ABSTRACT
Dendritic cell-cytokine-induced killer (DC-CIK) cell therapy has been experimentally implemented for enhancing anti-tumoral immunity in patients with hepatocellular carcinoma (HCC) undergoing postoperative transcatheter arterial chemoembolization (POTACE). We performed a retrospective study to evaluate the clinical efficacies of DC-CIK cell therapy and its correlations with several immune factors of the primary tumors. The overall survival time of HCC patients with HBV infection in the study group (POTACE plus DC-CIK cell therapy) was significantly longer than that of the control group (POTACE alone). The expression level of PD-L1 but not the tumor-infiltrated CD8 and CD4 T cells in the tumor tissues showed significant negative correlations with relapse-free survival (RFS) and overall survival (OS), which was also an independent prognostic factor for the five-years' suvival of patients with HCC receiving POTACE treatment. Furthermore, our study validated that PD-L1 expression was significantly inversely correlated with the survival time of HCC patients receiving POTACE plus DC-CIK cell therapy treatment. More importantly, DC-CIK cell therapy provided the best clinical benefits to HCC patients with the low PD-L1 expression receiving POTACE, which indicate that PD-L1 expression level can serve as a pivotal predictor for the therapeutic efficacy of DC-CIK cell therapy for HCC patients receiving POTACE treatment.
KEYWORDS: Hepatocellular carcinoma, Cytokine-induced killer cell immunotherapy, PD-L1, Tissue microarray, Immunohistochemistry, Transcatheter arterial chemoembolization
Introduction
Hepatocellular carcinoma (HCC) is the 3rd most common malignancy and has a very high cancer-related mortality rate worldwide.1 The incidence of HCC is strongly associated with geographic distributions, demographic characteristics, and gender. The major risk factors for HCC include hepatitis B or C infection and alcohol-related liver damage. There are several potential therapeutic strategies for the treatment of HCC, including liver resection or transplantation, radiofrequency ablation, transcatheter arterial chemoembolization (TACE), systemic chemotherapy and targeted molecular therapy. The choice of treatment usually depends on the tumor stage, accessible resources in the clinical center and the professional level of the clinician.2-4
Surgical resection is considered to be a curative therapy for very early stage HCCs, but this treatment is associated with a 5-year survival rate of merely 75% and a substantial recurrence rate of 50%.5 Due to the lack of effective systemic administration, localized treatments are still needed to reinforce the effect of or treating a recurrent nidus after liver resection. TACE, which involves injections of anticancer drugs and iodized oil into the hepatic artery, is an alternative adjuvant treatment for postoperative patients to eliminate residual or recurrent cancer cells. Nevertheless, tumor progression and metastasis are still frequent in many patients undergoing TACE.6-8 Therefore, a novel therapy with higher efficacy, potency and tolerability is required for patients with HCC subsequent to TACE treatment.
HCC has been recognized as a classical chronic inflammation-induced cancer, and the decreased anti-tumoral immunity is essential for tumor recurrence and metastasis.9 Various immune-based therapeutic strategies aiming to enhance the anti-tumoral response have been investigated in many clinical trials of HCC.10 DC-CIK cell therapy is an adoptive immunotherapy whereby anti-tumoral immune effectors mainly comprising of the CD3+CD8+ cells and CD3+CD56+ cell populations are infused into patients. DC-CIK cell therapy has been shown to be advantageous over standard cares for various malignances, including HCC, owing to an enhancement of anti-tumoral immunity, freedom from an immunological rejection response and reduced side effects.11-13 A few recent studies have shown that DC-CIK cell therapy improves the outcome and the quality of life of HCC patients undergoing TACE treatment.14 However, the efficacies of DC-CIK cell therapy for HCC patients receiving POTACE remains to be defined. Meanwhile, the correlations between the intrinsic immune status in patients’ primary tumor tissues and the outcomes of these treatments are still unknown.
In this study, we retrospectively analyzed the clinical efficacy of DC-CIK cell therapy on HCC patients receiving POTACE and explored correlations between these treatments and the intrinsic immune status of tumor tissues, including tumor-infiltrating T cells and the PD-L1 expression level.
Results
Basal characteristics of the patients
Based on the inclusion and exclusion criteria, a total of 52 patients were recruited for investigation, and the patients were divided into two groups: the study group, receiving the combination treatment of POTACE and DC-CIK cell therapy (n = 20), and the control group, receiving POTACE alone (n = 32). As shown in Table 1, the general characteristics of patients in two groups, such as age, gender, serum AFP levels, tumor size at first diagnosis, liver functional assessment with the Child-Pugh score, tumor tissue pathological stage and serum HBsAg levels, were uniformly distributed. Through a balanced detection test (χ2 test), we confirmed that there were no significant differences in the basal characteristics of two groups (p > 0.05) (Table 1), that suggested these populations were suitable to be comparisons.
Table 1.
Baseline characteristics of patients with HCC.
| Study group | Control group | ||
|---|---|---|---|
| Characteristic | (n = 20) | (n = 32) | P |
| Age/year | |||
| ≤60 | 12 | 22 | 0.519 |
| >60 | 8 | 10 | |
| Gender | |||
| Male | 17 | 27 | 0.952 |
| Female | 3 | 5 | |
| AFP | |||
| >400 | 5 | 7 | 0.795 |
| ≤400 | 15 | 25 | |
| Tuomr size | |||
| <5cm | 10 | 14 | 0.777 |
| ≥5cm | 10 | 18 | |
| Child-Pugh Stage | |||
| A | 18 | 30 | 0.622 |
| B | 2 | 2 | |
| TNM stage | |||
| I | 4 | 5 | |
| II | 6 | 17 | 0.227 |
| III | 9 | 10 | |
| IV | 1 | 0 | |
| HBsAg(±) | |||
| + | 16 | 27 | 0.685 |
| − | 4 | 5 |
P values are from Log-rank test. CI: confidence interval.
The clinical efficacies of DC-CIK cell therapy for patients treated with POTACE
We firstly investigated the effects of DC-CIK treatment on the overall survival (OS) of HCC patients receiving POTACE. As shown in Fig. 1A, the Kaplan-Meier curve indicated that the median survival time in the study group (n = 20) and control group (n = 32) was 36.8 ± 5.1 and 28.7 ± 3.8 months, respectively. The OS of the study group was slightly longer than that of the control group but there was no significant difference between two groups. It's well known that HBV infection is the main risk factor that plays the critical roles in the tumorigenesis, development, and prognosis of HCC patients. Besides of the carcinogenesis, HBV infection also affects the immunity of patients. We are thus interested in inputting the parameter of HBV infection to access the efficacies of DC-CIK cell therapy in this study. As shown in Fig. 1B, where the non-HBV infection patients of the two groups were excluded, the OS of the study group (n = 15) was significantly prolonged as compared with that of the control group (n = 27) (log rank, p = 0.044) (Fig. 1B); in this case, the median survival times of the study and control groups were 38.7 ± 5.5 and 26.7 ± 3.9 months, respectively. This data suggest that DC-CIK cell therapy prolongs the OS of HBV-infected HCC patients receiving postoperative TACE treatment, which should be attributable to the specific immune response of the DC-CIK cells to HBV-infected HCC cells.
Figure 1.
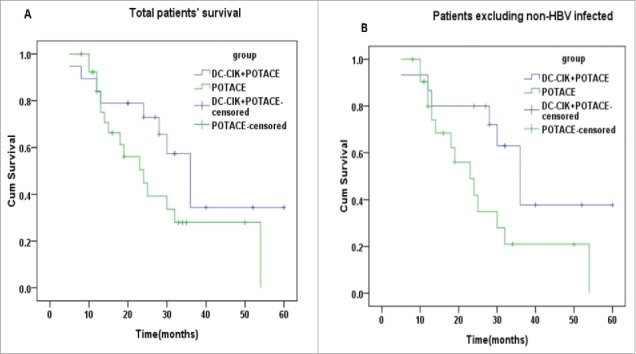
The prognosis of DC-CIK cell therapy for patients treated by POTACE. (A) The overall survival curves of total patients with HCC undergoing POTACE alone and POTACE plus DC-CIK cell therapy, respectively (n = 46, log rank, p = 0.118). (B) The overall survival curves of HCC patients without of HBV-infection undergoing POTACE alone (n = 22) and POTACE plus DC-CIK cell therapy (n = 15), respectively (log rank, p = 0.044). The Kaplan-Meier curves were used to analyze the OS of patients, log-rank test was used to check the significant difference between two groups; P < 0.05 signified statistical significance; blue and green line represents the low and high expression of PD-L1, respectively. Plus sign (+) on the line represents censored data.
Serum Alpha-fetoprotein (AFP) level is a well-defined marker for diagnosing primary cancer and the tumor recurrence of HCC.15 Therefore, we also determined the effects of DC-CIK cell therapy on the serum AFP levels of patients with HCC. As shown in Table 2, there were not significantly different of the AFP levels between the study group (n = 20) and the control group prior to the DC-CIK treatment (n = 32, t-test, p = 0.570). After DC-CIK cell therapy was performed, the AFP level of the study group (n = 20) was obviously reduced as compared with that of the control group (n = 32, t-test, p = 0.031), which indicate that DC-CIK cell therapy should decrease the relapse of tumor in HCC patients receiving POTACE.
Table 2.
Variations of AFP concentrations before and after treatment in two groups.
| Group | Before treatment AFP (ng/ml) | After treatment AFP(ng/ml) |
|---|---|---|
| DC-CIK+POTACE | 236.1 ± 389 | 50.3 ± 105.7 |
| POTACE | 332.4 ± 668.5 | 603 ± 1161.7 |
Correlations between the PD-L1 expression level and the OS and RFS times of HCC patients and the effect of PD-L1 expression on the therapeutic efficacy of DC-CIK cell therapy
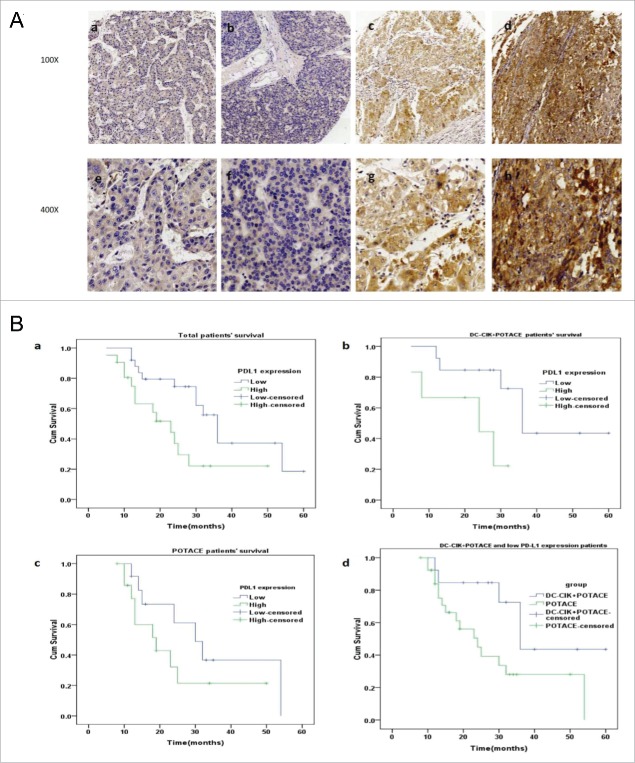
Programmed cell death-ligand 1 (PD-L1), a member of the B7 family of cell surface ligands, is expressed in both tumor cells and peritumoral stromal cells in the tumor microenvironment. PD-L1 plays a critical role in regulating the immunological escape of cancer cells by inhibiting the anti-tumor immunity of the T cells.16 To evaluate the intrinsic immunity of tumor cells, we assessed the PD-L1 expression level by immunohistochemistry in the TMAs (Tissue microarrays) of human HCC tissues. Representative photographs of the PD-L1 staining intensity scores are presented in Fig. 2A, whereby the score of each slide was independently assessed by two experienced pathologists. The survival analysis of all patients (Fig. 2Ba) indicated that patients with low PD-L1 expression levels had longer OS times than patients with high PD-L1 expression levels (n = 46, log rank, p = 0.015); the median survival times of the HCC patients with low and high PD-L1 expression were 37.5 ± 4.0 and 24.4 ± 3.7 months, respectively. This result suggested that the expression level of PD-L1 was significantly negatively correlated with OS for HCC patients treated with postoperative TACE. In the study group, patients with the low PD-L1 expression levels (n = 13) had longer OS times than patients with high PD-L1 expression levels (n = 6, log rank, p = 0.033); the median survival times of the patients with low and high PD-L1 expression levels were 42.1 ± 5.7 and 20.8 ± 4.3 months, respectively (Fig. 2Bb). However, in the control group, the OS time of the patients was not significantly correlated with the PD-L1 expression level (n = 27, log rank, p = 0.192) (Fig. 2Bc). Moreover, the OS of patients with the low PD-L1 expression level in the study group was significantly prolonged relative to the control group regardless of the PD-L1 expression level (n = 33, log rank, p = 0.033) (Fig. 2Bd). These data suggest that DC-CIK cell therapy is an effective adjuvant treatment for patients with low expression levels of PD-L1 who receive POTACE.
Figure 2.
PD-L1 expression in tumor tissues and its correlations with the prognosis of HCC patients undergoing POTACE and POTACE plus DC-CIK cell therapy, respectively. (A) Representative images of the immunohistochemical staining of PD-L1 expression in tumor tissues that were related to the physiological scores of IRS at 0,2,6 and 9. (a,e) represented IRS at 0. (b,f) represented IRS at 2. (c,g) represented IRS at 6. (d,h) represented IRS at9. (a-d,100x magnification; e-h, 400x magnification). (Ba) In total cohort, the Kaplan-Meier curves of patients with the high and low PD-L1 expression level in tumor tissues, respectively. As shown in this panel, the HCC patients with the low expression of PD-L1(blue line, IRS≦3, n = 25) have significant long OS as compared with that of patients with high expression of PD-L1(blue line, IRS>3, n = 21) (log rank, p = 0.015). (Bb) In DC-CIK cell therapy plus POTACE treatment group, the Kaplan-Meier curves of patients with the high and low PD-L1 expression level, respectively. The low expression of PD-L1(blue line, IRS≦3, n = 13) have significant long OS as compared with that of patients with high expression of PD-L1(blue line, IRS>3, n = 6) (log rank, p = 0.033) (Bc) In POTACE alone group, the Kaplan-Meier curves of patients with the high and low PD-L1 expression level, respectively. There was no significant difference in the overall survival between two groups (log rank, p = 0.192). (Bd) the Kaplan-Meier curves of the patients with low expression of PD-L1 undergoing POTACE+DC-CIK cell therapy and the patients undergoing POTACE alone. The overall survival duration in patients with underexpressed PD-L1 in study group(n = 6) was much longer than those in control group (n = 27, log rank, p = 0.033). The blue line represents low expression group and the green line represents high expression group. P < 0.05 signified statistical significance; blue and green line represents the low and high expression of PD-L1, respectively. Plus sign (+) on the line represents censored data.
In addition, we also studied the relationship between patients’ relapse-free survival (RFS) with PD-L1 expression levels via Spearman bivariate correlation analysis. As shown in Table 3, the median RFS of patients with the low and high PD-L1 expression levels were 11.3 ± 2.5 and 6.8 ± 2.1 months, respectively. Correlation is significant at the 0.05 level (n = 46, p = 0.038, 2-tailed), which suggested that the RFS of the total patients was negatively correlated with PD-L1 expression level. Since AFP level is used to be as a parameter to access the relapse of HCC, we also determined the corrections between PD-L1 expression with AFP level in these patients by spearman's rho analysis. As shown in Table 4, the AFP level of patients with HCC receiving POTACE was positively correlated with their PD-L1 expression (n = 46, p = 0.045, 2-tailed). This data suggests that the high expression of PD-L1 is associated with the poor prognosis. We also determined the corrections between the expression level of PD-L1 and the other clinicopathological features of patients. As shown in Table S1, the expression level of PD-L1 was not significantly associated with that age, gender, original AFP level, TNM stage, Child-pugh stage, tumor diameter, tumor multiplicity, treatment method and HBsAg.
Table 3.
The relationship between patients' RFS with PD-L1 expression levels.
| |
|
|
PD-L1 expression |
Rslapse-free survival |
|---|---|---|---|---|
| Spearman's rho | PD-L1expression | Correlation Coefficient | 1.000 | −.306* |
| Sig. (2-tailed) | . | .038 | ||
| N | 46 | 46 | ||
| Rslapse-free surviva | Correlation Coefficient | −.306* | 1.000 | |
| Sig. (2-tailed) | .038 | . | ||
| N | 46 | 46 |
Correlation is significant at the 0.05 level (2-tailed).
Table 4.
The relationship between PD-L1 expression and AFP level.
| |
|
|
PD-L1 expression |
AFP level |
|---|---|---|---|---|
| Spearman's rho | PD-L1 expression | Correlation Coefficient | 1.000 | .297* |
| Sig. (2-tailed) | . | .045 | ||
| N | 46 | 46 | ||
| AFP level | Correlation Coefficient | .297* | 1.000 | |
| Sig. (2-tailed) | .045 | . | ||
| N | 46 | 46 |
Correlation is significant at the 0.05 level (2-tailed).
Associations between tumor-infiltrating T cells and PD-L1 expression in tumor tissue and the effects of tumor-infiltrating T cells on OS and DC-CIK cell therapeutic benefits
To explore the immunological statuses of the patient tumor tissues, we examined tumor-infiltrated CD8 and CD4 T cells in the TMAs of the HCC tissues. Representative photos of CD8, CD4 immunohistochemistry staining in TMAs that were corrected with the indicated intensity scores were shown in Figure S1A&B, respectively. As shown in Figure S2, there was no significant difference between the infiltrating intensities of both CD8+ T cells (Figure S2a) (n = 46, log-rank, p = 0. 257) or CD4+ T cells (Figure S2b) (n = 46, log-rank, p = 0.162) and the OS times of all patients. We also didn't observe the significant correlations between the intensities of tumor-infiltrating T cells with the OS times of the DC-CIK cell-treated patients (data not shown). These results indicate that the infiltrating T cells in the primary tumor tissues exhibit an immunotolerant status for cancer development of HCC patients. Thus, activation of anti-tumoral immunity should improve the outcomes of patients with HCC.
Intriguingly, we showed that infiltrating intensity of CD8+ T cells was positively correlated with the expression of PD-L1 in TAMs of patients with HCC, whose correlation coefficient was 0.346 with statistical significance at the p < 0.05 level (n = 46, 2-tailed, p = 0.019, Table 5).
Table 5.
Correlations between PD-L1 expression level and infiltrating intensity of CD8+ CTLs.
| |
|
|
PD-L1 |
CD8 |
|---|---|---|---|---|
| Spearman's rho | PD-L1 | Correlation Coefficient | 1.000 | .346* |
| Sig. (2-tailed) | . | .019 | ||
| N | 46 | 46 | ||
| CD8 | Correlation Coefficient | .346* | 1.000 | |
| Sig. (2-tailed) | .019 | . | ||
| N | 46 | 46 |
Correlation is significant at the 0.05 level (2-tailed).
Prognostic factors for HCC patients receiving postoperative TACE treatment
To explore the independent prognostic factors for the survival of HCC patients receiving POTACE, we performed a univariate Cox regression analysis of PD-L1 expression, age, gender, tumor size and TNM stage. As shown in Table 6, in addition to tumor size and TNM stage, two known independent prognostic factors, PD-L1 expression was identified as an independent prognostic marker for HCC patients receiving the POTACE (n = 46, hazard ratio, 2.631; 95% CI, 1.155-5.990; P = 0.021). To determine the strongest impact factor affecting the 5-year survival time of patients with HCC treated with POTACE, we performed a multivariate Cox regression analysis of tumor size, TNM stage, PD-L1 expression level, gender and age. As shown in Table 7, the PD-L1 expression level was the only prognostic factor affecting the 5-year overall times of the HCC patients receiving POTACE treatment (n = 46, hazard ratio, 2.790; 95% CI, 1.097-7.094; P = 0.015). These results suggest that PD-L1 expression is the most valuable factor as compared with the known prognostic factors, including tumor size and TNM stages, for predicting the prognosis of HCC patients treated with POTACE.
Table 6.
Univariate Cox proportional regression analysis on 5-year overall.
| Variable | Hazard ratio | 95% CI | P* |
|---|---|---|---|
| Age/year | |||
| ≤60 | 1.000 | 0.945-1.029 | 0.520 |
| >60 | 0.986 | ||
| Gender | |||
| Female | 1.000 | 0.426-3.894 | 0.660 |
| Male | 1.281 | ||
| PD-L1 expression | |||
| Low | 1.000 | 1.155-5.990 | 0.021 |
| High | 2.631 | ||
| Tuomr size | |||
| <5cm | 1.000 | 1.436-8.908 | 0.006 |
| ≥5cm | 3.577 | ||
| TNM stage | |||
| I-II | 1.000 | 1.147-6.381 | 0.023 |
| III-IV | 2.705 | ||
| CD4 | |||
| Low | 1.000 | 0.751-14.133 | 0.115 |
| High | 3.259 | ||
| CD8 | |||
| Low | 1.000 | 0.684-3.264 | 0.363 |
| High | 1.455 | ||
| β-catenin | |||
| Low | 1.000 | 0.310-2.300 | 0.740 |
| High | 0.844 |
P values are from Log-rank test. CI: confidence interval.
Table 7.
Multivariate Cox regression analysis on 5-year overall.
| Variable* | Hazard ratio | 95% CI | P* |
|---|---|---|---|
| Tumor Size | 2.522 | 0.761–8.352 | 0.130 |
| TNM Stage | 2.006 | 0.616–6.528 | 0.248 |
| PD-L1 Expression | 2.900 | 1.231–6.833 | 0.015 |
| Gender | 1.330 | 0.401–4.414 | 0.641 |
| Age | 0.998 | 0.950–1.048 | 0.952 |
P values are from Log-rank test. CI: confidence interval.
Discussion
POTACE is recommended experimentally for patients with recurrent cancer because it can induce cell death by blocking vascular supply and improving chemotoxicity. Moreover, POTACE should affect patient's immunity by the following two aspects: 1) the dead tumor cells release tumor antigens that can be absorbed by antigen-presenting cells (APCs), which results in activation of the tumor-specific immune response, subsequently; 2) TACE treatment activates tissue hypoxia that stimulates tumor immunotolerance via hypoxia-induced PD-L1 overexpression in tumor cells and increasing the number of regulatory T cells.17 Therefore, the outcome of patients receiving postoperative TACE greatly depends on the balance between antitumoral immunity and tumor immunologic escape. In this study, DC-CIK cell therapy significantly improved the outcomes of patients with HBV infection but not of non-HBV infection patients, which should be attributable to DC cells-loaded by HBV epitopes that can induce the specific antitumoral immunity of CIK cells. We assumed that PBMCs should absorb the HBV-associated antigens that were released by the TACE-induced dead tumor cell and became the HBV-loaded PBMCs in vivo, which could present HBV-associated antigens to the T cells and produce the HBV-specific DC-CIK cells in vitro. It would predominantly contribute to the improved outcomes of the HCC patients with HBV. In addition to the HBV infection, a few of studies have also shown that the TACE treatment increases the frequency of the AFP-specific CD4 T cell response.18 Consistently, our study showed that DC-CIK cell therapy significantly decreased the AFP levels in the peripheral blood of the HCC patients, which indicated that the expanded CIK cells might contain the AFP-specific immune cells that could contribute to the decrease in the patient AFP levels. Taken together, DC-CIK cell therapy improves the outcomes of HCC patients receiving POTACE, which may be attributed to expansion of the POTACE-induced secondary HBV- and AFP-specific immune responses.
However, in addition to the tumor antigen-induced immune response, the immune states in the primary tumor microenvironments may also play critical roles in the prognoses of HCC patients receiving POTACE plus DC-CIK cell therapy. In this study, we observed that many CD4 and CD8 T cells frequently infiltrated into the tumor tissues of patients with HCC, but the high infiltrating intensities were not significantly associated with the better outcomes. By contrast, the lower intensity of CD4 was associated modestly with the improved prognosis of the HCC patients. These results suggested that the infiltrating T cells were a profoundly immunotolerant state in the tumor tissues of HCC, which should be caused by various factors, including cytokines, immune cells, and receptor or ligand expression of the antitumoral immunity inhibition.19,20 Among these factors, we mainly focused on the receptor and ligand level because interference of this interaction has provided a clinical benefit for various cancers including HCC.
At the receptor/ligand level, the PD-1/PD-L1 checkpoint pathway, the immune co-inhibitory checkpoint signal, is essential for tumor-induced T cell immune tolerance.21 In physiological conditions, PD-1 is kinetically expressed in the activated T cell within a finite window of time that functions in the inhibition of the T cell response to activation signals by binding to PD-L1 or PD-L2. PD-L1 is constitutively expressed on numerous cell types, including APCs and nonhematopoietic cells that can bind to PD-1 of the activated T cell to suppress the immune response and prevent damage from a T cell overreaction. Cancer cells usually overexpress PD-L1 and exploit the PD-1/PD-L1 inhibitory pathway to escape immune surveillance.22 In addition, PD-L1 overexpression also suppresses Fas-mediated apoptosis and up-regulates glycolytic metabolism and activation of the PI3K-Akt pathway, which promotes cancer cell survival.23,24 PD-L1 has been implicated in a variety of malignancies, including non-small-cell lung cancer,25-27 breast cancer,28 and renal cell carcinoma,29 for which its overexpression is significantly correlated with a poor outcome. In HCC, Qiang Gao et al., first reported that overexpression of PD-L1 in tumor tissues was significantly associated with a high risk of both cancer metastasis and tumor relapse.30 Zeng Z, et. al., also showed that upregulation of circulating PD-L1/PD-1 was associated with the poor post-cryoablation prognosis in patients with HBV-related hepatocellular carcinoma.31 Consistently, our study presented that the PD-L1 expression level in the primary tumor tissue was the only independent prognostic factor for the evaluation of patient survival; its high expression was significantly associated with shorter durations of relapse-free survival and overall survival relative to the control for HCC patients receiving POTACE treatment. These results indicated that the PD-L1 expression level was the most important prognostic predictor for HCC patients treated with POTACE plus DC-CIK cell therapy.
More importantly, our study validated that PD-L1 expression was significantly inversely correlated with the survival time in patients in the DC-CIK cell therapy group, which suggested that HCC cells with high PD-L1 expression levels could escape from DC-CIK cell-mediated death. A more recent study showed that the PD-1 expression by CIK cells was increased upon interactions with tumor cells and the overexpression of PD-L1 in the tumor cells inhibited the tumoricidal ability of the CIK cells.32 Therefore, we speculate that the relapsed tumor cells would retain the features of the primary tumor cells, of which the high expression of PD-L1 also provides a capacity to resist the tumoricidal effect of the DC-CIK cells that caused the shortened survival outcomes. We propose that a combination treatment between DC-CIK cell therapy and a reagent that interrupts the PD-1/PD-L1 interaction should improve the prognosis of HCC patients receiving POTACE plus DC-CIK cell therapy.
In addition, a more recent study showed that high PD-L1 expression was significantly correlated with improved OS benefits from CIK cell therapy and that low PD-L1 expression and PD-L1 expression did not reveal a direct impact on the survival time of patients in a surgery-alone group.33 The difference between this study and our results may be attributable to the following two aspects: 1) all patients in our study underwent both hepatectomy and TACE therapy, whereas patients in the other study received surgery alone; 2) the scoring system used for the determination of PD-L1 expression in the tissues by the authors of the previous study was based on unique criteria whereby positivity was based on a 5% threshold of PD-L1 expression on the cell membrane, whereas our scoring methods based on the staining intensity in multiple areas of the tissue that were more reasonable.
It should be noted that our findings are limited due to data collections from a single medical center with a small sample size. A multi-center trial and expanding sample size to reveal the clinical scenario with more accuracy and reliability warrant further study.
In summary, our results validate that DC-CIK cell therapy is advantageous for prolonging OS in HCC patients with HBV infection receiving POTACE. The PD-L1 expression level in tumor tissues is negatively associated with the RFS and OS times of HCC patients receiving POTACE plus DC-CIK cell therapy, which should serve as a pivotal predictor of the therapeutic efficacy of DC-CIK cell therapy in HCC patients receiving POTACE.
Materials and Methods
Clinical data collection
This study was conducted with a protocol approved by the medical ethics committee of Xuzhou Medical University (XYFY2012020). The medical records of 52 patients with primary HCCs, who were patients at the affiliated hospital of Xuzhou Medical University from June 2009 to January 2016, were collected.
The following inclusion criteria were applied: 1) cases conformed to the Guideline for Clinical Diagnosis and Staging of Primary Hepatocellular Carcinoma, Guangzhou, September 2001; 2) patients were treated with the combination therapy of liver resection and TACE; 3) the Karnofsky performance scale scores (KPSs) of the patients were higher than 60, with a survival expectancy of more than 6 months; 4) patients had no history of allergy or serious bacterial infections; and 5) female patients were not pregnant or lactating.
The following exclusion criteria were applied: 1) the presence of metastatic liver cancer, hepatic encephalopathy, gastrointestinal bleeding, massive ascites, other severe complications, viral infections other than HBV and HCV, and alcohol- or drug-induced liver disease; 2) severe coagulation disorder; 3) comorbidity of diseases of the heart, liver, kidneys, blood and metabolic system, or severe primary disease, concurrent with pneumonia or severe systemic infection; 4) systemic failure, intolerance or contraindication of femoral artery intubation; and 5) granulocytopenia or thrombocytopenia.
TACE procedures
Serum alpha-fetoprotein (AFP) level surveillance and noninvasive imaging tests were performed for postoperative HCC for evaluating disease progression. As a consequence of the increased AFP levels and typical imaging features of HCC, the postoperative patients needed to receive palliative treatments. Generally, abdominal computed tomography was conducted at 1-month post-resection to ensure complete tumor clearance. After the first scan, serum AFP measurements and repeat imaging evaluations (e.g., ultrasonography, CT, MRI) were performed every 3–6 months, according to the likelihood of recurrence. Once tumor recurrence was diagnosed, TACE treatment was performed within one week. Relapse-free survival (RFS) of the patients was defined in this study as the time from the resection date to the TACE initiation date. Experienced interventional radiologists performed all TACE procedures in Xuzhou Medical University. A 3-Fr microcatheter (Microferret; Cool, Bloomington, IN, USA) was advanced toward the tumor-feeding arteries depending on the size, arterial supply and location of the tumor for selective embolization. Further super-selective catheterization was performed as proximally as possible to the tumor for TACE of the tumor-feeding arteries. Iodized oil emulsion plus doxorubicin hydrochloride was used for chemoembolization. The dosing of the embolization agent was dependent on tumor number, tumor size, feeding-tumor vessels and liver functionality. To assess the hemodynamics in other arterial vessels and to ascertain the severity of vascular occlusion, angiography was performed subsequent to embolization.
DC-CIK cell preparation
Peripheral blood mononuclear cells (PBMCs) were collected from patients with advanced HCC using an apheresis system (COMTEC®, Fresenius-Kabi, Bad Homburg, Germany) and isolated by density gradient centrifugation with Lymphoprep (AXIS-SHIELD, Norway). Blood samples comprising approximately 1–3 × 109 PBMCs/50–60 ml were collected from each patient and cultured in GTT-551 serum-free medium (Takara, Japan) for 2 h. For the preparation of autologous DCs, the adherent cells were cultured in serum-free medium containing 1000 U/ml GM-CSF (PeproTech, USA) and 500 U/ml IL-4 (PeproTech, USA). To stimulate DC maturation, 50ng/ml TNF-α (PeproTech, USA) was added on day 6 and incubated for another 24 h. For preparation of CIK cells, PBMCs were cultured in fresh GTT-551 medium with 1000 U/ml IFN-γ (Shanghai KM, China) at 37°C with 5% CO2 for 24 h, followed by incubation with 50ng/ml anti-CD3 monoclonal antibody (BioLegend, USA) and 500 U/ml recombinant human IL-2 (rhIL-2, Beijing SL, China) in the medium in a cell culture bag (Takara, Japan) for another 7 days. Then, the CIK cells were cocultured with the DCs at a cell number ratio of 1:10 in the presence of the indicated stimulators for 6 days.
Collection and qualification of DC-induced CIK (DC-CIK) cells
The cells were sampled for sterility testing and phenotypic analysis (Figure S3A&B) . The phenotypes were analyzed by flow cytometry (BD Canto II, USA) with the following fluorescence-conjugated antibodies: CD3-PerCP, CD4-FITC, CD8-PE, and CD56-APC (BD Bioscience, USA). We determined the cell viability via trypan blue staining, and then the viable cells were automatically accounted by Cellometer Auto T4 (Nexcelom, USA). CIK cells were confirmed to be free of bacterial, fungal or mycoplasma contamination and met the release standard. Thereafter, cells were harvested and rinsed three times with normal saline, followed by supernatant removal and resuspension of the cell pellet in 300 ml of normal saline containing 500U/IL-2 for clinical infusions. Approximately 3∼5 × 1010 DC-CIK cells were intravenously infused. For a single course, DC-CIK cell therapy was conducted once every day and consecutively performed for three days. This procedure was repeated three times at an interval of three months. Patients receiving the infusion of DC-CIK cells were monitored by the senior physicians in our center daily for the occurrence of side effects, including cedrat acidosis, exothermic reactions, and redness, swelling, and induration at the injection site.
Fabrication of TMAs and immunohistochemistry
Due to the inaccessibility of the paraffin blocks of 6 patients, we collected paraffin blocks for 46 cases. The blocks were sectioned, and 3 typical pathological characterizations on each slide were selected to generate the tissue microarrays (TMAs). A total of 138 dots with 1.0 mm diameters were arrayed on one glass slide. Immunohistochemistry was performed as described by Bai et al.,.34 HPHT (high-pressure high temperature) was used for antigen retrieval. The anti-PD-L1 antibody (1:100 dilution, Cell Signaling Technology, USA), anti-CD8 antibody, and anti-CD4 antibody (1:200 dilution, Cell Signaling Technology) were employed as primary antibodies and incubated with the tissue sections at 4°C overnight. Afterward, the sections were incubated for another 2 h with a broad-spectrum secondary antibody (goat anti-rabbit/mouse IgG/HRP compound) (ZSGB-BIO, China).
Evaluation of immunostaining
The semi-quantitative immunoreactivity score (IRS) that was described by Shou et al., 35was implemented to determine the immunostaining intensities of the PD-L1 antibody. The value of the IRS equaled the percentage of positive cells multiplied by the staining intensity. The percentage of immunoreactive cells was cataloged into the following 5 levels: 0 (negative), 1 (0-10%), 2 (11-50%), 3 (51-80%) and 4 (80-100%). The immunostaining intensity was defined as follows: 0 (negative), 1 (mild), 2 (moderate) and 3 (intensive). Consequently, the value of the IRS of each dot was defined as low (0-3) and high (4-12). To determine the tumor infiltration intensity of the CD4 and CD8 T cells, the number of positively stained cells in each field of the light microscope with 100 × magnification was determined, and a total of 10 random fields was combined per slide to calculate the score, which comprised 4 levels: 0 (<5 positive cells), 1 (5-20 positive cells), 2 (20-100 positive cells), and 3 (>100 positive cells).36 Scores of 0–1 and 2–3 were defined as negative and positive, respectively. These analyses were conducted by two pathologists in a double-blinded manner.
Statistical analysis
Statistical analysis was performed using the SPSS 16 software package (SPSS Sciences, Chicago, IL, USA). The survival duration was estimated by the median survival duration, with a 95% confidence interval. The Pearson χ2 test or Fisher exact test was employed to determine the comparability of the clinical data. A Kaplan-Meier survival analysis was performed and verified using the log-rank test. The values prior and subsequent to treatment for each group were compared using the Student's t-test for the number of self-matched pairs. Bivariate correlations were used to detect correlations. p < 0.05 was considered to be statistically significant.
Supplementary Material
Funding Statement
This work was supported by The Major Programs of Nature Science Project of the Education Department of Jiangsu Province (14KJA320003), the Joint Program Between the Science and Technology Department of Yunnan Province and Kunming Medical University (2013FZ265), the National Natural Science Foundation of China (81673008), the Social Development Project of Jiangsu Province (BE2017642), and the Key Project For Industry Innovation of Xuzhou City (KC17012).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful for the assistance of the follow-up office in The Affiliated Hospital of Xuzhou Medical University. This research was supported by grants from the Major Programs of Natural Science Project of the Education Department of Jiangsu Province (14KJA320003), National Natural Science General Program (81673008), and the joint program between the Science and Technology Department of Yunnan Province and Kunming Medical University (2013FZ265).
References
- 1.de Lope CR Tremosini S, Forner A, Reig M, Bruix J. Management of HCC. J Hepatol. 2012;56(supplement 1):S75–S87. doi: 10.1016/S0168-8278(12)60009-9. PMID:22300468. [DOI] [PubMed] [Google Scholar]
- 2.Zhong J-H, Rodríguez AC, Ke Y, Wang Y-Y, Wang L, Li L-Q. Hepatic Resection as a Safe and Effective Treatment for Hepatocellular Carcinoma Involving a Single Large Tumor, Multiple Tumors, or Macrovascular Invasion. Medicine. 2015;94(3):e396. doi: 10.1097/MD.0000000000000396. PMID:25621684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Serag HB. Hepatocellular Carcinoma. N Engl J Med. 2011;365(12):1118–1127. doi: 10.1056/NEJMra1001683. PMID:21992124. [DOI] [PubMed] [Google Scholar]
- 4.Farazi PA, Depinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6(9):674. doi: 10.1038/nrc1934. PMID:16929323. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Liu Y, Ye N, Yao G. A Randomized Trial Comparing Radiofrequency Ablation and Surgical Resection for HCC Conforming to the Milan Criteria. Ann Surg. 2011;254(5):837; author reply 837. doi: 10.1097/SLA.0b013e318235e4eb. PMID:21997808. [DOI] [PubMed] [Google Scholar]
- 6.Qi X, Liu L, Wang D, Li H, Su C, Guo X. Hepatic resection alone versus in combination with pre- and post-operative transarterial chemoembolization for the treatment of hepatocellular carcinoma: A systematic review and meta-analysis. Oncotarget. 2015;6(34):36838–36859. doi: 10.18632/oncotarget.5426. PMID:26451613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu K, Min X-L, Peng J, Yang K, Yang L, Zhang X-M. The Changes of HIF-1α and VEGF Expression After TACE in Patients With Hepatocellular Carcinoma. J Clin Med Res. 2016;8(4):297–302. doi: 10.14740/jocmr2496w. PMID:26985249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X, Wang K, Wang M, Yang G, Ye X, Wu M, Cheng S. Transarterial chemoembolization (TACE) combined with sorafenib versus TACE for hepatocellular carcinoma with portal vein tumor thrombus: a systematic review and meta-analysis. Oncotarget. 2017;8(17):29416–29427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernandez-Gea V, Toffanin S, Friedman SL, Llovet JM. Role of the Microenvironment in the Pathogenesis and Treatment of Hepatocellular Carcinoma. Gastroenterology. 2013;144(3):512–527. doi: 10.1053/j.gastro.2013.01.002. PMID:23313965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsuchiya N, Sawada Y, Endo I, Uemura Y, Nakatsura T. Potentiality of immunotherapy against hepatocellular carcinoma. World Journal of Gastroenterology: WJG. 2015;21(36):10314–10326. doi: 10.3748/wjg.v21.i36.10314.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rai V, Abdo J, Alsuwaidan AN, Agrawal S, Sharma P, Agrawal DK. Cellular and molecular targets for the immunotherapy of hepatocellular carcinoma. Molecular and Cellular Biochemistry. 2018;437(1-2):13–36. [DOI] [PubMed] [Google Scholar]
- 12.Gattinoni L, Powell DJ, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6(5):383. doi: 10.1038/nri1842. PMID:16622476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8(4):299–308. doi: 10.1038/nrc2355. PMID:18354418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding M, Wang Y, Chi J, Wang T, Tang X, Cui D, Qian Q, Zhai B. Is Adjuvant Cellular Immunotherapy Essential after TACE-Predominant Minimally-Invasive Treatment for Hepatocellular Carcinoma? A Systematic Meta-Analysis of Studies Including 1774 Patients. PLoS One. 2016;11(12):e0168798. doi: 10.1371/journal.pone.0168798. PMID:28006010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siripongsakun S, Wei SH, Lin S, Chen J, Raman SS, Sayre J, Tong MJ, Lu DS. Evaluation of alpha-fetoprotein in detecting hepatocellular carcinoma recurrence after radiofrequency ablation. J Gastroenterol Hepatol. 2014;29(1):157. doi: 10.1111/jgh.12438.. [DOI] [PubMed] [Google Scholar]
- 16.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL. and others. Colocalization of Inflammatory Response with B7-H1 Expression in Human Melanocytic Lesions Supports an Adaptive Resistance Mechanism of Immune Escape. Sci Transl Med. 2012;4(127):127ra37–127ra37. doi: 10.1126/scitranslmed.3003689. PMID:22461641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Semaan A, Dietrich D, Bergheim D, Dietrich J, Kalff JC, Branchi V, Matthaei H, Kristiansen G, Fischer HP, Goltz D. CXCL12 expression and PD-L1 expression serve as prognostic biomarkers in HCC and are induced by hypoxia. Virchows Archiv An International Journal of Pathology. 2017;470(2):185–196. [DOI] [PubMed] [Google Scholar]
- 18.Greten TF, Duffy AG, Korangy F. Hepatocellular carcinoma from an immunological perspective. Clinical Cancer Research An Official Journal of the American Association for Cancer Research. 2013;19(24):6678–85. doi: 10.1158/1078-0432.CCR-13-1721. PMID:24030702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crispe IN, Matthew G, Ingo K, Beena J, Bradford S, Sherry W. Cellular and molecular mechanisms of liver tolerance. Immunological Reviews. 2006;213(1):101. doi: 10.1111/j.1600-065X.2006.00435.x. PMID:16972899. [DOI] [PubMed] [Google Scholar]
- 20.Fankhauser SC, Starnbach MN. PD-L1 limits the mucosal CD8(+) T cell response to Chlamydia trachomatis. Journal of immunology (Baltimore, Md.: 1950). 2014;192(3):1079–1090. doi: 10.4049/jimmunol.1301657. PMID:24353266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K. and others. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. doi: 10.1038/nm730. PMID:12091876. [DOI] [PubMed] [Google Scholar]
- 22.Patel SP, Kurzrock R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol Cancer Ther. 2015;14(4):847–856. doi: 10.1158/1535-7163.MCT-14-0983. PMID:25695955. [DOI] [PubMed] [Google Scholar]
- 23.Black M, Barsoum IB, Truesdell P, Cotechini T, Macdonald-Goodfellow SK, Petroff M, Siemens DR, Koti M, Craig AWB, Graham CH. Activation of the PD-1/PD-L1 immune checkpoint confers tumor cell chemoresistance associated with increased metastasis. Oncotarget. 2016;7(9):10557–10567. doi: 10.18632/oncotarget.7235. PMID:26859684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makarova-Rusher OV, Medina-Echeverz J, Duffy AG, Greten TF. The yin and yang of evasion and immune activation in HCC. J Hepatol. 2015;62(6):1420–1429. doi: 10.1016/j.jhep.2015.02.038. PMID:25733155. [DOI] [PubMed] [Google Scholar]
- 25.Sumimoto H, Takano A, Teramoto K, Daigo Y. RAS–Mitogen-Activated Protein Kinase Signal Is Required for Enhanced PD-L1 Expression in Human Lung Cancers. PLoS ONE. 2016;11(11):e0166626. doi: 10.1371/journal.pone.0166626. PMID:27846317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Akita H, Nishimura M. B7-H1 Expression on Non-Small Cell Lung Cancer Cells and Its Relationship with Tumor-Infiltrating Lymphocytes and Their PD-1 Expression. Clin Cancer Res. 2004;10(15):5094–5100. doi: 10.1158/1078-0432.CCR-04-0428. PMID:15297412. [DOI] [PubMed] [Google Scholar]
- 27.Mu C-Y, Huang J-A, Chen Y, Chen C, Zhang X-G. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol. 2011;28(3):682–688. doi: 10.1007/s12032-010-9515-2. PMID:20373055. [DOI] [PubMed] [Google Scholar]
- 28.Guo L, Li W, Zhu X, Ling Y, Qiu T, Dong L, Fang Y, Yang H, Ying J. PD-L1 expression and CD274 gene alteration in triple-negative breast cancer: implication for prognostic biomarker. SpringerPlus. 2016;5(1):805. doi: 10.1186/s40064-016-2513-x. PMID:27390646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abbas M, Steffens S, Bellut M, Eggers H, Großhennig A, Becker JU, Wegener G, Schrader AJ, Grünwald V, Ivanyi P. Intratumoral expression of programmed death ligand 1 (PD-L1) in patients with clear cell renal cell carcinoma (ccRCC). Med Oncol. 2016;33(7):80. doi: 10.1007/s12032-016-0794-0. PMID:27317388. [DOI] [PubMed] [Google Scholar]
- 30.Gao Q, Wang X-Y, Qiu S-J, Yamato I, Sho M, Nakajima Y, Zhou J, Li B-Z, Shi Y-H, Xiao Y-S. and others. Overexpression of PD-L1 Significantly Associates with Tumor Aggressiveness and Postoperative Recurrence in Human Hepatocellular Carcinoma. Clin Cancer Res. 2009;15(3):971–979. doi: 10.1158/1078-0432.CCR-08-1608. PMID:19188168. [DOI] [PubMed] [Google Scholar]
- 31.Zeng Z, Shi F, Zhou L, Zhang MN, Chen Y, Chang XJ, Lu YY, Bai WL, Qu JH, Wang CP. Upregulation of circulating PD-L1/PD-1 is associated with poor post-cryoablation prognosis in patients with HBV-related hepatocellular carcinoma. Plos One. 2011;6(9):e23621. doi: 10.1371/journal.pone.0023621. PMID:21912640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dai C, Lin F, Geng R, Ge X, Tang W, Chang J, Zheng W, Liu X, Ying L, Zhe Z. Implication of combined PD-L1/PD-1 blockade with cytokine-induced killer cells as a synergistic immunotherapy for gastrointestinal cancer. Oncotarget. 2016;7(9):10332–10344. doi: 10.18632/oncotarget.7243. PMID:26871284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen CL, Pan QZ, Zhao JJ, Wang Y, Li YQ, Wang QJ, Pan K, Weng DS, Jiang SS, Tang Y. PD-L1 Expression as a Predictive Biomarker for Cytokine-Induced Killer Cell Immunotherapy in Patients with Hepatocellular Carcinoma. Oncoimmunology. 2016;5(7):00–00. doi: 10.1080/2162402X.2016.1176653.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bai J, Mei PJ, Liu H, Li C, Li W, Wu YP, Yu ZQ, Zheng JN. BRG1 expression is increased in human glioma and controls glioma cell proliferation, migration and invasion in vitro. Journal of Cancer Research & Clinical Oncology. 2012;138(6):991. doi: 10.1007/s00432-012-1172-8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang S, Wu X, Chen Y, Zhang J, Ding J, Zhou Y, He S, Tan Y, Qiang F, Bai J. Prognostic and predictive role of JWA and XRCC1 expressions in gastric cancer. Clinical Cancer Research An Official Journal of the American Association for Cancer Research. 2012;18(10):2987. doi: 10.1158/1078-0432.CCR-11-2863. PMID:22452940. [DOI] [PubMed] [Google Scholar]
- 36.Guo X, Fan Y, Lang R, Gu F, Chen L, Cui L, Pringle GA, Zhang X, Fu L. Tumor infiltrating lymphocytes differ in invasive micropapillary carcinoma and medullary carcinoma of breast. Mod Pathol. 2008;21(9):1101–1107. doi: 10.1038/modpathol.2008.72. PMID:18469794. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.