Abstract
Electrophysiological experiments in the partial cortical isolation (“undercut” or “UC”) model of injury-induced neocortical epileptogenesis have shown alterations in GABAergic synaptic transmission attributable to abnormalities in presynaptic terminals. To determine whether the decreased inhibition was associated with structural abnormalities in GABAergic interneurons, we used immunocytochemical techniques, confocal microscopy and EM in UC and control sensorimotor rat cortex to analyze structural alterations in fast-spiking parvalbumin-containing interneurons and pyramidal (Pyr) cells of layer V. Principle findings were: 1) There were no decreases in counts of parvalbumin (PV)- or GABA-immunoreactive interneurons in UC cortex, however there were significant reductions in expression of VGAT and GAD-65 and -67 in halos of GABAergic terminals around Pyr somata in layer V. 2) Consistent with previous results, somatic size and density of Pyr cells was decreased in infragranular layers of UC cortex. 3) Dendrites of biocytin-filled FS interneurons were significantly decreased in volume. 4) There were decreases in the size and VGAT content of GABAergic boutons in axons of biocytin-filled FS cells in the UC, together with a decrease in colocalization with postsynaptic gephyrin, suggesting a reduction in GABAergic synapses. Quantitative EM of layer V Pyr somata confirmed the reduction in inhibitory synapses. 5) There were marked and lasting reductions in brain derived neurotrophic factor (BDNF)-IR and -mRNA in Pyr cells and decreased TrkB-IR on PV cells in UC cortex. 6) Results lead to the hypothesis that reduction in trophic support by BDNF derived from Pyr cells may contribute to the regressive changes in axonal terminals and dendrites of FS cells in the UC cortex and decreased GABAergic inhibition.
Keywords: Epilepsy, parvalbumin, axonal boutons, BDNF, TrkB, trophic, GABA, traumatic injury, inhibitory synapses
Introduction
Structural and functional abnormalities in GABAergic neurons and inhibitory circuits are prominent in animal models of epileptogenesis (Ribak and Reiffenstein, 1982);(Ribak, 1985); (Houser et al., 1986; Li and Prince, 2002; Chen and Roper, 2003); (Magloczky and Freund, 2005; Kumar and Buckmaster, 2006; Faria and Prince, 2010); (Faria et al., 2012); (Ma and Prince, 2012) and in human epileptogenic brain (De Lanerolle et al., 1989; Marco et al., 1996; DeFelipe, 1999). Disinhibition can result from actual loss of interneurons of various subtypes (De Lanerolle et al., 1989); (Sloviter, 1987); (Houser and Esclapez, 1996); (Rosen et al., 1998); (Wyeth et al., 2010); (Buckmaster and Dudek, 1997) and/or functional alterations in surviving interneurons. In the partial neocortical isolation, an injury/deafferentation model of posttraumatic epileptogenesis (“undercut “or “UC” below), we previously found impaired inhibitory synaptic transmission from fast-spiking (FS) parvalbumin (PV)-containing interneurons onto excitatory neurons, as well as to other FS cells (Ma and Prince, 2012). The decreased GABAergic inhibition appeared to be due to functional abnormalities in presynaptic terminals, as evidenced by increased failures of evoked monosynaptic and unitary IPSCs, decreased release probability (Pr) (Faria and Prince, 2010), increase in the coefficient of variation (CV) of the amplitude of unitary IPSCs (Ma and Prince, 2012) and decreases in presynaptic N-current calcium channels (Faria et al., 2012). Reductions in mIPSC frequency in layer V Pyr cells in UC cortex (Li and Prince, 2002), and topographic restriction of inhibitory connectivity (Jin et al., 2011) were also compatible with presynaptic interneuronal abnormalities. In the current experiments, we assessed potential structural alterations in FS interneurons that might underlie terminal dysfunction. Results show that the density of GABAergic neurons is not reduced in the UC cortex. However, there are significant reductions in vesicular GABA transporter (VGAT), and glutamic acid decarboxylase (GAD) -65 and -67 in their perisomatic GABAergic terminals. In addition, GABAergic boutons were decreased in size and fewer were closely associated with postsynaptic gephyrin, suggesting a reduction in GABAergic synapses, confirmed with electron microscopy that showed decreased density of symmetrical synapses on layer V Pyr cell somata. Similar abnormalities of GABAergic neurons are reported following cortical deafferent ation and as a consequence of decreased activity-dependent BDNF gene activation. Reductions in BDNF expression in layer V Pyr cells of the UC and the well-established dependence of interneuronal growth and development on activation of TrkB receptors, suggest that loss of trophic support contributes to functional and structural abnormalities of FS interneurons in the epileptogenic UC cortex.
Partial results of these experiments were presented in a review (Prince et al., 2012).
Methods
All procedures were conducted according to National Institutes of Health Guide for the Care and Use of Laboratory Animals and protocols approved by the Stanford Institutional Animal Care and Use Committee. We analyzed FS interneurons of neocortical layer V in the UC model to determine whether there were abnormalities in their axons and presynaptic terminals that could result in defects in GABAergic transmission. Interictal discharges originate in layer V of the UC (Prince and Tseng, 1993); (Hoffman et al., 1994) and alterations in FS interneurons in this lamina could contribute to hyperexcitability and epileptiform bursts in in vitro cortical slices (Prince and Tseng, 1993; Hoffman et al., 1994) and seizures in vivo (Chauvette et al., 2016; Ping and Jin, 2016a). We identified FS cells in whole cell recordings from their electrophysiological phenotype (Xiang et al., 1998) and, retrospectively, appearance following biocytin labeling. FS interneurons included predominately PV-positive cells (Uematsu et al., 2008)
Animals
A total of 21 naïve control and 22 lesioned Sprague-Dawley rats were used in these experiments, as detailed below.
Partial cortical isolations
These were prepared as previously described (Hoffman et al., 1994); see supplemental Fig. S5 from Graber and Prince 2006 for details (Graber and Prince, 2006). Male Sprague-Dawley rats aged postnatal day (P) 21–30 were anesthetized with ketamine/xylazine (80/8 mg/kg, ip), the skull exposed and a bone window opened over sensory-motor cortex. A 30 g needle, bent at a 90° angle ~3 mm from its tip, was inserted parasagitally, beneath the dura and pia, parallel to the cortical surface and 1 mm from the midline, with care to avoid large vessels. The needle was lowered ~2 mm to make a transcortical cut and rotated through ~135° to make an undercutting lesion, raised to just beneath the pia to make a second transcortical cut, and removed. A third transcortical cut was then made parallel and ~3 mm lateral to the initial one. The bone window was covered with sterile Saran Wrap® and the skin sutured. Animals were allowed to recover for 2–4 wks. prior to the terminal experiment. In previous experiments, more than 80% of animals with such lesions have had epileptogenic activity in at least one in vitro slice after 2 wks. (Hoffman et al., 1994); (Graber and Prince, 1999). Most animals underwent UC surgeries at P28-30, except those used for electron microscopy where UCs were performed at P21.
Biocytin labeling
Methods for preparing in vitro neocortical slices and identifying FS interneurons for biocytin filling were as previously described (Bacci et al., 2003); (Xiang et al., 2002). Eighteen well-filled FS interneurons from sensory-motor cortical layer V of 5 naïve control (7 cells) and 5 UC rats (11 cells) were used for confocal analysis of dendritic and axonal structures. Of these, 5 UC and 5 control FS cells were processed for PV immunoreactivity (IR) and all were positive for PV. Basic techniques were similar to those previously described (Xiang et al., 1998). Postnatal (P49-56) naïve rats and animals that had been subjected to a unilateral partial cortical isolation 3–4 wks. earlier (“undercut” or “UC” animals) were anesthetized with pentobarbital (55 mg/kg), decapitated, and brains removed and immersed in cold “cutting” solution (4°C) containing (in mM): 234 sucrose, 11 glucose, 24 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 10 MgSO4 and 0.5 CaCl2, gassed with 95% O2/5% CO2. The undercut cortical area was clearly visible at the pial surface. Coronal slices (300 μm) from naïve and undercut animals were cut with a vibratome from a block of brain containing the undercut, incubated for 1 hr. at 32°C and subsequently at room temperature in oxygenated artificial cerebrospinal fluid (ACSF) containing (in mM): 126 NaCl, 26 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 2 MgSO4, 2 CaCl2 and 10 glucose; pH 7.4. Slices were transferred one at a time to the recording chamber on the stage of an upright microscope, minimally submerged and perfused (~3 ml/min) with the above ACSF at 32°C. Recordings and cell labeling were begun after 1 hr. of incubation. Landmarks, including cortical laminae and sites of transcortical or undercutting lesions, were easily delineated in slices under low power with the upright microscope. Sites for recording and biocytin labeling in lesioned animals were from layer V inside the isolation, and within ~1–2 mm of the transcortical cut, as this is the most epileptogenic territory within the injured cortex (Hoffman et al., 1994). Comparable sites were selected in contralateral homotopic and naïve cortex. Pyramidal (Pyr) cells and interneurons were identified using a 40× or 63× water immersion lens, DIC optics, infrared microscopy and a CCD camera. Cells with a multipolar morphology, a round cell body and absence of a single emerging apical dendrite oriented towards the pial surface were selected for recordings and biocytin labeling (Bacci et al., 2003). (Xiang et al., 1998). Patch clamp techniques were used to obtain current clamp recordings with biocytin-filled electrodes, using a dual channel patch-clamp amplifier (Multiclamp 700A, Axon Instruments). A Digidata 1320 digitizer and PClamp9 (Axon Instruments) as well as locally generated software were used for data acquisition and analysis and for the generation of stimuli. Membrane input resistance was determined from responses to small current injections (25 to 30 pA, 250 ms, 0.2 Hz). Patch pipettes with a 1.5–2 μm tip diameter (3–10 MΩ resistance in the bath) were prepared from borosilicate glass on a two-stage puller and filled with an intracellular solution consisting of (in mM): 130 Kgluconate; 10 KCl; 2 NaCl; 10 HEPES; 10 EGTA; 0.05–0.1% biocytin; pH = 7.3 corrected with KOH; 290 mOsm. Neurons were classified based on their firing behavior in response to DC current injections (Beierlein et al., 2003) (Xiang et al., 1998). In contrast to Pyr neurons, FS cells generated high-frequency, non-accommodating action potential (AP) firing to depolarizing current pulses, lacked rebound spiking after hyperpolarizing current steps and had APs with prominent after-hyperpolarizations (Bacci et al., 2003). Intracellular labeling with biocytin was used to confirm the interneuronal morphology in FS cells. The patch electrode was left in place for 30–60 min. to allow filling of the neuron and processes, and the slice was processed as below. Only FS cells with a stable resting potential more negative than −60 mV and input resistance > 65 MΩ, were used for anatomical analysis.
After cells were electrophysiologically identified, slices were fixed between pieces of filter paper to ensure flatness and placed in 4% paraformaldehyde in 0.1 M phosphate buffer (PB, pH 7.4) at 4°C overnight. Slices were rinsed in PBS and 2% H2O2 to suppress endogenous peroxidases. Nonspecific binding was blocked by incubation in 10% normal goat serum with 2% BSA and 0.05% Triton X-100 in PBS, and slices exposed to fluorescein or Texas Red Avidin D (Vector Laboratories, Burlingame, CA) for visualizing biocytin filled cells. Sections were air-dried and cover slipped with Vectashield mounting medium (Vector Laboratories, Burlingame). Labeled cells were scanned using a confocal microscope (Zeiss, LSM 150; Carl Zeiss MicroImaging, Inc.) at appropriate magnification and intervals as needed for 3D reconstruction. Z-stack series images were assembled using Zeiss LSM Image Browser software to verify contiguous biocytin filling and to view general morphology. Images were analyzed as described below. Standard DAB labeling was also employed for cell tracing and reconstruction by using the Neurolucida software-controlled microscope system (Axioskope; Carl Zeiss, Inc., Germany).
Immunocytochemistry
Brain slices were fixed as above, followed by 30% sucrose for cryoprotection. Thick sections (35 μm) were obtained with a sliding microtome (Microm, HM 400). Using standard immunocytochemical techniques, free-floating sections were rinsed twice in PBS buffer followed by 50% alcohol. Non-specific binding was blocked as above and sections were exposed to Fluorescein Avidin D (Vector Laboratories) for visualizing biocytin-filled cells followed by primary antisera at 4°C for 12–48 hours with vesicular GABA transporter (VGAT) rabbit polyclonal, a gift from Dr. R Reimer, and gephyrin mouse monoclonal (Synaptic Systems). Some sections were exposed to secondary antibody-conjugates with indocarbocyanine and/or indodicarbocyanine (Cy3 and Cy5; 1:200 Jackson ImmunoResearch Lab), Alexa Fluor 568 and/or Alexa Fluor 488 (both Alexa fluorophores, 1:1000; Invitrogen Corporation) for 2 hours at room temperature to allow triple labeling when necessary. Table 1 provides a list of antibodies used, sources and concentrations. The specificity of BDNF and TrkB receptor antibodies used in this study has been supported in previous studies (Snapyan et al., 2009; Bergami et al., 2013).
Table 1.
| Antibodies | Source | Dilution |
|---|---|---|
| Rabbit polyclonal anti-VGAT | Dr. Richard Reimer | 1/1000 |
| Rabbit polyclonal anti-GAD65 | Milipore | 1/1000 |
| Rabbit polyclonal anti-GAD67 | Milipore | 1/1000 |
| Mouse monoclonal anti-Gephyrin | Synaptic Systems | 1/500 |
| Rabbit polyclonal anti-GABA | Sigma | 1/1000 |
| Rabbit polyclonal anti-Parvalbumin | Dr. Kenneth Baimbridge | 1/1000 |
| Mouse monoclonal anti-Parvalbumin | Sigma | 1/1000 |
| Mouse monoclonal anti-Synaptophysin | Milipore | 1/500 |
| Goat polyclonal anti-BDNF | Santa Cruz Biotech | 1/100 |
| Mouse monoclonal anti-GAD65 | Milipore | 1/500 |
| Mouse monoclonal anti-Neurofilament 68 KD | Sigma | 1/400 |
| Mouse monoclonal anti-Neurofilament 200KD | Sigma | 1/40 |
| Rabbit polyclonal anti-TrkB | Santa Cruz Biotech | 1/50 |
| Rabbit polyclonal anti-GFAP | Milipore | 1/1000 |
| Mouse monoclonal anti-NeuN | Milipore | 1/100 |
| Rabbit monoclonal anti-Cd11b | Abcam | 1/200 |
Cell counts
Five naïve control and 6 UC rats were deeply anaesthetized and perfused transcardially with 4% paraformaldehyde 4–6 weeks after UC. Cortical sections of 40 microns were obtained from both control and UC groups, and the following antibodies were used to label somata: NeuN mouse monoclonal (Millipore, Temecula CA; 1:500), GABA (Sigma, St Louis, MO; 1:500), and PV rabbit polyclonal (R301 antibody, gift of Kenneth Baimbridge, Vancouver, Canada;1:1,000) and PV monoclonal (PARV-19 clone, Sigma, St. Louis, MO; 1:1,000). Further details are provided in Supplemental Methods and Tables 1 and 2.
Table 2.
Cell counts in partially isolated neocortex
| Condition | IR | n | Mean | SEM | P-Value |
|---|---|---|---|---|---|
| Control | NeuN | 10 | 952.8 | 49.65 | 0.027 (p<0.05) |
| UC | NeuN | 10 | 793.3 | 44.01 | |
| Control | GABA | 10 | 65.4 | 4.17 | 0.47 (p>0.05) |
| UC | GABA | 10 | 59.8 | 6.37 | |
| Control | PV | 7 | 50.42 | 2.71 | 0.96 (p>0.05) |
| UC | PV | 7 | 50.14 | 4.00 |
IR: immunoreactivity; n: number of images used for cell counts
PV: parvalbumin-containing interneurons; NeuN: total neurons
Mean: total number of cells in 425 μm wide grids layer I–VI
Image analysis
Software included Neurolucida (MicroBrightField, version 5), Volocity (Volocity 2.6.1; Improvision Ltd) and Sigmascan pro (V5; SPSS, Inc.). Morphometric analysis for measuring lengths, areas etc. was done with NeuroExplorer software. In some experiments, Pyr neuron perisomatic VGAT-IR, glutamic acid decarboxylase (GAD) 65- or 67-IR was quantitatively assessed on enlarged images with Volocity software as described below and in detail elsewhere (Faria et al., 2012). Signals from each fluorophore were analyzed separately. Voxels occupied by biocytin-labeled dendrites or the perisomatic IR of interest were detected with consistent fluorescence signal threshold intensities throughout the analysis. Overlapping voxels from the images of VGAT- and GAD- channels were considered to indicate co-localization of VGAT in close apposition to labeled dendrites or, in the case of perisomatic labeling of VGAT-, GAD65- or 67-IR, to indicate colocalization of these proteins in presynaptic GABAergic boutons. Random image noise was reduced by disregarding any particle under 0.003 μm. Two parallel lines (“train tracks”) were drawn around somata so that the perisomatic immunoreactivity region of interest (ROI) was included between them. The image stack was scanned and cropped in the z-dimension to encompass 4–6 sequential 0.5 μm optical sections through the approximate center of the Pyr cell soma. VGAT or GAD65 immunoreactivity was used as a guide to demarcate the perisomatic region, as it associates closely with the somata of Pyr neurons (Chaudhry et al., 1998) The inner boundary of the ROI was drawn at the junction of positive perisomatic immunostaining for VGAT or GAD65 and the void representing the cell body. The outer boundary was drawn to include immunoreactive VGAT, GAD65 or GAD67 puncta in close apposition to the soma. This resulted in a ~3 μm wide “train track” around the perisomatic region that extended ~3 μm in the z-dimension. Within the ROI, object identification was restricted by voxel intensity (fluorescence intensity 2 standard deviations from the mean) and size (> 0.2 μm3). The cumulative volume of the identified objects, i.e. the cumulative volume of the perisomatic protein of interest within the ROI, was expressed as a percentage of the total volume of the ROI. The ratio of immunoreactive voxel volume/total volume of the ROI between the lines was measured using Volocity. In experiments dealing with colocalization of TrkB-IR over somata of PV interneurons, confocal images were obtained by using a Zeiss 880 confocal microscope. Z-stacks of optical sections (0.3 μm OD) were trimmed to 61.3 μm, y; 61.3 μm, z: 10.9 μm, allowing analysis of the midportion of the soma of each PV cell. Z stacks were rendered into Volocity program to determine the percent of colocalization of TrkB voxels over each PV soma. Mean BDNF mRNA or immunoreactivity (IR) per neuron was estimated from pixel number and area, assuming a pixel size of 0.2×0.2 μm. Mean soma area was obtained from NeuN immunoreacted confocal sections through the largest soma diameter in single optical sections from control and UC cortex. The cumulative area of the identified BDNF mRNA or IR pixels within ROI drawn along the boundary of cell bodies was compared to the area of the ROI (i.e. the area of the soma). Thus, the expression of BDNF in the cell was represented as a percentage of soma area.
Axon and bouton analysis
A Zeiss LSM 510 confocal Laser Scanning Microscope was used to visualize and capture images of well-filled axonal arbors of 9 FS control interneurons from 5 naive rats and 7 FS interneurons from 5 undercut rats 3–4 weeks after UC. Axons of biocytin-filled cells were imaged at 100× and z-stacks (optical distance of 0.37 um) were obtained. Nine z-stacks were necessary to capture soma, dendrites and axonal arborizations of each cell. Images were exported to Neurolucida software for reconstruction and analysis. Axonal processes were traced and manually examined with Neurolucida software by two blinded investigators. Boutons were marked for quantitative analysis of density, and classified according to their largest diameter in confocal sections (“big bouton” ≥ 1.0 μm; “small bouton” < 1.0 μm in diameter), presence of VGAT-IR and close apposition to postsynaptic gephyrin-IR.
Tissue processing for electron microscopy
A total of 6 Sprague Dawley rats (P35-42) were used for electron microscopic (EM) analysis of synapses onto lamina V Pyr neuronal somata. In 3 rats, partial neocortical isolations were placed in sensori-motor cortex, as described above, 2–3 weeks before the animal was perfused for EM. Samples from undercut cortex and “control” neocortex contralateral to the undercut were taken from these 3 rats. Samples from unlesioned neocortex corresponding to the site of the isolation were obtained from 2 other naïve control animals. An additional naïve rat was used to obtain cortical tissue for immunocytochemistry of GAD65 in presynaptic terminals by light and electron microscopy.
Animals were anesthetized with pentobarbital (100 mg/kg i.p.), then perfused with saline, followed by a solution consisting of 2% paraformaldehyde and 2% glutaraldehyde in 0.1 M sodium phosphate (PB), pH 7.4. After the initial perfusion fixation, the brains were immediately removed from the skull, and placed into the same fixative overnight at 4°C. After post-fixation, the brains were rinsed in PB, cryoprotected in 10% sucrose in 0.1 M PB, followed by 30% sucrose in 0.1 M PB for 48 hours at 4°C, and then frozen on dry ice. Before sectioning, the brains were blocked to isolate samples containing the region of the undercut cortex and the corresponding neocortex contralateral to the undercut (or the corresponding neocortex in naïve control animals). From these brains, 60 μm coronal sections were cut on a vibratome, and the region of interest (i.e., the undercut cortex or corresponding control neocortex) dissected out. Subsequent processing for EM included postfixation in 1% osmium tetroxide (0.15 M PB, pH7.4) for 1 hour at room temperature, alcohol dehydration, and flat embedding in Eponate12 resin (Ted Pella, Redding, CA) between two aclar sheets. Serial-thin sections from tissue of the various animals were cut, stained with uranyl acetate and Reynold’s lead citrate, and examined on a Philips CM120 electron microscope. To determine the chemical nature of synapses onto the somata of lamina V Pyr neurons, pre-embedding immunocytochemistry for glutamate decarboxylase (GAD65) was employed on selected sections using a protocol previously reported (Wenzel et al., 2001)).
Morphological EM data analyses
For each undercut rat used in this study, electron micrographs, at a magnification of 8,400, were obtained from 2 specimens from undercut cortex and 2 from contralateral cortex. Two specimens were also obtained from each naïve control at the same magnification. For each specimen, 25 micrographs were obtained covering random fields containing layer V Pyr neuronal somata that were identified based on lamina location (determined on semi-thin sections) and morphological characteristics (e.g., size and shape of the soma, and origin of axonal and dendritic processes). Electron micrographs were obtained from an average of 6 to 9 different somata per specimen; prints were generated from those at a final magnification of 21,000×, and coded for analysis. A total of 3,927 μm somatic membrane length of layer V Pyr neurons was analyzed, with a total of 1209 axonal boutons and 415 synapses classified. Synapses onto layer V Pyr neuronal somata were identified in two ways: First, presynaptic boutons (defined as axonal enlargements/terminals containing synaptic vesicles and mitochondria) located adjacent to the somatic membrane of the layer V neurons, were classified (and counted) as boutons associated with or without synaptic specializations. Second, synapses (defined as symmetric or asymmetric, based on appearance of pre- and postsynaptic specializations as well as the spherical or flat shape of vesicles) were classified and counted. All counts were related to the unit length of the somatic membrane. To quantify the somatic membrane length of lamina V Pyr neurons on electron micrographs, a digitizing graphics tablet and NIH software were used. In addition to counting synapses, the occurrence and frequency of puncta adherentia were determined for each bouton. For determining the statistical significance of the different experimental groups, means were calculated per animal and subjected to the paired Student’s t-test. P values were considered significant if less than 0.05 (identified in graphs).
Cell counts
We initially compared counts of NeuN- versus PV-immunoreactive cells in 3 control and 4 UC rats 4–6 weeks after the cortical lesion was made at P28-30. Rats were deeply anaesthetized and transcardially perfused with 4% paraformaldehyde for ICC 4–6 wks. after the UC. Fixed brains were removed and post-fixed overnight at 4°C. 40 μm-thick coronal sections were obtained with a sliding microtome (HM400, Microm) with a separation of 80 μm between sections. 1–3 sections were obtained from each brain (total: 7 control and 7 UC sections), and reacted with PV and NeuN antibodies-IR (above). Because of reported decreases in total GABA cells counts in UC cat cortex (Avramescu et al., 2009), we performed a second experiment in which counts of all GABA immunoreactive interneurons and all neurons labeled with NeuN (Mullen et al., 1992) were obtained from sensorimotor cortex in 2 additional control and 2 UC rats. We processed 2–3 sections from each rat with NeuN and GABA antibodies and 3 images per section were analyzed (10 images each from UC and control rat). Images for counts of PV and NeuN containing interneurons were analyzed using iLastik and Cell Profiler programs. Details are provided in Supplemental Methods. Counts were made in each tissue section within 425 μm wide grids extending from the pial surface to the junction of layer VI and white matter within the UC and a comparable area in sensorimotor control sections. Computer counts were verified visually in all images by a blinded observer to assess the accuracy of the computer detection software. Differences between computer and visual counts were < 10% in grids where both techniques were applied. GABA cell counts in UC vs. control were made in the same sections and areas by a blinded observer.
Results
Histopathology of the partially isolated cortex
3 control and 3 UC rats were perfused for histological examination of the UC cortex. The cortex in the area of the partial isolation was reduced in thickness (Supplemental Fig. S1A) with some loss of infragranular Pyr neurons (Gruner et al., 1974); (Prince and Tseng, 1993);; Figs. S3, S4). Immunoreactivity (IR) for 68 kD neurofilaments was increased in the neuropil and in both Pyr cells and interneurons in the undercut cortex 2–3 weeks after the lesion (Fig. S1A–B). Immunoreactivity for 200 kD neurofilament protein IR for 200 kD neurofilament protein was also markedly increased 3 weeks after the lesion (Fig. S1C, D). Although the onset and time course of these changes were not examined, IR for 200 kD neurofilament protein IR for 200 kD neurofilament protein was already markedly increased 3 days after the lesion (Fig. S1C, D), indicating that prominent structural changes were taking place early after the injury (See also (Takahashi et al., 2016). Immunoreactivity for glial fibrillary acidic protein in layer V was also apparent in hypertrophic astrocytes within the partial isolation (Li et al., 2012) (Fig. S2B, F) and there was also prominent activation of microglia (Cd11b-IR in Fig. S2D, F)
Parvalbumin-containing Interneurons in the UC cortex
Previous experiments in the UC model showed that the frequency of mIPSCs was decreased while the amplitude was not significantly affected (Li and Prince, 2002), a result compatible with presynaptic abnormalities such as decreased Pr (Faria and Prince, 2010; Ma and Prince, 2012), decreases in presynaptic Ca++ channels (Faria et al., 2012), and/or structural effects of injury such as decreases in inhibitory synapses and loss of interneurons (e.g. (Buckmaster and Dudek, 1997); (DeFelipe, 1999) (Avramescu et al., 2009) and references above). To assess possible interneuronal loss, we first counted PV-IR interneurons and total neurons reacted for NeuN in 425 μm-wide grids normal to the pial surface extending from layers I–VI of control vs. UC cortex. The mean number of PV cells counted per section (n = 10 sections) was not significantly decreased in the UC cortex (50.4 ± 2.7 PV-IR neurons; n = 5 control rats), and 50.1 ± 4.0 in UC cortex (n = 7; p = 0.96, unpaired t test), while the total cell count (NeuN profiles) was decreased significantly (mean 816.9 ± 11.6 NeuN-IR in control; n = 7; and 691 ± 36.5 in UC cortex; n = 7; p = 0.008, unpaired t test; Fig. S3), suggesting a decrease in Pyr neurons. This decrease was prominent in infragranular layers (e.g. Fig. S3 A1; S4A, A1) (Gruner et al., 1974; Prince and Tseng, 1993)) where counts of NeuN profiles were 493 ± 34.8 (control) and 347.7 ± 21.5 SEM (UC); 10 images from 2 rats for each count; (p = 0.002). There was no reduction in GABA cell counts (mean 65.4 ± 4.17 GABA-IR neurons in control, n = 10 sections, and 59.8 cells ± 6.4 in UC cortex (n = 10); p = 0.47, while NeuN counts in the same sections were significantly reduced in the UC compared to control (mean 952.8 ± 49.7 NeuN-IR neurons in control, n = 10 sections, and 793.3 cells ± 44.01 in UC cortex, n = 10, p = 0.027 unpaired t test; Methods, Fig. S4). Potential alterations in density of other interneuronal subtypes were not directly assessed; however, the GABA cell counts suggested that there were no significant decreases. Taken together, results of cell counts show significant reductions in Pyr cells in infragranular layers and sparing of PV- and total GABA-IR cells.
Structure of biocytin-labeled FS interneurons
To examine changes in interneuronal structure in the lesioned cortex, we measured morphological parameters of somata and dendrites of 9 electrophysiologically identified, biocytin-filled layer V FS interneurons from naïve cortex and 7 from partially isolated cortex, 3–4 weeks after UC. Immunoreactivity of axonal terminals for VGAT and GAD65 was assessed in perisomatic halos around layer V Pyr cells, sites of presumed termination of axons of FS PV-containing interneurons (Chaudhry et al., 1998). All of the filled neurons had anatomical characteristics of basket cells (Fig. 1A, B) and electrophysiological fast-spiking properties (Fig. 1C; (Ma and Prince, 2012). Sizes of somata of layer V basket cells are known to vary widely (Uematsu et al., 2008) and using Neurolucida we found no significant differences between somatic areas of filled control (200 ± 40 μm2, n = 5) vs undercut FS cells (196 μm2 ± 69 μm2, n = 5; p > 0.05). However, the dendrites in UC cells appeared to be thinner (Figs. 1, 2) and analysis with Volocity showed that the dendrites of filled undercut FS interneurons were decreased in volume (volume/μm of dendritic length = 1.35 ± 0.06 μm3 in control, n = 5, and 0.49 ± 0.14 μm3 in UC cells, n = 5; p < 0.001; Table 3).
Figure 1.
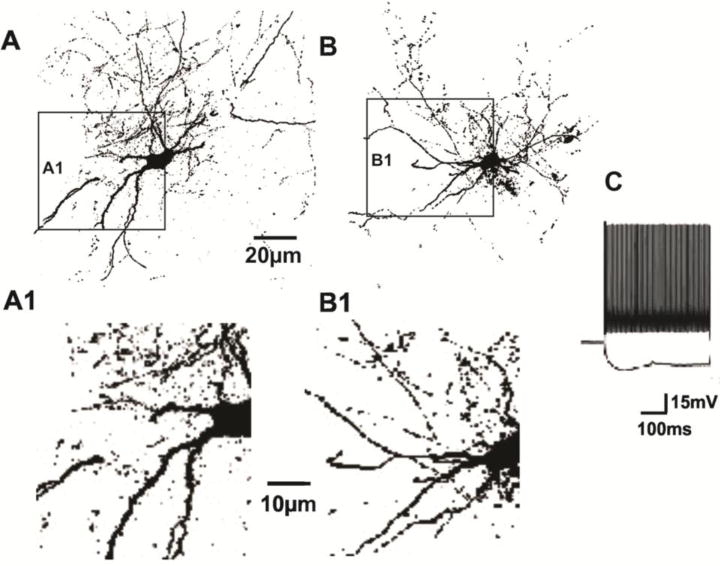
A, B Representative layer V biocytin-filled FS interneurons from control (A) and undercut rats (B). Scale bar: 20 μm for A, B. A1, B1: Images in rectangles of A, B enlarged ~2× to show decreases in thickness of dendrites in undercut 3 weeks after UC (B1) (see also Table 3). Calibration 10 μm for A1, B1. C: Responses of layer V FS interneuron to depolarizing and hyperpolarizing current pulses showing non-accommodating firing behavior.
Figure 2.
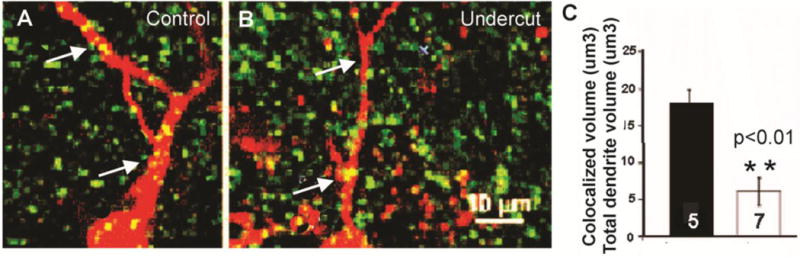
Close appositions of VGAT-IR with dendrites of FS cells. A–B: Confocal images of portions of biocytin-Texas Red-filled layer V FS cells in control (A) and undercut cortex (3 weeks after UC) (B). Green puncta: VGAT-IR. Yellow puncta: Sites of close apposition on somata and dendrites (arrows). Dendrites of UC cell (B) are thinner than those of control cell (A) (see also Fig. 1A1 vs. B1 and Table 3 for analysis of dendritic volume). C: Volume of closely apposed VGAT voxels as % of dendritic volume, analyzed for 5 control and 7 undercut layer V FS interneurons over 400–850 μm of primary/secondary dendritic length. Error bars: ±SEM. **: p < 0.01
Table 3.
Dendritic volumes
| Control | Undercut | ||||
|---|---|---|---|---|---|
|
| |||||
| Cell number | Dendritic length (um) | Dendritic volume/Length (vol/um) | Cell number | Dendritic length (um) | Dendritic volume/Length (vol/um) |
| Cell 1 | 823.4 | 1.45 | Cell 1 | 876.1 | 0.94 |
| Cell 2 | 659.6 | 1.34 | Cell 2 | 439 | 0.58 |
| Cell 3 | 389.5 | 1.15 | Cell 3 | 312.9 | 0.16 |
| Cell 4 | 832.1 | 1.5 | Cell 4 | 850.3 | 0.53 |
| Cell 5 | 799.2 | 1.3 | Cell 5 | 729.5 | 0.24 |
| Mean ± SEM | 700.8 ± 83.8 | 1.35 ± 0.06 | Mean ± SEM | 641.6 ± 113.0 | 0.49 ± 0.14 |
Dendritic length: p = 0.685, unpaired t test.
Dendritic volume/length: p < 0.001, unpaired t test.
N = 7 control, 5 UC rats
Fast-spiking interneurons provide GABAergic inhibitory synaptic input to other FS interneurons as well as Pyr cells (Galarreta and Hestrin, 1999; Gibson et al., 1999) and previous experiments have shown reductions in GABAergic inhibition onto FS cells in both the UC (Jin et al., 2011; Ma and Prince, 2012) and the freeze microgyrus model of developmental epileptogenesis (Jin et al., 2014). We therefore examined the incidence of close appositions between terminals containing VGAT and the membranes of biocytin-filled FS interneurons to determine whether there was anatomical evidence for loss of GABAergic inhibitory input. The images in Fig. 2 are representative of those obtained from confocal stacks of 7 biocytin-filled control FS cells (A) and 5 FS interneurons from undercut cortex (B) in sections reacted with VGAT antibody. The volume of voxels containing VGAT closely apposed to proximal dendritic membranes of biocytin-filled FS cells (arrows, yellow puncta in Fig. 2), was reduced significantly as a percent of dendritic volume in FS cells from UC versus control cortex (Fig. 2C), suggesting that inhibitory inputs to FS interneurons are reduced in UC cortex, as previously shown in laser scanning photostimulation experiments (Jin et al., 2011).
Axonal terminals of FS interneurons in UC cortex
FS GABAergic interneurons target somata, initial segments and proximal dendrites of pyramidal neurons in layer V (Chaudhry et al., 1998). We indirectly examined the FS interneuronal terminals onto Pyr cells immunocytochemically by reacting sections from control and undercut cortex for GAD 65, GAD67 and VGAT-IR and assessing the density of IR in perisomatic halos present around Pyr cell somata (see Methods and Fig. 3B, C). As shown in Figs. 3–6, and in other reports (Chu et al., 2009), (Tamas et al., 1997b)), halos of puncta of these GABAergic markers are prominent around Pyr cell somata. Double labeling with synaptophysin and PV confirmed that the perisomatic halos contained synaptic terminals of PV-containing FS interneurons (Fig. 3A, arrowheads). Analysis of the volume of VGAT-IR in the perisomatic ROI (Methods) showed that there were significant reductions in UC vs. contralateral layer V Pyr cells (Figs. 4, 5). In single high magnification images of layer V Pyr cells, control neurons showed almost continuous halos of VGAT/synaptophysin puncta closely applied to fluorescent Nissl-reacted somata (Fig. 4A–C), whereas there were gaps in halos of VGAT/synaptophysin puncta around Pyr cells from UC cortex 2 weeks after the undercut (c.f. Fig. 4A–C and D–F, arrows). Analysis with Volocity (Methods) showed that there were significant decreases in the perisomatic volume of VGAT-IR (−46%) and VGAT/synatophysin-IR (−67%) within the ROI in the UC (Fig. 4G). The time course of these changes was not examined, however similar reductions in perisomatic GAD65- and VGAT-IR were also present in UC vs. contralateral cortex 7 days after the UC (−39% VGAT; −61% GAD65; n = 20 cells from 4 control rats; 30 cells from 3 UC rats, p < 0.01; Fig. 5A, B; D, E; C, F). Because of the importance of GAD67 in regulating perisomatic inhibitory boutons and synapse formation during development (Chattopadhyaya et al., 2007), we used ICC to determine whether GAD67 might also be reduced in perisomatic halos of layer V Pyr cells in UC cortex. Analysis of sections reacted for PV and GAD67 showed a significant reduction of GAD67 in perisomatic PV halos in the UC cortex 2 weeks after UC (c.f. Figs. 6B, E and C, F). Taken together, these ICC results indicate that proteins/enzymes important in GABAergic transmission are depleted in perisomatic Pyr cell halos in the UC, either due to decreases in density of perisomatic synaptic axonal terminals of FS interneurons, as suggested by the images of Fig. 4D–F, and/or a decreased content of these substances in existing terminals.
Figure 3.
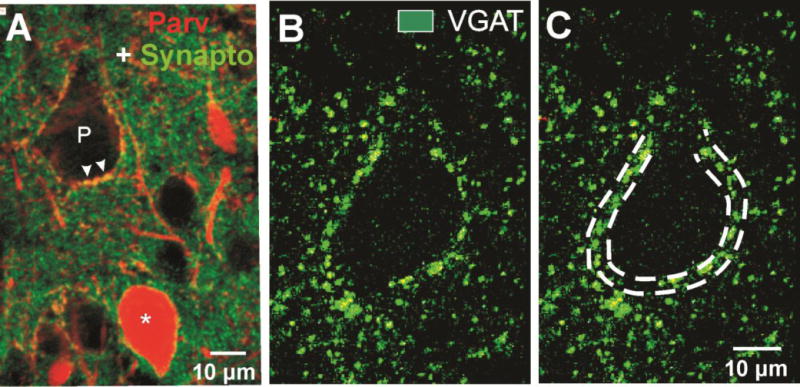
GABAergic terminals form perisomatic halos around layer V Pyr cell somata. A: Control section with dual immunoreactivity for parvalbumin (red) and synaptophysin (green). Perisomatic halo around Pyr cell (P) shows merged PV + synaptophysin IR (arrowheads). (*): PV interneuron. Scale bar: 10 μm. B–C: Perisomatic halos of VGAT-IR mark a layer V Pyr cell soma. Double dashed lines in C mark the ROI used for volumetric analysis of VGAT-IR in confocal sections (Methods).
Figure 6.
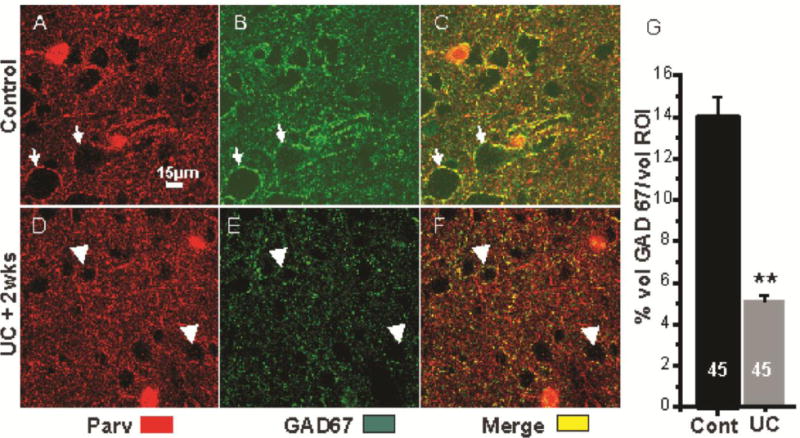
Reduced perisomatic GAD67-IR in UC. A–C: Immunoreactivity for PV (red), GAD67 (green) and PV+GAD67 (yellow) in 1 section through layer V of control cortex. Arrows: perisomatic halos around the same 2 Pyr cells in A–C. PV and GAD67 are colocalized in perisomatic terminals (yellow in C, merge). D–F: Comparable images to A–C in section from UC cortex 2 wks after lesion, processed with section of A–C. Arrows point to the same 2 cells in D–F. Perisomatic GAD67-IR is decreased (E vs. B, arrows). F: Merged D+E shows decreased GAD67 in PV-containing perisomatic halos (arrows). Somata of Pyr cells (voids) are smaller in UC sections (Prince and Tseng, 1993). Calibration in A for A–F. G: Percentage of GAD67-IR volume within the perisomatic ROI volume of layer V Pyr cells in control sections from 3 control and 3 UC rats, 15 cells/rat (see Methods).
Figure 4.
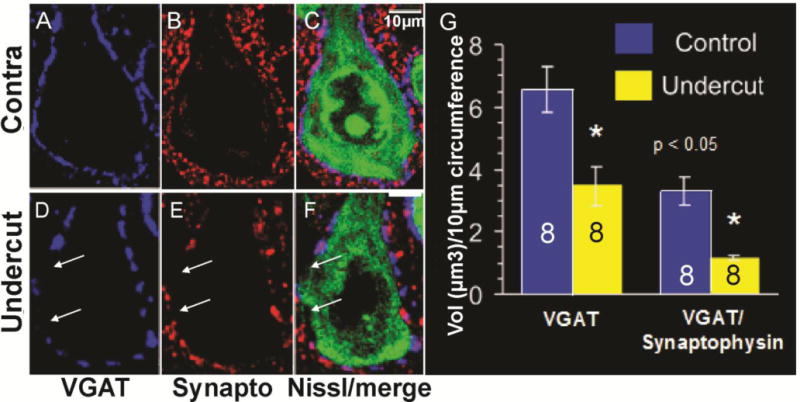
Perisomatic decreases in VGAT and synaptophysin around layer V Pyr cells in UC cortex. A–C: Control Pyr cell with halo of VGAT (A, blue) and synaptophysin (B, red) puncta around soma. C: Perisomatic magenta in merged image of A, B around fluorescent Nissl immunoreacted soma (green). D–F: As in A–C for a layer V Pyr neuron in UC cortex 2 weeks after UC. Arrows: Perisomatic areas with reduced VGAT/synaptophysin puncta. Scale bar in C: 10 μm for A–F. G: Volume (Vol) of voxels containing VGAT (left) and VGAT/synaptophysin (right) in perisomatic halos of control and UC layer V Pyr neurons per 10 μm somatic circumference, obtained using “train track” analysis (Fig. 2C) and Volocity (Methods). Numbers in bars: Data from 8 Pyr neurons in sections from 3 rats.
Figure 5.
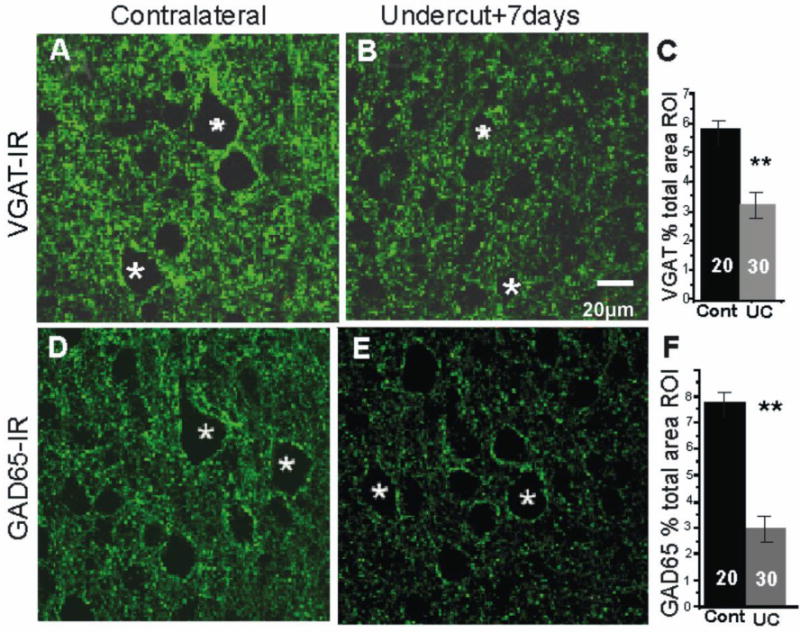
Pyramidal cell perisomatic VGAT- and GAD65-IR in layer V of undercut (UC) and contralateral cortex 7 days after UC. A, B: VGAT halos are less prominent around Pyr cells (asterisks) in UC (B) vs. contralateral cortex (A). D, E: GAD65-IR in representative sections from contralateral (D) and undercut cortex (E). Perisomatic halos of GAD65-IR around Pyr cells are also reduced in UC vs. contralateral (cont) cortex. C, F: Average volume of VGAT-IR-containing voxels (C) and GAD65-IR containing voxels (F) is significantly reduced in the UC cortex as a percentage of the ROI volume (Methods). Numbers in bars: number of Pyr cells. Data obtained from analysis of 10 Pyr cells from each of 3 UC rats and a total of 20 Pyr cells from 4 controls. **: p < 0.01. Scale bar in B for A, B and D, E.
To address these alternatives more directly, we next examined the expression of VGAT-IR in axonal boutons of biocytin-filled FS interneurons. Boutons of control biocytin-filled FS interneurons were identified as prominent swellings along their axons that were immunopositive for VGAT (Fig. 7A, B). We assumed that similar swellings, in which VGAT-IR was not detected on the same axons, were also GABAergic boutons. Multiple confocal 0.37 μm optical sections were used to categorize boutons based on maximum diameter, VGAT co-labeling and close apposition to postsynaptic gephyrin-IR. Boutons varied in size and were arbitrarily divided into groups with diameters > 1 μm (“big” boutons; arrows in Fig. 7) and those ≤ 1 μm (“small” boutons; arrowheads in Fig. 7D). Most boutons in control axons contained VGAT (e.g. Fig. 7C, arrows), and were often closely apposed to gephyrin-IR (not shown). However, in UC, axonal segments boutons with no obvious VGAT co-labeling were often seen in confocal sections 3–4 weeks after UC (Fig. 7D, arrowheads) and there were fewer profiles in which VGAT-containing boutons and gephyrin-IR were closely apposed (Fig. 7, graph D). Graphs A–D of Fig. 7 show an analysis of bouton size and close appositions to VGAT and gephyrin obtained 3 weeks after undercut. Graph A shows that big boutons make a significantly greater contribution to total boutons in control (~75%) vs. UC axons (~53%). By contrast, small boutons are increased as a percentage of the total in axonal segments of UC vs. control axons (Fig. 7, graph B; n = 9 control and 7 UC FS axons, each measured over axonal length of > 300 μm, p < 0.05). There were also significantly fewer small boutons containing VGAT-IR, or VGAT-IR in close apposition to gephyrin-IR in the axons from UC cortex (Fig. 7, graphs C, D). These results suggested that a portion of the reduction of VGAT-IR in perisomatic halos shown in Figs. 4D–G and 5A–C was due to reduced or absent VGAT content in individual boutons. In addition, the reduction in close approximations between presynaptic VGAT-containing boutons and postsynaptic gephyrin-IR indicated a possible reduction in inhibitory synapses in the undercut cortex. Glutamic acid decarboxylase 65- and 67-IR were also significantly reduced in the perisomatic halos on Pyr cells (Fig. 5D–F; Fig. 6B vs. E; Fig. 6G), suggesting that there might be altered capacity to synthesize GABA. Similar reductions in GAD65-IR in perisomatic terminals occur in the freeze microgyrus model of epileptogenesis following focal injury to developing neocortex (Chu et al., 2009).
Figure 7.
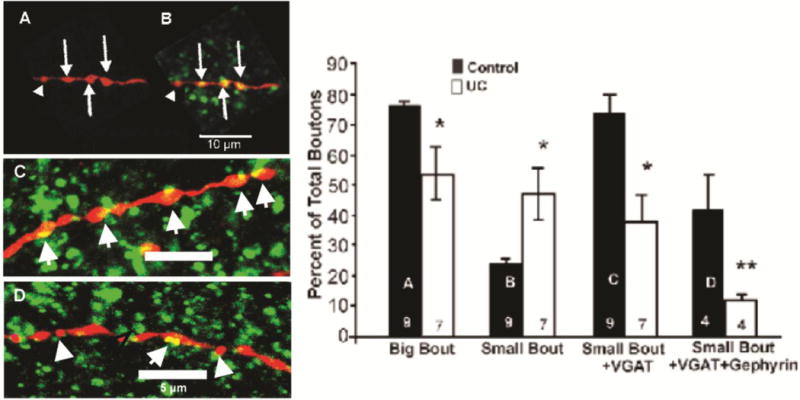
Analysis of axonal boutons of biocytin-filled FS interneurons. A: Confocal image of control axonal segment filled with biocytin (red) showing several big boutons (arrows) and small bouton (arrowhead). B: Same segment reacted for biocytin and VGAT (green) shows prominent colocalizations (yellow) of VGAT-IR in boutons. Arrows in merged image of B point to bigboutons > 1 μm diameter. C, D: Biocytin-filled axonal segments of FS interneurons from control (C) and UC cortex 3 weeks after lesion (D) (axonal segments red; VGAT-IR green; sites of close apposition: yellow; as in B. C: Arrows point to big boutons containing VGAT (yellow), D: Axonal segment from FS cell in UC cortex. Arrow points to bouton containing VGAT. Arrowheads: small boutons without VGAT-IR. Scale bar in A for A–B; bars in C, D: 5 μm.
Graphs A–D show distribution of bouton subclasses in FS interneuronal axons. A–B: Big boutons (A) and small boutons (B) as a percentage of total boutons in 9 control and 7 UC cells for total axonal lengths of 3321 μm and 2527 μm, respectively. C: Small boutons labeled with VGAT-IR as percentage of total along same axonal lengths as A, B. D: Boutons immunoreactive for VGAT in close apposition to gephyrin as percentage of total along axonal lengths of 2321 μm and 1927 μm analyzed in 4 control and 4 undercut FS cells, respectively. Error bars: ±SEM. *: p < 0.05; **: p < 0.01
Electron microscopy
The decreased numbers of close appositions of VGAT and gephyrin (Fig. 7, graphs C, D), and previous results showing decreased mIPSC frequency in layer V Pyr cells in the UC (Li and Prince, 2002), might have resulted from injury-induced decreases in inhibitory synapses. To more directly determine the density of inhibitory innervation of the somata of layer V Pyr cells, we obtained quantitative electron microscopic data from sensory-motor cortex in 2 naïve control rats and from undercut and contralateral cortex in 3 undercut rats, 2–3 weeks after UC. The most characteristic feature of the neocortical layer V in control animals was the presence of large Pyr neurons surrounded by normal appearing neuropil (i.e., without pathological signs of degenerating neurons and/or gliosis). Numerous axonal terminals were attached to the somatic membrane of layer V Pyr neurons where they made synaptic contacts, predominantly of the symmetric type (> 95% of all axo-somatic synapses) (Fig. 8D). The morphological features characteristic of symmetric synapses are commonly associated with an inhibitory synaptic function, as also demonstrated in this study by immuno-positivity for GAD65 in these synapses Fig. 8C).
Figure 8.
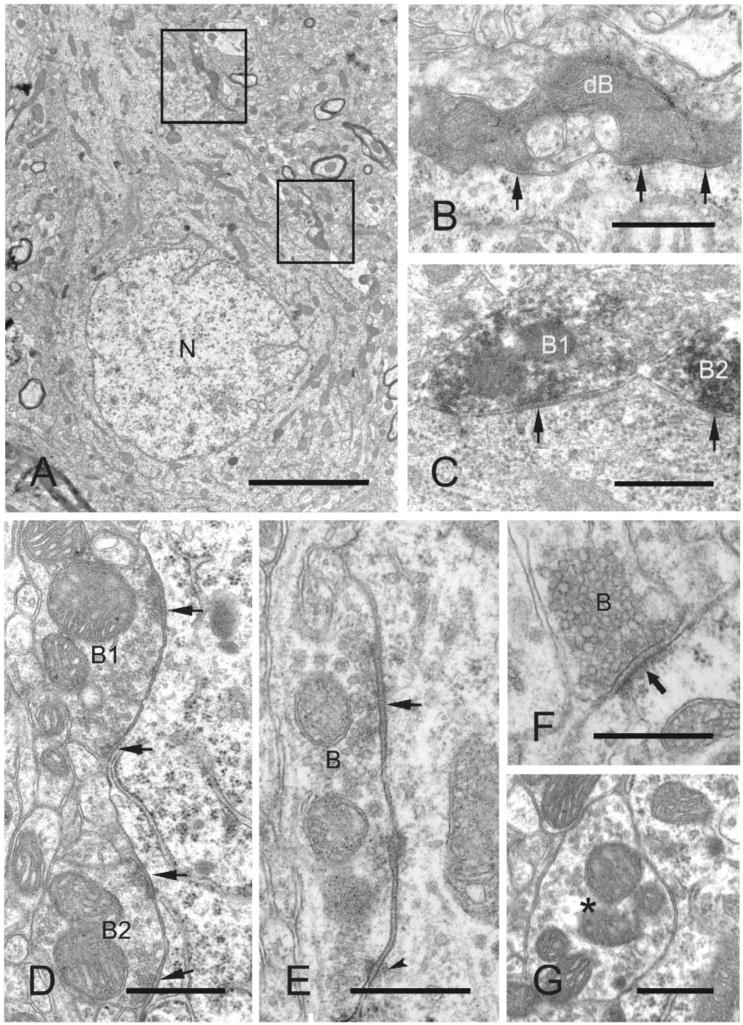
Electron microscopic analysis of symmetric (inhibitory) and asymmetric axosomatic synapses on layer V pyramidal neurons in naïve sensory-motor cortex (control, n = 2 rats) and undercut and contralateral cortex (undercut, undercut contra, n = 3 rats). 25 micrographs examined from each of 2 specimens/rat. A: Electron micrograph of a layer V pyramidal neuronal soma from undercut neocortex showing the nucleus (N), cytoplasm, and degenerated axonal boutons (boxed areas, one of which is shown enlarged in B) making synapses with the soma. B: Higher magnification of a degenerated axonal bouton (enpassant) exhibiting multiple synaptic contacts (arrows) with the soma. C: Electron micrograph of GAD65 immuno-positive axonal boutons (dark reaction product; B1, B2) exhibiting symmetric synapses (arrows) with the soma of a layer V pyramidal neuron. D: Electron micrograph of axonal boutons (B1, B2) making multiple symmetric synapses (arrows) with the soma of a neocortical layer V pyramidal neuron from a control rat. E: Electron micrograph of an axonal bouton (B) exhibiting a symmetric synapse (arrow) and puncta adherentia (arrowhead) with the soma of a layer V pyramidal neuron within the undercut neocortex 3 weeks after UC. F: Electron micrograph of an asymmetric synapse (arrow) between an axonal bouton (B) and the soma of a layer V pyramidal neuron from a control animal. G: Electron micrograph of a bouton (asterisk) containing mitochondria and synaptic vesicles adjacent to a layer V pyramidal neuronal soma, but without a synapse at this section plane. Scale bars: A, 4 μm; B–F, 500 nm; G, 1 μm.
In the chronically undercut cortex, there were histopathological features distinct from the normal neocortical ultrastructure. As reported earlier (Gruner et al., 1974); (Prince and Tseng, 1993) and confirmed in the present experiments (Supplemental Fig. S4A, A1), the total number of large Pyr neurons in layer V of the undercut cortex appeared to be reduced at 3 weeks post-lesion. Some degenerating neurons were still present. The glial reaction was particularly evident as microglial phagocytosis within the margins of the undercut cortex, but also observed as mild diffuse gliosis in all layers (Fig. S2B, D), (Li et al., 2012). Dark degenerating axonal terminals, with preserved ultrastructure of symmetric synapses, were occasionally seen on cell bodies of layer V Pyr neurons (Fig. 8A–C.)
A quantitative analysis of the distribution of axo-somatic synapses onto the somatic membrane of layer V Pyr neurons was carried out in control and undercut cortices. This analysis revealed that a small proportion of the somatic cell membrane in all experimental groups was in contact with axonal terminals/boutons (e.g., on average 0.33 boutons per μm membrane length in control animals, and 0.28 boutons per μm in undercut animals) (Fig. 9B). These boutons made synaptic contacts with the somatic membrane, primarily of the symmetric type. Axonal boutons attached to cell bodies of layer V Pyr neurons in the undercut cortex exhibited significantly fewer synapses (on average 0.08 boutons with synapses per μm membrane length) as compared with control cortices (0.12 in naïve controls and 0.11 in contralateral cortex controls) (Fig. 9A).
Figure 9.
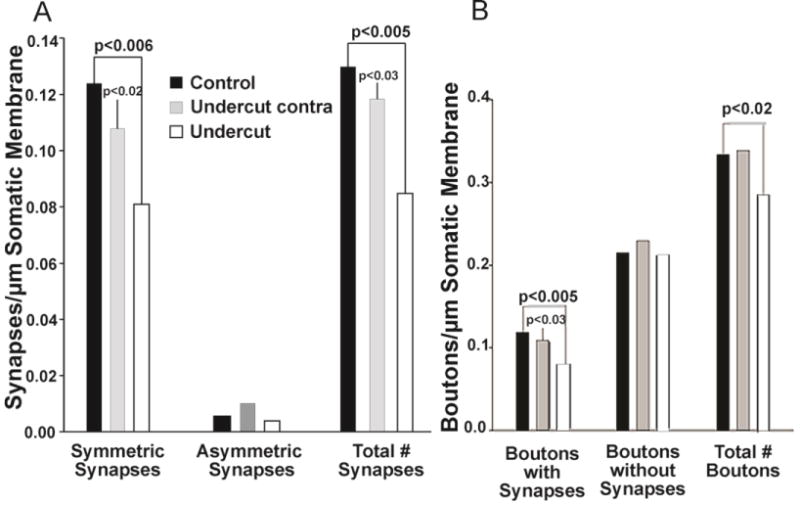
Quantitative assessment of synaptic pattern on layer V pyramidal neurons in the partially isolated undercut neocortex as compared with control cortices. A: Analysis of synapses on somata of layer V pyramidal neurons of both control and undercut animals revealed a majority of synapses of the symmetric type (right 2 graphs). The average number of symmetric synapses per μm somatic membrane length in undercut neocortex is significantly reduced as compared with control cortices (left graph). There are no significant differences in numbers of asymmetric synapses between the cortices of different animal groups (middle graph). These data suggest a selective reduction of inhibitory inputs on layer V pyramidal neurons within the undercut neocortex 2–3 weeks after UC. B: The quantitative analysis revealed that only about one third of boutons in contact with layer V pyramidal neuronal somata displayed synaptic specializations (left vs. right graph). There are no differences in the numbers of boutons without synapses across different treatment groups (middle graph). However, the number of boutons with synapses in undercut neocortex is significantly reduced as compared with control cortices.
As predicted from the immunocytochemical data, and previously reported at the margins of isolated cortical slabs in cat (Ribak et al., 1982), a more detailed analysis of the distribution of different synaptic types (i.e., symmetric or asymmetric synapses) revealed a very significant reduction in the density of symmetrical (inhibitory) synapses on somata of the layer V Pyr cells in undercut cortex vs. the corresponding neocortical region of control animals (~.082 vs 0.123 synapses/um somatic membrane, p = 0.006, 33% reduction; Fig. 9A) and a smaller, but still significant reduction compared to contralateral cortex of undercut rats (p = 0.02) (Fig. 9A, left graph). In addition, there was a trend toward reduced number of synapses (total and symmetric) in cortex contralateral to the undercut compared to control cortex, suggesting that the contralateral cortex was also involved in the process of deafferentation (Fig. 9A). Asymmetric synapses were rarely observed onto somata of layer V Pyr neurons in control and undercut cortex (non-significant differences) (Fig. 9A, middle graph). Finally, puncta adherentia were a common feature of neocortical Pyr neurons, and were frequently observed in axonal terminals near the synaptic region; there was a non-significant trend for more of these cellular contacts in axonal boutons in undercut cortex.
There were no significant changes in the density of asymmetric (excitatory) synapses on somata of Pyr cells, where they normally are sparse (Fig. 9A, middle graph), so that the significant reduction in total somatic synapses (Fig. 9A, right graph) mainly reflected the loss of symmetrical synapses. Counts of presynaptic boutons, defined as terminals containing vesicles, showed the expected reduction in boutons associated with postsynaptic structures (Fig. 9B, left graph) and total number of boutons in sections from the undercut (Fig. 9B, right graph). Measurements of the numbers of presynaptic terminals containing synaptic vesicles where no postsynaptic structure could be identified (Fig. 9B, middle graph), showed that there was no reduction in such structures in undercut tissue. Because serial EM was not done, it was not possible to determine whether presynaptic terminals without apparent postsynaptic structures were contacting the soma in an adjacent section, or to further examine small vs. big boutons.
Expression of BDNF/TrkB in UC cortex
Because of the known trophic effects of BDNF via TrkB receptors on interneuronal structure and function (Marty et al., 1996; McAllister et al., 1999); (Jin et al., 2003); (Rutherford et al., 1997); (Elmariah et al., 2004), and the possibility that the injury and deafferentation inherent in the UC model would decrease activity-driven BDNF expression (Jiao et al., 2011); (Lin et al., 2008); (Hong et al., 2008); (Sakata et al., 2009), we assessed effects of UC on BDNF-IR in layer V Pyr cells 7 days (15 cells from 3_sections/rat; 3 control and 3 UC rats) (Fig. 10) and 3 weeks after UC (20 cells from 3 sections from 3 control and 3 UC rats) (Fig. 11E, F, H). BDNF-IR was significantly reduced (p < 0.01) by about 43% 7 days after the UC (Fig. 10G); (compare Fig. 10B with E; C with F; C1 with F1). A similar reduction in BDNF-IR was present 21 days after the lesion (Fig. 11E vs. F; ~42% decrease in Fig. 11H; p < 0.01). To confirm the ICC results, in situ hybridization was used to measure BDNF mRNA in UC cortex 3 weeks after UC lesions in 23 layer V Pyr cells from 4 sections each in 3 control and 3 undercut rats (Fig. 11A–D; G). BDNF mRNA was reduced in UC versus control cortical Pyr neurons (p < 0.01) by ~50%/μm2 of soma area (Fig. 11G; Methods); (compare Fig. 11A with B; C with D). TrkB-IR was also decreased in 3 undercut vs. 3 control rats, 3 weeks after surgery (Fig. 12 A, C). Dual ICC showed that close appositions of TrkB-IR on PV interneuronal somata were significantly reduced (Fig. 12B, D, E; p < 0.005). Taken together, results indicate that significant prolonged reductions in BDNF and TrkB expression occur in the partially isolated cortex.
Figure 10.
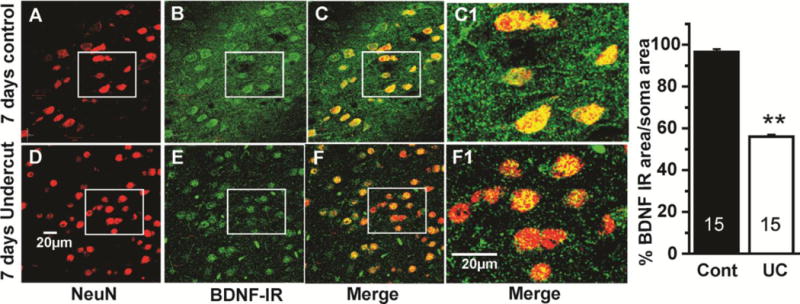
Decreased BDNF-IR in UC neocortex 7 days after injury. A–C: Confocal images from a section through layer V of control neocortex showing neurons with immunoreactivity for NeuN (red, A), BDNF (green, B) and merged A+B (yellow, C, C1). White boxes mark same group of control neurons in A–C, and an UC group in D–F below. D–F: Confocal images from a comparable section through layer V of undercut cortex, immunoreacted as in A–C. C1, F1: Boxes in C, F enlarged ~3.4×. BDNF-IR is decreased in neurons and in neuropil of UC cortex (c.f. yellow in C, F; C1, F1). Calibration in D: 20 μm for A–F; in F1: 20 μm for C1, F1. G: Graph of percentage of soma area occupied by BDNF IR in Pyr cells of control vs. UC cortex. Numbers in bars: number of cells. **: p < 0.01.
Figure 11.
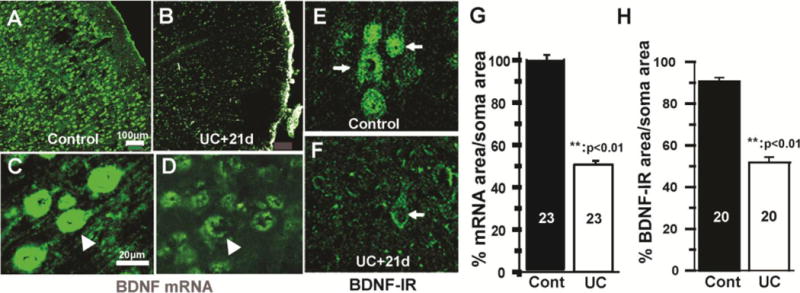
Decreases in BDNF mRNA and immunoreactivity in undercut cortex 21 days after lesion. A–B: Representative confocal images from control (A) and UC cortex (B) showing marked reductions in BDNF mRNA (green) in the UC. C–D: Higher magnification from control (C) and undercut layer V (D) showing decreased in BDNF mRNA in layer V Pyr cells (arrows) after undercut (D). Calibration in A for A–B and in C for C–F. E–F: Confocal images of BDNF-IR from layer Va in another UC rat showing marked reduction in BDNF expression in UC (F) compared to control cortex (E). Arrows: pyramidal cells. G–H: Graphs of BDNF mRNA expression (G) and IR (H) in Pyr cell somata of UC and control cortex (cont) (H). See Methods. **:p < 0.01
Figure 12.
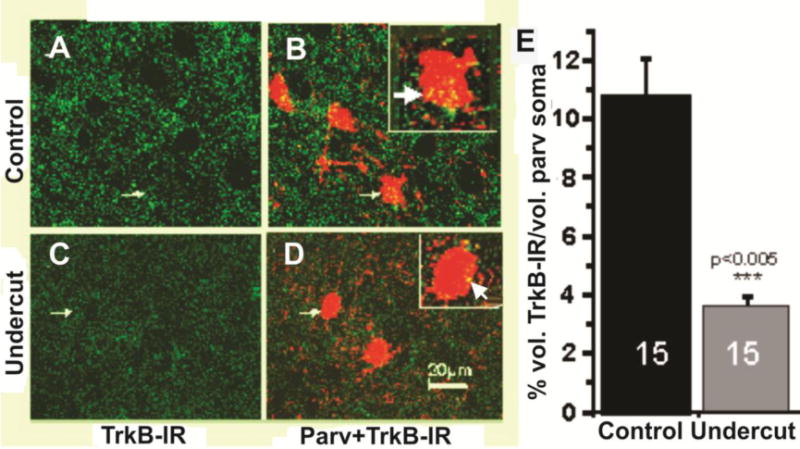
Decreases in TrkB in undercut cortex. A, C: Representative confocal 0.5 μm optical sections showing TrkB-IR (green) in layer V control (A) and within UC neocortex 3 weeks after undercut (C) showing marked reductions in UC. Arrows: PV-containing interneurons shown double labeled for PV-IR + TrkB-IR (yellow) in B, D. B,D: Confocal images of the sections of A,C from control (B) and undercut (D) immunoreacted for TrkB (green) + PV (red). Calibration in D: 20 μm for A–D. Small arrows: PV interneurons enlarged ~2× in white boxes. Yellow pixels: close appositions of TrkB- and PV-IR that are more prominent on somata of control (white arrow in box of B vs. D). E: Graph of mean colocalization volume of TrkB-IR as a percentage of somatic volume obtained from confocal image stacks of 15 UC layer V PV interneurons and 15 control interneurons in sections from 3 control vs. 3 undercut rats (Methods). Control: 11 ± 4% SD; undercut: 3.2 ± 1% SD. ***: p < 0.005.
Discussion and Conclusions
These results show that layer V PV-containing FS interneurons undergo structural alterations in their axonal terminals and dendrites, without a significant decrease in cell numbers, in the rat partial neocortical isolation model of posttraumatic epileptogenesis. Abnormalities are also present in infragranular Pyr cells, including decrease in soma size and cell loss. The abnormalities in axonal terminals and decreases in density of inhibitory synapses on Pyr cell somata found in these experiments would contribute to the previously-reported presynaptic alterations in GABAergic synaptic transmission onto both pyramidal cells and interneurons in this model, including decreases in Pr and mIPSC frequency, decreased uIPSC amplitude and increased failure rate (Ma and Prince, 2012);(Faria and Prince, 2010) (Li and Prince, 2002).
There were prominent decreases in intensity of perisomatic halos of VGAT-, GAD65- and GAD67-IR in the UC cortex. The analysis of boutons in filled axons (Figs. 7) and EM results (Figs. 8, 9) indicate that both reductions in expression of these GABA markers in individual boutons and a decrease in inhibitory synapses may contribute to the alterations in intensity of halos. The decreased expression of VGAT-, GAD65- and GAD67-IR or decreased bouton size in inhibitory terminals cannot be attributed to the enhanced excitation of interneurons present in the epileptogenic cortex of the UC model (Jin et al., 2014), as increases in activity levels can increase expression of these proteins (De Gois et al., 2005) and increase bouton size (Hanno-Iijima et al., 2015).
There was a shift in bouton size in UC vs. control axons, with increases in small (< 1 μm) and decreases in large (> 1 μm) boutons in the filled axons of FS cells in the UC cortex (Fig. 7). Because most large and some small swellings on a given axon contained VGAT and were closely apposed to postsynaptic gephyrin clusters, we assume that similar adjacent swellings unassociated with these markers are also boutons, rather than axonal beading resulting from traumatic injury (e.g. (Kilinc et al., 2008). Bouton size is an important structural variable at glutamatergic synapses, where it is directly correlated with other anatomical measures that predict synaptic efficacy, including the number of postsynaptic active zones, their size and total area, and the number of synaptic vesicles (Pierce and Lewin, 1994; Harris and Sultan, 1995; Tamas et al., 1997a). Recent results suggest that there is a similar relationship between bouton size and postsynaptic inhibition at inhibitory synapses (Jiao et al., 2006); (Villa et al., 2016). Alterations in short-term dynamics of transmitter release, such as decreased Pr and increased failure rate, might therefore be a consequence of activation of terminals with small boutons containing fewer vesicles. However, relationships between bouton size and parameters of postsynaptic GABAergic currents may not be as predicted in models of epileptogenesis (Buckmaster et al., 2016).
Previous reports suggest two mechanisms that might underlie the shift to a larger proportion of small axonal boutons in the UC FS interneurons. Deafferentation of immature neocortex can result in reductions in the density and size of perisomatic GABAergic boutons on Pyr cells (Figs. 4–7), that have been attributed to decreases in BDNF (Jiao et al., 2006; Jiao et al., 2011), such as are present in UC cortex in our experiments (Figs. 10, 11). Interneuronal axonal sprouting (e.g. (Bausch, 2005; Peng et al., 2013) might also be associated with decreases in bouton size, as occurs in sprouting Pyr cell axons in UC cortex (Salin et al., 1995). Additional experiments will be required to assess whether small boutons are associated with axonal sprouting in PV cells of the UC and the functional effects of bouton size on GABA release from single boutons in this injury model.
There are several potential mechanisms by which the UC injury may induce the alterations in interneuronal structure described here, leading to functional abnormalities found in previous electrophysiological experiments. Direct trauma to Interneurons might play a role, as FS interneurons have extensive local axonal and dendritic arbors within the neocortex (Uematsu et al., 2008), portions of which might be cut or stretched in the UC, resulting in a more extensive cellular reaction affecting axonal terminals and dendrites. Complete axonal and dendritic arbors were not analyzed, so such direct injury or amputation of processes would not have been detected. Preservation of total PV- and GABA-immunoreactive cell numbers in UCs (Figs. S3, 4) argues against significant direct injury. In this respect, our results differ from those reported in UC suprasylvian gyri of adult cat cortex where reductions in total GABAergic cell counts 2–6 weeks after UC lesions were reported (Avramescu et al., 2009). This may be due to species differences or other variables, such as the ages at which UCs were done and interval between injury and anatomical experiments.
Axotomy of Pyr cells and deafferentation inherent in the UC model provide two other mechanisms that likely underlie the structural and functional alterations in GABAergic inhibition in the UC cortex. Perisomatic “stripping” of somatic inhibitory synapses and decreased functional inhibition occur in axotomized motoneurons of spinal cord and brain stem (Kuno and Llinas, 1970) (Takata, 1981; Delgado-Garcia et al., 1988), and there is decreased incidence and efficacy of evoked IPSPs in spinal axotomized layer V corticospinal neurons (Tseng and Prince, 1996). Lesions made at the junction of layer VI and underlying white matter in the UC model (Graber and Prince, 2006) sever cortical efferent axons of layer V Pyr neurons and result in structural alterations in these cells, including decreases in their inhibitory innervation and size (Tseng and Prince, 1996) (Prince and Tseng, 1993). EM data in UC cortex show loss of somatic inhibitory synapses (Fig. 9A) and increases in numbers of presynaptic terminals that appear to be unassociated with postsynaptic structures (Fig. 9B). These changes might be a consequence of such stripping and are compatible with decreases in mIPSC frequency without amplitude changes in layer V Pyr neurons in UC cortex, Serial EM will be required to determine whether these presynaptic structural alterations are associated with inhibitory synaptic stripping or another process such as terminal withdrawal following injury to Pyr cells (Chen et al., 2014). The mechanisms leading to synaptic stripping may involve perineuronal activated astrocytes and microglia (Blinzinger and Kreutzberg, 1968; Chen, 1978); (Yamada et al., 2011); (Chen et al., 2014), such as are present in the UC (Fig. S2 B, D, F), as well as complement C3-mediated selective removal of inhibitory synapses (Berg et al., 2012).
Loss of trophic support by BDNF/TrkB signaling is another mechanism that could contribute to the structural and functional abnormalities in interneurons in the UCs. BDNF is a critical trophic factor for development and maintenance of interneurons; that also has protective effects on corticospinal Pyr neurons after axotomy (Brock et al., 2010); (Giehl et al., 1998; Jin et al., 2003). The partial neocortical isolation is an injury/deafferentation model of epileptogenesis in which there is interruption of thalamocortical, callosal and intracortical afferents that would result in decreased baseline activity. Such reductions are present in the first few days after UC lesions in partial isolations in cat (Sharpless and Halpern, 1962); (Timofeev et al., 2010) and in the controlled cortical impact model of traumatic injury in mice (Ping and Jin, 2016b). Loss of innervation in the UC might make interneurons vulnerable to structural and functional alterations, such as the loss and decrease in size of boutons on Pyr cell somata and decreases in parameters of inhibitory synaptic transmission as occurs in deafferented immature barrel cortex (Jiao et al., 2006). UCs are usually made at P21–28, ages when such lesions might affect ongoing maturation of PV-containing GABAergic interneurons (Bergmann et al., 1991);(Alcantara et al., 1993); (Chattopadhyaya et al., 2004). One mechanism underlying such effects is reduction in activity-driven BDNF expression (Jiao et al., 2011); (Lin et al., 2008); (Hong et al., 2008); (Sakata et al., 2009). Corticospinal Pyr neurons are a major source of BDNF and loss of BDNF in single Pyr cells due to deafferentation or axonal injury would reduce their GABAergic innervation and their mIPSC frequency (Kohara et al., 2007), presumably due to loss of paracrine effects of released BDNF on adjacent presynaptic terminals of interneurons. Both BDNF mRNA and IR were significantly reduced in injured layer V Pyr cells 3 weeks after UC, with IR reduced even 7 days after UC (Fig. 10, 11). TrkB-IR was also decreased on PV interneuronal somata in the UC (Fig. 12), which would result in a decrease the trophic BDNF actions on these neurons. Decreased BDNF activation of TrkB receptors might also contribute to decreased dendritic volume in UC interneurons (McAllister et al., 1995).
However, reductions in activity due to injury and loss of afferents to the UC appear to be time-limited and may not account for the decreases in BDNF expression present at later time points (Fig. 11C, D). Extensive increases in excitatory innervation of Pyr axons onto both Pyr cells and interneurons (Jin et al., 2011) (Jin et al., 2006) are present in the UC and contribute to enhanced excitation in cortical circuits. Epileptiform activity emerges as early as 3 hours (Topolnik et al., 2003) or 3 days after the cortical injury (Takahashi et al., 2016) (see also (Ping and Jin, 2016b); and persists for weeks or months in vitro (Prince and Tseng, 1993) (Li and Prince, 2002; Jin et al, 2006; Jin et al, 2010) and in vivo (Sharpless and Halpern, 1962) (Nita et al., 2007). This enhanced activity may be inadequate to maintain control levels of BDNF expression in the deafferented UC cortex. Finally, the reduction in GAD67 in UC cortex (Fig. 6) may also be related to some of the anatomical abnormalities, as decreases in this enzyme in developing neocortex result in defects in perisomatic GABAergic innervation of Pyr cells, including reductions in bouton density and size, similar to those described here (Chattopadhyaya et al., 2007); but see (Georgiev et al., 2016).
As emphasized in studies of interneurons in hippocampus (Magloczky and Freund, 2005; Houser, 2014), relationships between interneuronal structural alterations and epileptogenesis in cortical circuits are complex and hard to predict for a number of reasons, including differential effects of injury on subtypes of interneurons; axonal sprouting of surviving cells and reorganization of synaptic connectivity over time; potential alterations in postsynaptic GABA actions; and concurrent effects of injury on excitatory neurons and glia. Emphasis has often focused on epileptogenic effects of interneuronal loss, however decreased function of surviving interneurons may also contribute to abnormal network activity. As shown here, counts of interneurons are inadequate to determine whether GABAergic inhibition is affected in models of injury and epileptogenesis. The partial cortical isolation differs from other lesion-induced models of epileptogenesis, such as fluid percussion, controlled cortical impact, stroke, or prolonged status epilepticus, where there is usually cortical cavitation, substantial cell loss and involvement of hippocampus and other structures. However, structural and functional abnormalities in surviving interneurons due to decreases in trophic support may be a ubiquitous consequence of such injuries. The availability of a selective small molecule partial agonist at the TrkB receptor that can modify pathophysiology in other disease models (e.g.(Schmid et al., 2012); (Kron et al., 2014), (Massa et al., 2010) may provide an experimental approach to modify or reverse structural and functional abnormalities in PV interneurons and their pathophysiological consequences, such as cognitive disorders and epileptogenesis in cortical circuits.
Supplementary Material
Figure S1: Neuropathological alterations in UC cortex. A: Coronal section from rat 3 wks after UC reacted with 68 kD neurofilament antibody shows increased neurofilament-IR in undercut vs. contralateral hemisphere. Arrows point to edges of the partial cortical isolation. B: Neuropil and single pyramidal cells (double arrows), as well as presumptive interneuron (single arrow) from undercut area of A show increased neurofilament immunoreactivity (IR). C, D: Sections from layer V of partially isolated (D) and contralateral cortex (C) reacted with a 200 kD neurofilament antibody 3 days after UC surgery. Intense IR is present in lesioned area. Scale bar in C for C–D.
Figure S2: Activation of astrocytes and microglia in UC cortex. A, C, E: Immunoreactivity for GFAP (A), Cd11b (microglia) (C) and merged A + C (E) in layer V of control cortex. B, D, F: Images as in A, C, and E, 10 d after undercut (UC) showing reactive astrocytes and microglia (arrows). Calibration bar in A: 20 μm for A–F
Figure S3: Total number of neurons is decreased in UC cortex without concomitant reduction in PV- containing interneurons. Confocal images of NeuN (A) and PV neurons (B) in layers I–VI of control and UC cortex 5 weeks after injury from naive (control) and partially isolated (UC) cortex. A1–B1: Graphs of NeuN and parvalbumin (PV) cell counts (Methods) from control (black bars) and UC rats (white bars). Numbers in bars: number of grids counted. C: High magnification image of PV cells from undercut side (arrows). * p < 0.05. Calibration bars in control segments of A, B:100 μm for all segments.
Figure S4: Cell counts for NeuN- (A) and GABA-IR (B) in UC and control cortex. Images and cell counts show no reductions in GABA cell density in the presence of reduction in total NeuN-IR neurons 5 weeks after UC. A1: NeuN mean counts: 952.8 cells, SEM 49.65 in control and 793.3 cells, SEM 44.01 in UC cortex. p = 0.027. White circles in A: Comparable areas in UC and Control layer V show decreased size of NeuN profiles and decreased density of neurons in UC. Numbers in bars in A1, B1: numbers of grids counted. GABA mean counts: 65.4 cells, SEM 4.17 in control (n = 10) and 59.8 cells, SEM 6.37 in UC cortex (n = 10 grids).p = 0.47. PV mean counts were 50.42 cells, SEM 2.71 in control (n = 7) and 50.14 cells/grid, SEM 4.00 in UC cortex (n = 7 grids). P: 0.96
Figure S5: Schematic of undercutting methods. A: View of rodent neocortex and underlying white matter in the parasagittal plane. 1: Fine gauge needle, bent 90 degrees from the tip, is inserted at a near-tangential angle through dura and beneath the pia into superficial cortex. 2: Shaft is aligned normal to pial surface, raised slightly through layer I (not shown) sparing pial vasculature and then depressed through all cortical layers to underlying white matter, creating a transcortical cut (gray shading in 3). 3: Needle is rotated laterally 180 degrees to create a white matter undercut (dark line in 4, 5). 4: Needle is raised to extend the transcortical cut (gray shading). 5: Needle is removed through the point of insertion. B: Schematic of different types of cortical and white matter transactions. Bilateral lesions shown only for illustrative purposes. 1: Steps in A used to create a longer transcortical cut (dark line; small dark circle represents point of needle insertion) and underlying white matter undercut (gray shaded semicircle). 2: Associated lateral transcortical cut (dark line) placed without needle rotation can be used to create a more complete isolation (“U”-shaped in coronal brain slices. 3: Rotation of the depressed needle approximately 90 to 135 degrees can be used to create smaller lesion with two adjacent transcortical cuts. 4: Rostral (or caudal, not shown) transcortical cuts can also be used for more extensive isolation. C: Typical recording of field potentials from two electrodes spaced 1.5 mm apart in layer V demonstrates transcortical propagation of evoked interictal discharges. Dot: stimulus at white matter—layer VI junction. Actual slice contained only unilateral lesion. From (Graber and Prince, 2006) with permission.
Significance.
Injury to cortical structures is a major cause of epilepsy, accounting for about 20% of cases in the general population, with an incidence as high as ~50% among brain-injured personnel in wartime. Loss of GABAergic inhibitory interneurons is a significant pathophysiological factor associated with epileptogenesis following brain trauma and other etiologies. Results of these experiments show that the largest population of cortical interneurons, the parvalbumin-containing fast-spiking (FS) interneurons, are preserved in the partial neocortical isolation model of partial epilepsy. However, axonal terminals of these cells are structurally abnormal, have decreased content of GABA synthetic enzymes and vesicular GABA transporter and make fewer synapses onto pyramidal neurons. These structural abnormalities underlie defects in GABAergic neurotransmission that are a key pathophysiological factor in epileptogenesis found in electrophysiological experiments. BDNF, and its TrkB receptor, key factors for maintenance of interneurons and pyramidal neurons, are decreased in the injured cortex. Results suggest that supplying BDNF to the injured epileptogenic brain may reverse the structural and functional abnormalities in the parvalbumin FS interneurons and provide an antiepileptogenic therapy.
Highlights.
Numbers of pyramidal (Pyr) cells in infragranular layers and their soma sizes are decreased in epileptogenic partially isolated neocortex (undercut, “UC”), without a comparable reduction in GABAergic interneurons.
Structural alterations in terminals of fast-spiking parvalbumin (PV)-containing interneurons occur in UC, consistent with previously reported presynaptic functional abnormalities in GABAergic synaptic transmission.
Vesicular GABA transporter (VGAT) and GABA synthesizing enzymes GAD65 and 67 are decreased in perisomatic halos of terminals of FS interneurons on pyramidal somata.
Boutons of FS interneurons in UC are smaller and fewer contain VGAT in close apposition to postsynaptic gephyrin, suggesting a reduction in GABAergic synapses.
Quantitative EM shows a reduction in symmetrical (inhibitory) synapses on layer V Pyr somata in UC
Immunocytochemistry and in situ hybridization show significant decreases in BDNF in UC Pyr cells and reductions in TrkB-IR on PV cell somata.
We hypothesize that injury to Pyr cells and deafferentation in the UC lead to a decrease in BDNF activation of TrkB receptors on interneurons and consequent structural and functional alterations that decrease GABAergic inhibition.
Acknowledgments
Supported by Grants NS06477; NS12151; NS39579 from the NINDS (DAP) and the Edward and Irene Thiele Pimley Research Fund.
Abbreviations
- GABA
gamma-amino-butyric acid
- VGAT
vesicular GABA transporter
- GAD-65, -67
glutamic acid decarboxylase 65, 67
- UC
undercut or partial neocortical isolation
- FS
fast-spiking
- BDNF
brain derived neurotrophic factor
- PV
parvalbumin
- TrkB
tropomyosin receptor kinase B
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Methods: Counts of PV -containing interneurons: Images were analyzed using two computer programs: iLastik and Cell Profiler available at http://ilastik.org/documentation/pixelclassification/pixelclassification.html) and Cellprofiler.org. To enable iLastik to distinguish somata from neuropil and fibers, four raw confocal images of PV cells were used to “train” the pixel classification module of the program, enabling it to separate somata (foreground) from the background (fibers). After the program’s accuracy in detecting cell bodies was confirmed, the remaining images were analyzed automatically by the same module in iLastik, and the resulting data authenticated visually and imported into Cell Profiler for counting. In Cell Profiler, somata were detected and counted using “Enhance Edge” and “Detect Primary Objects” modules, and the program was instructed to only count objects with diameters between 22 to 60 pixels. This range of pixels encompasses the size of PV somata in confocal images, allowing the program to filter out unwanted objects for the second time and achieve higher accuracy. “Overlay Outlines” module, which marks the counted objects, was also incorporated into the program to again confirm the program’s precision.
Authors Contributions: IP, FS, DAP planned the experiments; IP, FS, JL, KS obtained and analyzed ICC images; JW, PS performed and analyzed EM; KG, RMT obtained cell counts; AB, FG identified and labeled interneurons; DAP, FG, IP wrote the manuscript.
References
- Alcantara S, Ferrer I, Soriano E. Postnatal development of parvalbumin and calbindin D28K immunoreactivities in the cerebral cortex of the rat. Anat Embryol (Berl) 1993;188:63–73. doi: 10.1007/BF00191452. [DOI] [PubMed] [Google Scholar]
- Avramescu S, Nita DA, Timofeev I. Neocortical post-traumatic epileptogenesis is associated with loss of GABAergic neurons. J Neurotrauma. 2009;26:799–812. doi: 10.1089/neu.2008.0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacci A, Rudolph U, Huguenard JR, Prince DA. Major differences in inhibitory synaptic transmission onto two neocortical interneuron subclasses. Journal of Neuroscience. 2003;23:9664–9674. doi: 10.1523/JNEUROSCI.23-29-09664.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bausch SB. Axonal sprouting of GABAergic interneurons in temporal lobe epilepsy. Epilepsy Behav. 2005;7:390–400. doi: 10.1016/j.yebeh.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Beierlein M, Gibson JR, Connors BW. Two dynamically distinct inhibitory networks in layer 4 of the neocortex. J Neurophysiol. 2003;90:2987–3000. doi: 10.1152/jn.00283.2003. [DOI] [PubMed] [Google Scholar]
- Berg A, Zelano J, Stephan A, Thams S, Barres BA, Pekny M, Pekna M, Cullheim S. Reduced removal of synaptic terminals from axotomized spinal motoneurons in the absence of complement C3. Exp Neurol. 2012;237:8–17. doi: 10.1016/j.expneurol.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Bergami M, Vignoli B, Motori E, Pifferi S, Zuccaro E, Menini A, Canossa M. TrkB signaling directs the incorporation of newly generated periglomerular cells in the adult olfactory bulb. J Neurosci. 2013;33:11464–11478. doi: 10.1523/JNEUROSCI.4812-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann M, Lahr G, Mayerhofer A, Gratzl M. Expression of synaptophysin during the prenatal development of the rat spinal cord: Correlation with basic differentiation processes of neurons. Neuroscience. 1991;42:569–582. doi: 10.1016/0306-4522(91)90399-9. [DOI] [PubMed] [Google Scholar]
- Blinzinger K, Kreutzberg G. Displacement of synaptic terminals from regenerating motoneurons by microglial cells. Z Zellforsch Mikrosk Anat. 1968;85:145–157. doi: 10.1007/BF00325030. [DOI] [PubMed] [Google Scholar]
- Brock JH, Rosenzweig ES, Blesch A, Moseanko R, Havton LA, Edgerton VR, Tuszynski MH. Local and remote growth factor effects after primate spinal cord injury. J Neurosci. 2010;30:9728–9737. doi: 10.1523/JNEUROSCI.1924-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster PS, Dudek FE. Neuron loss, granule cell axon reorganization, and functional changes in the dentate gyrus of epileptic kainate-treated rats [In Process Citation] J Comp Neurol. 1997;385:385–404. [PubMed] [Google Scholar]
- Buckmaster PS, Yamawaki R, Thind K. More Docked Vesicles and Larger Active Zones at Basket Cell-to-Granule Cell Synapses in a Rat Model of Temporal Lobe Epilepsy. J Neurosci. 2016;36:3295–3308. doi: 10.1523/JNEUROSCI.4049-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Higashiyama H, Knott GW, Kuhlman SJ, Welker E, Huang ZJ. Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. J Neurosci. 2004;24:9598–9611. doi: 10.1523/JNEUROSCI.1851-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Wu CZ, Knott G, Kuhlman S, Fu Y, Palmiter RD, Huang ZJ. GAD67-mediated GABA synthesis and signaling regulate inhibitory synaptic innervation in the visual cortex. Neuron. 2007;54:889–903. doi: 10.1016/j.neuron.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry FA, Reimer RJ, Bellocchio EE, Danbolt NC, Osen KK, Edwards RH, Storm-Mathisen J. The vesicular GABA transporter, VGAT, localizes to synaptic vesicles in sets of glycinergic as well as GABAergic neurons. J Neurosci. 1998;18:9733–9750. doi: 10.1523/JNEUROSCI.18-23-09733.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvette S, Soltani S, Seigneur J, Timofeev I. In vivo models of cortical acquired epilepsy. J Neurosci Methods. 2016;260:185–201. doi: 10.1016/j.jneumeth.2015.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DH. Qualitative and quantitative study of synaptic displacement in chromatolyzed spinal motoneurons of the cat. J Comp Neurol. 1978;177:635–664. doi: 10.1002/cne.901770407. [DOI] [PubMed] [Google Scholar]
- Chen HX, Roper SN. Reduction of spontaneous inhibitory synaptic activity in experimental heterotopic gray matter. J Neurophysiol. 2003;89:150–158. doi: 10.1152/jn.00325.2002. [DOI] [PubMed] [Google Scholar]
- Chen Z, Jalabi W, Hu W, Park HJ, Gale JT, Kidd GJ, Bernatowicz R, Gossman ZC, Chen JT, Dutta R, Trapp BD. Microglial displacement of inhibitory synapses provides neuroprotection in the adult brain. Nat Commun. 2014;5:4486. doi: 10.1038/ncomms5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y, Parada I, Prince DA. Temporal and topographic alterations in expression of the alpha3 isoform of Na+, K(+)-ATPase in the rat freeze lesion model of microgyria and epileptogenesis. Neuroscience. 2009;162:339–348. doi: 10.1016/j.neuroscience.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gois S, Schafer MK, Defamie N, Chen C, Ricci A, Weihe E, Varoqui H, Erickson JD. Homeostatic scaling of vesicular glutamate and GABA transporter expression in rat neocortical circuits. J Neurosci. 2005;25:7121–7133. doi: 10.1523/JNEUROSCI.5221-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lanerolle NC, Kim JH, Robbins RJ, Spencer DD. Hippocampal interneuron loss and plasticity in human temporal lobe epilepsy. Brain Res. 1989;495(2):387–395. doi: 10.1016/0006-8993(89)90234-5. [DOI] [PubMed] [Google Scholar]
- DeFelipe J. Chandelier cells and epilepsy. Brain. 1999;122:1807–1822. doi: 10.1093/brain/122.10.1807. [DOI] [PubMed] [Google Scholar]
- Delgado-Garcia JM, Del Pozo F, Spencer RF, Baker R. Behavior of neurons in the abducens nucleus of the alert cat - III. Axotomized motoneurons. Neuroscience. 1988;24:143–160. doi: 10.1016/0306-4522(88)90319-3. [DOI] [PubMed] [Google Scholar]
- Elmariah SB, Crumling MA, Parsons TD, Balice-Gordon RJ. Postsynaptic TrkB-mediated signaling modulates excitatory and inhibitory neurotransmitter receptor clustering at hippocampal synapses. J Neurosci. 2004;24:2380–2393. doi: 10.1523/JNEUROSCI.4112-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria LC, Prince DA. Presynaptic inhibitory terminals are functionally abnormal in a rat model of posttraumatic epilepsy. J Neurophysiol. 2010;104:280–290. doi: 10.1152/jn.00351.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria LC, Parada I, Prince DA. Interneuronal calcium channel abnormalities in posttraumatic epileptogenic neocortex. Neurobiol Dis. 2012;45:821–828. doi: 10.1016/j.nbd.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. A network of fast-spiking cells in the neocortex connected by electrical synapses. Nature. 1999;402:72–75. doi: 10.1038/47029. [DOI] [PubMed] [Google Scholar]
- Georgiev D, Yoshihara T, Kawabata R, Matsubara T, Tsubomoto M, Minabe Y, Lewis DA, Hashimoto T. Cortical Gene Expression After a Conditional Knockout of 67 kDa Glutamic Acid Decarboxylase in Parvalbumin Neurons. Schizophr Bull. 2016 doi: 10.1093/schbul/sbw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- Giehl KM, Schutte A, Mestres P, Yan Q. The survival-promoting effect of glial cell line-derived neurotrophic factor on axotomized corticospinal neurons in vivo is mediated by an endogenous brain-derived neurotrophic factor mechanism. J Neurosci. 1998;18:7351–7360. doi: 10.1523/JNEUROSCI.18-18-07351.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graber KD, Prince DA. Tetrodotoxin prevents posttraumatic epileptogenesis in rats. Ann Neurol. 1999;46:234–242. doi: 10.1002/1531-8249(199908)46:2<234::aid-ana13>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Graber KD, Prince DA. Chronic Partial Cortical Isolation. In: Pitkanen A, Schwartzchroin PA, Moshe SL, editors. Models of Seizures and Epilepsy. San Diego, CA: Elsevier; 2006. pp. 477–493. [Google Scholar]
- Gruner JE, Hirsch JC, Sotelo C. Ultrastructural features of the isolated suprasylvian gyrus of the cat. J Comp Neurol. 1974;154:1–28. doi: 10.1002/cne.901540102. [DOI] [PubMed] [Google Scholar]
- Hanno-Iijima Y, Tanaka M, Iijima T. Activity-Dependent Bidirectional Regulation of GAD Expression in a Homeostatic Fashion Is Mediated by BDNF-Dependent and Independent Pathways. PLoS ONE. 2015;10:e0134296. doi: 10.1371/journal.pone.0134296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Sultan P. Variation in the number, location and size of synaptic vesicles provides an anatomical basis for the nonuniform probability of release at hippocampal CA1 synapses. Neuropharmacology. 1995;34:1387–1395. doi: 10.1016/0028-3908(95)00142-s. [DOI] [PubMed] [Google Scholar]
- Hoffman SN, Salin PA, Prince DA. Chronic neocortical epileptogenesis in vitro. J Neurophysiol. 1994;71:1762–1773. doi: 10.1152/jn.1994.71.5.1762. [DOI] [PubMed] [Google Scholar]
- Hong EJ, McCord AE, Greenberg ME. A biological function for the neuronal activity-dependent component of Bdnf transcription in the development of cortical inhibition. Neuron. 2008;60:610–624. doi: 10.1016/j.neuron.2008.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser CR. Do structural changes in GABA neurons give rise to the epileptic state? Adv Exp Med Biol. 2014;813:151–160. doi: 10.1007/978-94-017-8914-1_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser CR, Esclapez M. Vulnerability and plasticity of the GABA system in the pilocarpine model of spontaneous recurrent seizures. Epilepsy Res. 1996;26:207–218. doi: 10.1016/s0920-1211(96)00054-x. [DOI] [PubMed] [Google Scholar]
- Houser CR, Harris AB, Vaughn JE. Time course of the reduction of GABA terminals in a model of focal epilepsy: a glutamic acid decarboxylase immunocytochemical study. Brain Res. 1986;383:129–145. doi: 10.1016/0006-8993(86)90014-4. [DOI] [PubMed] [Google Scholar]
- Jiao Y, Zhang C, Yanagawa Y, Sun QQ. Major effects of sensory experiences on the neocortical inhibitory circuits. J Neurosci. 2006;26:8691–8701. doi: 10.1523/JNEUROSCI.2478-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Zhang Z, Zhang C, Wang X, Sakata K, Lu B, Sun QQ. A key mechanism underlying sensory experience-dependent maturation of neocortical GABAergic circuits in vivo. Proc Natl Acad Sci U S A. 2011;108:12131–12136. doi: 10.1073/pnas.1105296108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Prince DA, Huguenard JR. Enhanced excitatory synaptic connectivity in layer v pyramidal neurons of chronically injured epileptogenic neocortex in rats. J Neurosci. 2006;26:4891–4900. doi: 10.1523/JNEUROSCI.4361-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Huguenard JR, Prince DA. Reorganization of inhibitory synaptic circuits in rodent chronically injured epileptogenic neocortex. Cereb Cortex. 2011;21:1094–1104. doi: 10.1093/cercor/bhq181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Jiang K, Prince DA. Excitatory and inhibitory synaptic connectivity to layer V fast-spiking interneurons in the freeze lesion model of cortical microgyria. J Neurophysiol. 2014;112:1703–1713. doi: 10.1152/jn.00854.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Hu H, Mathers PH, Agmon A. Brain-derived neurotrophic factor mediates activity-dependent dendritic growth in nonpyramidal neocortical interneurons in developing organotypic cultures. J Neurosci. 2003;23:5662–5673. doi: 10.1523/JNEUROSCI.23-13-05662.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilinc D, Gallo G, Barbee KA. Mechanically-induced membrane poration causes axonal beading and localized cytoskeletal damage. Exp Neurol. 2008;212:422–430. doi: 10.1016/j.expneurol.2008.04.025. [DOI] [PubMed] [Google Scholar]
- Kohara K, Yasuda H, Huang Y, Adachi N, Sohya K, Tsumoto T. A local reduction in cortical GABAergic synapses after a loss of endogenous brain-derived neurotrophic factor, as revealed by single-cell gene knock-out method. J Neurosci. 2007;27:7234–7244. doi: 10.1523/JNEUROSCI.1943-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kron M, Lang M, Adams IT, Sceniak M, Longo F, Katz DM. A BDNF loop-domain mimetic acutely reverses spontaneous apneas and respiratory abnormalities during behavioral arousal in a mouse model of Rett syndrome. Dis Model Mech. 2014;7:1047–1055. doi: 10.1242/dmm.016030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar SS, Buckmaster PS. Hyperexcitability, interneurons, and loss of GABAergic synapses in entorhinal cortex in a model of temporal lobe epilepsy. J Neurosci. 2006;26:4613–4623. doi: 10.1523/JNEUROSCI.0064-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M, Llinas R. Alterations of synaptic action in chromatolysed motoneurones of the cat. J Physiol. 1970;210:823–838. doi: 10.1113/jphysiol.1970.sp009244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Prince DA. Synaptic activity in chronically injured, epileptogenic sensory-motor neocortex. J Neurophysiol. 2002;88:2–12. doi: 10.1152/jn.00507.2001. [DOI] [PubMed] [Google Scholar]
- Li H, Graber KD, Jin S, McDonald W, Barres BA, Prince DA. Gabapentin decreases epileptiform discharges in a chronic model of neocortical trauma. Neurobiol Dis. 2012;48:429–438. doi: 10.1016/j.nbd.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Bloodgood BL, Hauser JL, Lapan AD, Koon AC, Kim TK, Hu LS, Malik AN, Greenberg ME. Activity-dependent regulation of inhibitory synapse development by Npas4. Nature. 2008;455:1198–1204. doi: 10.1038/nature07319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Prince DA. Functional alterations in GABAergic fast-spiking interneurons in chronically injured epileptogenic neocortex. Neurobiol Dis. 2012;47:102–113. doi: 10.1016/j.nbd.2012.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magloczky Z, Freund TF. Impaired and repaired inhibitory circuits in the epileptic human hippocampus. Trends Neurosci. 2005;28:334–340. doi: 10.1016/j.tins.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Marco P, Sola RG, Pulido P, Alijarde MT, Sánchez A, Cajal SRY, DeFelipe J. Inhibitory neurons in the human epileptogenic temporal neocortex - An immunocytochemical study. Brain. 1996;119:1327–1347. doi: 10.1093/brain/119.4.1327. [DOI] [PubMed] [Google Scholar]
- Marty S, Carroll P, Cellerino A, Castrén E, Staiger V, Thoenen H, Lindholm D. Brain-derived neurotrophic factor promotes the differentiation of various hippocampal nonpyramidal neurons, including Cajal- Retzius cells, in organotypic slice cultures. J Neurosci. 1996;16:675–687. doi: 10.1523/JNEUROSCI.16-02-00675.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massa SM, Yang T, Xie Y, Shi J, Bilgen M, Joyce JN, Nehama D, Rajadas J, Longo FM. Small molecule BDNF mimetics activate TrkB signaling and prevent neuronal degeneration in rodents. J Clin Invest. 2010;120:1774–1785. doi: 10.1172/JCI41356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister AK, Lo DC, Katz LC. Neurotrophins regulate dendritic growth in developing visual cortex. Neuron. 1995;15:791–803. doi: 10.1016/0896-6273(95)90171-x. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC. Neurotrophins and synaptic plasticity. Annu Rev Neurosci. 1999;22:295–318. doi: 10.1146/annurev.neuro.22.1.295. [DOI] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Nita DA, Cisse Y, Timofeev I, Steriade M. Waking-sleep modulation of paroxysmal activities induced by partial cortical deafferentation. Cereb Cortex. 2007;17:272–283. doi: 10.1093/cercor/bhj145. [DOI] [PubMed] [Google Scholar]
- Peng Z, Zhang N, Wei W, Huang CS, Cetina Y, Otis TS, Houser CR. A reorganized GABAergic circuit in a model of epilepsy: evidence from optogenetic labeling and stimulation of somatostatin interneurons. J Neurosci. 2013;33:14392–14405. doi: 10.1523/JNEUROSCI.2045-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce JP, Lewin GR. An ultrastructural size principle. Neuroscience. 1994;58:441–446. doi: 10.1016/0306-4522(94)90071-x. [DOI] [PubMed] [Google Scholar]
- Ping X, Jin X. Chronic Posttraumatic Epilepsy following Neocortical Undercut Lesion in Mice. PLoS One. 2016a;11:e0158231. doi: 10.1371/journal.pone.0158231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping X, Jin X. Transition from Initial Hypoactivity to Hyperactivity in Cortical Layer V Pyramidal Neurons after Traumatic Brain Injury In Vivo. J Neurotrauma. 2016b;33:354–361. doi: 10.1089/neu.2015.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince DA, Tseng G-F. Epileptogenesis in chronically injured cortex: in vitro studies. J Neurophysiol. 1993;69:1276–1291. doi: 10.1152/jn.1993.69.4.1276. [DOI] [PubMed] [Google Scholar]
- Prince DA, Parada I, Graber K. Traumatic Brain Injury and Posttraumatic Epilepsy. In: Noebels JL, et al., editors. Jasper’s Basic Mechanisms of the Epilepsies. 4. New York: Oxford; 2012. pp. 315–330. [Google Scholar]
- Ribak CE. Axon terminals of GABAergic chandelier cells are lost at epileptic foci. Brain Res. 1985;326:251–260. doi: 10.1016/0006-8993(85)90034-4. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Reiffenstein RJ. Selective inhibitory synapse loss in chronic cortical slabs: a morphological basis for epileptic susceptibility. Can J Physiol Pharmacol. 1982;60:864–870. doi: 10.1139/y82-122. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Bradburne M, Harris AB. A preferential loss of GABAergic, symmetric synapses in epileptic foci: a quantitative ultrastructural analysis of monkey neocortex. J Neurosci. 1982;2:1725–1735. doi: 10.1523/JNEUROSCI.02-12-01725.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen GD, Jacobs KM, Prince DA. Effects of neonatal freeze lesions on expression of parvalbumin in rat neocortex [In Process Citation] Cereb Cortex. 1998;8:753–761. doi: 10.1093/cercor/8.8.753. [DOI] [PubMed] [Google Scholar]
- Rutherford LC, DeWan A, Lauer HM, Turrigiano GG. Brain-derived neurotrophic factor mediates the activity-dependent regulation of inhibition in neocortical cultures. J Neurosci. 1997;17:4527–4535. doi: 10.1523/JNEUROSCI.17-12-04527.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata K, Woo NH, Martinowich K, Greene JS, Schloesser RJ, Shen L, Lu B. Critical role of promoter IV-driven BDNF transcription in GABAergic transmission and synaptic plasticity in the prefrontal cortex. Proc Natl Acad Sci U S A. 2009;106:5942–5947. doi: 10.1073/pnas.0811431106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin P, Tseng GF, Hoffman S, Parada I, Prince DA. Axonal sprouting in layer V pyramidal neurons of chronically injured cerebral cortex. J Neurosci. 1995;15:8234–8245. doi: 10.1523/JNEUROSCI.15-12-08234.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid DA, Yang T, Ogier M, Adams I, Mirakhur Y, Wang Q, Massa SM, Longo FM, Katz DM. A TrkB small molecule partial agonist rescues TrkB phosphorylation deficits and improves respiratory function in a mouse model of Rett syndrome. J Neurosci. 2012;32:1803–1810. doi: 10.1523/JNEUROSCI.0865-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpless SK, Halpern LM. The electrical excitability of chronically isolated cortex studied by means of permanently implanted electrodes. Electroencephalogr Clin Neurophysiol. 1962;14:244–255. doi: 10.1016/0013-4694(62)90034-2. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. Decreased hippocampal inhibition and a selective loss of interneurons in experimental epilepsy. Science. 1987;235:73–76. doi: 10.1126/science.2879352. [DOI] [PubMed] [Google Scholar]
- Snapyan M, Lemasson M, Brill MS, Blais M, Massouh M, Ninkovic J, Gravel C, Berthod F, Gotz M, Barker PA, Parent A, Saghatelyan A. Vasculature guides migrating neuronal precursors in the adult mammalian forebrain via brain-derived neurotrophic factor signaling. J Neurosci. 2009;29:4172–4188. doi: 10.1523/JNEUROSCI.4956-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi DK, Gu F, Parada I, Vyas S, Prince DA. Aberrant excitatory rewiring of layer V pyramidal neurons early after neocortical trauma. Neurobiol Dis. 2016;91:166–181. doi: 10.1016/j.nbd.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata M. Lingually induced inhibitory postsynaptic potentials in hypoglossal motoneurons after axotomy. Brain Res. 1981;224:165–169. doi: 10.1016/0006-8993(81)91127-6. [DOI] [PubMed] [Google Scholar]
- Tamas G, Buhl EH, Somogyi P. Massive autaptic self-innervation of GABAergic neurons in cat visual cortex. J Neurosci. 1997a;17:6352–6364. doi: 10.1523/JNEUROSCI.17-16-06352.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamas G, Buhl EH, Somogyi P. Fast IPSPs elicited via multiple synaptic release sites by different types of GABAergic neurone in the cat visual cortex. J Physiol (Lond) 1997b;500(Pt 3):715–738. doi: 10.1113/jphysiol.1997.sp022054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeev I, Bazhenov M, Avramescu S, Nita DA. Posttraumatic epilepsy: the roles of synaptic plasticity. Neuroscientist. 2010;16:19–27. doi: 10.1177/1073858409333545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topolnik L, Steriade M, Timofeev I. Hyperexcitability of intact neurons underlies acute development of trauma-related electrographic seizures in cats in vivo. Eur J Neurosci. 2003;18:486–496. doi: 10.1046/j.1460-9568.2003.02742.x. [DOI] [PubMed] [Google Scholar]
- Tseng G-F, Prince DA. Structural and funtional alterations in rat corticospinal neurons after axotomy. J Neurophysiol. 1996;75:248–267. doi: 10.1152/jn.1996.75.1.248. [DOI] [PubMed] [Google Scholar]
- Uematsu M, Hirai Y, Karube F, Ebihara S, Kato M, Abe K, Obata K, Yoshida S, Hirabayashi M, Yanagawa Y, Kawaguchi Y. Quantitative chemical composition of cortical GABAergic neurons revealed in transgenic venus-expressing rats. Cereb Cortex. 2008;18:315–330. doi: 10.1093/cercor/bhm056. [DOI] [PubMed] [Google Scholar]
- Villa KL, Berry KP, Subramanian J, Cha JW, Chan Oh W, Kwon HB, Kubota Y, So PT, Nedivi E. Inhibitory Synapses Are Repeatedly Assembled and Removed at Persistent Sites In Vivo. Neuron. 2016;90:662–664. doi: 10.1016/j.neuron.2016.03.035. [DOI] [PubMed] [Google Scholar]
- Wenzel HJ, Robbins CA, Tsai LH, Schwartzkroin PA. Abnormal morphological and functional organization of the hippocampus in a p35 mutant model of cortical dysplasia associated with spontaneous seizures. J Neurosci. 2001;21:983–998. doi: 10.1523/JNEUROSCI.21-03-00983.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyeth MS, Zhang N, Mody I, Houser CR. Selective Reduction of Cholecystokinin-Positive Basket Cell Innervation in a Model of Temporal Lobe Epilepsy. Journal of Neuroscience. 2010;30:8993–9006. doi: 10.1523/JNEUROSCI.1183-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Z, Huguenard JR, Prince DA. GABAA receptor-mediated currents in interneurons and pyramidal cells of rat visual cortex. J Physiol (Lond) 1998;506:715–730. doi: 10.1111/j.1469-7793.1998.715bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Z, Huguenard JR, Prince DA. Synaptic inhibition of pyramidal cells evoked by different interneuronal subtypes in layer v of rat visual cortex. J Neurophysiol. 2002;88:740–750. doi: 10.1152/jn.2002.88.2.740. [DOI] [PubMed] [Google Scholar]
- Yamada J, Nakanishi H, Jinno S. Differential involvement of perineuronal astrocytes and microglia in synaptic stripping after hypoglossal axotomy. Neuroscience. 2011;182:1–10. doi: 10.1016/j.neuroscience.2011.03.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Neuropathological alterations in UC cortex. A: Coronal section from rat 3 wks after UC reacted with 68 kD neurofilament antibody shows increased neurofilament-IR in undercut vs. contralateral hemisphere. Arrows point to edges of the partial cortical isolation. B: Neuropil and single pyramidal cells (double arrows), as well as presumptive interneuron (single arrow) from undercut area of A show increased neurofilament immunoreactivity (IR). C, D: Sections from layer V of partially isolated (D) and contralateral cortex (C) reacted with a 200 kD neurofilament antibody 3 days after UC surgery. Intense IR is present in lesioned area. Scale bar in C for C–D.
Figure S2: Activation of astrocytes and microglia in UC cortex. A, C, E: Immunoreactivity for GFAP (A), Cd11b (microglia) (C) and merged A + C (E) in layer V of control cortex. B, D, F: Images as in A, C, and E, 10 d after undercut (UC) showing reactive astrocytes and microglia (arrows). Calibration bar in A: 20 μm for A–F
Figure S3: Total number of neurons is decreased in UC cortex without concomitant reduction in PV- containing interneurons. Confocal images of NeuN (A) and PV neurons (B) in layers I–VI of control and UC cortex 5 weeks after injury from naive (control) and partially isolated (UC) cortex. A1–B1: Graphs of NeuN and parvalbumin (PV) cell counts (Methods) from control (black bars) and UC rats (white bars). Numbers in bars: number of grids counted. C: High magnification image of PV cells from undercut side (arrows). * p < 0.05. Calibration bars in control segments of A, B:100 μm for all segments.
Figure S4: Cell counts for NeuN- (A) and GABA-IR (B) in UC and control cortex. Images and cell counts show no reductions in GABA cell density in the presence of reduction in total NeuN-IR neurons 5 weeks after UC. A1: NeuN mean counts: 952.8 cells, SEM 49.65 in control and 793.3 cells, SEM 44.01 in UC cortex. p = 0.027. White circles in A: Comparable areas in UC and Control layer V show decreased size of NeuN profiles and decreased density of neurons in UC. Numbers in bars in A1, B1: numbers of grids counted. GABA mean counts: 65.4 cells, SEM 4.17 in control (n = 10) and 59.8 cells, SEM 6.37 in UC cortex (n = 10 grids).p = 0.47. PV mean counts were 50.42 cells, SEM 2.71 in control (n = 7) and 50.14 cells/grid, SEM 4.00 in UC cortex (n = 7 grids). P: 0.96
Figure S5: Schematic of undercutting methods. A: View of rodent neocortex and underlying white matter in the parasagittal plane. 1: Fine gauge needle, bent 90 degrees from the tip, is inserted at a near-tangential angle through dura and beneath the pia into superficial cortex. 2: Shaft is aligned normal to pial surface, raised slightly through layer I (not shown) sparing pial vasculature and then depressed through all cortical layers to underlying white matter, creating a transcortical cut (gray shading in 3). 3: Needle is rotated laterally 180 degrees to create a white matter undercut (dark line in 4, 5). 4: Needle is raised to extend the transcortical cut (gray shading). 5: Needle is removed through the point of insertion. B: Schematic of different types of cortical and white matter transactions. Bilateral lesions shown only for illustrative purposes. 1: Steps in A used to create a longer transcortical cut (dark line; small dark circle represents point of needle insertion) and underlying white matter undercut (gray shaded semicircle). 2: Associated lateral transcortical cut (dark line) placed without needle rotation can be used to create a more complete isolation (“U”-shaped in coronal brain slices. 3: Rotation of the depressed needle approximately 90 to 135 degrees can be used to create smaller lesion with two adjacent transcortical cuts. 4: Rostral (or caudal, not shown) transcortical cuts can also be used for more extensive isolation. C: Typical recording of field potentials from two electrodes spaced 1.5 mm apart in layer V demonstrates transcortical propagation of evoked interictal discharges. Dot: stimulus at white matter—layer VI junction. Actual slice contained only unilateral lesion. From (Graber and Prince, 2006) with permission.


