ABSTRACT
Cisplatin (DDP) -based chemotherapy is a standard strategy for cervical cancer, while chemoresistance remains a huge challenge. Copper transporter protein 1 (CTR1), a copper influx transporter required for high affinity copper (probably reduced Cu I) transport into the cell, reportedly promotes a significant fraction of DDP internalization in tumor cells. In the present study, we evaluated the function of CTR1 in the cell proliferation of cervical cancer upon DDP treatment. MicroRNAs (miRNAs) have been regarded as essential regulators of cell proliferation, apoptosis, migration, as well as chemoresistance. By using online tools, we screened for candidate miRNAs potentially regulate CTR1, among which miR-130a has been proved to promote cervical cancer cell proliferation through targeting PTEN in our previous study. In the present study, we investigated the role of miR-130a in cervical cancer chemoresistance to DDP, and confirmed the binding of miR-130a to CTR1. SOX9 also reportedly act on cancer chemoresistance. In the present study, we revealed that SOX9 inversely regulated miR-130a through direct targeting the promoter of miR-130a. Consistent with previous studies, SOX9 could affect cervical cancer chemoresistance to DDP. Taken together, we demonstrated a SOX9/miR-130a/CTR1 axis which modulated the chemoresistance of cervical cancer cell to DDP, and provided promising targets for dealing with the chemoresistance of cervical cancer.
KEYWORDS: cervical cancer, copper transporter protein 1 (CTR1), miR-130a, SOX9, chemoresistance, cisplatin (DDP)
Introduction
Cervical cancer, the second most common type of cancer in females worldwide, is one of the major causes of mortality in females [1]. Cisplatin (DDP) is one of the most widely used first line drugs for the treatment of solid organ cancers and are curative for most patients with germ cell tumors [2]. They are currently used in standard chemotherapy protocols for the treatment of patients with ovarian, bladder, cervical, head and neck and cervical cancers [3]. However, intrinsic and/or acquired resistance to DDP-based chemotherapy still remains a huge challenge for treatment of patients with cervical cancer.
Copper transporter 1 (CTR1, or hCtr1 encoded by SLC31A1), a copper influx transporter required for high affinity copper (probably reduced Cu I) transport into the cell, reportedly promotes a significant fraction of DDP internalization in tumor cells [4,5]. DDP resistance in cancers is associated with changes in CTR1 level, sub-cellular localization or functionality [6,7]. As the primary copper influx transporter, CTR1 controls cellular DDP accumulation. The correlation between higher CTR1 levels and higher platinum drug uptake in tumor cells has been confirmed in a number of studies [5,8]. CTR1 upregulation can sensitize tumor cells to platinum drugs, while CTR1 downregulation promotes resistance [5]. However, the detailed role of CTR1 in cervical cancer chemoresistance to DDP has not been revealed.
Non-coding RNAs (ncRNAs) are RNA transcripts of variable length, which are basically not transcribed and translated into proteins. One best studied example, especially in cancer, is microRNA (miRNA) [9]. The discovery of the miRNA has created additional layers of genomic regulatory functions [10–12]. Recent studies have developed further insight into the roles conducted by miRNAs in the development of innate and/or acquired chemoresistance properties of tumors [13]. We demonstrated that miR-130a promotes cervical cancer cell proliferation through targeting PTEN to inhibit its expression in our previous study [14]. In the present study, we further investigated its role and mechanism in cervical cancer chemoresistance.
In addition to miR-130a, SOX9, a transcription factor that may act as both oncogene and tumor suppressor depending on tumor origin, has been reported to play an essential role in cancer chemoresistance. SOX9 mediates SOX2-regulated proliferation, senescence, and self-renewal of glioma cells [15]; high levels of SOX2 and SOX9 correlate with TMZ resistance of glioma cell [15]. Although SOX9 might act on cancer chemoresistance, the function of SOX9 in the chemoresistance of cervical cancer remains to be uncovered.
In the present study, we assessed the detailed function of CTR1, miR-130a and SOX9 in the regulation of cervical cancer chemoresistance; further, we investigated the association among the indicated factors and the mechanism by which they interacted with each other to exert their functions. Taken together, we demonstrated a SOX9/miR-130a/CTR1 axis which modulated the chemoresistance of cervical cancer cell to DDP, and provided promising targets for dealing with the chemoresistance of cervical cancer.
Results
Effect of CTR1 in cervical cancer cell chemoresistance to DDP
To evaluate the role of CTR1 in the regulation of cervical cancer chemoresistance to DDP, we utilized HeLa/DDP and CaSki/DDP cells as cell models (Fig. S1). We transfected non-resistant HeLa and CaSki cells with si-CTR1 to achieve CTR1 knockdown, and transfected DDP-resistant HeLa/DDP and CaSki/DDP cells with CTR1 vector to achieve CTR1 overexpression, as verified using Western blot assays (Fig. 1A). After transfection, the indicated cells were exposed to different doses of DDP stimulation, 2 μg/ml for non-resistant cell lines and 8 μg/ml for DDP-resistant cell lines. The cell viability and DNA synthesis capability of the indicated cells was then determined using MTT and BrdU assays. Upon 2 μg/ml DDP treatment, the cell proliferation of si-CTR1-transfected HeLa and CaSki cell was significantly promoted, compared to the si-NC group (Fig. 1B, C and F). On the contrary, upon 8 μg/ml DDP treatment, the cell proliferation of CTR1-transfected DDP-resistant HeLa/DDP and CaSki/DDP cell was significantly suppressed (Fig. 1 D, E and G). These data indicated that CTR1 actually acts on the chemoresistance of cervical cancer cell to DDP.
Figure 1.
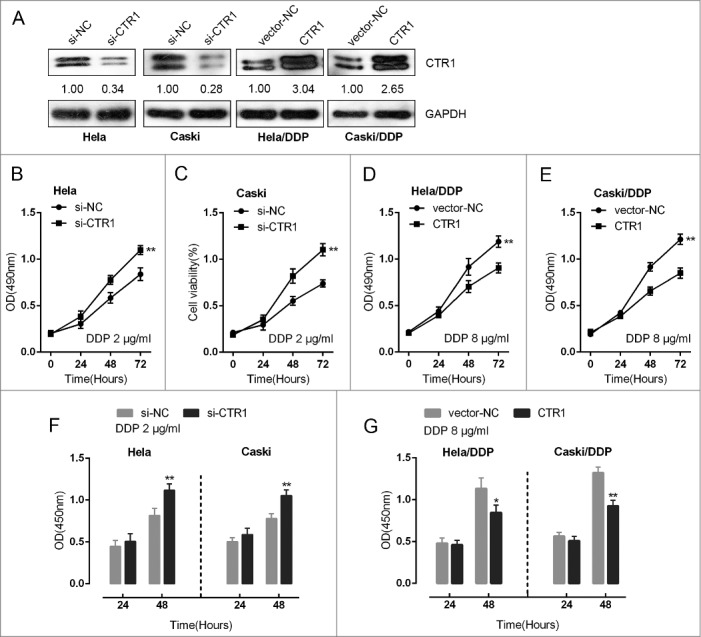
Effect of CTR1 in cervical cancer cell chemoresistance to DDP (A) HeLa and CaSki cells were transfected with si-CTR1; HeLa/DDP and CaSki/DDP cells were transfected with CTR1 vectors. CTR1 protein levels were verified using Western blot assays. (B)-(E) The cell viability of si-CTR1-transfected HeLa and CaSki cell upon 2 μg/ml DDP treatment and CTR1-transfected HeLa/DDP and CaSki/DDP cell upon 8 μg/ml DDP treatment was determined using MTT assays. (F)-(G) The DNA synthesis capability of si-CTR1-transfected HeLa and CaSki cell upon 2 μg/ml DDP treatment and CTR1-transfected HeLa/DDP and CaSki/DDP cell upon 8 μg/ml DDP treatment was determined using BrdU assays. The data are presented as mean ± SD of three independent experiments. *P<0.05, **P<0.01.
Effect of miR-130a in cervical cancer cell chemoresistance to DDP
Recent studies have developed further insight into the roles conducted by miRNAs in the development of innate and/or acquired chemoresistance properties of tumors [13]. To further investigate the mechanism of CTR1 acting on cervical cancer chemoresistance, we screened for the candidate miRNAs potentially target CTR1 by using online tools including miRWalk, miRanda, RNA22 and Targetscan (Fig. 2A). Among the candidate miRNAs, miR-130a has been reported to promote cervical cancer cell proliferation through direct targeting PTEN in our previous study [14]. To confirm the potential regulation of CTR1, ectopic miR-130a expression or miR-130a inhibition was first achieved by miR-130a mimics or miR-130a inhibitor transfection, as verified using real-time PCR assays (Fig. 2B). HeLa and CaSki cells were transfected with miR-130a mimics upon 2 μg/ml DDP stimulation; DDP-resistant HeLa/DDP and CaSki/DDP cells were transfected with miR-130a inhibitor upon 8 μg/ml DDP treatment. As exhibited by MTT and BrdU assays, ectopic miR-130a expression significantly promoted the proliferation of HeLa and CaSki cell (Fig. 2C, D and G); whereas miR-130a inhibition suppressed the proliferation of DDP-resistant HeLa/DDP and CaSki/DDP cell (Fig. 2E, F and H). These data indicated the potential role of miR-130a in cervical cancer chemoresistance.
Figure 2.
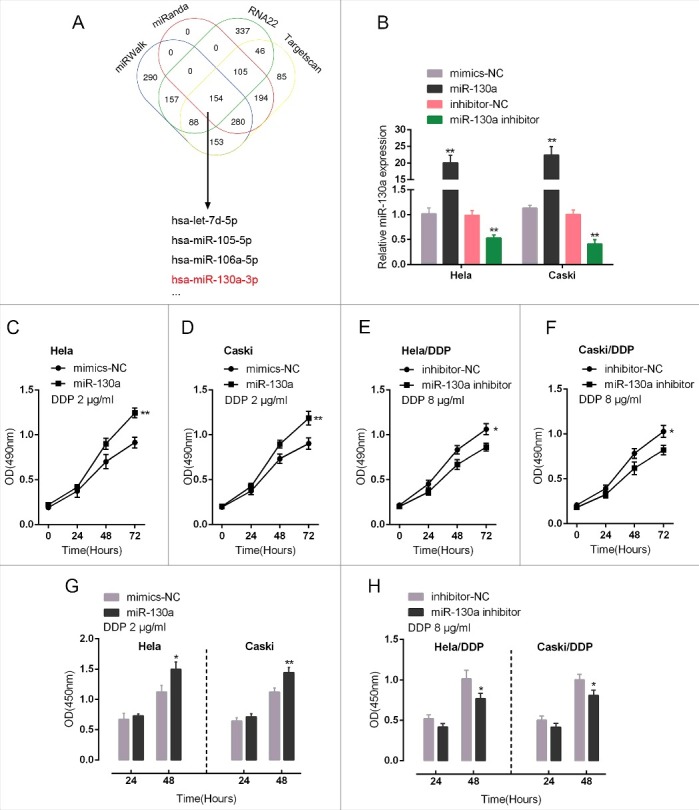
Effect of miR-130a in cervical cancer cell chemoresistance to DDP (A) Online tools including miRWalk, miRanda, RNA22 and Targetscan were used to screen for the candidate miRNAs potentially target CTR1, including let-7d, miR-105, miR-106a and miR-130a. (B) miR-130a mimics or miR-130a inhibitor was transfected into HeLa and CaSki cells to achieve miR-130a overexpression or miR-130a inhibition, as verified using real-time PCR assays. (C)-(F) The cell viability of miR-130a mimics-transfected HeLa and CaSki cell upon 2 μg/ml DDP treatment and miR-130a inhibitor-transfected HeLa/DDP and CaSki/DDP cell upon 8 μg/ml DDP treatment was determined using MTT assays. (F)-(G) The DNA synthesis capability of miR-130a mimics-transfected HeLa and CaSki cell upon 2 μg/ml DDP treatment and miR-130a inhibitor-transfected HeLa/DDP and CaSki/DDP cell upon 8 μg/ml DDP treatment was determined using BrdU assays. The data are presented as mean ± SD of three independent experiments. *P<0.05, **P<0.01.
MiR-130a direct binding to the 3′UTR of SCL31A1
We have demonstrated CTR1 and miR-130a regulation of cervical cancer cell chemoresistance to DDP; next, we validated the potential interaction between miR-130a and CTR1. In HeLa and CaSki cells, ectopic miR-130a expression reduced CTR1 protein levels, whereas miR-130a inhibition increased CTR1 protein (Fig. 3A). To confirm miR-130a binding to SCL31A1 (gene name of CTR1) predicted by online tools, we employed luciferase reporter gene and RNA immunoprecipitation assays. A wild-type and mutated SLC31A1 3′UTR luciferase reporter gene vector (wt-SLC31A1 3′UTR and mut-SLC31A1 3′UTR containing a 7 bp mutation in two predicted binding sites of miR-130a) was constructed (Fig. 3B). The indicated vectors were co-transfected with miR-130a mimics or miR-130a inhibitor into HEK293 cells; the luciferase activity was then determined using dual luciferase assays. Results showed that the luciferase activity of wt-SLC31A1 3′UTR vector was suppressed by miR-130a mimics, whereas amplified by miR-130a inhibitor; after mutation at either predicted miR-130a binding site, the changes of the luciferase activity were abolished (Fig. 3C). Further, the interaction between miR-130a and CTR1 in cervical cancer cells was validated using RNA immunoprecipitation assays with the AGO2 antibody. As exhibited by Western blot assays, AGO2 protein could be precipitated from the cellular extract (Fig. 3D). In RNA extracted from the precipitated AGO2 protein, we could detect both miR-130a and CTR1 with a 1.8∼2-folds enrichment compared to IgG (Fig. 3E), indicating that miR-130a and CTR1 existed in RISC. These data indicated that miR-130a might directly bind to the 3′UTR of SLC31A1 to regulate CTR1 expression.
Figure 3.
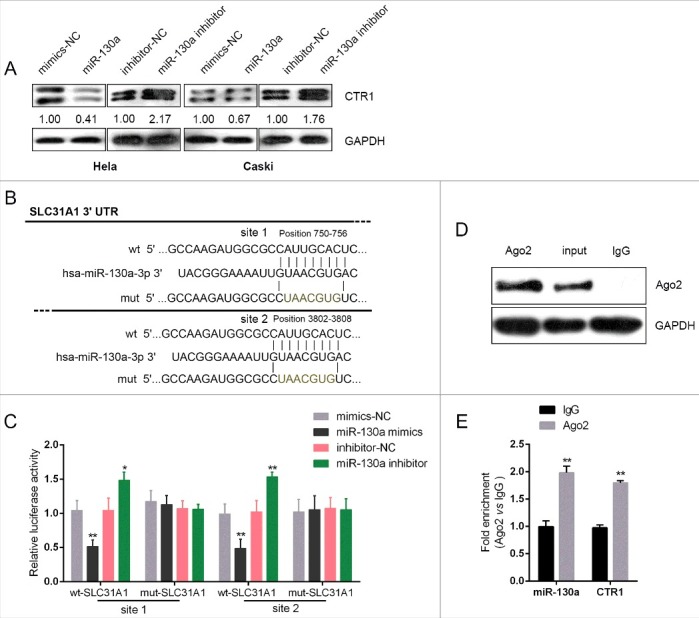
MiR-130a direct binding to the 3′UTR of SCL31A1 (A) HeLa and CaSki cells were transfected with miR-130a mimics or miR-130a inhibitor; the CTR1 protein levels in the indicated cells were determined using Western blot assays. (B) A wild-type and mutated SLC31A1 3′UTR luciferase reporter gene vector (wt-SLC31A1 3′UTR and mut-SLC31A1 3′UTR containing a 7 bp mutation in two predicted binding sites of miR-130a) was constructed. (C) The indicated vectors were co-transfected with miR-130a mimics or miR-130a inhibitor into HEK293 cells; the luciferase activity was then determined using dual luciferase assays. (D)-(E) Association of miR-130a and SOX9 with AGO2. HeLa cellular lysates were used for RNA immunoprecipitation with AGO2 antibody. Detection of AGO2 and IgG using Western blot (up), and detection of miR-130a or SOX9 using qRT-PCR (low). All data of SOX9 expression were normalized to β-actin mRNA expression levels. MiR-130a expression data was normalized to U6 small RNA expression. The data are presented as mean ± SD of three independent experiments. *P<0.05, **P<0.01.
SOX9 direct binding to the promoter of miR-130a to regulate its expression
In previous studies, SOX9 has been reported to be associated with cancer chemoresistance [15–17]. In addition, SOX9 could target miRNAs to regulate their expression [18,19]. Here, we achieved SOX9 knockdown or overexpression by transfection of si-SOX9 or SOX9 vector into HeLa and CaSki cells, as verified using Western blot assays (Fig. 4A). MiR-130a expression was significantly upregulated by SOX9 overexpression, whereas downregulated by SOX9 knockdown in both HeLa and CaSki cells (Fig. 4B). By using online tool Jaspar database, we predicted that miR-130a promoter possesses SOX9-reactive-element (SOX9RE, Fig. 4C). Through mutating two predicted binding sites, we constructed wt-miR-130a (without mutation) and mut-miR-130a (containing a 7 bp mutation in either of the two predicted binding sites) luciferase reporter gene vectors (Fig. 4C). These indicated vectors were co-transfected into HEK293 cells with SOX9 vector, and then the luciferase activity was determined. Results showed that the SOX9 significantly amplified the luciferase activity as compared to pcDNA3.1 when co-transfected with wt-miR-130a containing either site 1 or site 2. When binding element was mutated, luciferase activity was not changed, compared to that of the pcNDA3.1 (Fig. 4D). Furthermore, the real-time ChIP assay showed that the level of SOX9 antibody binding to either site 1 or site 2 of the binding elements in the miR-130a promoter was much greater than that of IgG (Fig. 4E), suggesting that SOX9 might bind to the promoter of miR-130a on the two predicted sites to activate its expression.
Figure 4.
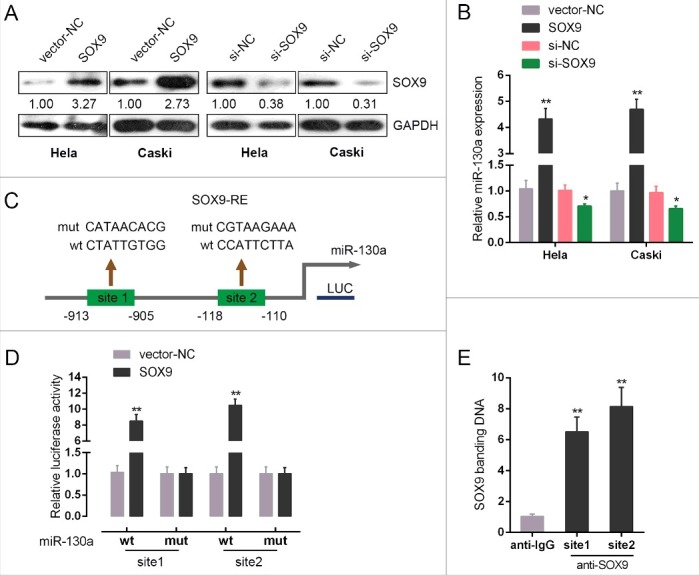
SOX9 direct binding to the promoter of miR-130a to regulate its expression (A) si-SOX9 or SOX9 vector was transfected into HeLa and CaSki cells to achieve SOX9 expression, as verified using Western blot assays. (B) miR-130a expression in the indicated cells was determined using real-time PCR assays. (C) A schematic diagram of potential SOX9 binding element (two possible binding sites) in the promoter region of miR-130a predicted by Jaspar database. A wt-miR-130a promoter luciferase reporter vector and a mut-miR-130a promoter luciferase reporter vector were constructed. (D) The indicated vectors were co-transfected into HEK293 cells with SOX9 vector; the luciferase activity was determined. (E) The real-time ChIP assay showed that the level of SOX9 antibody binding to miR-130a promoter was much greater than that of IgG. The data are presented as mean ± SD of three independent experiments. *P<0.05, **P<0.01.
SOX9 regulated the chemoresistance of cervical cancer through miR-130a/CTR1/PTEN
After confirmation of SOX9 binding to miR-130a promoter region, we next evaluated the role of SOX9 in the regulation of cervical cancer chemoresistance. DDP-resistant HeLa/DDP and CaSki/DDP cells were co-transfected with si-SOX9 and miR-130a mimics; further, the effects of co-processing si-SOX9 and miR-130a mimics on the chemoresistance of cervical cancer cell were evaluated. Results showed that SOX9 knockdown sensitize the cervical cancer cell to DDP (brought down the lC50 value of HeLa/DDP cell from 12.49 to 9.412, of CaSki/DDP cell from 13.93 to 8.522); miR-130a mimics promoted the chemoresistance of the cervical cancer cell to DDP (promoted the lC50 value of HeLa/DDP cell to 16.17, of CaSki/DDP cell to 17.23); moreover, the effect of SOX9 knockdown on the chemoresistance of the cervical cancer cell could be partially reversed by miR-130a mimics (reversed the lC50 value of HeLa/DDP cell from 16.17 to 11.77, of CaSki/DDP cell from 17.23 to 12.62) (Fig. 5A and B).
Figure 5.
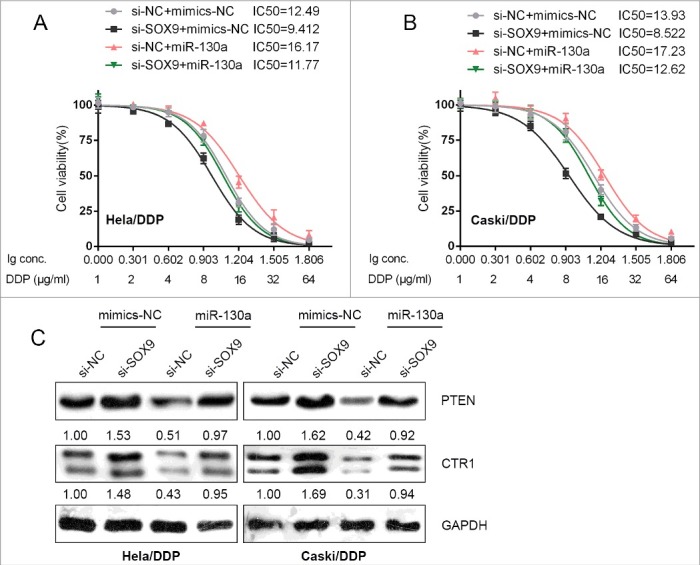
SOX9 regulated the chemoresistance of cervical cancer through miR-130a/CTR1/PTEN (A) and (B) HeLa/DDP and CaSki/DDP cells were co-transfected with si-SOX9 and miR-130a mimics, and were treated with a series of doses of DDP (1, 2, 4, 8, 16, 32, 64 μg/ml), and the cell viability of the indicated cells was determined using MTT assays. Data was displayed as a percentage normalized to the viability of cells with no DDP treatment. The abscissa was the logarithm of DDP concentration (log-conc.). LC50 represented the concentration of DDP when cell viability was reduced to 50%. (C) HeLa/DDP and CaSki/DDP cells were co-transfected with si-SOX9 and miR-130a mimics; the protein levels of PTEN and CTR1 in the indicated cells were determined using Western blot assays.
As we mentioned, miR-130a can direct target PTEN to promote cervical cancer cell proliferation [14]. In addition, miR-130a direct binds to the 3′UTR of SLC31A1 to inhibit CTR1 expression. Here, we evaluated the effect of co-processing si-SOX9 and miR-130a mimics on PTEN and CTR1 protein levels. Results from Western blot assays showed that SOX9 knockdown significantly increased PTEN and CTR1 protein levels, whereas miR-130a overexpression reduced PTEN and CTR1 protein levels; the promotive effect of SOX9 knockdown on PTEN and CTR1 proteins could be partially reversed by miR-130a overexpression (Fig. 5C). These data indicated that SOX9 acts on cervical cancer cell chemoresistance through miR-130a/PTEN/CTR1.
Expression of miR-130a, SOX9 and CTR1 in tumor tissues and their correlation
To further confirm the effect and mechanism of SOX9/miR-130a/CTR1 on cervical cancer chemoresistance, we evaluated the expression levels of miR-130a, SOX9 and CTR1 in DDP-sensitive and DDP-resistant cervical cancer tissues. Results from real-time PCR assays revealed that SOX9 and miR-130a expression was significantly upregulated in DDP-resistant tissues, compared to DDP-sensitive tissues; on the contrary, CTR1 expression was downregulated in DDP-resistant tissues, compared to DDP-sensitive tissues (Fig. 6A–C). In DDP-resistant tissues, SOX9 expression was positively correlated with miR-130a expression, CTR1 was inversely correlated with SOX9 and miR-130a expression, respectively (Fig. 6D-F). These data indicated that inhibiting SOX9 and miR-130a expression thus to rescue CTR1 expression in DDP-resistant cervical cancer cells present a promising strategy for dealing with cervical cancer chemoresistance.
Figure 6.
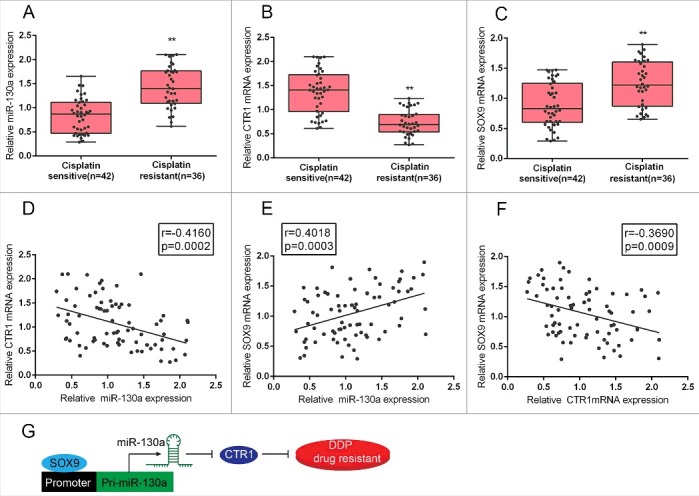
Expression of miR-130a, SOX9 and CTR1 in tumor tissues and their correlation (A)-(C) Expression levels of miR-130a, SOX9 and CTR1 in cisplatin resistant and cisplatin sensitive tissues (defined as described in Materials and methods section) were determined using real-time PCR assays. (E)-(F) The correlation between SOX9 and miR-130a, miR-130a and CTR1, SOX9 and CTR1 in cisplatin resistant tissues was analyzed using Spearman's rank correlation analysis. (G) A sketch map showing the mechanism by which SOX9 binds to the promoter region of miR-130a to activate its expression, thus to inhibit CTR1 expression, and finally promote the DDP drug resistance of cervical cancer cell.
Discussion
DDP-based chemotherapy is the most commonly used treatment for cervical cancer; however, due to the acquisition of chemoresistance of cervical cancer cells to DDP, the efficacy is very limited [20–22]. Although a number of mechanisms, such as reduction in the intracellular accumulation of the platinum compounds, increase in DNA damage repair, inactivation of apoptosis and so on are associated with the pathogenesis of cervical cancer and the chemoresistance of cervical cancer cells [21], the molecular biology of chemoresistance of cervical cancer cells to DDP needs further investigation.
Up to now, the complex molecular mechanism by which cisplatin enters cells remains poorly understood. Cisplatin is generally believed to pass the cell membrane via passive diffusion, and the diffusion rate is associated with cisplatin lipophilicity [23,24]. Recently, CTR1, which is a transmembrane protein and involved in the maintenance of copper homeostasis, has been recognized to regulate the influx of cisplatin and its analogs into the cells. CTR1 is downregulated in various CPR cell lines including HeLa cisplatin-resistant cells [25]. Du et al. found that the C-terminus of CTR1 protein was required for cisplatin uptake in HeLa cells; HeLa cells overexpressing CTR1 show 2.2-fold increase in cisplatin accumulation compared with the mock-transfected cells [26]. In a mouse model of cervical cancer, Ishida et al demonstrated that the level of DNA-cisplatin adducts correlated with CTR1 mRNA level in various organs, suggesting that CTR1 may regulate cisplatin uptake in vivo [27]. In the present study, we evaluated the detailed role of CTR1 in cervical cancer cell chemoresistance to DDP. Consistent with previous studies, CTR1 knockdown significantly increased the resistance of DDP-sensitive HeLa and CaSki cells to DDP, whereas CTR1 overexpression reduced the resistance of DDP-resistant HeLa/DDP and CaSki/DDP cells to DDP.
In addition to CTR1, miRNAs have been found to regulate multiple pathways that are involved in the cellular response to cisplatin [28]. The effects of miRNAs on the development of cisplatin resistance in cervical cancer have been investigated. Pouliot et al found that the miR-181 family members were overexpressed in cisplatin-resistant cells KB-CP5 and KB-CP20 compared with the parental KB-3-1 cells, and silencing the proteins that are essential for miRNA synthesis, such as DICER and TRBP2, reversed cisplatin resistance in the cells [29]. Chen et al found that in cervical squamous cell carcinoma, upregulation of miR-181 appeared to correlate with cisplatin resistance significantly and miR-181a inhibited apoptosis and enhanced cisplatin resistance by targeting protein kinase C-δ (PRKCD) [30]. In the present study, we used online tools to screen for the candidate miRNAs potentially regulate CTR1 by targeting, among which miR-130a has been reported to promote cervical cancer cell proliferation through targeting PTEN in our previous study [14]. Here, we observed that miR-130a overexpression reduced the DDP-sensitivity of DDP-sensitive HeLa and CaSki cells; on the contrary, miR-130a inhibition even aggravated the DDP-resistance of DDP-resistant HeLa/DDP and CaSki/DDP cells. Further, we confirmed that miR-130a directly bound to the 3′UTR of SLC31A1, which encoded CTR1, to inhibit the protein level of CTR1. These all indicated that miR-130a might act on DDP-resistance of cervical cancer cell through the regulation of CTR1.
It has been previously shown that SOX9, a transcription factor that may act as both oncogene and tumor suppressor depending on tumor origin, plays a key role in the regulation of cellular proliferation, senescence, and self-renewal [31–33]. In addition, SOX2-SOX9 has been reported to mediate glioma stem cell activity and temozolomide resistance [15. Here, we observed a positive regulation of miR-130a by SOX9, which inspired us to investigate the interaction between SOX9 and miR-130a, as well as the detailed role of SOX9 in cervical cancer cell chemoresistance. Through binding to the promoter region of miR-130a, SOX9 activated the expression of miR-130a; further, we also revealed that SOX9 knockdown obviously increased the DDP-sensitivity of DDP-resistant HeLa/DDP and CaSki/DDP cells, whereas miR-130a overexpression partially reversed the effect of SOX9 knockdown on cervical cancer cell chemoresistance. Co-processing si-SOX9 and miR-130a mimics also regulated PTEN and CTR1 protein levels, which further confirmed that SOX9 binds to the promoter region of miR-130a to activate its expression, thus inhibits the downstream genes of miR-130a, PTEN and CTR1, and finally promotes the chemoresistance of cervical cancer cells to DDP.
To validate our findings, we evaluated the expression levels of SOX9, miR-130a and CTR1 in DDP-sensitive and DDP-resistant tissues. In DDP-resistant tissues, SOX9 and miR-130a expression was significantly upregulated, whereas CTR1 expression was downregulated. In DDP-resistant tissues, SOX9 expression was positively correlated with miR-130a expression, CTR1 was inversely correlated with SOX9 and miR-130a expression, respectively, which further indicated that inhibiting SOX9 and miR-130a expression thus to rescue CTR1 expression in DDP-resistant cervical cancer cells present a promising strategy for dealing with cervical cancer chemoresistance.
Taken together, we revealed that SOX9/miR-130a/CTR1 axis regulated the chemoresistance of cervical cancer cell to DDP, and provided novel targets for dealing with the chemoresistance of cervical cancer.
Materials and methods
Tissue samples, cell lines and cell transfection
With the approval of the Ethic Committee of the Second Xiangya Hospital, Central South University, we collected 78 cases of cervical cancer tissues. All samples were obtained from patients who underwent surgical resection after combined radiotherapy and DDP-based chemotherapy at the Second Xiangya Hospital, Central South University (Changsha, China). All the tissue samples were snap-frozen and stored at −80°C in liquid nitrogen. After 2 cycles of DDP-based chemotherapy and radiotherapy [34-36], patients with no significant clinical efficacy (NC) or with progression disease (PD) were defined as cisplatin resistant (n = 36); the rest patients were defined as cisplatin sensitive (n = 42). All patients signed an informed consent approved by the institutional Review Board.
Human cervical cancer cell lines: HeLa and CaSki were obtained from the American Type Culture Collection (ATCC, USA). DDP-resistant cervical cancer cell lines: HeLa/DDP and CaSki/DDP, were purchased from Yrbio co., LTD, China, cultured in 10% fetal bovine serum (Gibco, USA) supplemented RPMI-1640 medium (Invitrogen, USA) at 37°C with 5% v/v CO2.
Si-CTR1 or CTR1 vector was used to achieve knockdown of CTR1 or CTR1 overexpression (GeneCopoecia, China). MiR-130a mimics or miR-130a inhibitor (Genepharma, China) was transfected into the indicated target cells to achieve miR-130a overexpression or miR-130a inhibition by using Lipofectamine 2000 (Invitrogen). Si-SOX9 was used to achieve knockdown of SOX9 (GeneCopoecia, China).
Real-time PCR
Trizol reagent (Invitrogen) was used for total RNA extraction following the manufacturer's instructions. By using miRNA-specific primer, total RNA was reverse transcribed and the miScript Reverse Transcription kit (Qiagen, Germany) was used for miR-130a qRT-PCR. The SYBR green PCR Master Mix (Qiagen) was used following the manufacturer's instructions. The Ct method was used to evaluate the relative expression and normalized to U6 expression.
Western blotting
RIPA buffer (Cell-Signaling Tech., US) was used to homogenize the cells. The expression of CTR1, SOX9 and PTEN in cervical cancer cells was detected by performing immunoblotting. Cells were lysed cultured, or transfected in 1% PMSF supplemented RIPA buffer. Proteins were loaded onto SDS-PAGE minigel, and then transferred onto PVDF membrane. The blots were probed with the following antibodies at 4°C overnight: anti-CTR1 (Cat# EPR7936, Abcam, USA), anti-PTEN (Cat# Y184, Abcam) and anti-SOX9 (Cat# EPR14335-78, Abcam), and incubated with HRP-conjugated secondary antibody (1:5000). Signals were visualized using ECL Substrates (Millipore, USA). The protein expression was normalized to endogenous GAPDH.
Luciferase activity
HEK293 cells (ATCC) were cultured overnight after being seeded into a 24-well plate. A wild-type and mutated SLC31A1 3′UTR (wt-SLC31A1 3′UTR and mut-SLC31A1 3′UTR containing a 7 bp mutation in two predicted binding sites of miR-130a) or miR-130a promoter (wt-130a and mut-130a containing a 7 bp mutation in two predicted sites of the SOX9 responsive element, SOX9RE) luciferase reporter gene vector was constructed. After cultured overnight, cells were co-transfected with the indicated vectors and miR-130a mimics and miR-130a inhibitor, respectively. Luciferase assays were performed 48 h after transfection using the Dual Luciferase Reporter Assay System (Promega, WI, USA).
MTT assay
24 h after seeding into 96-well plates (5000 cells per well), cells were transfected with si-CTR1, CTR1 vector, miR-130a mimics or miR-130a inhibitor. Medium with DDP (0, 1, 2, 4, 6, 8, 16, 32, 64 μg/ml) was applied at 24 h post-transfection. 48 h after transfection, 20 μl MTT (at a concentration of 5 mg/ml; Sigma-Aldrich) was added, and the cells were incubated for an additional 4 h in a humidified incubator. 200 μl DMSO was added after the supernatant discarded to dissolve the formazan. OD490 nm value was measured. The viability of the non-treated cells (control) was defined as 100%, and the viability of cells from all other groups was calculated separately from that of the control group.
BrdU incorporation assay
By measuring 5-Bromo-2-deoxyUridine (BrdU) incorporation, the DNA synthesis in proliferating cells was determined. BrdU assays were conducted at 24 h and 48 h after cervical cancer cells were transfected as indicated. Cells were seeded in 96-well culture plates at a density of 2 × 103 cells/well, cultured for 24 h or 48 h, then incubated with a final concentration of 10 μM BrdU (BD Pharmingen, San Diego, CA, USA) for 2 h. When the incubation period ended, the medium was removed, the cells were fixed for 30 min at RT, incubated with peroxidase-coupled anti-BrdU-antibody (Sigma-Aldrich) for 60 min at RT, washed three times with PBS, incubated with peroxidase substrate (tetramethylbenzidine) for 30 min, and the 450 nm absorbance values were measured for each well. Background BrdU immunofluorescence was determined in cells not exposed to BrdU but stained with the BrdU antibody.
RNA immunoprecipitation
RNA immunoprecipitation assays were performed by using the Imprint RNA Immunoprecipitation Kit (Sigma, St. Louis, USA) along with the AGO2 antibody (Cell signaling, Rockford, USA). The AGO2 antibody was then recovered by protein A/G beads. CTR1 and miR-130a RNA levels in the immunoprecipitates were measured by qRT-PCR.
Chromatin immunoprecipitation (ChIP)
Briefly, the treated cells were cross-linked with 1% formaldehyde, sheared to an average size of 400 bp DNA, and immunoprecipitated using antibodies against SOX9 (anti-SOX9, CL0639, monoclonal, Sigma-Aldrich). A positive control antibody (RNA polymerase II) and a negative control non-immune IgG were used to demonstrate the efficacy of the kit reagents (Epigentek Group Inc., NY, USA, P-2025-48). The immunoprecipitated DNA was subsequently cleaned, released, and eluted. The eluted DNA was used for downstream applications, such as ChIP-PCR. The fold-enrichment (FE) was calculated as the ratio of the amplification efficiency of the ChIP sample to that of the non-immune IgG. The amplification efficiency of RNA Polymerase II was used as a positive control. FE% = 2 (IgG CT-Sample CT) × 100%.
Statistical analysis
Data from three independent experiments were presented as mean ± SD, processed using SPSS 17.0 statistical software (SPSS, USA). Paired Student's t-test was used to compare the expression of miR-130a and CASC2 in cervical cancer tissues and normal tissues. P values of <0.05 were considered statistically significant.
Supplementary Material
Funding Statement
This work was supported by Hunan Provincial Natural Science Foundation of China [grant number 2016JJ4096]
Disclosure of potential conflict of interest
None to declare.
References
- [1].Joshi PK, Esko T, Mattsson H, et al.. Directional dominance on stature and cognition in diverse human populations. Nature 2015; 523:459–462. https://doi.org/10.1038/nature14618. PMID:26131930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ozols RF, Williams SD. Testicular cancer. Curr Probl Cancer 1989; 13:285–335. https://doi.org/10.1016/0147-0272(89)90020-2. PMID:2551577 [DOI] [PubMed] [Google Scholar]
- [3].Loehrer PJ, Einhorn LH. Drugs five years later. Cisplatin. Ann Intern Med 1984; 100:704–713. https://doi.org/10.7326/0003-4819-100-5-704. PMID:6370067 [DOI] [PubMed] [Google Scholar]
- [4].Larson CA, Blair BG, Safaei R, et al.. The role of the mammalian copper transporter 1 in the cellular accumulation of platinum-based drugs. Mol Pharmacol 2009; 75:324–330. https://doi.org/10.1124/mol.108.052381. PMID:18996970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kalayda GV, Wagner CH, Jaehde U. Relevance of copper transporter 1 for cisplatin resistance in human ovarian carcinoma cells. J Inorg Biochem 2012; 116:1–10. https://doi.org/10.1016/j.jinorgbio.2012.07.010. PMID:23010323 [DOI] [PubMed] [Google Scholar]
- [6].Yoshida H, Teramae M, Yamauchi M, et al. Association of copper transporter expression with platinum resistance in epithelial ovarian cancer. Anticancer Res 2013; 33:1409–1414. PMID:23564780 [PubMed] [Google Scholar]
- [7].Xu X, Duan L, Zhou B, et al. Genetic polymorphism of copper transporter protein 1 is related to platinum resistance in Chinese non-small cell lung carcinoma patients. Clin Exp Pharmacol Physiol 2012; 39:786–792. https://doi.org/10.1111/j.1440-1681.2012.05741.x. PMID:22725681 [DOI] [PubMed] [Google Scholar]
- [8].Kim ES, Tang X, Peterson DR, et al.. Copper transporter CTR1 expression and tissue platinum concentration in non-small cell lung cancer. Lung Cancer 2014; 85:88–93. https://doi.org/10.1016/j.lungcan.2014.04.005. PMID:24792335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zebisch A, Hatzl S, Pichler M, et al. Therapeutic Resistance in Acute Myeloid Leukemia: The Role of Non-Coding RNAs. Int J Mol Sci 2016; 17(12):2080. https://doi.org/10.3390/ijms17122080. PMID:27973410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ambros V. microRNAs: tiny regulators with great potential. Cell 2001; 107:823–826. https://doi.org/10.1016/S0092-8674(01)00616-X. PMID:11779458 [DOI] [PubMed] [Google Scholar]
- [11].Gibb EA, Brown CJ, Lam WL. The functional role of long non-coding RNA in human carcinomas. Mol Cancer 2011; 10:38.https://doi.org/10.1186/1476-4598-10-38. PMID:21489289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gibb EA, Enfield KS, Stewart GL, et al. Long non-coding RNAs are expressed in oral mucosa and altered in oral premalignant lesions. Oral Oncol 2011; 47:1055–1061. https://doi.org/10.1016/j.oraloncology.2011.07.008. PMID:21835683 [DOI] [PubMed] [Google Scholar]
- [13].Ayers D, Vandesompele J. Influence of microRNAs and Long Non-Coding RNAs in Cancer Chemoresistance. Genes (Basel) 2017; 8(3):95. https://doi.org/10.3390/genes8030095. PMID:28273813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Feng Y, Zhou S, Li G, et al. Nuclear factor-kappaB-dependent microRNA-130a upregulation promotes cervical cancer cell growth by targeting phosphatase and tensin homolog. Arch Biochem Biophys 2016; 598:57–65. https://doi.org/10.1016/j.abb.2016.03.019. PMID:27040383 [DOI] [PubMed] [Google Scholar]
- [15].Garros-Regulez L, Aldaz P, Arrizabalaga O, et al.. mTOR inhibition decreases SOX2-SOX9 mediated glioma stem cell activity and temozolomide resistance. Expert Opin Ther Targets 2016; 20:393–405. https://doi.org/10.1517/14728222.2016.1151002. PMID:26878385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ashkenazi S, Ortenberg R, Besser M, et al. SOX9 indirectly regulates CEACAM1 expression and immune resistance in melanoma cells. Oncotarget 2016; 7:30166–30177. https://doi.org/10.18632/oncotarget.7379. PMID:26885752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Li XL, Chen XQ, Zhang MN, et al.. SOX9 was involved in TKIs resistance in renal cell carcinoma via Raf/MEK/ERK signaling pathway. Int J Clin Exp Pathol 2015; 8:3871–3881. PMID:26097571 [PMC free article] [PubMed] [Google Scholar]
- [18].Wainwright EN, Jorgensen JS, Kim Y, et al.. SOX9 regulates microRNA miR-202-5p/3p expression during mouse testis differentiation. Biol Reprod 2013; 89:34. https://doi.org/10.1095/biolreprod.113.110155. PMID:23843232 [DOI] [PubMed] [Google Scholar]
- [19].Nakamura Y, He X, Kato H, et al.. Sox9 is upstream of microRNA-140 in cartilage. Appl Biochem Biotechnol 2012; 166:64–71. https://doi.org/10.1007/s12010-011-9404-y. PMID:22052544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Weng Y, Wang Y, Shi Y, et al. TLR9 expression and its role in chemosensitivity to DDP in human cervical cancer cells in vitro. J Huazhong Univ Sci Technolog Med Sci 2011; 31:550–554. https://doi.org/10.1007/s11596-011-0488-y. PMID:21823020 [DOI] [PubMed] [Google Scholar]
- [21].Zhu H, Luo H, Zhang W, et al. Molecular mechanisms of cisplatin resistance in cervical cancer. Drug Des Devel Ther 2016; 10:1885–1895. https://doi.org/10.2147/DDDT.S106412. PMID:27354763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chen J, Solomides C, Parekh H, et al. Cisplatin resistance in human cervical, ovarian and lung cancer cells. Cancer Chemother Pharmacol 2015; 75:1217–1227. https://doi.org/10.1007/s00280-015-2739-2. PMID:25894720 [DOI] [PubMed] [Google Scholar]
- [23].Ciarimboli G. Membrane transporters as mediators of cisplatin side-effects. Anticancer Res 2014; 34:547–550. PMID:24403515 [PubMed] [Google Scholar]
- [24].Galluzzi L, Vitale I, Michels J, et al. Systems biology of cisplatin resistance: past, present and future. Cell Death Dis 2014; 5:e1257. https://doi.org/10.1038/cddis.2013.428. PMID:24874729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zisowsky J, Koegel S, Leyers S, et al. Relevance of drug uptake and efflux for cisplatin sensitivity of tumor cells. Biochem Pharmacol 2007; 73:298–307. https://doi.org/10.1016/j.bcp.2006.10.003. PMID:17097621 [DOI] [PubMed] [Google Scholar]
- [26].Du X, Wang X, Li H, et al. Comparison between copper and cisplatin transport mediated by human copper transporter 1 (hCTR1). Metallomics 2012; 4:679–685. https://doi.org/10.1039/c2mt20021j. PMID:22552365 [DOI] [PubMed] [Google Scholar]
- [27].Ishida S, McCormick F, Smith-McCune K, et al. Enhancing tumor-specific uptake of the anticancer drug cisplatin with a copper chelator. Cancer Cell 2010; 17:574–583. https://doi.org/10.1016/j.ccr.2010.04.011. PMID:20541702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Drayton RM. The role of microRNA in the response to cisplatin treatment. Biochem Soc Trans 2012; 40:821–825. https://doi.org/10.1042/BST20120055. PMID:22817741 [DOI] [PubMed] [Google Scholar]
- [29].Pouliot LM, Shen DW, Suzuki T, et al. Contributions of microRNA dysregulation to cisplatin resistance in adenocarcinoma cells. Exp Cell Res 2013; 319:566–574. https://doi.org/10.1016/j.yexcr.2012.10.012. PMID:23137650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chen Y, Ke G, Han D, et al. MicroRNA-181a enhances the chemoresistance of human cervical squamous cell carcinoma to cisplatin by targeting PRKCD. Exp Cell Res 2014; 320:12–20. https://doi.org/10.1016/j.yexcr.2013.10.014. PMID:24183997 [DOI] [PubMed] [Google Scholar]
- [31].Swartling FJ, Savov V, Persson AI, et al.. Distinct neural stem cell populations give rise to disparate brain tumors in response to N-MYC. Cancer Cell 2012; 21:601–613. https://doi.org/10.1016/j.ccr.2012.04.012. PMID:22624711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Matheu A, Collado M, Wise C, et al.. Oncogenicity of the developmental transcription factor Sox9. Cancer Res 2012; 72:1301–1315. https://doi.org/10.1158/0008-5472.CAN-11-3660. PMID:22246670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wang G, Lunardi A, Zhang J, et al.. Zbtb7a suppresses prostate cancer through repression of a Sox9-dependent pathway for cellular senescence bypass and tumor invasion. Nat Genet 2013; 45:739–746. https://doi.org/10.1038/ng.2654. PMID:23727861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Li XY, Liu L, Xie XM, et al.. The role of raltitrexed/cisplatin with concurrent radiation therapy in treating advanced cervical cancer. Eur Rev Med Pharmacol Sci 2014; 18:3491–3496. PMID:25491626 [PubMed] [Google Scholar]
- [35].Yang Z, Chen D, Zhang J, et al. The efficacy and safety of neoadjuvant chemotherapy in the treatment of locally advanced cervical cancer: A randomized multicenter study. Gynecol Oncol 2016; 141:231–239. https://doi.org/10.1016/j.ygyno.2015.06.027. PMID:26115978 [DOI] [PubMed] [Google Scholar]
- [36].Xiong Y, Liang LZ, Cao LP, et al. Clinical effects of irinotecan hydrochloride in combination with cisplatin as neoadjuvant chemotherapy in locally advanced cervical cancer. Gynecol Oncol 2011; 123:99–104. https://doi.org/10.1016/j.ygyno.2011.06.011. PMID:21741694 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


