin binding and function as costimulatory molecules for human thymocyte proliferation. J Immunol 1995; 154:500-10; PMID:7814863 [PubMed] [Google Scholar]
- [26].Stimamiglio MA, Jiménez E, Silva-Barbosa SD, Alfaro D, García-Ceca JJ, Muñoz JJ, Cejalvo T, Savino W, Zapata A. EphB2-mediated interactions are essential for proper migration of T cell progenitors during fetal thymus colonization. J Leukoc Biol [Internet] 2010. [cited 2015 Aug 5]; 88:483-94. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20504947; PMID:20504947; https://doi.org/ 10.1189/jlb.0210079 [DOI] [PubMed] [Google Scholar]
- [27].Mendes-Da-Cruz DA, Stimamiglio MA, Muñoz JJ, Alfaro D, Terra-Granado E, Garcia-Ceca J, Alonso-Colmenar LM, Savino W, Zapata AG. Developing T-cell migration: Role of semaphorins and ephrins. FASEB J 2012; 26:4390-9; PMID:22815386; https://doi.org/ 10.1096/fj.11-202952 [DOI] [PubMed] [Google Scholar]
- [28].Golbert DCF, Correa-de-Santana E, Ribeiro-Alves M, de Vasconcelos ATR, Savino W. ITGA6 gene silencing by RNA interference modulates the expression of a large number of cell migration-related genes in human thymic epithelial cells. BMC Genomics 2013; 14(Suppl 6):S3. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3909006&tool=pmcentrez&rendertype=abstract; PMID:24564203; https://doi.org/ 10.1186/1471-2164-14-S6-S3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhang X, Rozengurt E, Reed EF. HLA class I molecules partner with integrin β4 to stimulate endothelial cell proliferation and migration. Sci Signal [Internet] 2010. [cited 2016 Sep 8]; 3:ra85. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21098729; PMID:21098729; https://doi.org/ 10.1126/scisignal.200067810.1126/scisignal.2001158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhang X, Reed EF. HLA class I: an unexpected role in integrin β4 signaling in endothelial cells. Hum Immunol [Internet] 2012. [cited 2016 Sep 8]; 73:1239-44. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22789625; PMID:22789625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Fernández E, Vicente A, Zapata A, Brera B, Lozano JJ, Martínez C, Toribio ML. Establishment and characterization of cloned human thymic epithelial cell lines. Analysis of adhesion molecule expression and cytokine production. Blood [Internet] 1994. [cited 2016 Sep 8]; 83:3245-54. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7514905; PMID:7514905 [PubMed] [Google Scholar]
- [32].Linhares-Lacerda L, Ribeiro-Alves M, Nogueira ACMDA, Mendes-da-Cruz DA, Magalhães DA, Dardenne M, Passos GA, Savino W. RNA interference-mediated knockdown of CD49e (α5 integrin chain) in human thymic epithelial cells modulates the expression of multiple genes and decreases thymocyte adhesion. BMC Genomics [Internet] 2010; 11(Suppl 5):S2 Available from: http://www.biomedcentral.com/1471-2164-11-S5; https://doi.org/ 10.1186/1471-2164-11-S5-S2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ritz C, Spiess AN. qpcR: an R package for sigmoidal model selection in quantitative real-time polymerase chain reaction analysis. Bioinformatics [Internet] 2008. [cited 2016 Sep 8]; 24:1549-51. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18482995; PMID:18482995; https://doi.org/ 10.1093/bioinformatics/btn227 [DOI] [PubMed] [Google Scholar]
- [34].R Development Core Team R: A language and environment for statistical computing. [Internet] R Foundation for Statistical Computing 2009. Available from: http://www.r-project.org/ [Google Scholar]
- [35].Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol [Internet] 2002. [cited 2016 Sep 8]; 3:RESEARCH0034. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12184808; PMID:12184808; https://doi.org/ 10.1186/gb-2002-3-7-research0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Eswar N, Webb B, Marti-Renom MA, Madhusudhan MS, Eramian D, Shen M-Y, Pieper U, Sali A. Comparative protein structure modeling using Modeller. Curr Protoc Bioinformatics [Internet] 2006. [cited 2016 Sep 8]; Chapter 5:Unit 5.6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18428767; PMID:18428767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bernstein FC, Koetzle TF, Williams GJ, Meyer EF, Brice MD, Rodgers JR, Kennard O, Shimanouchi T, Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol [Internet] 1977. [cited 2016 Sep 8]; 112:535-42. Available from: http://www.ncbi.nlm.nih.gov/pubmed/875032; PMID:875032; https://doi.org/ 10.1016/S0022-2836(77)80200-3 [DOI] [PubMed] [Google Scholar]
- [38].de Vries SJ, van Dijk M, Bonvin AMJJ. The HADDOCK web server for data-driven biomolecular docking. Nat Protoc [Internet] 2010. [cited 2016 Sep 8]; 5:883-97. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20431534; PMID:20431534; https://doi.org/ 10.1038/nprot.2010.32 [DOI] [PubMed] [Google Scholar]
- [39].Kligys KR, Wu Y, Hopkinson SB, Kaur S, Platanias LC, Jones JCR. α6β4 integrin, a master regulator of expression of integrins in human keratinocytes. J Biol Chem [Internet] 2012. [cited 2016 Sep 8]; 287:17975-84. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22493440; PMID:22493440; https://doi.org/ 10.1074/jbc.M111.310458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lannes-Vieira J, Chammas R, Villa-Verde DM, Vannier-dos-Santos MA, Mello-Coelho V, de Souza SJ, Brentani RR, Savino W. Extracellular matrix components of the mouse thymic microenvironment. III. Thymic epithelial cells express the VLA6 complex that is involved in laminin-mediated interactions with thymocytes. Int Immunol [Internet] 1993. [cited 2015 Aug 5]; 5:1421-30. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8260456; PMID:8260456; https://doi.org/ 10.1093/intimm/5.11.1421 [DOI] [PubMed] [Google Scholar]
- [41].Villa-Verde DM, Lagrota-Candido JM, Vannier-Santos MA, Chammas R, Brentani RR, Savino W. Extracellular matrix components of the mouse thymus microenvironment. IV. Modulation of thymic nurse cells by extracellular matrix ligands and receptors. Eur J Immunol [Internet] 1994. [cited 2015 Aug 5]; 24:659-64. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7510239; PMID:7510239; https://doi.org/ 10.1002/eji.1830240326 [DOI] [PubMed] [Google Scholar]
- [42].Ocampo JSP, Brito JMD, Corrêa-de-Santana E, Borojevic R, Villa-Verde DMS, Savino W. Laminin-211 controls thymocyte-thymic epithelial cell interactions. Cell Immunol [Internet] 2008; 254:1-9. Available from: https://doi.org/10.1016/j.cellimm.2008.06.005; PMID:18644586; https://doi.org/ 10.1016/j.cellimm.2008.06.005 [DOI] [PubMed] [Google Scholar]
- [43].Viola A, Contento RL, Molon B. T cells and their partners: the chemokine dating agency. Trends Immunol [Internet] 2006. [cited 2016 Dec 7]; 27:421-7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16860609; PMID:16860609; https://doi.org/ 10.1016/j.it.2006.07.004 [DOI] [PubMed] [Google Scholar]
- [44].Gómez-Moutón C, Lacalle RA, Mira E, Jiménez-Baranda S, Barber DF, Carrera AC, Martínez-A C, Mañes S. Dynamic redistribution of raft domains as an organizing platform for signaling during cell chemotaxis. J Cell Biol [Internet] 2004. [cited 2016 Dec 7]; 164:759-68. Available from: http://www.ncbi.nlm.nih.gov/pubmed/14981096; PMID:14981096; https://doi.org/ 10.1083/jcb.200309101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Negulescu PA, Krasieva TB, Khan A, Kerschbaum HH, Cahalan MD. Polarity of T cell shape, motility, and sensitivity to antigen. Immunity [Internet] 1996. [cited 2016 Dec 7]; 4:421-30. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8630728; PMID:8630728; https://doi.org/ 10.1016/S1074-7613(00)80409-4 [DOI] [PubMed] [Google Scholar]
- [46].Constantin G, Majeed M, Giagulli C, Piccio L, Kim JY, Butcher EC, Laudanna C. Chemokines trigger immediate beta2 integrin affinity and mobility changes: differential regulation and roles in lymphocyte arrest under flow. Immunity [Internet] 2000. [cited 2016 Dec 7]; 13:759-69. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11163192; PMID:11163192; https://doi.org/ 10.1016/S1074-7613(00)00074-1 [DOI] [PubMed] [Google Scholar]
- [47].Shamri R, Grabovsky V, Gauguet J-M, Feigelson S, Manevich E, Kolanus W, Robinson MK, Staunton DE, von Andrian UH, Alon R. Lymphocyte arrest requires instantaneous induction of an extended LFA-1 conformation mediated by endothelium-bound chemokines. Nat Immunol [Internet] 2005. [cited 2016 Dec 7]; 6:497-506. Available from: http://www.nature.com/doifinder/10.1038/ni1194; PMID:15834409; https://doi.org/ 10.1038/ni1194 [DOI] [PubMed] [Google Scholar]
- [48].Paessens LC, Singh SK, Fernandes RJ, van Kooyk Y. Vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1) provide co-stimulation in positive selection along with survival of selected thymocytes. Mol Immunol [Internet] 2008. [cited 2016 Sep 8]; 45:42-8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17604837; PMID:17604837; https://doi.org/ 10.1016/j.molimm.2007.05.016 [DOI] [PubMed] [Google Scholar]
- [49].Salomon DR, Crisa L, Mojcik CF, Ishii JK, Klier G, Shevach EM. Vascular cell adhesion molecule-1 is expressed by cortical thymic epithelial cells and mediates thymocyte adhesion. Implications for the function of alpha4beta1 (VLA4) integrin in T-cell development. Blood [Internet] 1997. [cited 2016 Sep 8]; 89:2461-71. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9116290; PMID:9116290 [PubMed] [Google Scholar]
- [50].Porter JC, Hogg N. Integrin cross talk: activation of lymphocyte function-associated antigen-1 on human T cells alters alpha4beta1- and alpha5beta1-mediated function. J Cell Biol [Internet] 1997. [cited 2016 Sep 8]; 138:1437-47. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9298996; PMID:9298996; https://doi.org/ 10.1083/jcb.138.6.1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Cascio G, Martín-Cófreces NB, Rodríguez-Frade JM, López-Cotarelo P, Criado G, Pablos JL, Rodríguez-Fernández JL, Sánchez-Madrid F, Mellado M. CXCL12 Regulates through JAK1 and JAK2 Formation of Productive Immunological Synapses. J Immunol [Internet] 2015. [cited 2016 Sep 8]; 194:5509-19. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25917087; PMID:25917087; https://doi.org/ 10.4049/jimmunol.1402419 [DOI] [PubMed] [Google Scholar]
- [52].Kallikourdis M, Trovato AE, Anselmi F, Sarukhan A, Roselli G, Tassone L, Badolato R, Viola A. The CXCR4 mutations in WHIM syndrome impair the stability of the T-cell immunologic synapse. Blood [Internet] 2013. [cited 2016 Sep 8]; 122:666-73. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23794067; PMID:23794067; https://doi.org/ 10.1182/blood-2012-10-461830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kallikourdis M, Viola A, Benvenuti F. Human Immunodeficiencies Related to Defective APC/T Cell Interaction. Front Immunol [Internet] 2015. [cited 2016 Sep 8]; 6:433. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26379669; PMID:26379669; https://doi.org/ 10.3389/fimmu.2015.00433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Ayres-Martins S, Lannes-Vieira J, Farias-De-Oliveira DA, Brito JM, Villa-Verde DMS, Savino W. Phagocytic cells of the thymic reticulum interact with thymocytes via extracellular matrix ligands and receptors. Cell Immunol [Internet] 2004. [cited 2015 Aug 5]; 229:21-30. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15331325; PMID:15331325; https://doi.org/ 10.1016/j.cellimm.2004.06.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


