ABSTRACT
Although the cross-talk between auxin and ethylene has been described during plant development, the role played by auxin upon gene expression during aerenchyma formation is poorly understood. Root aerenchyma formation results from the opening of gas spaces in the cortex. It is part of a developmental program (constitutive) or due to ethylene treatment or abiotic stress (induced) such as flooding and nutrient starvation. This process relies on programmed cell death and cell wall modifications. Here we followed development of aerenchyma formation in sugarcane along 5 cm from the root apex. As a constitutive process, the aerenchyma formation was observed in the cortex from the 3rd cm onwards. This occurred despite 1-methylcyclepropene (1-MCP) treatment, an inhibitor of ethylene perception. However, this process occurred while ethylene (and auxin) levels decreased. Within the aerenchyma formation zone, the concentration of ethylene is lower in comparison to the concentration in maize. Besides, the ratio between both hormones (ethylene and auxin) was around 1:1. These pieces of evidence suggest that ethylene sensitivity and ethylene-auxin balance may play a role in the formation of aerenchyma. Furthermore, the transcriptional analysis showed that genes related to cell expansion are up-regulated due to 1-MCP treatment. Our results help explaining the regulation of the formation constitutive aerenchyma in sugarcane.
KEYWORDS: 1-MCP, aerenchyma, auxin-ethylene interplay, cell wall degradation, sugarcane
The aerenchyma is characterized by enlarged and interconnected intercellular spaces filled with gas.1,2 They can be schizogenous and lysigenous. Whereas in the former, cells separate from one another without cell death, the second includes cell expansion, separation, cell death and cell wall modifications.2,3,4 Aerenchyma can be constitutive, i.e., its formation is included in the developmental program, or induced by abiotic stresses such as nutritional starvation and hypoxia.5 In sugarcane, the development of aerenchyma is constitutive.2,6 Its formation seems to be a result of different combinations of developmental modules,7 which are activated by plant hormones and the environment. Leite et al. (2017) reported that cell wall modifications in sugarcane roots produce a composite that apparently seals the gas spaces. They seem to function as chambers where gasses can be stored and used for respiration by the remaining living cells in the roots.
The level and tissue sensitivity to ethylene are responsible for triggering aerenchyma formation.8 In at least two species possessing constitutive aerenchyma (Juncus effusus and Oryza sativa) the role of ethylene has been demonstrated through the use of 1-methylcyclopropene (1-MCP), a substance that blocks the ethylene receptors in plants.9,10 Justin & Armstrong, (1991) reported the effect of the exogenous application of a synthetic auxin on aerenchyma formation in maize roots. However, the auxin interplay with ethylene has been neglected so far.
Here we report the levels of ethylene and auxin in root segments of sugarcane and discuss the possibility that their interplay has a role in aerenchyma formation in sugarcane. To our knowledge, this is the first report that shows both hormones measured at the same time during aerenchyma formation.
Roots were sectioned into five segments,2 and aerenchyma was present from S3 onwards in control and 1-MCP treatment (Fig. 1). No significant differences were found. Ethylene and auxin levels were measured for all five root segments (Table 1).12,13
Figure 1.
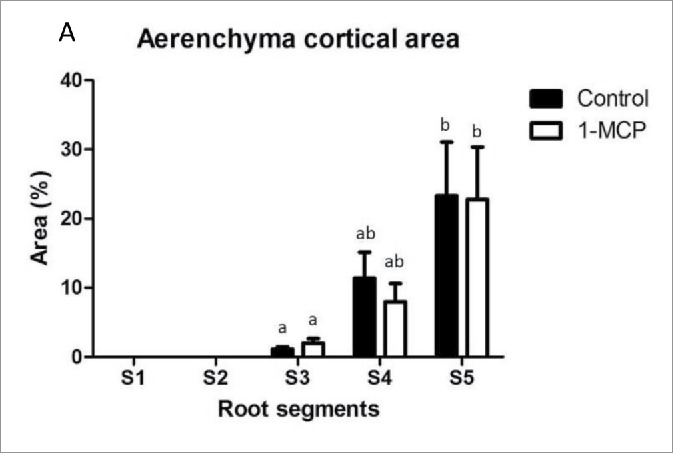
(A) Cortex cross sectional area occupied by the aerenchyma upon 1-MCP treatment. Measures were done in five different roots in three pools of five plants each. Different letters denote statistically significant differences (p < 0.1) between root segments and treatments.
Table 1.
Ethylene and free IAA levels along the segments in plants treated with 1-MCP.
| Ethylene1 |
Free-iAA2 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Segment | Control | 1-MCP | Control | 1-MCP | ||||
| S1 | 0,25 ± 0,02 | a | 0,12 ± 0,01 | a | 12,16 ± 2,04 | a | 18,85 ± 2,60 | a |
| S2 | 0,23 ± 0,04 | a | 0,05 ± 0,02 | b | 11,66 ± 0,70 | b | 7,63 ± 1,30 | b |
| S3 | 0,12 ± 0,02 | b | 0,10 ± 0,03 | b | 7,61 ± 0,51 | b | 9,82 ± 1,00 | b |
| S4 | 0,07 ± 0,01 | b | 0,07 ± 0,02 | c | 4,75 ± 0,39 | c | 4,87 ± 0,33 | b |
| S5 | 0,06 ± 0,01 | b | 0,06 ± 0,01 | c | 5,39 ± 1,02 | c | 5,90 ± 0,20 | b |
a,b,c significant differences (p < 0.1) between root segments. Bold numbers indicate significant differences (p < 0.1) between treatments.
1nL.g−1.h−1
2ng.g−1
To assess the role of ethylene in aerenchyma formation in sugarcane root development, the inhibitor 1- methylcyclopropene (1-MCP) (EthylBloc, AgroFresh) was applied to roots at every 48 h from May/2012 to August/2012. Plant growth, root harvesting, and aerenchyma area calculation were performed as reported by Leite et al. (2017).
1-MCP affected the proportions between ethylene and auxin within the root segments. In S1 and S2, 1-MCP treated roots displayed proportionally lower ethylene concentration. Ethylene and auxin levels decreased 2.5-fold during the S1-S2 transition in 1-MCP treated roots, whereas within control plants they were kept constant (Table 1). These differences led to a higher auxin-ethylene ratio on S1 and S2 in 1-MCP plants compared to control (Fig. S1). On the following root segments (S3-S5), the level of both hormones decreased, reaching an auxin:ethylene ratio closer to 1:1. Indeed, Gunawardena et al. (2001) highlighted that in maize, aerenchyma does not develop in lateral roots emergence sites – we confirmed that for sugarcane (results not shown) -, where high auxin and ethylene levels are required.14
There is much literature on the interplay between ethylene and auxin. Ethylene signaling increases auxin biosynthesis and transport during root growth.15 As a result, auxin tends to repress root elongation and affect cell wall extensibility.16 Ethylene levels can be decreased by 1-MCP treatment17 what results in the mediation of IAA-aminoacid conjugation.18,19 Thus, it is likely that the relatively lower ethylene levels observed in S1 and S2 in 1-MCP treated sugarcane roots could lead to increased free auxin concentration in S1 (Fig. S1 and Table 1). As both hormones decrease at slightly different rates and reach a ratio near to 1:1 from S3 onwards, it is possible that hormone balance could be important for aerenchyma development.
Whereas the ethylene levels in maize correlate positively with the aerenchyma area,20,21 in sugarcane, aerenchyma development, and ethylene levels are inversely correlated (r2 = −0.96). Thus, it is possible that the constitutive nature of sugarcane root aerenchyma might be due to an intrinsic higher sensitivity to ethylene. This possibly explains our finding that 1-MCP failed to block aerenchyma formation in roots of sugarcane.
Microarray and qPCR validation22 showed transcriptional alterations. The primers used are listed in Table S2. Around 89% of the qPCR profiles confirmed the microarray results (Table S3-S7). Three expansins were among the up-regulated transcripts in 1-MCP treatment (Table S3, S4 & S5). These proteins are related to the stability of hydrogen bonds between xyloglucan and cellulose.23 Thus, expansin action plays a key role in cell expansion.
Although xyloglucan occurs in small proportions in sugarcane walls,2,24 it might be important in the control of cell wall architecture by holding macrofibrils together in the wall.25 It is, therefore, possible that expansins play a role in the cortex cell expansion in maize and sugarcane. However, the fact that 1-MCP treated roots displayed higher expression of expansins is contradictory, since this protein normally leads to expansion due to some cell wall loosening during development. The higher auxin level observed closer to the root tip (S1 and S2) suggests that this hormone might be involved in triggering the expression of cell expansion-related genes that are key elements in the cellular processes that take place before aerenchyma formation.7,26 Indeed, Yoo et al., (2015) identified several transcripts related to auxin-mediated gene regulation during aerenchyma formation. However, differently from the previously observed positive correlation between auxin and aerenchyma in maize roots,11 in sugarcane, we found an inverse correlation (r2 = −0.96) between the hormone and aerenchyma formation.
Although more evidence has to be produced, our result suggest that the ratio between the hormones might be important in aerenchyma development of sugarcane roots. Such an interplay probably occurs through the expression of target genes related to cell expansion and programed cell death. Sensitivity to ethylene, rather than its concentration, is probably responsible for the sugarcane aerenchyma constitutive character.
Supplementary Material
Funding Statement
This study was funded by FAPESP (2008/57908-6, 2009/52840-7, 2010/17104-5) and CNPq (574002/2008-1, 490022/2009-0). EQPT was recipient of FAPESP fellowships (2011/07586-5 and 2013/16225-1).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Evans DE. Aerenchyma formation. New Phytol. 2003;161:35–49. doi: 10.1046/j.1469-8137.2003.00907.x. [DOI] [Google Scholar]
- 2.Leite DCC, Grandis A, Tavares EQP, Piovezani A, Pattathil S, Avci U, Rossini A, Cambler A, De Souza AP Hahn MG, Buckeridge MS. Cell wall changes during the formation of aerenchyma in sugarcane roots. Ann Bot. 2017;120:693–708. doi: 10.1093/aob/mcx050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gunawardena A, Pearce DME, Jackson MB, Hawes CR, Evans DE. Rapid changes in cell wall pectic polysaccharides are closely associated with early stages of aerenchyma formation, a spatially localized form of programmed cell death in roots of maize (Zea mays L.) promoted by ethylene. Plant Cell Environ. 2001;24:1369–75. doi: 10.1046/j.1365-3040.2001.00774.x. [DOI] [Google Scholar]
- 4.Rajhi I, Yamauchi T, Takahashi H, Nishiuchi S, Shiono K, Watanabe R, Mliki A, Nagamura Y, Tsutsumi N, Nishizawa NK, et al.. Identification of genes expressed in maize root cortical cells during lysigenous aerenchyma formation using laser microdissection and microarray analyses. New Phytol. 2011;190:351–68. doi: 10.1111/j.1469-8137.2010.03535.x. [DOI] [PubMed] [Google Scholar]
- 5.Fagerstedt KV. Programmed cell death and aerenchyma formation under hypoxia, in: Mancuso S., Shabala S. (Eds.). Waterlogging signaling and tolerance in plants: Springer Science, Heidelberg: 2010, pp. 99–118. [Google Scholar]
- 6.Tetsushi H, Karim MA. Flooding tolerance of sugarcane in relation to growth, physiology and root structure. South Pacific Studies. 2007;28:9–22 [Google Scholar]
- 7.Grandis A, De Souza AP Tavares EQP, Buckeridge MS. Using natural plant cell wall degradation mechanisms to improve second generation bioethanol, in: McCann MC, Buckeridge MS, Carpita NC (Eds.) Plants and Bioenergy, Springer, New York, 2014, 211–230. [Google Scholar]
- 8.Yamauchi T, Shimamura S, Nakazono M, Mochizukic T. Aerenchyma formation in crop species: A review. Field Crops Research. 2013;152:8–16. doi: 10.1016/j.fcr.2012.12.008. [DOI] [Google Scholar]
- 9.Visser EJ, Bögemann GM. Aerenchyma formation in the wetland plant Juncus effusus is independent of ethylene. New Phytol. 2006;171(2):305–314. doi: 10.1111/j.1469-8137.2006.01764.x. [DOI] [PubMed] [Google Scholar]
- 10.Yukiyoshi K, Karahara I. Role of ethylene signalling in the formation of constitutive aerenchyma in primary roots of rice. AoB PLANTS. 2014;6:plu043 doi: 10.1093/aobpla/plu043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Justin SHF, Armstrong W. A reassessment of the influence of NAA on aerenchyma formation in maize roots. New Phytol. 1991;117:607–18. doi: 10.1111/j.1469-8137.1991.tb00965.x. [DOI] [Google Scholar]
- 12.Mainardi JA, Purgatto E, Vieira Jr A, Bastos WA, Cordenunsi BR, Nascimento JR, Lajolo. Effects of ethylene and 1-methylciclopropene (1-MCP) on gene expression and activity of α-1,4-glucan-phosphorylase during banana ripening. J Agric Food Chem. 2006;54:7294–9. doi: 10.1021/jf061180k. [DOI] [PubMed] [Google Scholar]
- 13.Ludwig-Müller J, Georgiev M, Bley T. Metabolite and hormonal status of hairy root cultures of Devil's claw (Harpagophytum procumbens) in flasks and in a bubble column bioreactor. Process Biochem. 2008;43:15–23. doi: 10.1016/j.procbio.2007.10.006. [DOI] [Google Scholar]
- 14.Jung JKH, McCouch S. Getting to the roots of it: Genetic and hormonal control of root architecture. Frontiers Plant Sci. 2013;4:186. doi: 10.3389/fpls.2013.0018.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Růžička K, Ljung K, Vanneste S, Podhorská R, Beeckman T, Friml J, Benková E. Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell. 2007;19:2197–12. doi: 10.1105/tpc.107.052126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Staal M, Cnodder T, Simon D, Vandenbussche F, Van Der Straeten D Verbelen JP, Elzenga T, Vissenberg K. Apoplastic alkalinization is instrumental for the inhibition of cell elongation in the Arabidopsis root by the ethylene precursor 1-aminocyclopropane-1-carboxylic acid. Plant Physiol. 2011;155:2049–55. doi: 10.1104/pp.110.168476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blakenship SM, Dole JM. 1-Methylcyclopropene: A review, Postharvest. Biol Tec. 2003;28:1–25. doi: 10.1016/S0925-5214(02)00246-6. [DOI] [Google Scholar]
- 18.Liu K, Kang BC, Jiang H, Moore SL, Li H, Watkins CB, Setter TL, Jahn MM. A GH3-like gene, CcGH3, isolated from Capsicum chinense L. fruit is regulated by auxin and ethylene. Plant Mol Biol. 2005;58:447–64. doi: 10.1007/s11103-005-6505-4. [DOI] [PubMed] [Google Scholar]
- 19.Böttcher C, Keyzers RA, Boss PK, Davies C. Sequestration of auxin by the indole-3-acetic acid-amido synthetase GH3-1 in grape berry (Vitis vinifera L.) and the proposed role of auxin conjugation during ripening. J Exp Bot. 2010;61:3615–25. doi: 10.1093/jxb/erq174. [DOI] [PubMed] [Google Scholar]
- 21.He CJ, Morgan PW, Drew MC. Transduction of an ethylene signal is required for cell death and lysis in the root cortex of maize during aerenchyma formation induced by hypoxia. J Plant Physiol. 1996;112:463–472. doi: 10.1104/pp.112.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Souza AP Leite DCC, Pattathil S, Hahn MG, Buckeridge MS. Composition and structure of sugarcane cell wall polysaccharides: Implications for second-generation bioethanol production. BioEnerg. 2013;6:564–79. doi: 10.1007/s12155-012-9268-1. [DOI] [Google Scholar]
- 25.Buckeridge M.S., Santos W.D., Tiné M.S., De Souza A.P. The cell wall architecture of sugarcane and its implications to cell wall recalcitrance. In: Lam E, Carrer H, Silva J.A(Eds). Compendium of Bioenergy Plants: Sugarcane. Wilsonville: CRC Press; 2015. p. 125. [Google Scholar]
- 26.Tavares EQP, De Souza AP Buckeridge MS. How endogenous plant cell-wall degradation mechanisms can help achieve higher efficiency in saccharification of biomass. J Exp Bot. 2015;66:4133–43. doi: 10.1093/jxb/erv171. [DOI] [PubMed] [Google Scholar]
- 27.Yoo YH, Choi HK, Jung KH. Genome-wide identification and analysis of genes associated with lysigenous aerenchyma formation in rice roots. J Plant Biol. 2015;58:117–27. doi: 10.1007/s12374-014-0486-2. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


