ABSTRACT
Physiological responses to changes in environmental conditions such as temperature may partly arise from the resident microbial community that integrates a wide range of bio-physiological aspects of the host. In the present study, we assessed the effect of developmental temperature on the thermal tolerance and microbial community of Drosophila melanogaster. We also developed a bacterial transplantation protocol in order to examine the possibility of reshaping the host bacterial composition and assessed its influence on the thermotolerance phenotype. We found that the temperature during development affected thermal tolerance and the microbial composition of male D. melanogaster. Flies that developed at low temperature (13°C) were the most cold resistant and showed the highest abundance of Wolbachia, while flies that developed at high temperature (31°C) were the most heat tolerant and had the highest abundance of Acetobacter. In addition, feeding newly eclosed flies with bacterial suspensions from intestines of flies developed at low temperatures changed the heat tolerance of recipient flies. However, we were not able to link this directly to a change in the host bacterial composition.
KEYWORDS: climate change, developmental temperature, Drosophila, microbiota, thermal tolerance, transplantation
Introduction
Investigating physiological and genetic responses to variable and sometimes stressful temperatures in ectotherm animals is an important research field in ecology and evolution, and the knowledge obtained from such studies provides important information with regard to a species' distributions under changing environmental conditions.1–6 Alterations in phenotype and geographical distribution as part of adaptive responses to climate-driven changes, such as increasing and fluctuating temperatures, have been observed in various organisms, including ectothermic plants and animals.7–9 In addition, the ambient temperature considerably affects molecular processes and pathways that contribute to the maintenance of physiological homeostasis of these organisms. Recent studies provide examples on adaptive acclimation responses of insects to thermal stress through changes in the transcriptome,10 proteome,11 lipidome,12 and metabolome13,14 profiles. These plastic responses are proposed to be important for fitness of insects in variable thermal environments.15
Physiological responses to changes in environmental conditions such as temperatures may partly be a consequence of the resident microbial community.16–18 The host microbial community integrates a wide range of bio-physiological aspects of the host, including nutritional supplementation, stress resistance, and modulation of immune responses.19 Among the impacts of bacteria on their hosts, food digestion, and thereby changing nutrient availability, affects a wide range of fitness components, including growth rate, developmental time, survival, and stress resistance.20,21 Slower development and changes in the nutrient profile of germ-free Drosophila melanogaster (axenic) compared to untreated flies suggest an association between the resident microbiota and host traits.22 In another study carried out in D. melanogaster, elimination of the microbial community through sterilising the eggs changed the allocation pattern of carbohydrates in a gender specific pattern, which was explained by the trehalose levels.23 The protective role of trehalose as a stress-responsive factor in D. melanogaster and its importance for coping with environmental stressors such as cold and starvation highlights the significance of microbial symbionts for survival of ectotherms in extreme environments.24,25
Over the last few decades, studies have provided some support for an association between the host microbial population and thermal tolerance in a range of organisms. For example, short exposure to high temperature reduced the frequency of the bacterial symbiont Buchnera aphidicola in pea aphids. This led to reduced thermal resistance and affected the reproductive success of aphid hosts due to a deficiency in the biosynthesis of Buchnera-derived essential amino acids.26 The gut microbiota of cold acclimated mice has also been shown to be dramatically different from mice raised at benign temperature.27 In addition, the microbiota transplantation from cold acclimated to germ-free mice through co-habitation transferred the high insulin sensitivity phenotype of donor to the recipient mice. These results suggest that remodelling of the host microbiome composition through transplantation may alter the host responses to temperature changes.
The focus of the present study was to investigate the importance of the microbiota in mediating the relationship between the host phenotype and the environmental thermal conditions. We aimed to evaluate the influence of developmental temperature on the microbial community profile of D. melanogaster by assessing the microbiota of flies developed at three different temperatures: 13°C, 23°C or 31°C. In addition, we examined the possibility of reshaping the gut microbiome composition of flies developed at benign temperature (23°C) by transplanting microbiomes from intestines of flies developed at 13°C, 23°C or 31°C, using the Capillary Feeder (CAFÉ) system.28 In both experiments, the thermal resistance of treatment groups and their microbial community composition were assessed to investigate the relationship between developmental temperature, microbiota, and thermal resistance of flies.
Materials and methods
Stock population
The D. melanogaster population used in the present study was established in 2010 as a mass bred population, using the offspring of 589 inseminated females.29 The population was maintained on standard Drosophila agar-sugar-yeast medium at 25 ± 1°C and on a 12:12 L:D cycle. Prior to the experiment, the population was cultured in 300 mL plastic bottles (6 bottles per generation, approx. 300 flies per bottle) containing 70 mL standard Drosophila medium seeded with live yeast and developed at 23°C on a 12:12 L:D cycle. Adult flies (5 to 6 days of age) were allowed over a 12 h window to lay eggs on small spoons filled with 1 mL Drosophila standard medium enriched with live yeast. Eggs were collected at a controlled density (50 eggs per vial) and placed into 35 mL plastic vials containing 7 mL standard Drosophila medium. The eggs developed at three different developmental temperatures; 13°C, 23°C and 31°C. These temperatures were chosen based on previous studies with the same D. melanogaster population, showing that these temperatures led to differentiated thermal resistances and developmental times, with little effect on egg-to-adult viability.14 To synchronize the age of eclosed flies, eggs exposed to 13°C and 23°C developmental temperatures were collected 40 and 2 days before the high temperature treatment group (31°C), respectively. Eclosed flies from each developmental temperature were collected separately within 24 h of the first eclosion and sexed under CO2 anesthesia. Unless stated otherwise, males were transferred to 35 mL plastic vials with fresh medium (50 males per vial) and maintained at the temperature at which they had been developed. In the study, we performed two consecutive experiments as follows:
Assessing the influence of developmental temperature on thermal resistance and microbial community
The impact of developmental temperature on flies' resistance to low and high temperatures was assessed using ramping assays, in which flies were exposed to a gradual increase or decrease of temperature.30 The Critical Thermal maximum (CTmax) and Critical Thermal minimum (CTmin) were measured on males 3 days old (15 individuals per developmental temperature) by placing them individually into 5 mL screw cap glass vials without the use of anesthetics. The vials were placed randomly in a metal rack and then submerged in a water bath set at benign temperature (23°C). Thereafter, the water temperature was increased (CTmax) or decreased (CTmin) at a rate of 0.1°C/min, and the temperature at which a fly was totally immobilized was scored, until all flies went into coma. Along with the assessment of CTmax and CTmin for individual flies, 6 replicates of 50 males per developmental temperature were evenly subjected to heat and cold ramping assays, and when the temperature reached 35°C and 13°C, respectively, the samples were immediately snap frozen in liquid nitrogen and stored at −80°C for further assessments of their microbiomes.
To evaluate the possible effect of developmental temperature on the microbial community of flies, males were snap frozen in liquid nitrogen at 3 days of age and stored at −80°C. The assay was performed in triplicate for each thermal regime so that 150 males were frozen per developmental temperature (50 males per replicate). Concomitantly, 3 replicate medium samples (medium from vials in which larvae had been developing) were frozen per thermal regime and stored at −80°C for microbial assessment. The medium samples were collected from freshly eclosed flies to avoid the presence of larvae and pupae in the medium.
Reshaping the gut microbial community through bacterial transplantation
To test for the possibility of reshaping the gut microbial community of newly eclosed flies and to assess their influence on thermal tolerance of D. melanogaster, a transplantation protocol was developed in order to transfer microbiota between flies. Three bacterial suspensions (BS) were prepared from donor flies raised at different developmental temperatures (13°C, 23°C and 31°C). The flies were collected separately and sexed under CO2 anesthesia within 24 h of the first eclosion. Flies were placed in 35 mL plastic vials with fresh medium (10 males per vial) and kept at their respective temperatures. To increase the likelihood that all male flies had mated, 2 females were added to each vial. After 24 h of recovery time, the gastrointestinal tract of flies from each developmental temperature was dissected separately, following a previously described protocol31 with minor modifications. Briefly, 48 h old males were anesthetized with CO2 (10 flies at a time), decapitated using a sterilized razor blade and transferred individually to a petri dish filled with 2% agar solution and blue food dye. Each individual fly was placed next to a drop of 5% sucrose solution, the gastrointestinal tract including the crop, foregut, midgut, hindgut, and sexual organs were gently pulled out of the abdomen, using a pair of forceps, and placed into a 2 mL Eppendorf tube (30 gastrointestinal tracts per developmental temperature), which was kept on ice during the entire process. The gastrointestinal tracts were homogenized with disposable plastic pestles. Thereafter, 1000 µL of 5% sucrose solution and 100 µL green food dye were added to each suspension and homogenized by vortexing for 2 minutes. The supernatant was transferred to new, cooled Eppendorf tubes, which were labeled as BS-13 (bacterial suspension containing gut microbiota of flies developed at 13°C), BS-23 (bacterial suspension containing gut microbiota of flies developed at 23°C), and BS-31 (bacterial suspension containing gut microbiota of flies developed at 31°C). In addition, a suspension expected to be free of bacteria (FBS) was prepared, using a mixture of 1000 µL 5% sucrose solution and 100 µL green food dye, and labeled as FBS-suc.
Gut microbiota transplantation from donor to recipient flies was carried out by collecting flies developed at 23°C within 24 h post-eclosion. The flies were sexed under CO2 anesthesia and transferred into empty 35 mL plastic vials (10 male flies per vial). Flies from each vial received one of the treatments (suspension with microbiomes from intestines of flies developed at 13°C, 23°C or 31°C or a sucrose suspension without bacteria), using the CAFÉ system28 so that three glass micropipettes (Drummond) filled with suspension of one treatment were placed in a 35 mL plastic vial containing 10 males (Fig. 1). The feeding period lasted only 24 h to minimize negative influences of feeding through the CAFÉ system on fitness components.32 To quantify the effect of evaporation of the suspension on food intake, three glass micropipettes filled with sucrose suspension were placed in a 35 mL plastic vial with no flies and considered as a control vial (4 control vials in total). All vials were kept at 23°C, 60% humidity on a 12:12 L:D cycle during the experiment. Thermal resistance (CTmin and CTmax) and microbial community of recipient flies were assessed 24 h post treatment. CTmin and CTmax were assessed on 96 male flies (24 flies per treatment) according to the protocol applied in the first experiment. To assess the microbial community of recipient flies, 30 males of each treatment were frozen in liquid nitrogen 24 h post treatment and preserved at −80°C. The food intake and evaporation were calculated based on the height of remnant suspension in the glass micropipettes, which was measured using a digital Vernier Caliper (± 0.01 mm). The volume of food intake was corrected according to evaporation rate of the micropipettes kept in vials with no flies. The results showed that, on average, flies consumed more than 30% of the available suspension (supplementary Fig S1).
Figure 1.
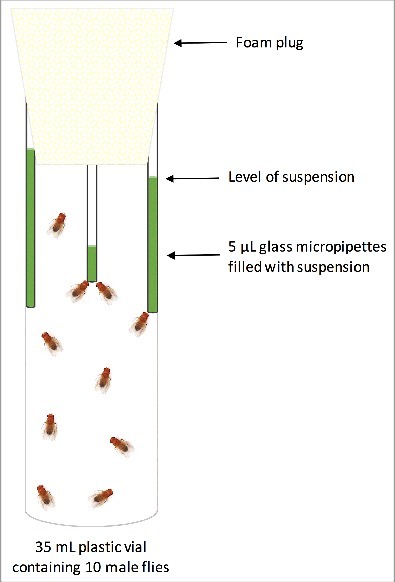
Illustration of the experimental set-up used to feed the newly eclosed flies with microbial suspensions. Three glass micropipettes filled with suspension of one treatment were placed in a 35 mL plastic vial containing 10 males. The level of suspension in the micropipettes was used to calculate the food intake.
DNA extraction & 16S rRNA gene amplicon sequencing
In both experiments and for all samples, total DNA was extracted from fly samples using DNeasy® Blood & Tissue Kit (Qiagen). FastDNA™ SPIN Kit (MP Biochemicals) was used to extract the total DNA from media samples. The V1-V3 region of the bacterial 16S rRNA gene was amplified, using 10 ng template DNA per sample, as described elsewhere.33 Amplicon size and quality were assessed, using 2% agarose gel electrophoresis and Qubit® dsDNA HS Assay Kit (Thermo Fischer Scientific), respectively. Sequencing was performed on the MiSeq platform (Illumina), using MiSeq reagent kit v3 (2 × 300PE).
All sequenced sample libraries were subsampled to 50,000 raw reads. Low quality reads were trimmed, using trimmomatic (version 0.32),34 and merged, using FLASH (version 1.2.7).35 The reads were formatted for use with the UPARSE workflow and screened for chimeric sequences.36 Usearch7 was used to de-replicate reads and clustering of OTUs (Operational Taxonomic Units) at 97% sequence similarity. Taxonomy was assigned, using RDP classifier37 as implemented in QIIME,38 using GreenGenes as reference database.39 All raw sequence data is available at European Nucleotide Archive (ENA) under accession numbers PRJEB14535 and PRJEB18474.
Statistical analyses
One-way ANOVA along with Tukey post-hoc tests were applied in JMP (version 12.1) to assess the effects of developmental temperature and microbial transplantation on the thermotolerance of flies. All amplicon data were analysed with R (version 3.3) and RStudio (version 1.0.44).40 Alpha diversity was assessed with the R package phyloseq,41 using the Chao1 and Shannon-Weaver diversity indices. The R package ampvis33 was used to perform Principal Component Analysis (PCA) on square-root transformed OTU abundances, to apply the parametric Wald test to test for significant OTU abundance differences between treatments after bacterial transplantation, and to visualize microbial community composition with heat maps. The envfit function from the R package vegan42 was used to investigate correlations between the microbial community and temperature. Only samples with at least 7000 and 5000 reads were considered for analysis in the thermal and gut transplantation assays, respectively. One sample from the 13°C group in the first experiment did not reach this criteria and was therefore omitted from the analysis. The microbiota were separated into two fractions according to the different host compartments, i.e. Wolbachia as intracellular and all others as gut residents in order to evaluate the effect of the transplantation, as described elsewhere.64
Results
Developmental temperature and thermal tolerance
Developmental temperature had a significant influence on flies' CTmax (F(2, 44) = 61.1, p <0.0001) and CTmin (F(2, 44) = 2620.5, p <0.0001). The highest heat tolerance (CTmax) was observed for flies developed at 31°C, and the lowest at 13°C flies with a range of 41.2°C to 38.9°C (Fig. 2A). Moreover, the flies developed at 13°C showed the highest cold tolerance, and flies developed at 31°C showed the lowest cold resistance (CTmin), ranging from 0.1°C to 8.2°C (Fig. 2B).
Figure 2.
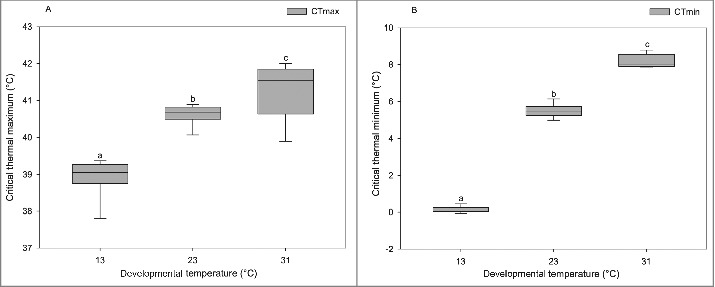
Boxplots representing CTmax (A) and CTmin (B) of male D. melanogaster developed at different temperatures, including 13°C (n = 15), 23°C (n = 15) and 31°C (n = 15). Dissimilar letters indicate significant differences between treatment groups. Boxplot graphs show median values, and whiskers extend up to 1.5 * interquartile range (IQR).
Microbial transplantation and thermal tolerance
The microbial transplantation had a significant influence on the heat tolerance of recipient flies (F(3, 95) = 8.5, p <0.0001; Fig. 3A), while no difference was observed between the treatment groups in the CTmin assay (F(3, 95) = 0.5, p = 0.6; Fig. 3B). According to pairwise post-hoc comparisons among all treatments, flies fed with the BS-13 suspension had the lowest heat resistance, whereas no significant differences in heat tolerance were observed between flies that consumed BS-23, BS-31, or FBS-suc suspensions (Fig. 3A, Table 1).
Figure 3.
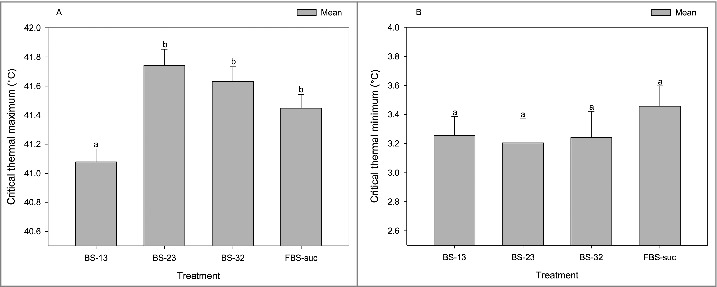
The barplots representing CTmax (A) and CTmin of male D. melanogaster 24 h post microbial transplantation. Each column shows the mean CTmax or CTmin of each treatment group, including BS-13 (flies that received the suspension containing the gut microbiota of flies developed at 13°C; n = 24), BS-23 (flies that received the suspension containing the gut microbiota of flies developed at 23°C; n = 24), BS-31 (flies that received the suspension containing the gut microbiota of flies developed at 31°C; n = 24), and FBS-suc (flies that received a sucrose suspension without bacteria; n = 24). Error bars indicate the standard error of the mean. Dissimilar letters indicate significant differences between treatment groups.
Table 1.
The p-values for pairwise comparisons between treatment groups. The thermal tolerance of flies 24 h post microbial transplantation was compared using Tukey post-hoc tests to identify significant differences between treatment groups. ND, not determined.
| Treatments | BS-13 | BS-23 | BS-31 | FBS-suc |
|---|---|---|---|---|
| BS-13 | p < 0.0001 | p < 0.001 | p < 0.01 | |
| BS-23 | p < 0.0001 | ND | ND | |
| BS-31 | p = 0.0 | p = 0.9 | ND | |
| FBS-suc | p = 0.0 | p = 0.2 | p = 0.6 |
Microbiome analysis of flies developed at three different temperatures
The microbial composition of flies exposed to a gradual increase (CTmax) or decrease (CTmin) of temperature (ramping assay) was not significantly different from the composition in flies sampled directly from the respective developmental temperatures (Goodness of fit, p = 1; Fig. 4). Therefore, the microbial analysis was performed on 8 – 9 replicates (5 – 6 replicates from the ramping assay and 3 replicates from the developmental temperature assay) to improve the statistical power of analyses. The developmental temperature had a considerable influence on the microbiota of D. melanogaster (p <0.001). Samples differed in microbial diversity with higher richness (alpha diversity index Chao1) detected in flies developed at 13°C (Fig. 4A). More than 100 operational taxonomic units (OTUs) were detected in multiple samples from 13°C, while the samples from 23°C and 31°C contained no more than 50 OTUs. In the samples with low microbial diversity, it is notable that one OTU was responsible for more than 50%, and in some cases 90% of the total read abundance (Table 2), which is supported by a low evenness (Shannon-Weaver index) score for these samples (between 0.5 and 1.5). Three bacterial genera shaped the majority of the sequences in both experiments: Acetobacter, Wolbachia, and Leuconostoc. In flies developed at 23°C and 31°C, Acetobacter constituted 60.2 to 98.6% of the total read abundance, respectively, while this genus accounted for 0.1 – 2.2% of the reads in flies developed at 13°C. The most dominant genus in 13°C developed flies was Wolbachia, which accounted for 80.6 to 89.2% of the total reads. However, the relative abundance of Wolbachia in flies developed at 23°C and 31°C averaged 7.2% (1 – 35.2%) of the total reads. The genus Leuconostoc showed a relatively low abundance in all treatment groups (0.3 – 5.6%).
Figure 4.
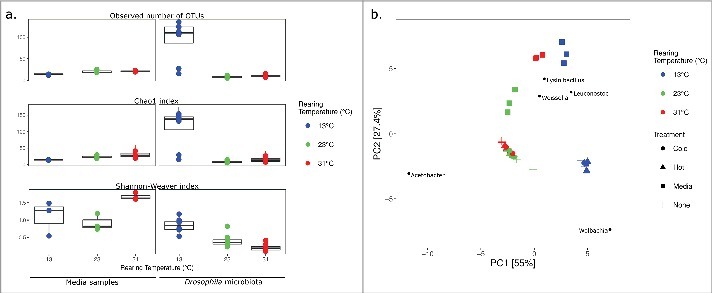
Bacterial within-community (alpha) diversity of the media samples and the microbiome in D. melanogaster developed at three different temperatures were investigated, using observed numbers of OTUs and diversity indices (a). The principal component analysis (PCA) shows the (beta) diversity of the microbiome in male D. melanogaster after rearing at 13°C, 25°C and 31°C (+), and related media samples (▪)(b). Microbiomes deriving from ramping experiments, in which flies reared at these temperatures were exposed to increasing (▴) or decreasing (•) temperatures. The 5 most loaded species are shown on the plot at their highest possible taxonomic classification.
Table 2.
Heatmap of the 12 most abundant OTUs detected in flies developed at three different temperatures (13, 23, and 31 ˚C), showing average read abundance ± standard deviation for each development temperature, per sample type. OTUs are listed with their highest possible taxonomic classification.
| Media samples |
Drosophila microbiota |
|||||
|---|---|---|---|---|---|---|
| 13 °C | 23 °C | 31 °C | 13 °C | 23 °C | 31 °C | |
| Proteobacteria; Acetobacter; | 0.5 ± 0.3 | 75.4 ± 12.2 | 14.0 ± 4.3 | 0.7 ± 0.7 | 87.0 ± 10.5 | 95.7 ± 3.0 |
| Proteobacteria; Wolbachia; | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 85.0 ± 2.9 | 11.4 ± 9.4 | 3.3 ± 2.6 |
| Firmicutes; Leuconostoc; | 60.9 ± 24.1 | 4.7 ± 1.5 | 0.6 ± 0.3 | 5.6 ± 2.5 | 1.4 ± 1.2 | 0.3 ± 0.3 |
| Firmicutes; Lysinibacillus; | 6.1 ± 6.5 | 6.1 ± 3.5 | 32.7 ± 3.5 | 0.7 ± 0.7 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Firmicutes; Weissella; | 1.0 ± 0.8 | 7.9 ± 12.3 | 24.4 ± 20.5 | 0.3 ± 0.2 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Firmicutes; Enterococcus; | 7.7 ± 8.2 | 0.0 ± 0.0 | 9.2 ± 15.7 | 0.1 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Firmicutes; Lactobacillus; | 9.4 ± 11.7 | 2.0 ± 1.2 | 3.8 ± 2.9 | 0.5 ± 0.2 | 0.0 ± 0.0 | 0.0 ± 0.1 |
| Firmicutes; Enterococcus; | 12.6 ± 16.7 | 0.0 ± 0.0 | 0.0 ± 0.0 | 1.3 ± 1.0 | 0.0 ± 0.0 | 0.1 ± 0.1 |
| Firmicutes; f__Lactobacillaceae_OTU_11; | 0.7 ± 0.4 | 1.7 ± 0.7 | 5.2 ± 0.9 | 0.3 ± 0.2 | 0.1 ± 0.1 | 0.4 ± 0.1 |
| Firmicutes; Lactococcus; | 0.0 ± 0.0 | 0.2 ± 0.4 | 3.1 ± 2.7 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Actinobacteria; f__Microbacteriaceae_OTU_8; | 0.1 ± 0.2 | 0.1 ± 0.1 | 2.9 ± 4.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Firmicutes; Paenibacillus; OTU_14; | 0.0 ± 0.0 | 0.2 ± 0.2 | 1.8 ± 1.6 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
The effect of developmental temperature on the microbial community of flies and medium samples was assessed using principal component analysis (PCA). Samples from flies developed at 23°C and 31°C formed a cluster separately from the 13°C treatment group. Moreover, the media samples clustered together by developmental temperature (Fig. 4B). Fitting of the temperature as an environmental factor presented a relationship along the horizontal axis (PC1), indicating that developmental temperature was likely responsible for 95% of the variance between the samples (p <0.001, R2 = 0.8). Among the 5 OTUs with the heaviest loadings in the model, Wolbachia clustered near the samples developed at low temperature (13°C) and Acetobacter grouped with the samples developed at higher temperatures (23 and 31°C). The genera Leuconostoc, Weissella, and Lysinibacillus appeared to be dominant in the media samples.
Microbiome analysis of flies after microbial transplantation
Microbial community analysis of flies subjected to the microbiome transplantation revealed a similar structure to that detected in the first experiment (Table 3), with the genera Wolbachia, Acetobacter, and Leuconostoc representing the dominant fraction of microorganisms. Wolbachia was the most dominant organism detected in all treatment groups, making up an average of 84.2 ± 9.8% of total reads. Treatment via microbiome transplants did not appear to cause a significant effect on overall microbial composition (p = 0.7), and we found no significant differences in OTUs detected in the different treatment groups (parametric Wald-tests). The microbiomes of flies after transplantation were significantly different from the control (23°C), which did not receive any transplantation (p <0.001). But the microbiomes of the three temperature microbiome transplants (13–23, 23–23, and 31–23) were not significantly different from each other (p = 0.414) as evaluated using a MANOVA (adonis) test. Separation of the gut microbiome and the somatic Wolbachia did not change these conclusions.
Table 3.
Heatmap of the 12 most abundant OTUs detected in bacterial recipient flies, showing average read abundance ± standard deviation for each development temperature, per sample type. OTUs are listed with their highest possible taxonomic classification.
| BS-13 | BS-23 | BS-31 | FBS-suc | |
|---|---|---|---|---|
| Proteobacteria; Wolbachia; | 83.6 ± 9 | 82.8 ± 13.1 | 82.5 ± 14.7 | 88.1 ± 5.9 |
| Proteobacteria; Acetobacter; | 6 ± 4.2 | 8.2 ± 10.1 | 7.8 ± 9.9 | 3.6 ± 3.7 |
| Firmicutes; Leuconostoc; | 7.4 ± 4 | 5.2 ± 2.2 | 5.2 ± 1.3 | 5 ± 2.1 |
| Proteobacteria; Acetobacter; | 1.4 ± 0.7 | 2.8 ± 2 | 3.6 ± 3.6 | 2 ± 1.2 |
| Firmicutes; f__Leuconostocaceae_OTU_5; | 0.7 ± 0.9 | 0.2 ± 0.2 | 0.4 ± 0.4 | 0.2 ± 0.2 |
| Firmicutes; Lactococcus; | 0.2 ± 0.3 | 0.4 ± 0.2 | 0.1 ± 0.1 | 0.2 ± 0.1 |
| Firmicutes; Lysinibacillus; | 0.1 ± 0.2 | 0.1 ± 0.1 | 0.2 ± 0.3 | 0.2 ± 0.4 |
| Actinobacteria; Corynebacterium; | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.2 | 0.3 ± 0.3 |
| Firmicutes; f__Lactobacillaceae_OTU_10; | 0.3 ± 0.2 | 0.2 ± 0.2 | 0 ± 0.1 | 0 ± 0 |
| Firmicutes; Paenibacillus; | 0 ± 0 | 0.1 ± 0.1 | 0 ± 0 | 0.1 ± 0.2 |
| Actinobacteria; Corynebacterium; | 0.1 ± 0.1 | 0 ± 0 | 0 ± 0 | 0.1 ± 0.2 |
| Firmicutes; Paenibacillus; | 0.1 ± 0.2 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
Discussion
The present study addressed the influence of resident microbial community and its structure on shaping the thermal phenotype of D. melanogaster. We assessed the bacterial population and thermal tolerance of flies developed at three different temperatures (13°C, 23°C and 31°C), and, in addition, we evaluated the possible effects of transferring microbiota between flies, using a novel protocol.
Developmental temperature, thermal tolerance, and microbial community composition
The results of the present study verified previous findings, showing strong associations between developmental temperature and heat and cold resistance.14,17,43,44 Thus, development at low temperature increased cold tolerance at the cost of lower heat tolerance and development at high temperature increased heat tolerance at the expense of reduced cold tolerance. Consistent with previous studies on D. melanogaster, the composition and diversity of microbiota of flies developed at different temperatures were simple and consisted mainly of three genera; Acetobacter, Wolbachia, and Leuconostoc.45–48 Although causality is not provided, the relatively higher abundance of Wolbachia and Acetobacter in flies developed at low (13°C) and high (23°C and 31°C) temperatures, respectively, supports the presence of a relationship between ambient temperature and host microbial composition. Moreover, this link is in agreement with studies showing high sensitivity of Wolbachia to high temperature.49,50 Thus, the temperature-specific abundance of bacteria may affect the thermotolerance of flies. Given this, we hypothesized that transplantation of microbiota from flies developed at extremely low and high (13°C and 31°C) temperatures to flies developed at intermediate (23 °C) temperatures would affect their temperature tolerance.
Microbial transplantation and thermal tolerance
Flies developed at 23°C and fed with the suspension containing the gut microbiota of flies developed at 13°C had significantly lower heat tolerance, compared to flies from the other treatment groups (Fig. 3A). Although further studies are needed to provide a causative link, this suggests an influence of the microbiome on shaping heat tolerance and that changing the microbiome alters upper thermal tolerance limits. Feeding flies with bacterial suspension from flies developed at different temperatures did not affect the cold tolerance of recipient flies (Fig. 3B). Assuming that we have been succesful in changing the microbiome of the flies through transplantation, this suggests a small impact of the microbiome on cold tolerance. The importance of developmental temperature on cold tolerance is well documented in previous studies,51–53 which indicates the complex and multifactorial nature of cold stress responses that must be considered collectively to obtain a better understanding of the mechanism involved in coping with low temperature stress.
The role of resident microbiota in D. melanogaster
The bacterial community of flies developed at different temperatures and flies that received bacteria through transplantation consisted mainly of the three genera Acetobacter, Wolbachia, and Leuconostoc. However, the abundance of these bacteria in flies developed at different temperatures was not comparable with flies that received bacterial suspension through the CAFÉ system. Given the considerable influence of dietary composition on the host microbial population, this discrepancy could have arisen from the nutritive environments applied in the experiments.55 To assess the effect of developmental temperature on the microbial composition, larvae were cultured on standard Drosophila medium that provides all essential macronutrients, including protein, carbohydrates, and fat. However, in the second experiment, the gastrointestinal tracts of flies were homogenized in 5% sucrose solution, providing a carbohydrate rich diet for recipient flies. A positive correlation between the concentration of dietary sucrose and relative abundance of Wolbachia has been observed in D. melanogaster.56 The interactive influence of sucrose on the relative abundance of Wolbachia supports the increased presence of this bacterium in recipient flies that are fed diets containing sucrose. The Wolbachia strains are mainly present in flies' reproductive organs of both sexes and numerous somatic tissues, including the brain.57 The relative abundance of these intracellular bacteria in the host varies according to the Wolbachia strain, temperature, and their interaction.50,60 For example, high temperature was found to reduce the occurrence of Wolbachia-associated effects on infected D. melanogaster, such as cytoplasmic incompatibility or parthenogenesis due to the low survival of Leptopilina heterotoma strain under high temperature.59 In the present study, the lower abundance of Wolbachia in flies developed at 23°C and 31°C (Table 2), which is seemingly opposite to previous observations,60 supports a key involvement of temperature in regulating the presence of this genus in flies and also suggests that Wolbachia have an impact on host temperature tolerance. A clear separation between the presence of Wolbachia and gut microbiota has been shown to increase the relative levels of Leuconostocaceae and Acetobacteraceae families in the gut flora of flies infected with Wolbachia.61 If we assume similar separate compartments of the Wolbachia and the gut residents Acetobacter and Leuconostoc, we find increased support that the transplantation of microbiota was succesful (Table 3). Thus, the abundance of Acetobacter and Leuconostoc was, respectively, high and low in flies developed at higher temperatures (23°C and 31°C) in experiment 1 (Table 2). The same trend was observed in experiment 2 (Table 3). Further studies are needed to investigate the significance of this trend.
Among the identified bacteria, Acetobacter is one of the most common genera associated with Drosophila.17,23,48,61 This genus affects a wide variety of physiological and biological aspects in Drosophila, including metabolism, immunity, gut homeostasis, growth rate, and developmental time.22,61,62 For example, the presence of Acetobacter pomorum promoted D. melanogaster larval development through modulating the insulin signaling pathway.62 The influence of Acetobacter on flies' development is in agreement with the results of the present study, in which the lowest abundance of Acetobacter was observed in flies raised at 13°C, which had a much longer developmental time (37 days), compared to flies developed at 23°C (9 days) and 31°C (10 days). In addition, the highest abundance of Acetobacter in flies developed at 31°C may be caused by providing more optimal growth conditions for acetic acid bacteria.63 The discrepancy between the microbial community of flies and those sampled in the medium used to develop the flies (Fig. 4b) indicates that, between dietary-associated microbiota and developmental temperature, the latter factor plays a pivotal role in shaping the flies' microbial structure.
The bacterial transplantation did not significantly change the microbial community of recipient flies, although a phenotypic effect on the heat tolerance of treated individuals was observed (Table 3; Fig. 3A). An explanation for this is the use of whole flies in the assessment of bacterial community that may mask alterations in the gut bacterial composition of recipient flies in response to microbial transplantation. Also, the feeding period might have been too short or the microbial concentrations of the suspensions too low, for there to be an effect. According to previous studies, less than 50% of Drosophila-associated microbiota are located in a fly intestine, and the rest is unevenly distributed among other internal tissues and on the surface of the fly.46,47 Thus, assessing the bacterial population of whole organisms may have reduced the ability to detect the small changes in the gut microbiota of flies after transplantation. Another possibility is that the concentration of transferred bacteria was not high enough in the transplants for the bacteria to establish themselves in the recipient flies, or that the microbiota from flies raised at 13°C had lower fitness at higher temperatures.
Conclusions
The developmental temperature strongly affected the thermal tolerance and microbial composition of male D. melanogaster. The flies developed at low temperature (13°C) were the most cold resistant and showed the highest abundance of Wolbachia, while the flies developed at high temperature were the most heat tolerant and had the highest abundance of Acetobacter. The observed temperature-induced alteration in the bacterial community is a novel observation in insects. In addition, feeding the newly eclosed flies with bacterial suspensions to reshape the gut bacterial community and transmit the microbiota between flies indicated that it is possible to change phenotypes such as the heat tolerance of recipient flies, although we were not able to link this directly to a change in the host bacterial composition. Based on the results obtained, we propose that insects might be used as model organisms for the study of how microbial compositions can affect fitness components.
Supplementary Material
Acknowledgements
We thank Helle Blendstrup, Susan Marie Hansen, Temesgen Alemneh, and Tibebu Alemu for their excellent technical assistance.
Funding
This work was supported by the Danish Research Council for Strategic Research via the Centre ‘EcoDesign’ (grant number 09–067230) to JLN and the Danish Natural Research Council through a sapere aude grant to TNK (grant number DFF-4002-00036).
Bibliography
- 1.Colinet H, Sinclair BJ, Vernon P, Renault D. Insects in fluctuating thermal environments. Annu Rev Entomol. 2015;60(1):123–140. doi: 10.1146/annurev-ento-010814-021017. [DOI] [PubMed] [Google Scholar]
- 2.Donnelly A, Caffarra A, Kelleher CT, O'Neill BF, Diskin E, Pletsers A, Proctor H, Stirnemann R, O'Halloran J, Penuelas J, et al.. Surviving in a warmer world: environmental and genetic responses. Climate Research. 2012;53:245–262. doi: 10.3354/cr01102. [DOI] [Google Scholar]
- 3.Helmuth B, Kingsolver JG, Carrington E. Biophysics, physiological ecology, and climate change: does mechanism matter? Annu Rev Physiol. 2005;67:177–201. doi: 10.1146/annurev.physiol.67.040403.105027. [DOI] [PubMed] [Google Scholar]
- 4.Pacifici M, Foden WB, Visconti P, Watson JEM, Butchart SHM, Kovacs KM, Scheffers BR, Hole DG, Martin TG, Akçakaya HR, et al.. Assessing species vulnerability to climate change. Nature Climate Change. 2015;5:215–224. doi: 10.1038/nclimate2448. [DOI] [Google Scholar]
- 5.Sørensen JG, Kristensen TN, Overgaard J. Evolutionary and ecological patterns of thermal acclimation capacity in Drosophila: is it important for keeping up with climate change? Current Opinion in Insect Science. 2016;17:98–104. doi: 10.1016/j.cois.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Parmesan C. Ecological and evolutionary responses to recent climate change. Annual Review of Ecology, Evolution, and Systematics. 2006;37:637–669. doi: 10.1146/annurev.ecolsys.37.091305.110100. [DOI] [Google Scholar]
- 7.Bakkenes M, Alkemade JRM, Ihle F, Leemans R, Latour JB. Assessing effects of forecasted climate change on the diversity and distribution of European higher plants for 2050. Global Change Biology. 2002;8:390–407. doi: 10.1046/j.1354-1013.2001.00467.x. [DOI] [Google Scholar]
- 8.Chen I-C, Hill JK, Ohlemüller R, Roy DB, Thomas CD. Rapid range shifts of species associated with high levels of climate warming. Science. 2011;333:1024–1026. doi: 10.1126/science.1206432. [DOI] [PubMed] [Google Scholar]
- 9.Thomas CD, Lennon JJ. Birds extend their ranges northwards. Nature. 1999;399:213–213. doi: 10.1038/20335. [DOI] [Google Scholar]
- 10.Sørensen JG, Schou MF, Kristensen TN, Loeschcke V. Thermal fluctuations affect the transcriptome through mechanisms independent of average temperature. Sci Rep. 2016;6:30975. doi: 10.1038/srep30975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kristensen TN, Kjeldal H, Schou MF, Nielsen JL. Proteomic data reveal a physiological basis for costs and benefits associated with thermal acclimation. J Exp Biol. 2016;219:969–976. doi: 10.1242/jeb.132696. [DOI] [PubMed] [Google Scholar]
- 12.Colinet H, Renault D, Javal M, Berková P, Šimek P, Koštál V. Uncovering the benefits of fluctuating thermal regimes on cold tolerance of Drosophila flies by combined metabolomic and lipidomic approach. Biochimica Et Biophysica Acta. 2016;1861:1736–1745. [DOI] [PubMed] [Google Scholar]
- 13.Hariharan R, Hoffman JM, Thomas AS, Soltow QA, Jones DP, Promislow DEL. Invariance and plasticity in the Drosophila melanogaster metabolomic network in response to temperature. BMC Systems Biology. 2014;8:139. doi: 10.1186/s12918-014-0139-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schou MF, Kristensen TN, Pedersen A, Karlsson GB, Loeschcke V, Malmendal A. Metabolic and functional characterization of effects of developmental temperature in Drosophila melanogaster. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. Am J Physiol – Regul, Integr Comp Physiol. 2017;312: R211–R222.15. doi: 10.1152/ajpregu.00268.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bahrndorff S, Loeschcke V, Pertoldi C, Beier C, Holmstrup M. The rapid cold hardening response of Collembola is influenced by thermal variability of the habitat. Functional Ecology. 2009;23:340–347. doi: 10.1111/j.1365-2435.2008.01503.x. [DOI] [Google Scholar]
- 16.Osei-Poku J, Mbogo CM, Palmer WJ, Jiggins FM. Deep sequencing reveals extensive variation in the gut microbiota of wild mosquitoes from Kenya. Mol Ecol. 2012;21:5138–5150. doi: 10.1111/j.1365-294X.2012.05759.x. [DOI] [PubMed] [Google Scholar]
- 17.Wong AC-N, Chaston JM, Douglas AE. The inconstant gut microbiota of Drosophila species revealed by 16S rRNA gene analysis. ISME J. 2013;7:1922–32. doi: 10.1038/ismej.2013.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bahrndorff S, de Jonge N, Skovgård H, Nielsen JL. Bacterial communities associated with houseflies (Musca domestica L.) sampled within and between farms. PLoS ONE. 2016;12(1):e0169753. doi: 10.1371/journal.pone.0169753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bahrndorff S, Alemu T, Alemneh T, Nielsen JL. The microbiome of animals: implications for conservation biology. Int J Genomics. 2016;2016:1–7. doi: 10.1155/2016/5304028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krishnan M, Bharathiraja C, Pandiarajan J, Prasanna VA, Rajendhran J, Gunasekaran P. Insect gut microbiome – An unexploited reserve for biotechnological application. Asian Pacific Journal of Tropical Biomedicine. 2014;4:S16–S21. doi: 10.12980/APJTB.4.2014C95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaston JM, Newell PD, Douglas AE. Metagenome-wide association of microbial determinants of host phenotype in Drosophila melanogaster. mBio. 2014;5:e01631–14. doi: 10.1128/mBio.01631-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newell PD, Douglas AE. Interspecies interactions determine the impact of the gut microbiota on nutrient allocation in Drosophila melanogaster. Applied and Environmental Microbiology. 2014;80:788–796. doi: 10.1128/AEM.02742-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ridley EV, Wong ACN, Westmiller S, Douglas AE. Impact of the resident microbiota on the nutritional phenotype of Drosophila melanogaster. PLoS ONE. 2012;7:e36765. doi: 10.1371/journal.pone.0036765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Overgaard J, Malmendal A, Sørensen JG, Bundy JG, Loeschcke V, Nielsen NC, Holmstrup M. Metabolomic profiling of rapid cold hardening and cold shock in Drosophila melanogaster. J Insect Physiol. 2007;53:1218–32. doi: 10.1016/j.jinsphys.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 25.Schwasinger-Schmidt TE, Kachman SD, Harshman LG. Evolution of starvation resistance in Drosophila melanogaster: measurement of direct and correlated responses to artificial selection. J Evol Biol. 2012;25:378–87. doi: 10.1111/j.1420-9101.2011.02428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunbar HE, Wilson ACC, Ferguson NR, Moran NA. Aphid thermal tolerance is governed by a point mutation in bacterial symbionts. PLoS Biol. 2007;5:e96. doi: 10.1371/journal.pbio.0050096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chevalier C, Stojanović O, Colin DJ, Suarez-Zamorano N, Tarallo V, Veyrat-Durebex C, Rigo D, Fabbiano S, Stevanović A, Hagemann S, et al.. Gut microbiota orchestrates energy homeostasis during Cold. Cell. 2015;163:1360–1374. doi: 10.1016/j.cell.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Wong R, Piper MDW, Wertheim B, Partridge L. Quantification of food intake in Drosophila. PLoS ONE. 2009;4:e6063. doi: 10.1371/journal.pone.0006063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schou MF, Kristensen TN, Kellermann V, Schlötterer C, Loeschcke V. A Drosophila laboratory evolution experiment points to low evolutionary potential under increased temperatures likely to be experienced in the future. Journal of Evolutionary Biology. 2014;27:1859–1868. doi: 10.1111/jeb.12436. [DOI] [PubMed] [Google Scholar]
- 30.Overgaard J, Kristensen TN, Sørensen JG. Validity of thermal ramping assays used to assess thermal tolerance in arthropods. PLoS ONE. 2012;7:1–7. doi: 10.1371/journal.pone.0032758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tauc HM, Tasdogan A, Pandur P. Isolating intestinal stem cells from adult Drosophila midguts by FACS to study stem cell behavior during aging. J Vis Exp: JoVE. 2014;94:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deshpande SA, Carvalho GB, Amador A, Phillips AM, Hoxha S, Lizotte KJ, Ja WW. Quantifying Drosophila food intake: comparative analysis of current methodology. Nature Methods. 2014;11(5):535–540. doi: 10.1038/nmeth.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Albertsen M, Karst SM, Ziegler AS, Kirkegaard RH, Nielsen PH. Back to basics:the influence of DNA extraction and primer choice on phylogenetic analysis of activated sludge communities. PLoS ONE. 2015;10:e0132783–e0132783. doi: 10.1371/journal.pone.0132783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics (Oxford, England). 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Magoč T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics (Oxford, England). 2011;27:2957–2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013;10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- 37.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Applied and Environmental Microbiology. 2007;73:5261–7. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, et al.. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–6. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.R Core Team R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. http://www.R-project.org/. [Google Scholar]
- 41.McMurdie PJ, Holmes S. Phyloseq: a bioconductor package for handling and analysis of high-throughput phylogenetic sequence data. Pacific Symposium on Biocomputing. 2012;235–246. [PMC free article] [PubMed] [Google Scholar]
- 42.Oksanen J, Kindt R, Legendre P, BO’ Hara, Henry M, Stevens H. Vegan Community Ecology Package version. 2016. http://cran. r-project.org/ [Google Scholar]
- 43.Kristensen TN, Hoffmann AA, Overgaard J, Sørensen JG, Hallas R, Loeschcke V. Costs and benefits of cold acclimation in field-released Drosophila. Proceedings of the National Academy of Sciences. 2008;105(1):216–221. doi: 10.1073/pnas.0708074105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schou MF, Loeschcke V, Kristensen TN. Strong Costs and Benefits of Winter Acclimatization in Drosophila melanogaster. PLoS ONE. 2015;10(6):e0130307. doi: 10.1371/journal.pone.0130307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buchon N, Broderick NA, Lemaitre B. Gut homeostasis in a microbial world: insights from Drosophila melanogaster. Nature Reviews Microbiology. 2013;11:615–626. doi: 10.1038/nrmicro3074. [DOI] [PubMed] [Google Scholar]
- 46.Chandler JA, Lang JM, Bhatnagar S, Eisen JA, Kopp A. Bacterial communities of diverse Drosophila species: Ecological Context of a Host–Microbe Model System. PLoS Genetics. 2011;7:e1002272. doi: 10.1371/journal.pgen.1002272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Staubach F, Baines JF, Künzel S, Bik EM, Petrov DA. Host species and environmental effects on bacterial communities associated with Drosophila in the laboratory and in the natural environment. PLoS ONE. 2013;8:e70749. doi: 10.1371/journal.pone.0070749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wong CNA, Ng P, Douglas AE. Low-diversity bacterial community in the gut of the fruitfly Drosophila melanogaster. Environ Microbiol. 2011;13:1889–1900. doi: 10.1111/j.1462-2920.2011.02511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomas MB, Blanford S. Thermal biology in insect-parasite interactions. Trends in Ecology & Evolution. 2003;18:344–350. doi: 10.1016/S0169-5347(03)00069-7. [DOI] [Google Scholar]
- 50.Wiwatanaratanabutr I, Kittayapong P. Effects of crowding and temperature on Wolbachia infection density among life cycle stages of Aedes albopictus. Journal of Invertebrate Pathology. 2009;102:220–4. doi: 10.1016/j.jip.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 51.Ayrinhac A, Gibert P, Legout H, Moreteau B, Vergilino R. Cold adaptation in geographical populations of Drosophila melanogaster: phenotypic plasticity is more important than genetic variability. Functional Ecology 2004;18:700–706. doi: 10.1111/j.0269-8463.2004.00904.x. [DOI] [Google Scholar]
- 52.Gibert P, Huey RB. Chill‐coma temperature in Drosophila: effects of developmental temperature, latitude, and phylogeny. Physiological and Biochemical Zoology: Ecological and Evolutionary Approaches. 2001;74:429–434. doi: 10.1086/320429. [DOI] [PubMed] [Google Scholar]
- 53.Sisodia S, Singh BN. Influence of developmental temperature on cold shock and chill coma recovery in Drosophila ananassae: Acclimation and latitudinal variations among Indian populations. Journal of Thermal Biology. 2010;35:117–124. doi: 10.1016/j.jtherbio.2010.01.001. [DOI] [Google Scholar]
- 54.Rako L, Hoffmann AA. Complexity of the cold acclimation response in Drosophila melanogaster. J Insect Physiol. 2006;52:94–104. doi: 10.1016/j.jinsphys.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 55.Colman DR, Toolson EC, Takacs-Vesbach CD. Do diet and taxonomy influence insect gut bacterial communities? Molecular Ecology. 2012;21:5124–5137. doi: 10.1111/j.1365-294X.2012.05752.x. [DOI] [PubMed] [Google Scholar]
- 56.Ponton F, Wilson K, Holmes A, Raubenheimer D, Robinson KL, Simpson SJ. Macronutrients mediate the functional relationship between Drosophila and Wolbachia. Proceedings of the Royal Society of London B: Biological Sciences. 2015;282:20142029. doi: 10.1098/rspb.2014.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clark ME, Anderson CL, Cande J, Karr TL. Widespread prevalence of Wolbachia in laboratory stocks and the implications for Drosophila research. Genetics. 2005;170:1667–1675. doi: 10.1534/genetics.104.038901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoffmann ARYA Acclimation for desiccation resistance in Drosophila melanogaster and the association between acclimation responses and genetic variation. Journal of Insect Physiology. 1990;36:885–891. doi: 10.1016/0022-1910(90)90176-G. [DOI] [Google Scholar]
- 59.Mouton L, Henri H, Bouletreau M, Vavre F. Effect of temperature on Wolbachia density and impact on cytoplasmic incompatibility. Parasitology. 2006;132:49–56. doi: 10.1017/S0031182005008723. [DOI] [PubMed] [Google Scholar]
- 60.Rohrscheib CE, Frentiu FD, Horn E, Ritchie FK, van Swinderen B, Weible II MW, O'Neill SL, Brownlie JC. Intensity of mutualism breakdown is determined by temperature not amplification of Wolbachia genes. PLoS Pathogens. 2016;12:e1005888. doi: 10.1371/journal.ppat.1005888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Crotti E, Rizzi A, Chouaia B, Ricci I, Favia G, Alma A, Sacchi L, Bourtzis K, Mandrioli M, Cherif A, et al.. Acetic acid bacteria, newly emerging symbionts of insects. Applied and Environmental Microbiology. 2010;76:6963–6970. doi: 10.1128/AEM.01336-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shin SC, Kim S-HS-HS-H, You H, Kim B, Kim AC, Lee K-AK-A, Yoon J-HJ-H, Ryu J-H, Lee W-JW-J. Drosophila microbiome modulates host developmental and metabolic homeostasis via insulin signaling. Science. 2011;334:670–4. doi: 10.1126/science.1212782. [DOI] [PubMed] [Google Scholar]
- 63.Sharafi S, Rasooli I, Beheshti-Maal K. Isolation, characterization and optimization of indigenous acetic acid bacteria and evaluation of their preservation methods. Iran J Microbiol. 2010;2:38–45. [PMC free article] [PubMed] [Google Scholar]
- 64.Ye YH, Seleznev A, Flores HA, Woolfit M, McGraw EA. Gut microbiota in Drosophila melanogaster interacts with Wolbachia but does not contribute to Wolbachia-mediated antiviral protection. J Invertebr Pathol. 2017; 143:18–25. doi: 10.1016/j.jip.2016.11.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


