ABSTRACT
The mixed treatment comparison study was performed in order to compare the toxicities of Gemcitabine and different targeted drug combinations in the treatment of advanced/metastatic pancreatic cancer (PC). Searches were performed from the inception of PubMed and Cochrane Library databases to February 2017. This study included randomized controlled trials (RCTs) of Gemcitabine and different targeted drug combinations in the treatment of advanced/metastatic PC. Odds ratio (OR) values were calculated by direct and indirect comparisons, and the surface under the cumulative ranking curves (SUCRA) were drawn. A total of six RCTs were finally incorporated into the study. These studies included six therapy regimens: Gemcitabine + Axitinib, Gemcitabine + Trametinib, Gemcitabine + Sorafenib, Gemcitabine + Bevacizumab, Gemcitabine + Erlotinib and Gemcitabine + Tipifarnib. The results showed that Gemcitabine + Axitinib combinations showed lower incidence rates of rashes (all grades) in comparison to Gemcitabine + Trametinib and Gemcitabine + Erlotinib combinations. Compared with Gemcitabine+ Trametinib combinations, Gemcitabine + Axitinib combinations showed lower incidence rates of diarrhea (grade ≥ 3). Moreover, the cluster analyses results revealed that Gemcitabine + Axitinib combinations and Gemcitabine + Sorafenib combinations showed lower incidence rates of hematotoxicity, while Gemcitabine + Axitinib combinations showed lower incidence rates of non-hematotoxicity. Collectively, the data provided strong evidence of Gemcitabine + Axitinib combinations showing lower incidence rates of non-hematotoxicity, and Gemcitabine + Axitinib and Gemcitabine + Sorafenib combinations may have lower incidence rates of hematotoxicity in the treatment of advanced/metastatic PC.
KEYWORDS: Bayesian network meta-analysis, Hematotoxicity, Non-hematotoxicity, Pancreatic cancer, Randomized controlled trials, Targeted drugs
Introduction
Pancreatic cancer (PC) is a devastating disease with the highest mortality rate in solid tumors.1 It is the fourth leading cause of cancer deaths.2 In 2012, PC resulted in 42,885 and 41,509 incidences and deaths in USA, 79,331 and 78,651 incidences and deaths in The European Union and 32,899 and 31,046 incidences and deaths in Japan.3 The high mortality rate reasons include early diagnostic rates, low eradication rates, poor radiotherapy and chemotherapy response rates.4 Nearly half the patients with early stage pancreatic cancer are asymptomatic.5 PC is deadly and aggressive, patients suffering from PC only survive for 4–8 months and only about 4% of patients survive for 5 years post diagnosis.6 PC has various therapies that involve surgery and chemotherapy.7
The standard treatment of advanced or metastatic PC remains to be 5-fluorouracil (5-FU) chemotherapy,8 and the efficacy of 5-FU for the treatment with advanced PC is not satisfactory.4 Gemcitabine is more effective than 5-FU for alleviating some disease-related symptoms in patients suffering from advanced, symptomatic PC.9 At the present time, Gemcitabine has become the care standard after a small randomized trial showed statistically significant improvements in cancer-related symptoms and a modest improvement in overall survival.10 Gemcitabine is expected to increase the antitumor efficacy as well as improve the clinical benefit response.11,12 A recent clinical study proved that several new combination chemotherapy regimens are superior to single Gemcitabine chemotherapy and extends the overall survival.13 It was demonstrated that the survival benefits of combination therapies were better than single Gemcitabine regimens.2 The combination of Gemcitabine and Tipifarnib has an acceptable toxicity profile but does not prolong overall survival in advanced PC compared with single-agent Gemcitabine.10 The combination of Gemcitabine with Erlotinib, a small tyrosine kinase inhibitor targeting epidermal growth factor receptor (EGFR), has been demonstrated to significantly prolong patient survival rates, leading to approval of the combined regimen by regulatory agencies.1 Anti-EGFR monoclonal antibodies are related to a higher risk of high-grade infection and febrile neutropenia in cancer patients.14 The target of each molecular targeted drug is shown in supplementary Fig. 1. However, the comparative benefits and harms of available combination chemotherapy treatments remain unclear.2
Previous studies worked on the different regimens of targeted drugs combined with gemcitabine in the treatment of PC. The network meta-analysis incorporates both direct and indirect comparisons of diverse regimens.2 Therefore, we aim to provide significant insight for patients and physicians alike by performing a network meta-analysis in order to compare the toxicity of different targeted drugs combined with Gemcitabine in the treatment of advanced or metastatic PC, and collecting clinical data to fill in the blanks.
Materials and methods
Search strategy
PubMed, Cochrane Library, and other databases were searched since the inception of the first database to February 2017. Searches were conducted using free words and keyword combinations as: pancreatic cancer, targeted drugs, gemcitabine and randomized controlled trial, etc.
Inclusion and exclusion criteria
The inclusion criteria was as follows: (1) study design must be a randomized controlled trial (RCT); (2) the interventions were Gemcitabine + Placebo, Gemcitabine + Axitinib, Gemcitabine + Trametinib, Gemcitabine + Sorafenib, Gemcitabine + Bevacizumab, Gemcitabine + Erlotinib and Gemcitabine + Tipifarnib; (3) patients with advanced or metastatic PC aging from 26 to 93; (4) the end outcomes of studies included Anemia, Neutropenia, Thrombocytopenia, Rash, Diarrhea and Stomatitis. The exclusion criteria was as follows: (1) PC patients who previously undergone chemotherapy, gemcitabine, targeted drugs and radiotherapy in the last two weeks; (2) PC patients suffering from brain metastases, interstitial lung disease or pneumonia; (3) PC patients suffering from serious heart disease conditions or CNS disease; (4) incomplete literature data; (5) non-RCTs, duplications, conference reports, meta-analysis and summaries, non-English references.
Data extraction and quality assessment
The date of included literatures was extracted independently by two researchers according to a unified data collection form. If there were disputes, a number of researchers would discuss until consensus. Two or more researchers used the Cochrane Collaboration's tool for assessing the risk of bias in the RCTs. The aforementioned tool included six domains, namely random assignment, allocation concealment, blinding, loss outcome data, choosing the outcome reports and other biases. The assessment included assignment of a “yes,” “no,” or “unclear” judgement for each domain to designate a low, high, or unclear risk of bias, respectively. A study was classified as low risk of bias, if one or no domains were regarded as “unclear” or “no”. A study was classified as high risk of bias, if four or more domains were regarded as “unclear” or “no”. A study was classified as moderate risk of bias, if two or three domains were regarded as “unclear” or “no”.15 Quality assessment and investigation of publication bias were conducted by the Review Manager 5 (RevMan 5.2.3, Cochrane Collaboration, Oxford, UK).
Statistical analysis
Firstly, traditional pairwise meta-analyses were performed for studies that directly compared different treatment arms. The pooled estimates of odds ratio (OR) and 95% confidence intervals (CIs) were recorded in the results. The Chi-square test and I-square test were employed to test heterogeneity amongst the studies.16 Secondly, the network graphs were drawn using the R version 3.2.1 and network package. Moreover, each node represented a variety of interventions, the node size represented the sample size, and the lines between the nodes represented the included number of researches. Thirdly, Bayesian network meta-analyses were performed to compare different interventions to each other. Each analysis was conducted on the basis of non-informative priors for effect sizes and precision. Lack of auto correlation and convergence were checked and confirmed after four chains and a 20,000-simulation burn-in phase. Finally, direct probability statements were derived from an additional 50,000-simulation phase.17 To assist the interpretation of ORs, the probability of each intervention being the safest treatment method was calculated on the basis of a Bayesian approach using probability values summarized as surface under the cumulative ranking curve (SUCRA). The SUCRA value was directly proportional to the rank of intervention.18,19 Cluster analyses were used in order to group the treatments according to their similarities regarding both outcomes.18 All computations were performed using the R (V.3.2.2) package gemtc (V.0.6) as well as the Markov Chain Monte Carlo engine Open BUGS (V.3.4.0).
Results
Baseline characteristics of included studies
The study retrieved a total of 417 related literatures. A total of 6 repeated literatures, 86 letters or reviews, 105 non-human literatures and 25 non-English literatures were eliminated. Out of the remaining 195 literatures, 31 non-cohort literatures, 78 literatures unrelated to advanced/metastatic or PC subjects, 76 literatures without non-targeted drugs combined with gemcitabine and 4 literatures without complete data were further eliminated. The included literatures were published from 2004 to 2015. Lastly, six RCTs were incorporated in the network meta-analysis.1,3,4,10,20,21 (Fig. 1). The study included 2,753 advanced or metastatic PC patients and a majority of the patients took Gemcitabine + placebo. Five RCTs were conducted in the Caucasian population and one RCT was conducted in the Asian population. Furthermore, six included studies were conducted by two-arm trials. The baseline characteristics of the included literatures were shown in Supplementary Table 1 and the Cochrane bias evaluation was shown in Fig. 2.
Figure 1.
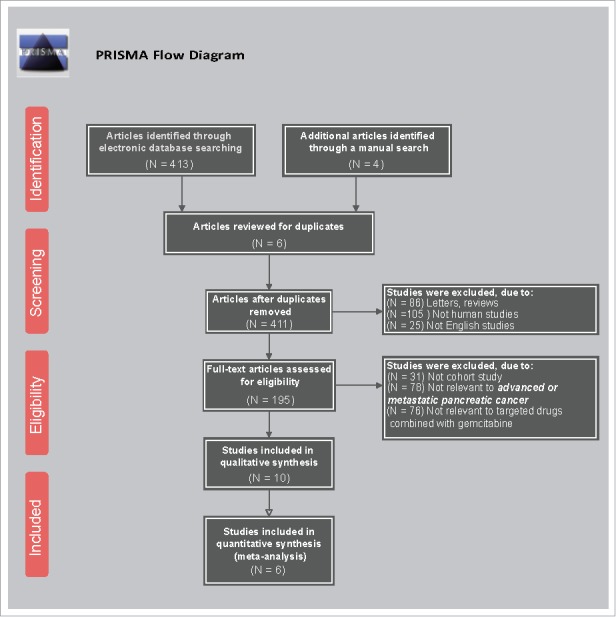
Flow chart showing literature search and study selection.
Figure 2.
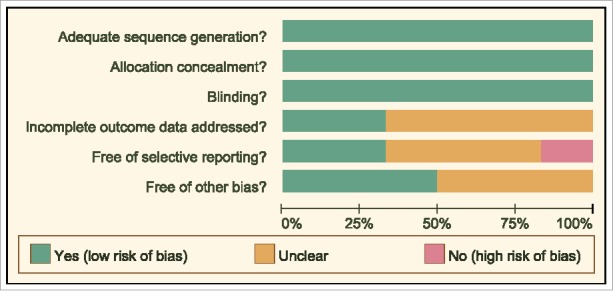
The Cochrane system bias evaluation of included literatures.
Paired Meta-analyses of six Gemcitabine combinations with different target drugs in the treatment of advanced/metastatic PC
The paired comparisons of the targeted drugs combined with chemotherapy for the treatment of PC found that Gemcitabine + Placebo combination showed lower incidence rates of toxicity. Furthermore, Gemcitabine + Axitnib showed lower incidence rates of anemia (all grades) and neutropenia (grade ≥ 3). Gemcitabine + Sorafenib combinations showed lower incidence rates of neutropenia (all grades) and anemia (grade ≥ 3). However, there were no significant differences in the incidence rates of hematotoxicity among thrombcytopenia (all grades) and thrombocytopenia (grade ≥ 3)] (Table 1).
Table 1.
Estimated OR and 95%CI of pairwise meta-analysis for hematologic toxicity events in advanced or metastatic pancreatic cancer patients.
| Toxicity events |
Pairwise meta-analysis | |||
|---|---|---|---|---|
| Included studies | Comparisons | Treatment1 | Treatment2 | OR (95%CI) |
| Hematologic (all grades) | ||||
| Anemia | ||||
| 1 study | A vs. B | 55/308 | 27/305 | 2.24 (1.37–3.66) |
| 1 study | A vs. C | 36/80 | 39/80 | 0.86 (0.46–1.60) |
| 1 study | A vs. D | 50/52 | 20/50 | 37.50 (8.18–171.87) |
| 1 study | A vs. G | 97/342 | 97/331 | 0.96 (0.68–1.33) |
| Neutropenia | ||||
| 1 study | A vs. B | 55/308 | 73/305 | 0.69 (0.47–1.02) |
| 1 study | A vs. C | 35/80 | 37/80 | 0.90 (0.40–1.69) |
| 1 study | A vs. D | 48/52 | 32/50 | 6.75 (2.09–21.79) |
| 1 study | A vs. G | 63/342 | 73/331 | 0.80 (0.55–1.16) |
| Thrombocytopenia | ||||
| 1 study | A vs. B | 37/308 | 49/305 | 0.71 (0.45–1.13) |
| 1 study | A vs. C | 37/80 | 49/80 | 0.54 (0.29–1.02) |
| 1 study | A vs. D | 38/52 | 40/50 | 0.68 (0.27–1.71) |
| 1 study | A vs. G | 66/342 | 78/331 | 0.78 (0.54–1.12) |
| Hematologic (grade≥3) | ||||
| Anemia | ||||
| 1 study | A vs. B | 9/308 | 3/305 | 3.03 (0.81–11.30) |
| 1 study | A vs. C | 9/80 | 18/80 | 0.44 (0.18–1.04) |
| 1 study | A vs. D | 15/52 | 2/50 | 9.73 (2.09–45.22) |
| 1 study | A vs. E | 8/263 | 5/277 | 1.71 (0.55–5.29) |
| 1 study | A vs. G | 16/342 | 20/331 | 0.76 (0.39–1.50) |
| Neutropenia | ||||
| 1 study | A vs. B | 34/308 | 52/305 | 0.60 (0.38–0.96) |
| 1 study | A vs. C | 24/80 | 26/80 | 0.89 (0.46–1.74) |
| 1 study | A vs. D | 29/52 | 26/50 | 1.16 (0.53–2.54) |
| 1 study | A vs. E | 29/263 | 33/277 | 0.92 (0.54–1.56) |
| 1 study | A vs. G | 30/342 | 40/331 | 0.70 (0.42–1.15) |
| Thrombocytopenia | ||||
| 1 study | A vs. B | 9/308 | 15/305 | 0.58 (0.25–1.35) |
| 1 study | A vs. C | 13/80 | 12/80 | 1.10 (0.47–2.58) |
| 1 study | A vs. D | 12/52 | 6/50 | 2.20 (0.76–6.41) |
| 1 study | A vs. E | 12/263 | 12/277 | 1.06 (0.47–2.39) |
| 1 study | A vs. G | 12/342 | 15/331 | 0.77 (0.35–1.66) |
Notes: OR = odds ratio; 95%CI = 95% confidence intervals; A = Gemcitabine+Placebo; B = Gemcitabine+Axitinib; C = Gemcitabine+Trametinib; D = Gemcitabine+Sorafenib; E = Gemcitabine+Bevacizumab; G = Gemcitabine+Tipifarnib.
Gemcitabine + Trametinib and Gemcitabine + Erlotinib combinations showed higher incidence rates of rash (all grades) in comparison to Gemcitabine + Placebo combinations. Additionally, Gemcitabine + Axitinib, Gemcitabine + Trametinib and Gemcitabine + Sorafenib combinations showed higher incidence rates of diarrhea (all grades) and stomatitis (all grades). There were no significant differences in the incidence rates of non-hematotoxicity among rash (grade ≥ 3), diarrhea (grade ≥ 3) and stomatitis (grade ≥ 3) (Table 2).
Table 2.
Estimated OR and 95%CI of pairwise meta-analysis for non-hematologic toxicity events in advanced or metastatic pancreatic cancer patients.
| Toxicity events |
Pairwise meta-analysis | |||
|---|---|---|---|---|
| Included studies | Comparisons | Treatment1 | Treatment2 | OR (95%CI) |
| Non-hematologic (all grades) | ||||
| Rash | ||||
| 1 study | A vs. B | 43/308 | 43/305 | 0.99 (0.63–1.56) |
| 1 study | A vs. C | 20/80 | 38/80 | 0.37 (0.19–0.72) |
| 1 study | A vs. F | 29/280 | 72/282 | 0.34 (0.21–0.54) |
| 1 study | A vs. G | 15/342 | 18/331 | 0.80 (0.40–1.61) |
| Diarrhea | ||||
| 1 study | A vs. B | 68/308 | 101/305 | 0.57 (0.40–0.82) |
| 1 study | A vs. C | 22/80 | 43/80 | 0.33 (0.17–0.63) |
| 1 study | A vs. D | 29/52 | 49/50 | 0.03 (0.00–0.20) |
| 1 study | A vs. F | 41/280 | 56/282 | 0.69 (0.44–1.08) |
| 1 study | A vs. G | 25/342 | 37/331 | 0.63 (0.37–1.07) |
| Stomatitis | ||||
| 1 study | A vs. B | 12/308 | 52/305 | 0.20 (0.10–0.38) |
| 1 study | A vs. C | 22/80 | 43/80 | 0.17 (0.07–0.37) |
| 1 study | A vs. D | 29/52 | 49/50 | 0.06 (0.02–0.18) |
| 1 study | A vs. F | 41/280 | 56/282 | 0.59 (0.30–1.18) |
| Non-hematologic (grade≥3) | ||||
| Rash | ||||
| 1 study | A vs. B | 3/308 | 3/305 | 0.99 (0.20–4.94) |
| 1 study | A vs. C | 0/80 | 5/80 | 0.09 (0.00–1.57) |
| 1 study | A vs. F | 1/280 | 6/282 | 0.16 (0.02–1.38) |
| 1 study | A vs. G | 1/342 | 2/331 | 0.48 (0.04–5.35) |
| Diarrhea | ||||
| 1 study | A vs. B | 6/308 | 3/305 | 2.00 (0.50–8.07) |
| 1 study | A vs. C | 1/80 | 6/80 | 0.16 (0.02–1.33) |
| 1 study | A vs. D | 6/52 | 4/50 | 1.50 (0.40–5.67) |
| 1 study | A vs. F | 2/280 | 6/282 | 0.33 (0.07–1.65) |
| 1 study | A vs. G | 3/342 | 4/331 | 0.72 (0.16–3.26) |
| Stomatitis | ||||
| 1 study | A vs. B | 1/308 | 1/305 | 0.99 (0.06–15.90) |
| 1 study | A vs. C | 0/80 | 2/80 | 0.20 (0.01–4.13) |
| 1 study | A vs. D | 0/52 | 4/50 | 0.10 (0.01–1.88) |
| 1 study | A vs. F | 1/280 | 1/282 | 1.01 (0.06–1.18) |
Notes: OR = odds ratio; 95%CI = 95% confidence intervals; A = Gemcitabine+Placebo; B = Gemcitabine+Axitinib; C = Gemcitabine+Trametinib; D = Gemcitabine+Sorafenib; F = Gemcitabine+Erlotinib; G = Gemcitabine+Tipifarnib.
The evidence of network relationship
Seven Gemcitabine combinations with different target drugs were included in the study. They were as follows: Gemcitabine + placebo; Gemcitabine + Axitinib; Gemcitabine + Trametinib; Gemcitabine + Sorafenib; Gemcitabine + Bevacizumab; Gemcitabine + Erlotinib and Gemcitabine + Tipifarnib. The majority of patients received Gemcitabine + placebo, a few received Gemcitabine + Tipifarnib, Gemcitabine + Axitinib and Gemcitabine + Bevacizumab, while the least number of patients received Gemcitabine + Sorafenib combinations (Fig. 3).
Figure 3.
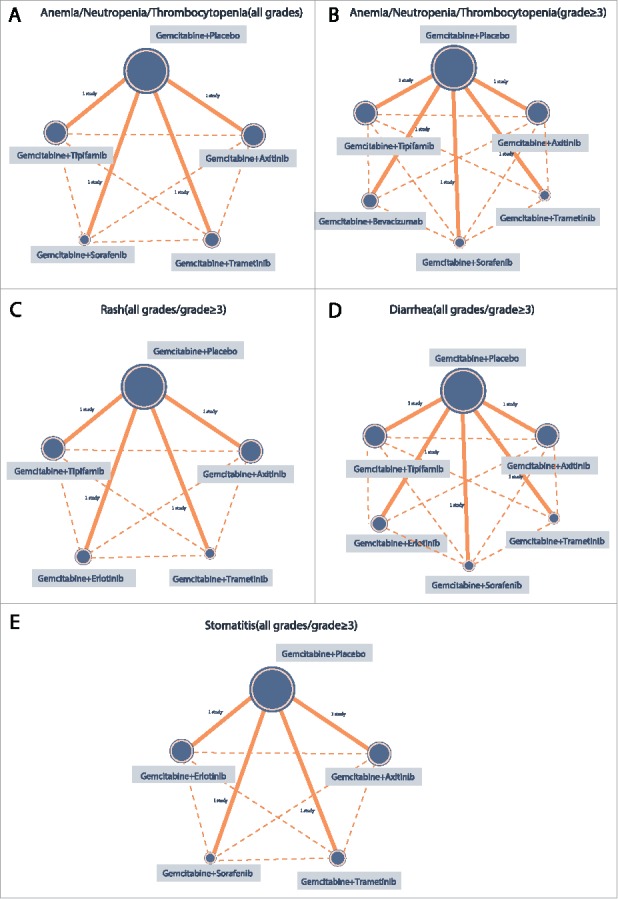
The network evidence graphs of anemia, neutropenia, thrombocytopenia, rash, diarrhea and stomatitis (all grades, grade ≥ 3) (Gemcitabine + Placebo is taken as the reference for all targeted therapies; The size of the circle represents the sample size. The larger the circle, the larger the sample size is).
The main results of network meta-analysis
The network meta-analysis showed the following results for non-hematologic toxicities: compared The Gemcitabine + Axitinib patients showed lower incidence of rash rates compared to the patients receiving the Gemcitabine + Trametinib and Gemcitabine + Erlotinib combinations (all grades) (95%CI = 0.01∼0.79, OR = 0.11, 95%CI = 0.01∼0.98; OR = 0.10, 95%, respectively). Whereas patients receiving Gemcitabine + Axitinib combinations showed lower incidence rates of diarrhea compared to the patients receiving Gemcitabine + Trametinib combinations (grades ≥3). None of the Gemcitabine and varied drug combination patient groups showed any significant difference in terms of anemia, neutropenia and thrombocytopenia in hematologic toxicities (Table 3,Table 4 and Supplementary Fig. 2).
Table 3.
OR and 95%CI of six treatment modalities of six hematologic endpoint outcomes.
| OR (95%CI) | |||||
|---|---|---|---|---|---|
| Hematologic (all grades) | |||||
| Anemia | |||||
| A | 0.14 (0.00, 8.15) | 1.17 (0.02, 81.35) | 0.02 (0.00, 2.03) | 1.09 (0.02, 69.54) | |
| 7.16 (0.12, 572.91) | B | 8.60 (0.02, 3494.09) | 0.18 (0.00, 82.00) | 7.97 (0.02, 3123.86) | |
| 0.85 (0.01, 65.16) | 0.12 (0.00, 44.41) | C | 0.02 (0.00, 8.45) | 0.92 (0.00, 342.54) | |
| 43.12 (0.49, 4169.92) | 5.68 (0.01, 2891.05) | 47.49 (0.12, 27841.94) | D | 45.57 (0.11, 22388.71) | |
| 0.92 (0.01, 59.19) | 0.13 (0.00, 44.59) | 1.08 (0.00, 462.62) | 0.02 (0.00, 9.31) | G | |
| Neutropenia | |||||
| A | 0.38 (0.04, 3.49) | 1.10 (0.11, 11.19) | 0.13 (0.01, 1.50) | 1.27 (0.13, 12.42) | |
| 2.63 (0.29, 24.32) | B | 2.90 (0.12, 73.62) | 0.34 (0.01, 8.66) | 3.33 (0.13, 89.14) | |
| 0.91 (0.09, 8.88) | 0.34 (0.01, 8.51) | C | 0.12 (0.00, 3.19) | 1.16 (0.05, 29.09) | |
| 7.66 (0.67, 99.25) | 2.94 (0.12, 94.27) | 8.52 (0.31, 278.91) | D | 10.05 (0.38, 306.85) | |
| 0.79 (0.08, 8.00) | 0.30 (0.01, 7.45) | 0.86 (0.03, 22.05) | 0.10 (0.00, 2.65) | G | |
| Thrombocytopenia | |||||
| A | 0.40 (0.12, 1.40) | 1.88 (0.55, 6.38) | 1.49 (0.37, 6.22) | 1.28 (0.41, 4.07) | |
| 2.50 (0.71, 8.53) | B | 4.71 (0.81, 26.25) | 3.76 (0.60, 24.32) | 3.20 (0.59, 17.18) | |
| 0.53 (0.16, 1.82) | 0.21 (0.04, 1.23) | C | 0.81 (0.13, 5.22) | 0.68 (0.13, 3.92) | |
| 0.67 (0.16, 2.69) | 0.27 (0.04, 1.66) | 1.24 (0.19, 7.99) | D | 0.87 (0.14, 4.96) | |
| 0.78 (0.25, 2.45) | 0.31 (0.06, 1.68) | 1.46 (0.25, 7.73) | 1.15 (0.20, 7.12) | G | |
| Hematologic (grade≥3) | |||||
| Anemia | |||||
| A | 0.08 (0.00, 1.87) | 2.32 (0.15, 37.97) | 0.08 (0.00, 1.71) | 0.58 (0.03, 9.64) | 1.22 (0.09, 18.18) |
| 12.62 (0.53, 581.06) | B | 30.19 (0.48, 3188.84) | 1.07 (0.01, 136.24) | 7.51 (0.12, 829.90) | 16.98 (0.27, 1652.03) |
| 0.43 (0.03, 6.49) | 0.03 (0.00, 2.10) | C | 0.03 (0.00, 2.06) | 0.25 (0.00, 12.43) | 0.56 (0.01, 24.31) |
| 12.03 (0.59, 268.43) | 0.93 (0.01, 81.93) | 29.80 (0.49, 1710.19) | D | 7.10 (0.11, 365.84) | 14.92 (0.28, 901.17) |
| 1.72 (0.10, 28.67) | 0.13 (0.00, 8.29) | 4.08 (0.08, 213.72) | 0.14 (0.00, 8.80) | E | 2.26 (0.04, 106.91) |
| 0.82 (0.06, 11.62) | 0.06 (0.00, 3.73) | 1.79 (0.04, 101.93) | 0.07 (0.00, 3.60) | 0.44 (0.01, 23.35) | G |
| Neutropenia | |||||
| A | 0.46 (0.17, 1.39) | 1.16 (0.39, 3.38) | 0.90 (0.27, 2.67) | 1.11 (0.39, 2.98) | 1.45 (0.51, 3.89) |
| 2.17 (0.72, 6.05) | B | 2.44 (0.52, 11.47) | 1.92 (0.37, 8.51) | 2.36 (0.53, 9.90) | 3.13 (0.67, 12.77) |
| 0.86 (0.30, 2.56) | 0.41 (0.09, 1.91) | C | 0.75 (0.15, 3.62) | 0.92 (0.23, 4.26) | 1.29 (0.28, 5.46) |
| 1.11 (0.37, 3.66) | 0.52 (0.12, 2.67) | 1.34 (0.28, 6.48) | D | 1.20 (0.28, 5.93) | 1.69 (0.37, 7.62) |
| 0.90 (0.34, 2.55) | 0.42 (0.10, 1.90) | 1.09 (0.23, 4.44) | 0.84 (0.17, 3.53) | E | 1.37 (0.30, 5.33) |
| 0.69 (0.26, 1.97) | 0.32 (0.08, 1.50) | 0.78 (0.18, 3.52) | 0.59 (0.13, 2.67) | 0.73 (0.19, 3.30) | G |
| Thrombocytopenia | |||||
| A | 0.53 (0.12, 2.03) | 0.89 (0.26, 3.13) | 0.43 (0.11, 1.72) | 0.95 (0.28, 3.04) | 1.29 (0.39, 4.23) |
| 1.89 (0.49, 8.66) | B | 1.69 (0.29, 12.58) | 0.82 (0.13, 6.31) | 1.82 (0.29, 12.47) | 2.53 (0.42, 16.83) |
| 1.12 (0.32, 3.80) | 0.59 (0.08, 3.46) | C | 0.48 (0.07, 3.01) | 1.05 (0.19, 5.84) | 1.45 (0.26, 7.95) |
| 2.35 (0.58, 9.38) | 1.22 (0.16, 7.98) | 2.09 (0.33, 14.01) | D | 2.23 (0.35, 13.74) | 3.02 (0.49, 18.93) |
| 1.05 (0.33, 3.52) | 0.55 (0.08, 3.46) | 0.96 (0.17, 5.33) | 0.45 (0.07, 2.86) | E | 1.37 (0.26, 7.53) |
| 0.77 (0.24, 2.55) | 0.40 (0.06, 2.36) | 0.69 (0.13, 3.90) | 0.33 (0.05, 2.04) | 0.73 (0.13, 3.87) | G |
Notes: OR and 95%CI below the treatments should be read from row to column while above the treatments should be read from column to row. OR = odds ratio; 95%CI = 95% confidence intervals; A = Gemcitabine+Placebo; B = Gemcitabine+Axitinib; C = Gemcitabine+Trametinib; D = Gemcitabine+Sorafenib; E = Gemcitabine+Bevacizumab; G = Gemcitabine+Tipifarnib.
Table 4.
OR and 95%CI of six treatment modalities of six non-hematologic endpoint outcomes.
| OR (95%CI) | |||||
|---|---|---|---|---|---|
| Non-hematologic (all grades) | |||||
| Rash | |||||
| A | 0.29 (0.06, 1.36) | 2.71 (0.57, 13.27) | 2.99 (0.67, 14.14) | 1.23 (0.24, 6.52) | |
| 3.50 (0.74, 17.16) | B | 9.36 (1.02, 92.76) | 10.33 (1.26, 90.71) | 4.21 (0.45, 41.90) | |
| 0.37 (0.08, 1.76) | 0.11 (0.01, 0.98) | C | 1.08 (0.12, 10.36) | 0.44 (0.05, 4.68) | |
| 0.33 (0.07, 1.50) | 0.10 (0.01, 0.79) | 0.93 (0.10, 8.41) | F | 0.41 (0.04, 3.77) | |
| 0.81 (0.15, 4.14) | 0.24 (0.02, 2.21) | 2.25 (0.21, 21.84) | 2.47 (0.27, 24.25) | G | |
| Diarrhea | |||||
| A | 0.42 (0.01, 26.80) | 3.15 (0.05, 208.59) | 64.04 (0.61, 7704.44) | 1.41 (0.02, 82.20) | 1.58 (0.03, 88.60) |
| 2.39 (0.04, 131.89) | B | 7.39 (0.02, 2343.83) | 148.77 (0.31, 79234.08) | 3.32 (0.01, 948.13) | 3.80 (0.01, 1189.69) |
| 0.32 (0.00, 19.63) | 0.14 (0.00, 41.70) | C | 20.62 (0.04, 10762.48) | 0.47 (0.00, 140.96) | 0.50 (0.00, 173.64) |
| 0.02 (0.00, 1.63) | 0.01 (0.00, 3.27) | 0.05 (0.00, 26.26) | D | 0.02 (0.00, 12.55) | 0.02 (0.00, 10.07) |
| 0.71 (0.01, 45.20) | 0.30 (0.00, 114.65) | 2.15 (0.01, 795.46) | 46.81 (0.08, 25274.19) | F | 1.12 (0.00, 345.72) |
| 0.63 (0.01, 37.35) | 0.26 (0.00, 91.23) | 1.99 (0.01, 692.07) | 41.16 (0.10, 18905.66) | 0.89 (0.00, 294.60) | G |
| Stomatitis | |||||
| A | 1.48 (0.05, 54.85) | 6.13 (0.18, 221.89) | 19.76 (0.46, 798.39) | 1.64 (0.05, 57.51) | |
| 0.68 (0.02, 22.11) | B | 4.17 (0.03, 610.35) | 13.37 (0.07, 2238.47) | 1.11 (0.01, 156.96) | |
| 0.16 (0.00, 5.56) | 0.24 (0.00, 34.47) | C | 3.21 (0.02, 611.15) | 0.27 (0.00, 39.83) | |
| 0.05 (0.00, 2.16) | 0.07 (0.00, 13.80) | 0.31 (0.00, 50.19) | D | 0.08 (0.00, 11.77) | |
| 0.61 (0.02, 21.86) | 0.90 (0.01, 146.27) | 3.73 (0.03, 577.73) | 12.03 (0.08, 2208.64) | F | |
| Non-hematologic (grade≥3) | |||||
| Rash | |||||
| A | 0.99 (0.08, 11.05) | 6.81 (0.46, 292.28) | 9.07 (0.62, 370.88) | 2.30 (0.12, 97.36) | |
| 1.01 (0.09, 12.15) | B | 7.28 (0.16, 533.29) | 9.84 (0.23, 733.82) | 2.37 (0.06, 192.09) | |
| 0.15 (0.00, 2.18) | 0.14 (0.00, 6.15) | C | 1.38 (0.01, 110.68) | 0.35 (0.00, 35.15) | |
| 0.11 (0.00, 1.61) | 0.10 (0.00, 4.26) | 0.73 (0.01, 70.12) | F | 0.24 (0.00, 24.51) | |
| 0.44 (0.01, 8.54) | 0.42 (0.01, 17.58) | 2.86 (0.03, 305.85) | 4.19 (0.04, 419.48) | G | |
| Diarrhea | |||||
| A | 0.11 (0.00, 1.60) | 8.64 (0.62, 370.49) | 0.63 (0.06, 5.82) | 3.51 (0.32, 55.29) | 1.32 (0.13, 15.29) |
| 9.10 (0.63, 526.96) | B | 91.68 (1.78, 13496.48) | 5.80 (0.18, 652.52) | 33.71 (0.86, 3857.15) | 12.35 (0.32, 1109.38) |
| 0.12 (0.00, 1.61) | 0.01 (0.00, 0.56) | C | 0.07 (0.00, 2.38) | 0.39 (0.01, 17.62) | 0.15 (0.00, 5.27) |
| 1.60 (0.17, 15.69) | 0.17 (0.00, 5.66) | 15.23 (0.42, 1064.71) | D | 5.86 (0.20, 192.49) | 2.18 (0.08, 61.23) |
| 0.28 (0.02, 3.16) | 0.03 (0.00, 1.16) | 2.58 (0.06, 187.55) | 0.17 (0.01, 4.95) | F | 0.37 (0.01, 12.76) |
| 0.76 (0.07, 7.77) | 0.08 (0.00, 3.11) | 6.86 (0.19, 513.41) | 0.46 (0.02, 12.78) | 2.72 (0.08, 92.84) | G |
| Stomatitis | |||||
| A | 0.92 (0.02, 33.25) | 2.44 (0.13, 96.04) | 5.59 (0.42, 224.30) | 0.85 (0.02, 28.95) | |
| 1.09 (0.03, 46.55) | B | 2.79 (0.02, 455.98) | 7.23 (0.06, 925.05) | 0.90 (0.00, 178.08) | |
| 0.41 (0.01, 7.73) | 0.36 (0.00, 41.16) | C | 2.26 (0.03, 238.49) | 0.30 (0.00, 37.95) | |
| 0.18 (0.00, 2.35) | 0.14 (0.00, 17.48) | 0.44 (0.00, 33.19) | D | 0.14 (0.00, 12.52) | |
| 1.18 (0.03, 51.95) | 1.11 (0.01, 220.96) | 3.36 (0.03, 728.13) | 7.36 (0.08, 1096.69) | F | |
Notes: OR and 95%CI below the treatments should be read from row to column while above the treatments should be read from column to row. OR = odds ratio; 95%CI = 95% confidence intervals; A = Gemcitabine+Placebo; B = Gemcitabine+Axitinib; C = Gemcitabine+Trametinib; D = Gemcitabine+Sorafenib; F = Gemcitabine+Erlotinib; G = Gemcitabine+Tipifarnib.
The SUCRA values of six targeted drugs combined with gemcitabine in the treatment of advanced/metastatic PC
Table 5 shows the SUCRA value results for hematologic toxicities. The SUCRA value of cumulative probability sorting of seven regimens showed that Gemcitabine + Sorafenib combination showed the highest incidence rates of anemia (all grades), neutropenia (all grades), anemia (grade ≥ 3) and thrombocytopenia (grade ≥ 3) [anemia (all grades): 92.4%; neutropenia (all grades): 91.8%, anemia (grade ≥ 3): 86.8%, thrombocytopenia (grade ≥ 3): 83.3%]. Gemcitabine + Axitinib combination showed the highest incidence rates of thrombocytopenia (all grades) and neutropenia (grade ≥ 3) [thrombocytopenia (all grades): 95.2%, neutropenia (grade ≥ 3): 91.5%]. Gemcitabine + Trametinib combination showed the lowest incidence rates of for anemia (all grades), thrombocytopenia (all grades) and anemia (grades ≥ 3) [anemia (all grades): 43.2%, thrombocytopenia (all grades): 36.8%, anemia (grade ≥ 3): 30.2%]. Gemcitabine + Tipifarnib combination showed the lowest incidence rates of neutropenia (all grades), neutropenia (grade ≥ 3) and thrombocytopenia (grade ≥ 3) [neutropenia (all grades): 40.4%, neutropenia (grade ≥ 3): 35.5%, thrombocytopenia (grade ≥ 3): 37.8%].
Table 5.
SUCRA values of seven treatment modalities under twelve endpoint outcomes.
| Treatments |
||||||||
|---|---|---|---|---|---|---|---|---|
| SUCRA values (%) | A | B | C | D | E | F | G | |
| Hematologic (all grades) | ||||||||
| Anemia | 44.6 | 75.2 | 43.2 | 92.4 | NR | NR | 45.0 | |
| Neutropenia | 47.0 | 74.2 | 45.6 | 91.8 | NR | NR | 40.4 | |
| Thrombocytopenia | 68.0 | 95.2 | 36.8 | 47.2 | NR | NR | 53.0 | |
| Hematologic (grade≥3) | ||||||||
| Anemia | 47.2 | 86.7 | 30.2 | 86.8 | 58.0 | NR | 41.8 | |
| Neutropenia | 57.3 | 91.5 | 49.5 | 65.2 | 51.7 | NR | 35.5 | |
| Thrombocytopenia | 47.3 | 76.3 | 53.8 | 83.3 | 52.2 | NR | 37.8 | |
| Non-hematologic (all grades) | ||||||||
| Rash | 70.6 | 96.8 | 36.8 | NR | NR | 34.4 | 61.8 | |
| Diarrhea | 72.3 | 84.5 | 48.5 | 22.7 | NR | 62.0 | 60.2 | |
| Stomatitis | 83.0 | 71.0 | 45.8 | 30.2 | NR | 69.0 | NR | |
| Non-hematologic (grade≥3) | ||||||||
| Rash | 81.6 | 78.6 | 41.8 | NR | NR | 36.8 | 60.8 | |
| Diarrhea | 63.67 | 94.2 | 26.5 | 72.0 | NR | 37.3 | 55.8 | |
| Stomatitis | 72.0 | 69.2 | 51.4 | 36.4 | NR | 71.0 | NR | |
Notes: SUCRA = surface under the cumulative ranking curves; NR = not report; A = Gemcitabine+Placebo; B = Gemcitabine+Axitinib; C = Gemcitabine+Trametinib; D = Gemcitabine+Sorafenib; E = Gemcitabine+Bevacizumab; F = Gemcitabine+Erlotinib; G = Gemcitabine+Tipifarnib.
The SUCRA value results for non-hematologic toxicities were also recorded. Gemcitabine + Axitinib combination showed the highest incidence rate of rash (all grades), diarrhea (all grades) and diarrhea (grade ≥ 3)[rash (all grades):96.8%, diarrhea (all grades): 84.5%, diarrhea (grade ≥ 3): 94.2%]. Gemcitabine + Trametinib combination showed the lowest incidence rates of diarrhea (grade ≥ 3), (26.5%). Gemcitabine + Sorafenib combination showed the lowest incidence rates of diarrhea (all grades), stomatitis (all grades) and stomatitis (grade ≥ 3) lowest [diarrhea (all grades): 22.7%, stomatitis (all grades): 30.2%, stomatitis (grade ≥ 3): 36.4%]. Gemcitabine + Erlotinib combination showed the lowest incidence rates of rash (all grades) and rash (grade ≥ 3) [rash (all grade): 34.4%, rash (grade ≥ 3): 36.8%].
Cluster analysis of hematologic and non-hematologic toxicities of six targeted drugs combined with gemcitabine in the treatment of advanced/metastatic PC
Gemcitabine + Axitinib and Gemcitabine + Sorafenib combinations show lower incidence rates of hematologic toxicities in the treatment of advanced or metastatic PC, whereas Gemcitabine + Trametinib and Gemcitabine + Tipifarnib combinations show higher incidence rates. Gemcitabine + Axitinib combinations shows lower incidence rates of non-hematologic toxicities, whereas Gemcitabine + Trametinib and Gemcitabine + Erlotinib combinations show higher incidence rates (Fig. 4).
Figure 4.
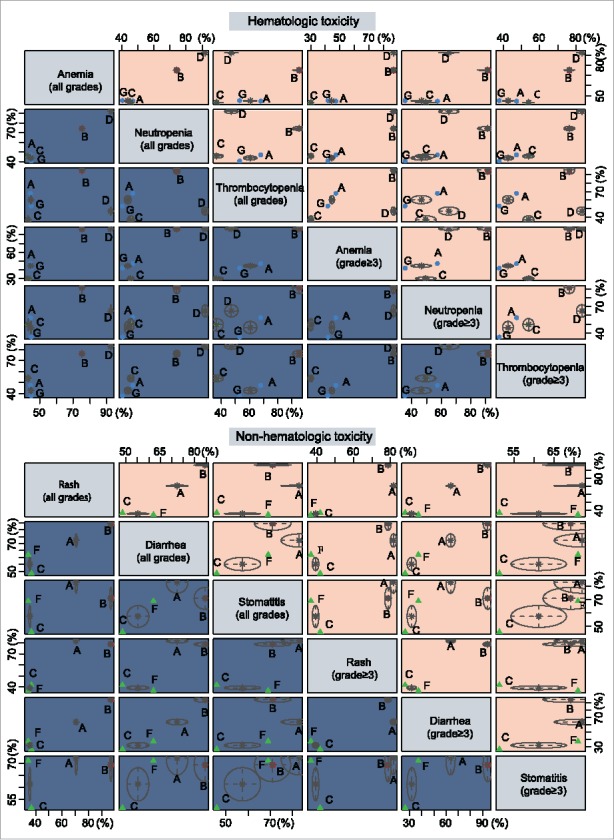
Cluster analyses of anemia, neutropenia, thrombocytopenia, rash, diarrhea and stomatitis. (The 6 outcome indexes are considered, and the advantages and disadvantages of each intervention are compared; In each small square, the intervention measures in the upper right corner are “superior”, and the intervention measures in the lower left corner are “inferior”.).
Discussion
The study aims to summarize clinical data, and further evaluated six commonly used chemotherapy regimens by paired meta-analysis and network meta-analysis. The obtained results confirm previous observations and suggest that Gemcitabine + Axitinib and Gemcitabine + Sorafenib combination regimens may have lower incidence rates of hematotoxicity in the treatment of advanced or metastatic PC, and additionally, Gemcitabine + Axitinib, Gemcitabine + Trametinib, Gemcitabine + Sorafenib combination regimens may have higher incidence rates of non-hematotoxicity. Studies suggest that Axitinib behaves as a powerful, selective oral inhibitor of vascular endothelial growth factor.22 Axitinib safety profile was typically same in patients of all three domains, although differences in incidence rates of some adverse events were noted.3 Gemcitabine + Axitinib combination showed a similar safety profile to individual Gemcitabine, although the non-statistical significant gain in overall survival needs to be further assessed in a randomized phase III trial.22 Furthermore, studies suggest that Sorafenib showed activity against PC in pre-clinical models, and Gemcitabine + Sorafenib combination is invalid in advanced PC.23
The network meta-analysis results showed that Gemcitabine + Axitinib combination regimen showed lower incidence rate of rash (all grades) in comparison to Gemcitabine + Trametinib and Gemcitabine + Erlotinib combination regimens. Gemcitabine + Axitinib combination regimen showed lower incidence rate of diarrhea (grade ≥ 3) in comparison to Gemcitabine + Trametinib combination regimen. In all targeted therapies, merely the EGFR tyrosine kinase inhibitor, Erlotinib showed activity and fringe in the overall population, but was clinically relevant in patients developing skin rashes.24 Gemcitabine + Axitinib combination treatment showed a higher incidence rate (>20%) of all-causality fatigue, diarrhea, hypertension, stomatitis and hand-foot syndrome in comparison with Placebo or Gemcitabine treatment.3 The addition of Gemcitabine + Trametinib combination did not improve the rates of OS, PFS, ORR or DCR in patients with formerly untreated metastatic PC.4 Inhibition of MEK prevents cell proliferation and induces apoptosis; and Trametinib is a MEK1/2 inhibitor.25 EGFR inhibitors and MEK inhibitors are well-known for causing papulopustular rash.26 Escalation of Erlotinib dosage induced rashes (grade ≥2) in 29 out of 71 (41.4%) patients, compared with 7 out of 75 (9.3%) patients receiving standard dosage, and no significant differences in efficacy was observed between escalated and standard dosage regimen.27 Gemcitabine + Erlotinib combination showed acceptable toxicity, and efficacy that was comparable with western patients.28
The cluster analyses results show that the SUCRA values of Gemcitabine + Axitinib and Gemcitabine + Sorafenib were higher, while Gemcitabine + Trametinib and Gemcitabine + Tipifarnib were lower in hemotoxicity. The SUCRA values of Gemcitabine + Axitinib were higher in non-hemotoxicity, whereas Gemcitabine + Sorafenib and Gemcitabine + Erlotinib were lower in comparison. Gemcitabine + Erlotinib combination results demonstrate that it significantly prolonged the patients' survival rates. It is a small tyrosine kinase inhibitor that targets EGFR.1 Patients were treated by Gemcitabine + Trametinib combinations or Placebo constantly until disease progression, unacceptable toxicity or withdrawal of consent. If toxicity was attributed to one drug but not the other, patients continued treatment with monotherapy.4
The study integrated the current number of chemotherapy drugs, which were compared and analyzed without yielding obvious conclusions on ideal drugs for the progression or metastasis of advanced or metastatic PC due to limited references and data. Insufficient evaluation data resulted in deviations in network meta-analysis. Correction of the aforementioned deviations relied on optimization of the new algorithms and additional clinical and basic research data. Therefore, the presented study showed a difference in toxicity of six Gemcitabine combinations with different target drugs regimens (Gemcitabine + Axitinib, Gemcitabine + Trametinib, Gemcitabine + Sorafenib, Gemcitabine + Bevacizumab, Gemcitabine + Erlotinib and Gemcitabine + Tipifarnib) with Gemcitabine + Placebo in the treatment of advanced or PC by general comparison.
Conclusion
In conclusion, the results indicate that Gemcitabine combinations with different target drugs regimens may show more frequent toxicities in the treatment of advanced or metastatic PC, which provides us with significant insight for their clinical use and treatment of advanced or metastatic PC.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to thank the reviewers for their constructive comments.
References
- 1.Goncalves A, Gilabert M, Francois E, Dahan L, Perrier H, Lamy R, Re D, Largillier R, Gasmi M, Tchiknavorian X, et al.. BAYPAN study: a double-blind phase III randomized trial comparing gemcitabine plus sorafenib and gemcitabine plus placebo in patients with advanced pancreatic cancer. Ann Oncol. 2012;23(11):2799–805. doi: 10.1093/annonc/mds135. PMID:22771827. [DOI] [PubMed] [Google Scholar]
- 2.Gresham GK, Wells GA, Gill S, Cameron C, Jonker DJ. Chemotherapy regimens for advanced pancreatic cancer: A systematic review and network meta-analysis. BMC Cancer. 2014;14:471. doi: 10.1186/1471-2407-14-471. PMID:24972449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ioka T, Okusaka T, Ohkawa S, Boku N, Sawaki A, Fujii Y, Kamei Y, Takahashi S, Namazu K, Umeyama Y, et al.. Efficacy and safety of axitinib in combination with gemcitabine in advanced pancreatic cancer: subgroup analyses by region, including Japan, from the global randomized Phase III trial. Jpn J Clin Oncol. 2015;45(5):439–48. doi: 10.1093/jjco/hyv011. PMID:25647781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Infante JR, Somer BG, Park JO, Li CP, Scheulen ME, Kasubhai SM, Oh DY, Liu Y, Redhu S, Steplewski K, et al.. A randomised, double-blind, placebo-controlled trial of trametinib, an oral MEK inhibitor, in combination with gemcitabine for patients with untreated metastatic adenocarcinoma of the pancreas. Eur J Cancer. 2014;50(12):2072–81. doi: 10.1016/j.ejca.2014.04.024. PMID:24915778. [DOI] [PubMed] [Google Scholar]
- 5.Lu C, Xu CF, Wan XY, Zhu HT, Yu CH, Li YM. Screening for pancreatic cancer in familial high-risk individuals: A systematic review. World J Gastroenterol. 2015;21(28):8678–86. doi: 10.3748/wjg.v21.i28.8678. PMID:26229410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valenzuela MM, Neidigh JW, Wall NR. Antimetabolite Treatment for Pancreatic Cancer. Chemotherapy (Los Angel). 2014;3(3):137. PMID:26161298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asuthkar S, Rao JS, Gondi CS. Drugs in preclinical and early-stage clinical development for pancreatic cancer. Expert Opin Investig Drugs. 2012;21(2):143–52. doi: 10.1517/13543784.2012.651124. PMID:22217246. [DOI] [PubMed] [Google Scholar]
- 8.Oettle H. Progress in the knowledge and treatment of advanced pancreatic cancer: from benchside to bedside. Cancer Treat Rev. 2014;40(9):1039–47. doi: 10.1016/j.ctrv.2014.07.003. PMID:25087471. [DOI] [PubMed] [Google Scholar]
- 9.r Burris HA, MJ Moore, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, et al.. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J Clin Oncol. 1997;15:2403–13. doi: 10.1200/JCO.1997.15.6.2403. PMID:9196156. [DOI] [PubMed] [Google Scholar]
- 10.Van Cutsem E, van de Velde H, Karasek P, Oettle H, Vervenne WL, Szawlowski A, Schoffski P, Post S, Verslype C, Neumann H, et al.. Phase III trial of gemcitabine plus tipifarnib compared with gemcitabine plus placebo in advanced pancreatic cancer. J Clin Oncol. 2004;22(8):1430–8. doi: 10.1200/JCO.2004.10.112. PMID:15084616. [DOI] [PubMed] [Google Scholar]
- 11.Sofuni A, Itoi T, Itokawa F, Tsuchiya T, Kurihara T, Ishii K, Tsuji S, Ikeuchi N, Moriyasu F. Usefulness of contrast-enhanced ultrasonography in determining treatment efficacy and outcome after pancreatic cancer chemotherapy. World J Gastroenterol. 2008;14(47):7183–91. doi: 10.3748/wjg.14.7183. PMID:19084932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee HS, Park SW. Systemic Chemotherapy in Advanced Pancreatic Cancer. Gut Liver. 2016;10(3):340–7. doi: 10.5009/gnl15465. PMID:27114434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Loon K EA, Fogelman DR, Wolff RA, Javle MM, Iyer RV, Picozzi VJ, Martin LK, Bekaii-Saab T, Tempero MA, Foster NR, et al.. Should combination chemotherapy serve as the backbone in clinical trials of advanced pancreatic cancer? A pooled analysis of phase ii trials of gemcitabine-containing doublets plus bevacizumab. Pancreas. 2014;43:343–9. doi: 10.1097/MPA.0000000000000095. PMID:24622062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Funakoshi T, Suzuki M, Tamura K. Infectious complications in cancer patients treated with anti-EGFR monoclonal antibodies cetuximab and panitumumab: A systematic review and meta-analysis. Cancer Treat Rev. 2014;40(10):1221–9. doi: 10.1016/j.ctrv.2014.09.002. PMID:25288497. [DOI] [PubMed] [Google Scholar]
- 15.Chung JH, Lee SW. Assessing the quality of randomized controlled urological trials conducted by korean medical institutions. Korean J Urol. 2013;54(5):289–96. doi: 10.4111/kju.2013.54.5.289. PMID:23700493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen LX, Li YL, Ning GZ, Li Y, Wu QL, Guo JX, Shi HY, Wang XB, Zhou Y, Feng SQ. Comparative efficacy and tolerability of three treatments in old people with osteoporotic vertebral compression fracture: a network meta-analysis and systematic review. PLoS One. 2015;10(4):e0123153. doi: 10.1371/journal.pone.0123153. PMID:25874802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tu YK NI, Chambrone L, Lu HK, Faggion CM Jr. A bayesian network meta-analysis on comparisons of enamel matrix derivatives, guided tissue regeneration and their combination therapies. Journal of clinical periodontology. 2012;39:303–14. doi: 10.1111/j.1600-051X.2011.01844.x. PMID:22393565. [DOI] [PubMed] [Google Scholar]
- 18.Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS One. 2013;8(10):e76654. doi: 10.1371/journal.pone.0076654. PMID:24098547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64(2):163–71. doi: 10.1016/j.jclinepi.2010.03.016. PMID:20688472. [DOI] [PubMed] [Google Scholar]
- 20.Kindler HL, Niedzwiecki D, Hollis D, Sutherland S, Schrag D, Hurwitz H, Innocenti F, Mulcahy MF, O'Reilly E, Wozniak TF, et al.. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol. 2010;28(22):3617–22. doi: 10.1200/JCO.2010.28.1386. PMID:20606091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, et al.. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25(15):1960–6. doi: 10.1200/JCO.2006.07.9525. PMID:17452677. [DOI] [PubMed] [Google Scholar]
- 22.Spano JP, Chodkiewicz C, Maurel J, Wong R, Wasan H, Barone C, Letourneau R, Bajetta E, Pithavala Y, Bycott P, et al.. Efficacy of gemcitabine plus axitinib compared with gemcitabine alone in patients with advanced pancreatic cancer: An open-label randomised phase II study. Lancet. 2008;371(9630):2101–8. doi: 10.1016/S0140-6736(08)60661-3. PMID:18514303. [DOI] [PubMed] [Google Scholar]
- 23.Kindler HL, Wroblewski K, Wallace JA, Hall MJ, Locker G, Nattam S, Agamah E, Stadler WM, Vokes EE. Gemcitabine plus sorafenib in patients with advanced pancreatic cancer: a phase II trial of the University of Chicago Phase II Consortium. Invest New Drugs. 2012;30(1):382–6. doi: 10.1007/s10637-010-9526-z. PMID:20803052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heinemann V, Haas M, Boeck S. Systemic treatment of advanced pancreatic cancer. Cancer Treat Rev. 2012;38(7):843–53. doi: 10.1016/j.ctrv.2011.12.004. PMID:22226241. [DOI] [PubMed] [Google Scholar]
- 25.Infante JR, Fecher LA, Falchook GS, Nallapareddy S, Gordon MS, Becerra C, DeMarini DJ, Cox DS, Xu Y, Morris SR, et al.. Safety, pharmacokinetic, pharmacodynamic, and efficacy data for the oral MEK inhibitor trametinib: A phase 1 dose-escalation trial. Lancet Oncol. 2012;13(8):773–81. doi: 10.1016/S1470-2045(12)70270-X. PMID:22805291. [DOI] [PubMed] [Google Scholar]
- 26.Vaubel J, Livingstone E, Schadendorf D, Zimmer L. Retarded low-dose doxycycline for EGFR or MEK inhibitor-induced papulopustular rash. J Eur Acad Dermatol Venereol. 2014;28(12):1685–9. doi: 10.1111/jdv.12365. PMID:24422792. [DOI] [PubMed] [Google Scholar]
- 27.Van Cutsem E Li CP, Nowara E, Aprile G, Moore M, Federowicz I, Van Laethem JL Hsu C, Tham CK, Stemmer SM, et al.. Dose escalation to rash for erlotinib plus gemcitabine for metastatic pancreatic cancer: the phase II RACHEL study. Br J Cancer. 2014;111(11):2067–75. doi: 10.1038/bjc.2014.494. PMID:25247318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okusaka T, Furuse J, Funakoshi A, Ioka T, Yamao K, Ohkawa S, Boku N, Komatsu Y, Nakamori S, Iguchi H, et al.. Phase II study of erlotinib plus gemcitabine in Japanese patients with unresectable pancreatic cancer. Cancer Sci. 2011;102(2):425–31. doi: 10.1111/j.1349-7006.2010.01810.x. PMID:21175992. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


