ABSTRACT
Extracellular matrix (ECM) stiffness influences gene expression, leading to modulation of various cellular functions. While ROCK2 regulates actomyosin activity as well as cell migration and proliferation, expression of ROCK2 is increased in response to stiffening ECM. However, the mechanism underlying rigidity-dependent ROCK2 expression remains elusive. Here, we show that YAP, a mechanically regulated transcription coactivator, upregulates ROCK2 expression in an ECM rigidity-dependent manner. YAP interacted with the ROCK2 promoter region in an actomyosin activity-dependent manner. Knockdown of YAP decreased ROCK2 expression while activity of the ROCK2 promoter was upregulated by expressing constitutively active YAP. Furthermore, we found that ROCK2 expression promotes transcriptional activation by YAP. Our results reveal a novel positive feedback loop between YAP and ROCK2, which is modulated by ECM stiffness.
KEYWORDS: actin, ECM, cancer, myosin, ROCK2, YAP
Introduction
Actin filaments (F-actin), the polymerized form of globular actin (G-actin), are major components of the cytoskeleton. The actin cytoskeleton adopts various architectures—including stress fibers, filopodia, and lamellipodia—that underlie cellular morphogenesis and migration.1,2
Cells bind to the ECM through integrin receptors. This binding initiates the recruitment of focal adhesion (FA) proteins to the cell-ECM adhesion sites. Subsequent activation of Rho GTPases including Rho, Rac, and Cdc42 promotes actin polymerization.3,4 Rho then increases actin-myosin contractile force generation via Rho-associated coiled coil-containing protein kinase (ROCK)-mediated myosin activation, which leads to further activation of integrin signaling.5
The mechanical environment surrounding the cell, such as ECM stiffness and cell density, has a significant impact on cell properties and behaviors.6,7 Stiffening of the ECM promotes cellular migration and invasion via integrin signal-dependent alteration of actin cytoskeletal architectures.8,9 Furthermore, ECM stiffness affects the differentiation and proliferation of cells by modulating gene expression, in which remodeling of the actin cytoskeleton plays a significant role.10,11,12 Thus, the actin cytoskeleton works as a key mediator in mechanical cue-induced signal transduction.
Yes-associated protein (YAP) is a “mechanotransducer” and a major downstream effector of the Hippo pathway.12 YAP translocates into the nucleus and binds to TEA domain (TEAD) transcription factors, thereby inducing the expression of target genes, such as CTGF.13 Upon cell-cell adhesion mediated by adherens junctions and tight junctions, YAP is phosphorylated by large tumor suppressor kinases (LATS) and subsequently binds to 14–3–3 proteins, resulting in the sequestration of YAP in the cytoplasm. On the other hand, inhibition of actin polymerization causes the inactivation of YAP by both LATS-dependent and -independent mechanisms.12 The YAP binding protein angiomotin (AMOT) is involved in the LATS-independent regulation of YAP localization. While the interaction of AMOT with YAP sequesters YAP in the cytoplasm, F-actin binding of AMOT attenuates this interaction, leading to nuclear translocation of YAP.12,14,15
While ROCK2 is a key regulator of actomyosin, it also regulates cell proliferation, invasion, and metastasis.16,17 We have recently reported that, in human breast cancer MCF-7 cells, stiffened ECM increases ROCK2 expression, which causes an increase in cellular sensitivity against anti-tumor drugs.18 Thus, ROCK2 is likely to be a mechanical cue-dependent molecule that might affect cancer therapy outcomes. However, the mechanism by which ECM stiffness alters ROCK2 expression remains unknown.
In the present study, we examine the molecular mechanisms underlying the regulation of ROCK2 expression by ECM stiffness. Our results suggest that, in response to ECM rigidity, YAP induces ROCK2 expression in an actomyosin-dependent manner, and ROCK2, in turn, enhances the activation of YAP.
Materials and methods
Cell culture
MCF-7 human breast cancer cells, 293T human embryonic kidney cells, C2C12 mouse myoblasts were cultured in Dulbecco's modified Eagle's medium (Nissui Pharmaceutical) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. N-acryloyl-6-aminocaproic acid (ACA)-copolymerized acrylamide gels for polyacrylamide culture substrates were prepared as described previously.19,20
Retroviral vectors and retroviral infection
Retroviral infection was performed as described previously.21 Briefly, pSuper-YAP puro was cotransfected with the pAmpho into 293T cells using the Polyethylenimine as a transfection reagent (Polysciences, Inc.). At 48 h after transfection, the supernatant was collected and then used to infect MCF-7 cells in the presence of 8 μg/mL Polybrene. At 24 h after infection, the cells were selected using puromycin (1.5 μg/mL) for 3 d.
Antibodies and materials
Anti-ROCK2 mouse monoclonal (D-11; Santa Cruz) and anti-α-tubulin mouse monoclonal (DM1A; Sigma-Aldrich) antibodies were used for immunoblot analysis. Anti-YAP rabbit monoclonal antibody (D8H1X; Cell Signaling Technology) was used for immunoblot and immunofluorescence analyses. Latrunculin A was purchased from Sigma-Aldrich. Blebbistatin and Y-27632 were purchased from Merck Millipore.
Fluorescence microscopy
To immunostain for YAP, cells were fixed with 4% PFA, permeabilized with 0.2% Triton X-100, and blocked with 2% BSA in PBS. The cells were then incubated with the anti-YAP rabbit polyclonal antibody. Alexa Fluor 488-conjugated goat anti-rabbit IgG (Molecular Probes) was used as a secondary antibody. DAPI (Vector Laboratories) was used to stain nuclei. Images were acquired using a confocal microscope (LSM700; Zeiss) and then analyzed with ImageJ software (NIH).
Plasmids
To generate retroviruses encoding small hairpin RNAs (shRNAs) against human YAP and human ROCK2, the YAP target sequences #1: 5′- GCCACCAAGCTAGATAAAGAA −3′, #2: 5′-GACATCTTCTGGTCAGAGA-3′ and ROCK2 target sequences #1: 5′-GGTTTATGCTATGAAGCTT-3′, #2: 5′-GGATAAACATGGACATCTA-3′ were cloned into the pSuper retro hygro vector and pSuper retro puro vector (Oligoengine, Seattle, WA), respectively. pCS2-YAP 5SA gift from Dr. Hiroshi Nishina contains the insert phosphorylation-defective YAP 5SA mutant.
Luciferase assay
The reporter construct ROCK2-WT-luc was generated by subcloning the 0.8 kb fragment encompassing the promoter region (−815 to +20) of the human ROCK2 gene into the pGL3-basic vector. The control plasmid phRL-TK (Renilla luciferase reporter) was obtained from Toyobo (Osaka, Japan). Luciferase activity was determined using the Dual-Luciferase Reporter Assay System (Promega).
Quantitative real-time PCR
Total RNA was purified using NucleoSpin RNA kit (Takara). cDNA was prepared using PrimeScript 1st strand cDNA Synthesis kit (Takara). Quantitative real-time PCR analysis was performed with Thunderbird SYBR qPCR Mix (Toyobo) under the following conditions: 1 min at 95°C, followed by 40 cycles of 95°C for 15 sec and 55°C for 1 min using StepOne Plus Real-Time PCR system (Applied Biosystems). The following primers were used: human YAP forward 5′- AGGAGAGACTGCGGTTGAAA-3′ and reverse 5′-CCCAGGAGAAGACACTGCAT −3′; human CTGF forward 5′- ACCGACTGGAAGACACGTTTG-3′ and reverse 5′- CCAGGTCAGCTTCGCAAGG-3′; human ROCK1 forward 5′- GACCTGTAACCCAAGGAGAT-3′ and reverse 5′- GGAAAGTGGTAGAGTGTAGG-3′; human ROCK2 forward 5′- CAACTGTGAGGCTTGTATGAAG-3′ and reverse 5′- TGCAAGGTGCTATAATCTCCTC-3′; and human ubiquitin forward 5′- TGACTACAACATCCAGAA-3′ and reverse 5′- ATCTTTGCCTTGACATTC −3′.
Immunoblot analysis
Immunoblot analysis was performed as described previously.19 Briefly, cells were solubilized with the lysis buffer (50 mM Tris pH 7.4, 150 mM NaCl, 1% Triton X-100, 1% SDS, 10 mM EDTA, 1 mM Na3VO4, 10 mM NaF, and protease inhibitor cocktail [PIC; Nacalai Tesque]) and then centrifuged at 20,000 × g for 20 min after sonication. The supernatants were used as total cell extracts and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Chromatin immunoprecipitation (ChIP) assay
The ChIP assay was performed using a SimpleChIP Plus Enzymatic Chromatin IP Kit following the manufacturer's protocol provided by Cell Signaling Technology. Cross-linked chromatin was immunoprecipitated with anti-YAP rabbit polyclonal antibody (D8H1X; Cell Signaling Technology) or normal rabbit IgG (Cell Signaling Technology) antibodies. Precipitated DNA was analyzed by quantitative real-time PCR. The following primers were used: human ROCK2 promoter forward 5′- TGGGTTTACTGGGTCAAAGG −3′ and reverse 5′- AAGAGAACGGGAGAGCAC −3′; and human CTGF promoter forward 5′- GCCAATGAGCTGAATGGAGT −3′ and reverse 5′- CAATCCGGTGTGAGTTGATG −3′ as a positive control.
Statistical analysis
Statistical analysis of data was performed using the unpaired Student's 2-sided t-test.
Results and discussion
To elucidate the mechanism underlying rigidity-dependent ROCK2 expression, we focused on YAP as it is known to be a mechanically regulated transcription coactivator. We found that the expression of both ROCK2 and CTGF, a well-recognized transcriptional target of the YAP-TEAD complex, was considerably higher on the 30 kPa substrate than on the 2 kPa substrate (Fig. 1A). We then tested whether YAP-TEAD regulated ROCK2 expression by examining the effect of shRNA-mediated depletion of YAP on ROCK2 expression. Knockdown of YAP decreased mRNA expressions of both ROCK2 and CTGF (Fig. 1B). The ROCK2 expression on a protein level was also reduced upon the YAP knockdown (Fig. 1C).
Figure 1.
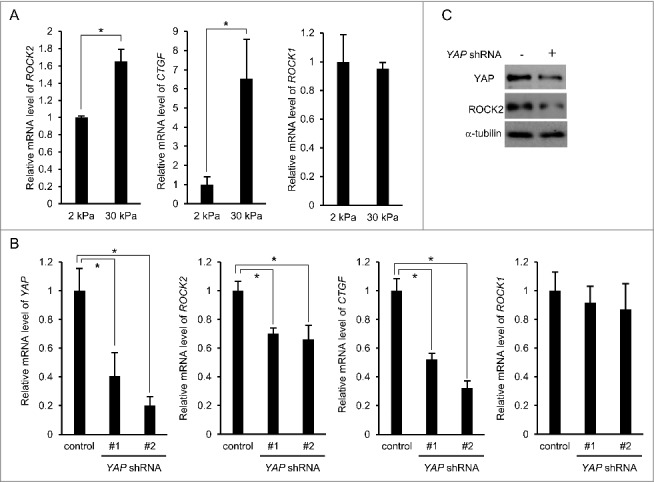
YAP is involved in ROCK2 expression. (A) MCF-7 cells were cultured on substrates with elasticities of 2 and 30 kPa. The expression of ROCK2, CTGF, and ROCK1 were evaluated by quantitative real-time PCR. Each bar represents the mean ± SD; n = 3. Asterisks, p < 0.05. (B, C) Cells were infected with a control, YAP shRNA-#1, or -#2 -expressing retrovirus. (B) The expression of YAP, ROCK2, CTGF, and ROCK1 were evaluated by quantitative real-time PCR. Each bar represents the mean ± SD; n = 3. Asterisks, p < 0.01. (C) Extracts from control and YAP shRNA-#1-expressing cells cultured on plates were subjected to immunoblot analysis with antibodies against YAP ROCK2 and α-tubulin as a loading control.
The ROCK family consists of ROCK1 and ROCK2, which are functionally redundant in part.22, 23 We previously reported that, in fibroblasts, ROCK2 expression is downregulated when cells are cultured on soft (<4 kPa) substrates, whereas ROCK1 expression remains unaffected.24 Consistently, ROCK1 expression in MCF-7 cells on the 2 kPa substrate was comparable to that on the 30 kPa substrate (Fig. 1A). Furthermore, ROCK1 expression was not decreased by YAP knockdown (Fig. 1B). These results indicate that, in contrast to the case of ROCK2, expression of ROCK1 is regulated by neither substrate rigidity nor YAP.
We identified a potential TEAD-binding sequence (GGAATG) located at -806 to -801 in the promoter region of ROCK2 (Fig. 2A). To examine whether YAP-TEAD directly activates the ROCK2 promoter, we constructed a luciferase reporter gene expressed under the ROCK2 promoter (ROCK2-WT-luc in Fig. 2A). ROCK2 promoter activity was increased by expressing the constitutively active YAP mutant, YAP 5SA (Fig. 2B). In contrast, expression of YAP 5SA did not increase the activity of the reporter gene carrying point mutations in the TEAD binding sequence (ROCK2-MT-luc in Fig. 2A and 2B). Furthermore, the ChIP assay revealed that YAP bound to the ROCK2 promoter as well as the CTGF promoter (Fig. 3). These results reveal that YAP-TEAD induces ROCK2 expression by directly activating the ROCK2 promoter.
Figure 2.

YAP induces activation of the ROCK2 promoter. (A) The ROCK2 promoter and luciferase reporter gene constructs. The TEAD-recognition sequence in the ROCK2 promoter is shown. The following reporter plasmids used in this assay are also indicated: ROCK2-WT-luc containing ROCK2 promoter and putative TEAD-recognition sequence, and ROCK2-MT-luc in which 4 critical nucleotide residues for TEAD binding were altered (indicated by the underlined letters). (B) The pCS2-YAP 5SA expression vector was transfected into C2C12 cells with each reporter plasmid. The luciferase activity was measured 24 hours after transfection. The activity was normalized with the average value of the cells transfected with control vector. Each bar represents the mean ± SD; n = 3. Asterisks, p < 0.01.
Figure 3.

Binding of YAP to the ROCK2 promoter is decreased by treatment with latrunculin A or blebbistatin. MCF-7 cells were treated with latrunculin A (200 nM) or blebbistatin (50 μM) for 40 min. The binding of YAP to the ROCK2 and CTGF promoters was evaluated by chromatin immunoprecipitation assay and quantitative real-time PCR. Each bar represents the mean ± SD; n = 3. Asterisks, p < 0.05.
Stiff ECM increases actin polymerization and myosin activity, which then promotes the nuclear accumulation of YAP.12,25,26 We therefore tested whether actomyosin was involved in YAP-dependent ROCK2 expression. Treatment of cells with latrunculin A, an inhibitor of actin polymerization, or blebbistatin, a myosin II inhibitor, diminished YAP binding to the ROCK2 promoter as well as the CTGF promoter (Fig. 3), suggesting that YAP-TEAD-mediated ROCK2 expression depends on actomyosin activity.
ROCK increases actomyosin activity through phosphorylation of myosin light chain (MLC) and myosin phosphatase (MYPT).23 Since actomyosin activity promotes YAP-mediated gene expression,12,25,26 we next asked whether YAP-dependent regulation of ROCK2 in turn affects YAP activity. Inhibition of ROCK with Y-27632 diminished nuclear localization of YAP in MCF-7 cells (Fig. 4A and B), which is consistent with previous reports using other cell lines.27 Furthermore, knockdown of ROCK2 decreased both expression of CTGF (Fig. 4C, and Supplementary Fig. 1A) and nuclear accumulation of YAP (Fig. 4D, E, and Supplementary Fig. 1B and C). These results indicate that ROCK2 is involved in activation of YAP.
Figure 4.

Knockdown of ROCK2 decreases nuclear accumulation of YAP. (A, B) MCF-7 cells were treated with Y-27632 (10 μM) for 3 h. (C–E) MCF-7 cells were infected with either a control or ROCK2 shRNA-expressing retrovirus. (A, D) Confocal images of cells stained for YAP (green) and DAPI (blue). Z-stack images with an interval of 1.0 μm were obtained using a confocal microscope, and projected images are shown. Scale bars, 20 μm. (B, E) The mean nuclear fluorescence intensity for the stained YAP was quantified using ImageJ software. The relative fluorescence intensities are shown. Each bar represents the mean ± SD; n = 10 (B) or n = 130 (E). Asterisks, p < 0.01. (C) The expression of ROCK2 and CTGF were evaluated by quantitative real-time PCR. Each bar represents the mean ± SD; n = 3. Asterisks, p < 0.01.
Shi et al. used MEFs derived from ROCK1−/− and ROCK2−/− mice to demonstrate that ROCK2, but not ROCK1, regulates MLC2 phosphorylation in cells cultured on stiff substrates (plastic plates; ∼106 kPa).28,29 Thus, ROCK2, but not ROCK1, appears to be crucial for the activation of actomyosin on stiff ECM, which might underlie ROCK2-dependent YAP activation (Fig. 4).
Cell proliferation is largely affected by ECM rigidity.30-32 While YAP promotes cell cycle progression and cell proliferation through the expression of TEAD-target genes,13,26,33-35 ROCK is also indispensable for these cellular behaviors.17,36,37 Our results in this study suggest that YAP and ROCK2 form a positive feedback loop that is modulated by ECM rigidity. This feedback loop may be involved in the rigidity-dependent regulation of cell proliferation.
ECM stiffness, ROCK2, and YAP all affect the susceptibility of cancer cells against chemotherapy, even though their effects are somewhat controversial. We have reported that stiff ECM upregulates ROCK2 expression, which enhances the susceptibility of breast cancer cells against the antitumor drug doxorubicin in a p53-dependent manner.18 Furthermore, while overexpression of YAP is observed in many cancers,33,38,39 antitumor drug treatments activate YAP and thereby induce apoptosis through transcriptional activation of p73, a p53 family tumor suppressor.40 Therefore, although its effect may be dependent on cellular context, the YAP-ROCK2 axis found in this study is likely to be a critical factor that determines the susceptibility of cancer cells against chemotherapy. Further studies on the mechanism underlying the mechanical regulation of YAP and ROCK2 would contribute to the development of effective chemotherapy treatments of cancer.
Supplementary Material
Funding Statement
This work was supported by The Naito Foundation and Grant for Collaborative Research from Nagoya University (2614Dj-02b).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr. Hiroshi Nishina for the YAP 5SA expression vector and Drs. Takahito Nishikata, Junji Kawakami, Koji Nagahama for discussion.
References
- [1].Fletcher DA, Mullins RD. Cell mechanics and the cytoskeleton. Nature 2010; 463:485-92; PMID:20110992; https://doi.org/ 10.1038/nature08908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Machesky LM. Lamellipodia and filopodia in metastasis and invasion. FEBS Lett 2008; 582:2102-11; PMID:18396168; https://doi.org/ 10.1016/j.febslet.2008.03.039 [DOI] [PubMed] [Google Scholar]
- [3].Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell 2002; 110:673-87; PMID:12297042 [DOI] [PubMed] [Google Scholar]
- [4].Huveneers S, Danen EH. Adhesion signaling - crosstalk between integrins, Src and Rho. J Cell Sci 2009; 122:1059-69; PMID:19339545; https://doi.org/ 10.1242/jcs.039446 [DOI] [PubMed] [Google Scholar]
- [5].Moore SW, Roca-Cusachs P, Sheetz MP. Stretchy proteins on stretchy substrates: the important elements of integrin-mediated rigidity sensing. Dev Cell 2010; 19:194-206; PMID:20708583; https://doi.org/ 10.1016/j.devcel.2010.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Iskratsch T, Wolfenson H, Sheetz MP. Appreciating force and shape - the rise of mechanotransduction in cell biology. Nat Rev Mol Cell Biol 2014; 15:825-33; PMID:25355507; https://doi.org/ 10.1038/nrm3903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Humphrey JD, Dufresne ER, Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol 2014; 15:802-12; PMID:25355505; https://doi.org/ 10.1038/nrm3896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, et al.. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 2009; 139:891-906; PMID:19931152; https://doi.org/ 10.1016/j.cell.2009.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Trappmann B, Chen CS. How cells sense extracellular matrix stiffness: a material's perspective. Curr Opin Biotechnol 2013; 24:948-53; PMID:23611564; https://doi.org/ 10.1016/j.copbio.2013.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell 2006; 126:677-89; PMID:16923388; https://doi.org/ 10.1016/j.cell.2006.06.044 [DOI] [PubMed] [Google Scholar]
- [11].Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol 2014; 15:786-801; PMID:25415508; https://doi.org/ 10.1038/nrm3904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Low BC, Pan CQ, Shivashankar GV, Bershadsky A, Sudol M, Sheetz M. YAP/TAZ as mechanosensors and mechanotransducers in regulating organ size and tumor growth. FEBS Lett 2014; 588:2663-70; PMID:24747426; https://doi.org/ 10.1016/j.febslet.2014.04.012 [DOI] [PubMed] [Google Scholar]
- [13].Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev 2014; 94:1287-312; PMID:25287865; https://doi.org/ 10.1152/physrev.00005.2014 [DOI] [PubMed] [Google Scholar]
- [14].Zhao B, Li L, Lu Q, Wang LH, Liu CY, Lei Q, Guan KL. Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes Dev 2011; 25:51-63; PMID:21205866; https://doi.org/ 10.1101/gad.2000111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Moleirinho S, Guerrant W, Kissil JL. The Angiomotins–from discovery to function. FEBS Lett 2014; 588:2693-703; PMID:24548561; https://doi.org/ 10.1016/j.febslet.2014.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Li M, Ke J, Wang Q, Qian H, Yang L, Zhang X, Xiao J, Ding H, Shan X, Liu Q, et al.. Upregulation of ROCK2 in gastric cancer cell promotes tumor cell proliferation, metastasis and invasion. Clin Exp Med 2016; https://doi.org/ 10.1007/s10238-016-0444-z [DOI] [PubMed] [Google Scholar]
- [17].Kumper S, Mardakheh FK, McCarthy A, Yeo M, Stamp GW, Paul A, , Worboys J, Sadok A, Jørgensen C, Guichard S, et al.. Rho-associated kinase (ROCK) function is essential for cell cycle progression, senescence and tumorigenesis. Elife 2016; 5:e12994; PMID:26765561; https://doi.org/ 10.7554/eLife.12203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ebata T, Mitsui Y, Sugimoto W, Maeda M, Araki K, Machiyama H, Harada I, Sawada Y, Fujita H, Hirata H, et al.. Substrate Stiffness Influences Doxorubicin-Induced p53 Activation via ROCK2 Expression. Biomed Res Int 2017; 2017:5158961; PMID:28191463; https://doi.org/ 10.1155/2017/5158961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Guo AK, Hou YY, Hirata H, Yamauchi S, Yip AK, Chiam KH, Tanaka N, Sawada Y, Kawauchi K. Loss of p53 enhances NF-κB-dependent lamellipodia formation. J Cell Physiol 2014; 229:696-704; PMID:24647813; https://doi.org/ 10.1002/jcp.24505 [DOI] [PubMed] [Google Scholar]
- [20].Yip AK, Iwasaki K, Ursekar C, Machiyama H, Saxena M, Chen H, Harada I, Chiam KH, Sawada Y. Cellular response to substrate rigidity is governed by either stress or strain. Biophys J 2013; 104:19-29; PMID:23332055; https://doi.org/ 10.1016/j.bpj.2012.11.3805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kawauchi K, Araki K, Tobiume K, Tanaka N. p53 regulates glucose metabolism through an IKK-NF-kappaB pathway and inhibits cell transformation. Nat Cell Biol 2008; 10:611-8; PMID:18391940; https://doi.org/ 10.1038/ncb1724 [DOI] [PubMed] [Google Scholar]
- [22].Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, et al.. Role of YAP/TAZ in mechanotransduction. Nature 2011; 474:179-83; PMID:21654799; https://doi.org/ 10.1038/nature10137 [DOI] [PubMed] [Google Scholar]
- [23].Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, Elvassore N, Dupont S, Piccolo S. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 2013; 154:1047-59; PMID:23954413; https://doi.org/ 10.1016/j.cell.2013.07.042 [DOI] [PubMed] [Google Scholar]
- [24].Amano M, Nakayama M, Kaibuchi K. Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton (Hoboken) 2010; 67:545-54; PMID:20803696; https://doi.org/ 10.1002/cm.20472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Vigil D, Kim TY, Plachco A, Garton AJ, Castaldo L, Pachter JA, Dong H, Chen X, Tokar B, Campbell SL, et al.. ROCK1 and ROCK2 are required for non-small cell lung cancer anchorage-independent growth and invasion. Cancer Res 2012; 72:5338-47; PMID:22942252; https://doi.org/ 10.1158/0008-5472.CAN-11-2373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Higuchi S, Watanabe TM, Kawauchi K, Ichimura T, Fujita H. Culturing of mouse and human cells on soft substrates promote the expression of stem cell markers. J Biosci Bioeng 2014; 117:749-55; PMID:24360205; https://doi.org/ 10.1016/j.jbiosc.2013.11.011 [DOI] [PubMed] [Google Scholar]
- [27].Johnson R, Halder G. The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat Rev Drug Discov 2014; 13:63-79; PMID:24336504; https://doi.org/ 10.1038/nrd4161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Shi J, Wu X, Surma M, Vemula S, Zhang L, Yang Y, Kapur R, Wei L. Distinct roles for ROCK1 and ROCK2 in the regulation of cell detachment. Cell Death Dis 2013; 4:e483; PMID:23392171; https://doi.org/ 10.1038/cddis.2013.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Shi J, Surma M, Zhang L, Wei L. Dissecting the roles of ROCK isoforms in stress-induced cell detachment. Cell Cycle 2013; 12:1492-500; PMID:23598717; https://doi.org/ 10.4161/cc.24699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Assoian RK, Klein EA. Growth control by intracellular tension and extracellular stiffness. Trends Cell Biol 2008; 18:347-52; PMID:18514521; https://doi.org/ 10.1016/j.tcb.2008.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Klein EA, Yin L, Kothapalli D, Castagnino P, Byfield FJ, Xu T, Levental I, Hawthorne E, Janmey PA, Assoian RK. Cell-cycle control by physiological matrix elasticity and in vivo tissue stiffening. Curr Biol 2009; 19:1511-8; PMID:19765988; https://doi.org/ 10.1016/j.cub.2009.07.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kumar S, Weaver VM. Mechanics, malignancy, and metastasis: the force journey of a tumor cell. Cancer Metastasis Rev 2009; 28:113-27; PMID:19153673; https://doi.org/ 10.1007/s10555-008-9173-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ehmer U, Sage J. Control of Proliferation and Cancer Growth by the Hippo Signaling Pathway. Mol Cancer Res 2016; 14:127-40; PMID:26432795; https://doi.org/ 10.1158/1541-7786.MCR-15-0305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lamar JM, Stern P, Liu H, Schindler JW, Jiang ZG, Hynes RO. The Hippo pathway target, YAP, promotes metastasis through its TEAD-interaction domain. Proc Natl Acad Sci U S A 2012; 109:E2441-50; PMID:22891335; https://doi.org/ 10.1073/pnas.1212021109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Liu X, Li H, Rajurkar M, Li Q, Cotton JL, Ou J, Zhu LJ, Goel HL, Mercurio AM, Park JS, et al.. Tead and AP1 Coordinate Transcription and Motility. Cell Rep 2016; 14:1169-80; PMID:26832411; https://doi.org/ 10.1016/j.celrep.2015.12.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Croft DR, Olson MF. The Rho GTPase effector ROCK regulates cyclin A, cyclin D1, and p27Kip1 levels by distinct mechanisms. Mol Cell Biol 2006; 26:4612-27; PMID:16738326; https://doi.org/ 10.1128/MCB.02061-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhang S, Tang Q, Xu F, Xue Y, Zhen Z, Deng Y, Liu M, Chen J, Liu S, Qiu M, et al.. RhoA regulates G1-S progression of gastric cancer cells by modulation of multiple INK4 family tumor suppressors. Mol Cancer Res 2009; 7:570-80; PMID:19372585; https://doi.org/ 10.1158/1541-7786.MCR-08-0248 [DOI] [PubMed] [Google Scholar]
- [38].Zender L, Spector MS, Xue W, Flemming P, Cordon-Cardo C, Silke J, Fan ST, Luk JM, Wigler M, Hannon GJ, et al.. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell 2006; 125:1253-67; PMID:16814713; https://doi.org/ 10.1016/j.cell.2006.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].S Steinhardt AA, Gayyed MF, Klein AP, Dong J, Maitra A, Pan D, Montgomery EA, Anders RA. Expression of Yes-associated protein in common solid tumors. Hum Pathol 2008; 39:1582-9; PMID:18703216; https://doi.org/ 10.1016/j.humpath.2008.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Strano S, Monti O, Pediconi N, Baccarini A, Fontemaggi G, Lapi E, Mantovani F, Damalas A, Citro G, Sacchi A, et al.. The transcriptional coactivator Yes-associated protein drives p73 gene-target specificity in response to DNA Damage. Mol Cell 2005; 18:447-59; PMID:15893728; https://doi.org/ 10.1016/j.molcel.2005.04.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


