ABSTRACT
Afatinib exhibits therapeutic efficacy for lung adenocarcinoma patients harboring HER2 exon 20 insertions. HER2 S310Y single site substitution was discovered in recent years and afatinib efficacy for adenocarcinoma patients harboring S310Y mutation has not been reported. We presented a case of a 41-year-old male patient with lung adenocarcinoma harboring the HER2 S310Y mutation obtained clinical response to the treatment of afatinib, an oral HER family blocker. After the treatment of afatinib, the patient achieved partial response (PR) in chest lesions and almost complete response (CR) in intracranial lesions. He experienced progressive disease (PD) with liver metastasis and achieved a progression-free survival (PFS) of 5 months. He continually treated with afatinib after CT guided percutaneous radiofrequency ablation to eradicate the hepatic tumor cells and achieved stable disease (SD). In this study, we reported the first clinical evidence of efficacy generated by afatinib, the irreversible HER family inhibitor, targeting HER2 S310Y single site mutation in lung adenocarcinoma.
KEYWORDS: adenocarcinoma, HER2 S310Y, afatinib, next-generation sequencing, targeted therapy
Introduction
Gene alterations of human epidermal growth factor 2 (HER2/ERBB2) occur in 1–4% of lung adenocarcinomas as oncogenic driver mutations.1 Median overall survival of HER2 gene variations in lung cancer patients is 1.6–1.9 years from the time of stage IV diagnosis. HER2 mutations found in clinical patients so far are commonly consisting of an in-frame insertion YVMA (p.A775_G776insYVMA) in exon 20, located in kinase domain of HER2 protein.2 Afatinib is an oral HER family blocker, which covalently binds and irreversibly blocks all kinase-competent HER family members.3 Afatinib displays a manageable toxicity profile and promising results in several retrospective studies targeting mutated HER2 exon 20 in non-small-cell lung cancer (NSCLC).4
Although the exon 20 mutation in HER2 kinase domain has been already well characterized, mutations in extracellular domain (ECD) were poorly investigated. HER2 S310 single site substitution was located in the ECD of HER2 and first discovered by a comprehensive genetic study.5 S310Y mutation has been identified in a few NSCLC patients,6,7 but no clinical efficacious drug targeting this mutation has been reported. Herein, we reported the first clinical evidence of efficacy generated by afatinib targeting HER2 exon 8 S310Y mutation in a lung adenocarcinoma patient.
Case presentation
A 41-year-old male with a smoking index of 200 (20 cigarettes/day for 10 years) presented to the outpatient clinic in May 2015, with intermittent cough and left chest pain for 2 months. Chest computed tomography (CT) revealed a mass arising from the lingual segment in the left upper lobe of lung. Neither mediastinal lymph nodes enlargement nor pleural thickening was observed. No bilateral pulmonary effusion appeared. Wedge resection was performed in left upper lung under double lumen intubation thoracoscopy. During the operation, he was founded the invasive adenocarcinoma in left lung and pleural nodules and diaphragm. The patient was diagnosed as stage IV left upper lung adenocarcinoma.
This patient was treated with chemotherapy by gemcitabine (1600 mg, D1, D8) and carboplatin (500 mg, D2) for 4 cycle, followed by gemcitabine alone (1600 mg, D1, D8) for 3 cycle after operation. He presented with chemotherapy-induced side effects of anorexia and myelosuppression. He achieved stable disease (SD) and experienced progressive disease (PD) 11 months later with enlarged left lung lesion (Figure 1A) and brain metastasis (Figure 2A). Positron emission tomography-computed tomography (PET-CT) revealed an increase in pulmonary lesion number, accompanied with the involvement of supraclavicular fossae lymph node and left pleura and thoracic vertebra. Magnetic resonance imaging (MRI) revealed intracerebral metastasis.
Figure 1.
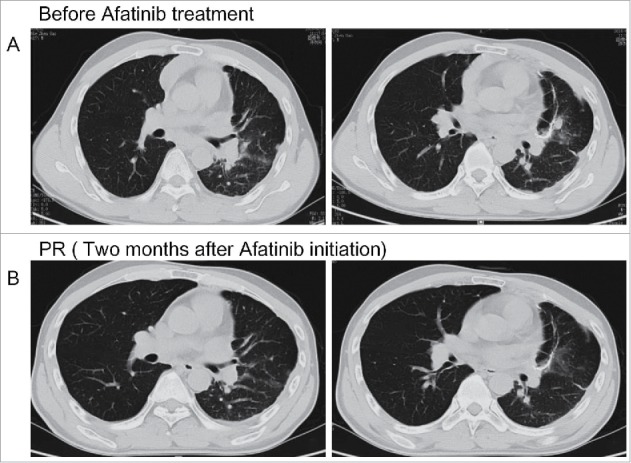
Chest computed tomography scan revealed the tumor response to afatinib. (A) The progressive disease status of lung lesion before afatinib treatment. (B) The partial response of lung lesions after afatinib treatment.
Figure 2.
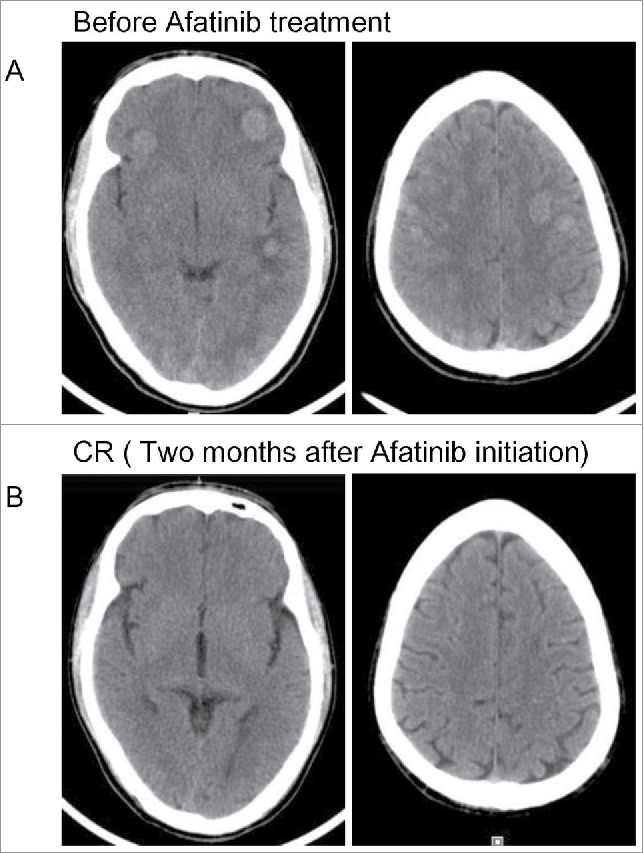
Head computed tomography scan revealed the tumor response to afatinib. (A) The progressive disease status of intracranial lesions before afatinib treatment. (B) Complete response of intracranial lesions after afatinib treatment.
Capture-based targeted sequencing of assessing plasma circulating tumor DNA (ctDNA) and tissue biopsy were performed. NGS revealed the harboring of HER2 exon 8 S310Y missense mutation NM_004448.3(HER2):c.929C>A(p.Ser310Tyr) with allelic fraction (AF) of 0.57% in liquid biopsy and 38.9% in tissue sample (Figure 3). No other afatinib targeted mutations were identified. From May 2016, the patient was treated with afatinib (40 mg), the irreversible HER family inhibitor. Shortly after initiation of treatment, the patient experienced rapid clinical symptom relief. He displayed side effects of mild skin rash, fatigue and loss of appetite during the treatment of afatinib. He achieved partial response (PR) in lung lesion and almost complete response (CR) in intracranial lesions two months after the initiation of afatinib. Head and chest CT scan revealed decreased number of left lung lesions, shrinkage of pleura nodules and mediastinum lymph nodes (Figure 1B), accompanied with nearly tumor-free in brain (Figure 2B).
Figure 3.
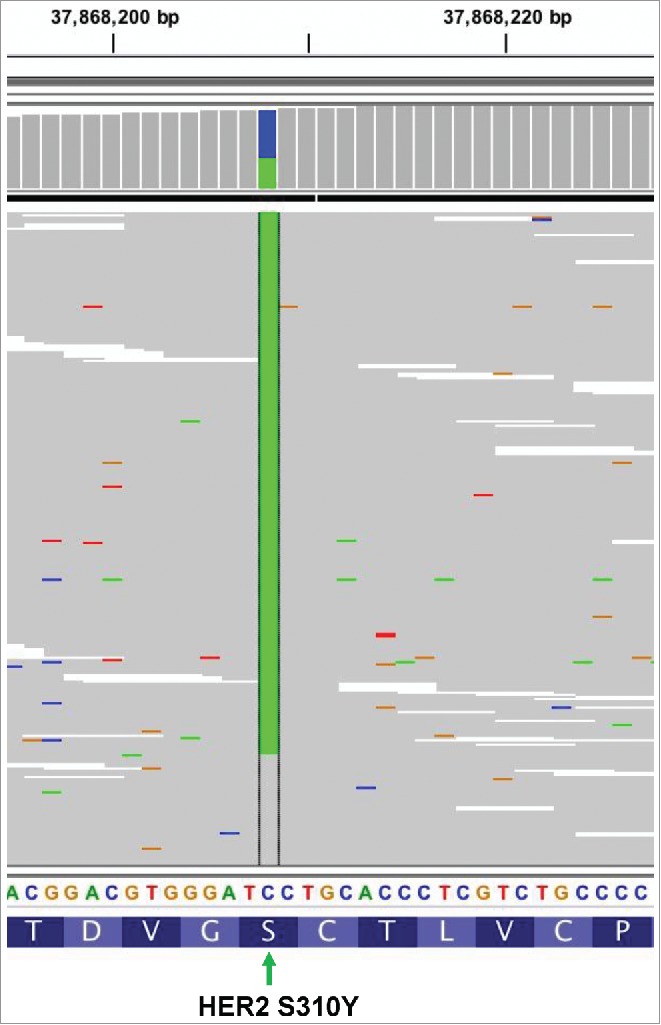
The Intergrative Genomics Viewer (IGV) screenshots displayed the reads from ctDNA sequencing and revealed the harboring of HER2 S310Y. Different nucleobase types were presented with different colors. Adenine (A) is presented by green, cytosine (C) is indicated by blue, guanine (G) is yellow, and thymine (T) is red. The green column in the middle indicates position of the mutated nucleobase (929C>A). The mutation of HER2 S310Y in terms of nucleobase and amino acid level occurred as 929C>A and Serine310 to Tyrosine, respectively [NM_004448.3(HER2):c.929C>A(p.Ser310Tyr)].
However, this patient experienced progressive disease (PD) with enlarged lung lesion and increasing pleural effusion in October 2016. He also experienced hepatic metastasis, resulting a progression-free survival (PFS) of 5 months. Capture-based NGS revealed HER2 exon 8 S310Y mutation in hepatic puncture biopsy (AF = 15.2%). The hepatic tumor was treated with CT guided percutaneous radiofrequency ablation. At the same time, he was continued with ongoing afatinib treatment (40 mg, QD) and his extra-hepatic lesion achieved stable disease (SD) with slow progression. Unfortunately, he experienced enlarged lung lesion and developed brain metastasis 5 months later in March 2017. The patient passed away a few weeks later.
Discussion
HER2 aberrances, which can lead to phosphatase inhibition or structure change of receptors, can be oncogenic and been identified in several malignancies.8 Afatinib, can covalently binds to HER2 and irreversibly inhibits its enzymatic activity, provides an effective, long-lasting blockade of aberrant HER2 receptor signaling in multiple types of cancer.9-12 Here, we presented the first case of a lung adenocarcinoma patient harboring HER2 S310Y mutation, effectively treated with afatinib.
With the rapid development of targeted therapy, HER2 inhibitors has dramatically revolutionized and widely applied to HER2 mutant lung cancer patients. HER2 exon 20 insertions were the most common subtype accounting for the majority (50–80%) of HER2 mutant lung cancer.13,14 This tyrosine kinase domain mutation can cause constitutive phosphorylation and activation of HER2 receptor by altering the ATP-binding pocket, resulting in oncogenic activity of HER2.15 The responses to afatinib have been reported in several studies with tumors harboring the YVMA exon 20 insertion.16
Extracellular domain mutations of HER2, S310F and S310Y, were discovered in cancer patients recent years and speculated to result in hydrophobic interactions and non-covalent dimerization, thus subsequently activate the downstream signaling pathways.7 To date, limited number of targeted therapy studies focusing on HER2 S310F have been reported. A breast cancer patient with HER2 S310F mutation responded well to trastuzumab monotherapy with decrease in the size of liver and bone metastases within 3 months of initiating treatment.17 An extramammary paget's disease patient associated with adnexal adenocarcinoma harboring HER2 S310F was reported to achieve continuing partial response to lapatinib and capecitabine therapy.18
Nevertheless, to the best of our knowledge, the efficacy of afatinib to HER2 S310Y mutation in NSCLC patients has not been reported before. In our case, after afatinib treatment, the lung adenocarcinoma patient obtained PR in chest lesion and almost CR in intracranial lesions, accompanied with significant symptoms relief. Although the patient experienced PD five months later, he continually achieved longer duration of treatment with afatinib in other lesions after the local ablative therapy. In this study, we provided the first clinical evidence of afatinib to be efficacious for lung adenocarcinoma patients harboring HER2 S310Y mutation, which served as guidance for HER2-associated targeted therapy. Further investigation of highly effective and tolerable HER2 therapies will be highly expected to carry out in order to expand treatment options for HER2 ECD mutant cancer patients.
In our presented case, the patient harboring HER2 S310Y mutation was negative for HER2 amplification, assessed by both NGS and fluorescence in situ hybridization (FISH). In addition, HER2 protein overexpression was also negative in this patient detected by immunohistochemical staining (IHC). This case demonstrated that HER2 S310Y was independent of HER2 amplification and protein overexpression in this patient, acting as a functional driver mutation for tumorigenesis and responded to afatinib treatment. Many researches also focus on the coincidence of the gene mutation, amplification and protein overexpression. Li et al. reported that HER2 mutation was not overlapped with HER2 amplification and HER2 protein overexpression.19 However, the co-existence of HER2 amplification was observed in four of eight,20 three of thirty-four,21 and two of six22 of HER2 mutated lung cancer patients. Previous studies of the underlying associations of HER2 mutation, copy number amplification and HER2 protein overexpression have not reached a unanimous conclusion. Therefore, further efforts are still needed to interrogate the complexity of HER2 alterations and the associations of HER2 mutation, amplification and protein overexpression in cancers.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Informed consent statement
This study was approved by the Institutional Review Board at the Shenzhen People's Hospital. The patient provided informed consent and gave permission to this study.
Acknowledgments
The authors would like to thank Guangzhou Burning Rock for the next-generation sequencing.
References
- 1.Shigematsu H, Takahashi T, Nomura M, Majmudar K, Suzuki M, Lee H, Wistuba II, Fong KM, Toyooka S, Shimizu N, et al.. Somatic mutations of the HER2 kinase domain in lung adenocarcinomas. Cancer Res. 2005;65:1642–6. doi: 10.1158/0008-5472.CAN-04-4235. PMID:15753357. [DOI] [PubMed] [Google Scholar]
- 2.Arcila ME, Chaft JE, Nafa K, Roy-Chowdhuri S, Lau C, Zaidinski M, Paik PK, Zakowski MF, Kris MG, Ladanyi M. Prevalence, clinicopathologic associations, and molecular spectrum of ERBB2 (HER2) tyrosine kinase mutations in lung adenocarcinomas. Clin Cancer Res. 2012;18:4910–8. doi: 10.1158/1078-0432.CCR-12-0912. PMID:22761469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solca F, Dahl G, Zoephel A, Bader G, Sanderson M, Klein C, Kraemer O, Himmelsbach F, Haaksma E, Adolf GR. Target binding properties and cellular activity of afatinib (BIBW 2992), an irreversible ErbB family blocker. J Pharmacol Exp Ther. 2012;343:342–50. doi: 10.1124/jpet.112.197756. PMID:22888144. [DOI] [PubMed] [Google Scholar]
- 4.Peters S, Zimmermann S. Targeted therapy in NSCLC driven by HER2 insertions. Transl Lung Cancer Res. 2014;3:84–8. PMID:25806285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang MT, Asthana S, Gao SP, Lee BH, Chapman JS, Kandoth C, Gao J, Socci ND, Solit DB, Olshen AB, et al.. Identifying recurrent mutations in cancer reveals widespread lineage diversity and mutational specificity. Nat Biotechnol. 2016;34:155–63. doi: 10.1038/nbt.3391. PMID:26619011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kan Z, Jaiswal BS, Stinson J, Janakiraman V, Bhatt D, Stern HM, Yue P, Haverty PM, Bourgon R, Zheng J, et al.. Diverse somatic mutation patterns and pathway alterations in human cancers. Nature. 2010;466:869–73. doi: 10.1038/nature09208. PMID:20668451. [DOI] [PubMed] [Google Scholar]
- 7.Greulich H, Kaplan B, Mertins P, Chen TH, Tanaka KE, Yun CH, Zhang X, Lee SH, Cho J, Ambrogio L, et al.. Functional analysis of receptor tyrosine kinase mutations in lung cancer identifies oncogenic extracellular domain mutations of ERBB2. Proc Natl Acad Sci U S A. 2012;109:14476–81. doi: 10.1073/pnas.1203201109. PMID:22908275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moasser MM. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene. 2007;26:6469–87. doi: 10.1038/sj.onc.1210477. PMID:17471238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Genova C, Rijavec E, Barletta G, Burrafato G, Biello F, Dal Bello MG, Coco S, Truini A, Alama A, Boccardo F, et al.. Afatinib for the treatment of advanced non-small-cell lung cancer. Expert Opin Pharmacother. 2014;15:889–903. doi: 10.1517/14656566.2014.902445. PMID:24646054. [DOI] [PubMed] [Google Scholar]
- 10.Hurwitz JL, Scullin P, Campbell L. Afatinib treatment in advanced non-small cell lung cancer. Lung Cancer (Auckl). 2011;2:47–57. PMID:28210118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keating GM. Afatinib: a review of its use in the treatment of advanced non-small cell lung cancer. Drugs. 2014;74:207–21. doi: 10.1007/s40265-013-0170-8. PMID:24435321. [DOI] [PubMed] [Google Scholar]
- 12.Geuna E, Montemurro F, Aglietta M, Valabrega G. Potential of afatinib in the treatment of patients with HER2-positive breast cancer. Breast Cancer (Dove Med Press). 2012;4:131–7. PMID:24367201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kris MG, Camidge DR, Giaccone G, Hida T, Li BT, O'Connell J, Taylor I, Zhang H, Arcila ME, Goldberg Z, et al.. Targeting HER2 aberrations as actionable drivers in lung cancers: phase II trial of the pan-HER tyrosine kinase inhibitor dacomitinib in patients with HER2-mutant or amplified tumors. Ann Oncol. 2015;26:1421–7. doi: 10.1093/annonc/mdv186. PMID:25899785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arcila ME, Nafa K, Chaft JE, Rekhtman N, Lau C, Reva BA, Zakowski MF, Kris MG, Ladanyi M. EGFR exon 20 insertion mutations in lung adenocarcinomas: prevalence, molecular heterogeneity, and clinicopathologic characteristics. Mol Cancer Ther. 2013;12:220–9. doi: 10.1158/1535-7163.MCT-12-0620. PMID:23371856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herter-Sprie GS, Greulich H, Wong KK. Activating mutations in ERBB2 and their impact on diagnostics and treatment. Front Oncol. 2013;3:86. doi: 10.3389/fonc.2013.00086. PMID:23630663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li BT, Lee A, O'Toole S, Cooper W, Yu B, Chaft JE, Arcila ME, Kris MG, Pavlakis N. HER2 insertion YVMA mutant lung cancer: Long natural history and response to afatinib. Lung Cancer. 2015;90:617–9. doi: 10.1016/j.lungcan.2015.10.025. PMID:26559459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jasra S, Opyrchal M, Norton L, Mehta R. A Rare Case of S310F Somatic ERBB2 Mutation in a HER2-Nonamplified Breast Cancer. Clin Breast Cancer. 2017;17:e37–41. doi: 10.1016/j.clbc.2016.08.001. PMID:27665021. [DOI] [PubMed] [Google Scholar]
- 18.Vornicova O, Hershkovitz D, Yablonski-Peretz T, Ben-Itzhak O, Keidar Z, Bar-Sela G. Treatment of metastatic extramammary Paget's disease associated with adnexal adenocarcinoma, with anti-HER2 drugs based on genomic alteration ERBB2 S310F. Oncologist. 2014;19:1006–7. doi: 10.1634/theoncologist.2014-0054. PMID:25085898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li BT, Ross DS, Aisner DL, Chaft JE, Hsu M, Kako SL, Kris MG, Varella-Garcia M, Arcila ME. HER2 Amplification and HER2 mutation are distinct molecular targets in lung cancers. J Thorac Oncol. 2016;11:414–9. doi: 10.1016/j.jtho.2015.10.025. PMID:26723242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li C, Sun Y, Fang R, Han X, Luo X, Wang R, Pan Y, Hu H, Zhang Y, Pao W, et al.. Lung adenocarcinomas with HER2-activating mutations are associated with distinct clinical features and HER2/EGFR copy number gains. J Thorac Oncol. 2012;7:85–9. doi: 10.1097/JTO.0b013e318234f0a2. PMID:22071781. [DOI] [PubMed] [Google Scholar]
- 21.Mazieres J, Peters S, Lepage B, Cortot AB, Barlesi F, Beau-Faller M, Besse B, Blons H, Mansuet-Lupo A, Urban T, et al.. Lung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectives. J Clin Oncol. 2013;31:1997–2003. doi: 10.1200/JCO.2012.45.6095. PMID:23610105. [DOI] [PubMed] [Google Scholar]
- 22.Yoshizawa A, Sumiyoshi S, Sonobe M, Kobayashi M, Uehara T, Fujimoto M, Tsuruyama T, Date H, Haga H. HER2 status in lung adenocarcinoma: a comparison of immunohistochemistry, fluorescence in situ hybridization (FISH), dual-ISH, and gene mutations. Lung Cancer. 2014;85:373–8. doi: 10.1016/j.lungcan.2014.06.007. PMID:25047676. [DOI] [PubMed] [Google Scholar]


