Abstract
Background
Clinical trials are vital for evidence-based cancer care. Oncologist engagement in clinical trials has an effect on patient recruitment, which in turn can affect trial success. Identifying barriers to clinical trial participation might enable interventions that could help to increase physician participation.
Methods
To assess factors affecting physician engagement in oncology trials, a national survey was conducted using the online SurveyMonkey tool (SurveyMonkey, San Mateo, CA, U.S.A.; http://www.surveymonkey.com). Physicians associated with the Canadian Cancer Clinical Trials Network and the Canadian Cancer Trials Group were asked about their specialty, years of experience, barriers to participation, and motivating interventions, which included an open-ended question inviting survey takers to suggest interventions.
Results
The survey collected 207 anonymous responses. Respondents were predominantly medical oncologists (46.4%), followed by radiation oncologists (24.6%). Almost 70% of the respondents had more than 10 years of experience. Significant time constraints included extra paperwork (77%), patient education (54%), and extended follow-up or clinic visits (53%). Timing of events within trials was also a barrier to participation (55%). Most respondents favoured clinical work credits (72%), academic credits (67%), a clinical trial alert system (75%), a regular meeting to review trial protocols (65%), and a screening log to aid in patient accrual (67%) as motivational strategies. Suggested interventions included increased support staff, streamlined regulatory burden, and provision of greater funding for trials and easier access to ancillary services.
Conclusions
The present study confirms that Canadian oncologists are willing to participate in clinical research, but face multiple barriers to trial participation. Those barriers could be mitigated by the implementation of several interventions identified in the study.
Keywords: Clinical trials, health operations research, cross-sectional studies, physician engagement surveys, oncologists, trial participation, barriers, motivating interventions
INTRODUCTION
Clinical trials are the backbone of modern evidence-based medicine, and trial outcomes inform clinical decision-making along the entire spectrum of care. However, clinical trials are complex and expensive, and have significant time and resource implications. Trials require participation, coordination, and collaboration by health care providers, patients, administrators, and government and private stakeholders. Barriers to engagement of any one of those groups could be detrimental to attempts at improving disease-related outcomes, resulting in loss of invested resources and reduced awareness among health care providers about standards of care.
In a cross-sectional study of prematurely terminated clinical trials1, 905 (12%) of the identified clinical trials were closed early. Of those 905 trials, 619 (68.4%) closed for reasons other than the scientific data related to the trial, with 350 (38.7%) having been closed because of an insufficient accrual rate—the leading cause of trial closure. Another 8.5% of closures were attributable to an unspecified business decision or strategic reasons, and a further 6.4%, to problems with trial administration or conduct, including personnel issues. Those proportions compare with the 193 closures (21%) related to scientific data, 3.8% to funding, and 5.6% to results of other trials or to changes in the standard of care.
Physicians are a key human resource in conducting clinical trials. Their level of engagement can have a significant effect on patient recruitment and retention, on the quality of the data collected, and on follow-up, which can affect the overall success of a trial2–4. The engagement of clinical investigators is therefore key to ensuring the successful conduct and completion of clinical trials. To assess factors affecting physician engagement in clinical trials, including physician demographics, barriers to engagement, motivating interventions, and system and resource issues, we implemented a national cross-sectional survey of Canadian physicians working directly in oncology or in affiliated disciplines through the Canadian Cancer Clinical Trials Network and the Canadian Cancer Trials Group (cctg). Based on the results of the survey, we propose interventions that might help with physician engagement in clinical trials.
METHODS
The open-access online SurveyMonkey tool (SurveyMonkey, San Mateo, CA, U.S.A.; http://www.surveymonkey.com) was used to create and administer our anonymous survey on physician engagement in clinical trials. A link to the survey was embedded in an e-mail message sent to all Canadian Cancer Clinical Trials Network principal investigators (n = 75) and the cctg disease site chairs and centre representatives (n = 110), with a request to circulate the survey broadly to appropriate clinicians within their respective organizations. The timeline to respond was set at 3 weeks. Reminder e-mail messages were sent at 10 days and at 18 days.
The survey had questions concerning six topics: medical specialty, years of experience practicing medicine, factors related to time constraints in clinical trials, barriers to trial participation as an investigator, motivating interventions for participation, and an open-ended question to identify other interventions that might motivate a physician to participate in clinical trials. For the time constraints, the barriers, and the motivating interventions, survey-takers were asked rank their level of agreement with each statement (strongly disagree = 1 to strongly agree = 5); results were pooled and a weighted average score out of 5 was assigned.
The final section of the survey, which asked for the physicians to propose their own interventions, was included to help mitigate author bias with regard to the formulation of survey questions, and to capture barriers and motivations not considered by the authors during survey design. Table i lists the questions as found in the survey. With respect to determining the questions that were relevant for inclusion in the survey, a literature review was performed to look at physician-related barriers to trial participation and the reasons underlying premature trial termination; however, physician surveys on the topic were found to be relatively scarce.
TABLE I.
The survey questions
| 1 | Please choose one of the following: I am a ... | ||||||
| □ Medical oncologist □ Surgical oncologist □ Radiation oncologist | |||||||
| □ Hematologist □ Other specialist | |||||||
| 2 | I have been practicing medicine for ... | ||||||
| □ 1–5 years □ 6–10 years □ 11–15 years □16–20 years □ >20 years | |||||||
|
| |||||||
| Strongly disagree | Disagree | Neutral | Agree | Strongly agree | |||
| 1 | 2 | 3 | 4 | 5 | |||
|
| |||||||
| 3 | Please rank, on a 5-point scale, the aspects of clinical trials that you consider to be time-constraining | ||||||
| a) | Extra paperwork associated with clinical trials | ||||||
| b) | Finding patients that meet all of the eligibility criteria of a clinical trial | ||||||
| c) | Explaining the clinical trial to the patient | ||||||
| d) | Obtaining informed consent from patients | ||||||
| e) | Additional clinic visits (extended follow-up for clinical trial patients) | ||||||
| f) | Nonstandard scans and scanning frequency | ||||||
| 4. | Please identify the barriers to your participation as a clinical trial investigator | ||||||
| a) | I find the timing of events within trials to be a problem (for example, must start treatment within 3 weeks of diagnosis) | ||||||
| b) | I lack a complete awareness of available trials | ||||||
| c) | I believe that some clinical trials deviate significantly from the standard of care | ||||||
| d) | I think that the process of having third-party quality assurance review for radiation oncology trials is a deterrent | ||||||
| 5. | Please rank which of the following interventions would motivate you to participate in clinical trials | ||||||
| a) | Attendance at an annual conference where new trials are presented and discussed | ||||||
| b) | A regular session held locally reviewing trial protocols | ||||||
| c) | A clinical trial alert system to draw my attention to new trials | ||||||
| d) | A screening log to aid in the accrual of patients | ||||||
| e) | Software that could assist you to plan the journey of a patient on a clinical trial | ||||||
| f) | Work load credits (clinical) allocated to clinical trial participation | ||||||
| g) | Additional payments toward educational activities (for example: meetings where trials are discussed) | ||||||
| h) | A system of academic credits allocated based on accruing patients on clinical trials | ||||||
| 6. | Please state any other interventions that would motivate you to participate in clinical trials | ||||||
Given that all study participants were physicians who gave their responses anonymously and voluntarily and who did not receive any incentive for taking part in the survey, the survey was not submitted for ethics board review. No confidential or sensitive information was shared or used, and the survey did not make use of patient-related data at any time.
RESULTS
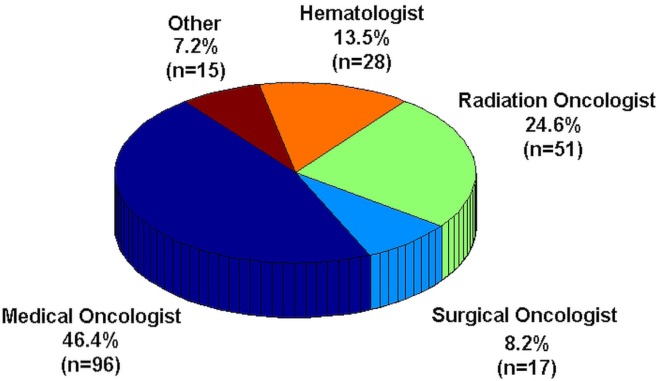
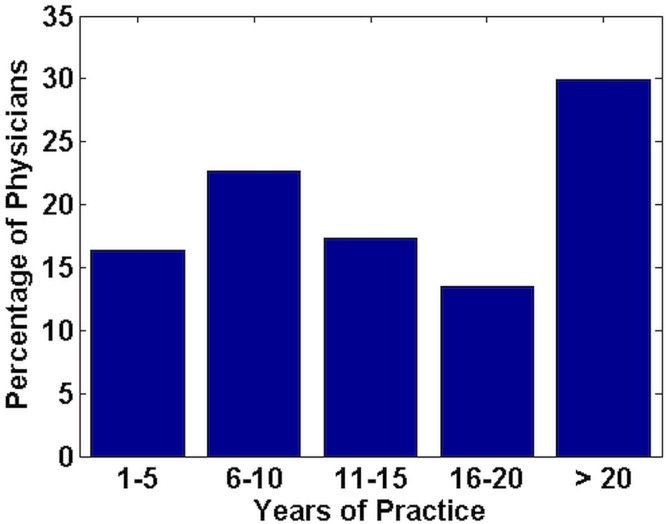
A total of 207 anonymous responses were received. Figure 1 shows the breakdown of the physicians by specialty. Figure 2 shows the breakdown of physicians by years of practice experience, grouped in 5-year segments. Approximately one third of survey respondents had more than 20 years of experience. Almost 70% had more than 10 years of experience.
FIGURE 1.
Medical specializations of the 207 survey respondents.
FIGURE 2.
Years of practice for the 207 survey respondents.
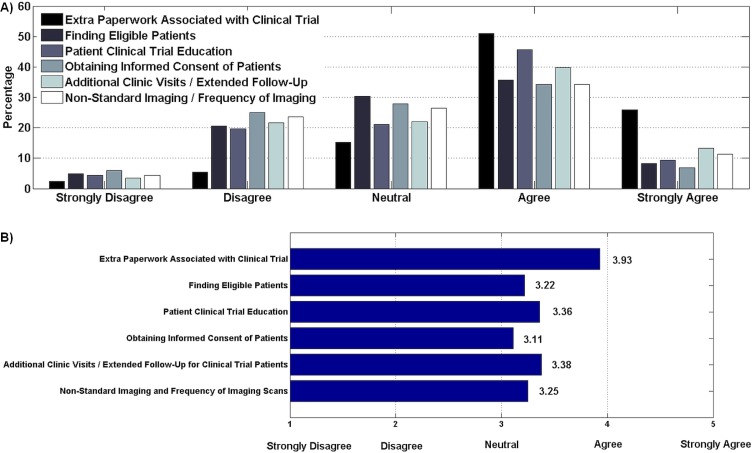
With regard to time constraints to participation, most respondents ranked extra paperwork in clinical trials as a significant time constraint (Figure 3). Additional clinic visits (or extended follow-up) and patient education were the next most significant factors. Identifying patients who meet eligibility criteria, obtaining informed consent, and performing nonstandard imaging tests or frequent imaging were not considered significant time-consuming factors.
FIGURE 3.
(A) Time constraints to participation in clinical trials (207 respondents). (B) Weighted agreement score. Of the respondents, 76% ranked extra paperwork in clinical trials as a significant time constraint. More than 50% of respondents agreed that additional clinic visits or extended follow-up and patient education were barriers, but more respondents strongly agreed that, compared with educating the patient about the trial (9%), additional clinic visits constituted a barrier (13%).
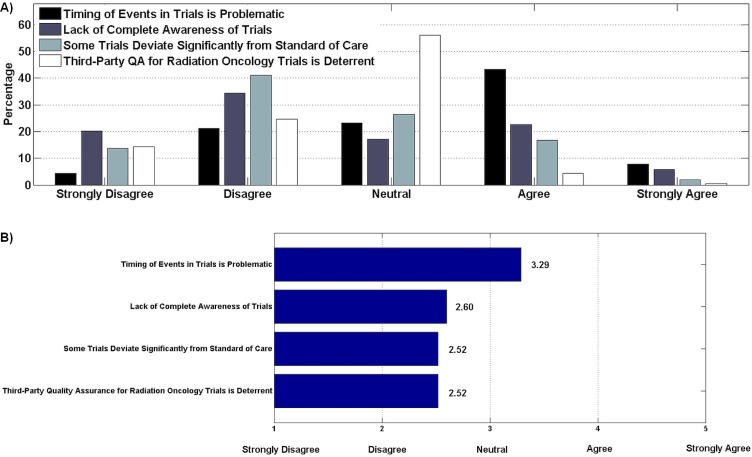
With respect to other barriers to trial participation, most physicians agreed that the timing of events such as requisite imaging and investigations within trials (as dictated by trial protocol) can pose a barrier to participation (Figure 4). However, there was disagreement that lack of awareness of trials or that significant deviation in some trials from the standard of care were important barriers. Most respondents were neutral about third-party quality assurance for radiation oncology trials.
FIGURE 4.
(A) Other barriers to participation in clinical trials (204 respondents). (B) Weighted agreement score. Of the respondents, 51% agreed that the timing of events in trials was a barrier; however, only 8% strongly agreed. More than 50% of respondents disagreed that trials deviated from standard of care or that lack of complete awareness of trials was problematic, with 20% strongly disagreeing that complete awareness constituted a barrier.
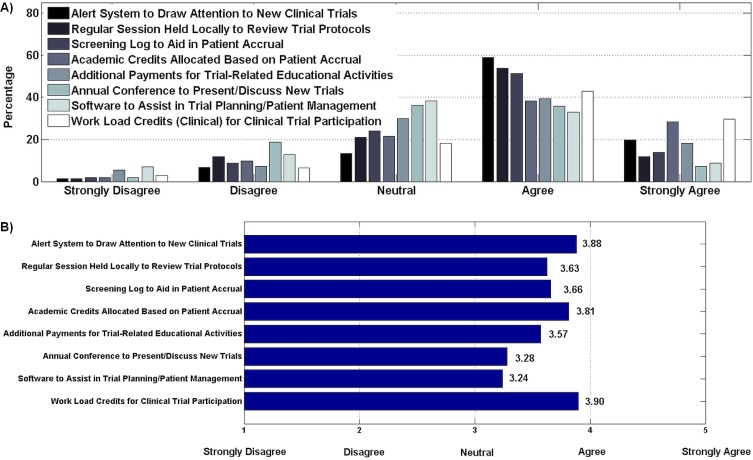
With respect to motivating interventions that might improve trial participation, clinical and academic work credits were the highest ranked option (Figure 5). Other interventions that were favoured were to keep physicians alerted to new trials and trial protocols, and to provide a method to screen patients for accrual purposes.
FIGURE 5.
(A) Motivating interventions for clinical trial participation (204 respondents). (B) Weighted agreement score. Clinical and academic work credits were highly ranked by respondents (29.6% and 19.6% expressing strong agreement respectively), with 72% and 67% expressing overall agreement respectively. Most respondents agreed with using a clinical trial alert system to draw attention to new trials (78%), a regular meeting to review trial protocols (66%), and a screening log to aid in patient accrual (65%). Attendance at an annual conference presenting new trials and trial planning software attracted less agreement (43% and 42% agreement respectively).
The final section of the survey asked respondents about their own ideas for motivating interventions that might improve participation in clinical trials. Many of the 149 responses that were received had commonalities with the interventions already suggested in the survey: greater funding for clinical trials; streamlining paperwork and regulatory burden; streamlining trial contracts and ethics; creating a more unified system; allocating extra credits and protected research time; prioritization of research by the cancer centre or hospital administrative leaders; hiring more support staff; increasing the availability of patient screening and eligibility information; increasing information about the availability of trials; obtaining easier access to ancillary services that make trials easier to conduct; creating intellectually stimulating trials with potential to have a significant effect on patient care; and revamping the structure of disease site groups, both at their centre locally and at the cctg, to facilitate participation.
DISCUSSION
The present study provides a high-level overview of some of the key factors affecting participation by physician–investigators in oncology clinical trials and proposes some interventions to increase motivation.
The proportion of survey respondents grouped by oncology specialization within Canada generally reflects patterns seen in physician groups studied elsewhere5. In our survey, medical oncologists provided the most responses. One reason for that observation could be that a greater proportion of oncology trials involve some form of systemic therapy. Furthermore, in recent oncology research, the emphasis has been on immunotherapies and targeted agents, which is predominantly the domain of medical oncology. Medical oncologists also constitute the largest proportion of practicing oncologists6, and some data suggest that, compared with other oncologic specialties, medical oncologists might be more involved in research7.
The duration of clinical practice was fairly uniformly distributed among survey respondents, although with some tendency toward longer practice times. Almost one third of respondents (30%) had been in practice for more than 20 years, and about 61%, for more than 10 years. However, our results are generally consistent with the findings of other studies showing that neither age nor years in practice are significant factors with respect to interest in participating in clinical trials8.
The most significant time constraint affecting trial participation in our study was the extra paperwork associated with clinical trials. A 2005 study of 22 oncologists likewise cited paperwork as the greatest barrier to participation in clinical trials9. Other time-constraining factors assessed in our survey ranked more evenly, although extended follow-up for clinical trial patients and explanation of the trial to the patient (that is, patient education) ranked some-what higher than the other factors assessed. Those findings are consistent with the literature about how trial follow-up time and time spent discussing trials with patients represent significant clinician-related barriers to trial involvement10. Another study also cited eligibility criteria and informed consent as barriers8, in keeping with the data we collected. However, of all the factors we examined, streamlining the paperwork involved in clinical trials seems to be both the most practical and straightforward intervention for lessening the time commitment involved in clinical trials.
Timing of events within trials was also ranked as a significant barrier to participation, because timing entails adhering to trial protocol and coordinating activities with other participating centres. The literature suggests that the timing of trial events might be even more difficult for a community hospital than for a specialist centre. For example, “in a [community hospital,] there might only be one histopathologist dealing with multiple sites [who might] not have protected time to deal with ‘trial’ tissue blocks in the tight timelines that some trials have”7.
A small proportion of the oncologists polled agreed or strongly agreed that they lack awareness of available trials and consider that lack of awareness to be a barrier to acting as a clinical trial investigator. That finding could be a reflection of trial oncologists in Canada having proportionally more academic appointments than their colleagues elsewhere11,12. Likewise, most respondents disagreed or strongly disagreed that clinical trials deviate significantly from the standard of care and that such deviation poses a barrier to being a clinical trial investigator. Historically, that question might have generated more agreement, but most clinical trials today are carefully planned and typically compare the experimental arm with the standard of care, in the expectation that the experimental arm will not be unacceptably worse than the comparator, such as in a noninferiority trial13, or might be superior in performance to the accepted standard.
Only 10 respondents agreed or strongly agreed that having third-party quality assurance review for radiation oncology trials is a barrier to being an investigator. That finding might partly reflect distribution of respondent specialities, because most were not radiation oncologists and do not have a significant role or interest in trials involving radiation oncology quality assurance or review.
Looking at motiving interventions for trial participation, the survey intervention that attracted the most respondent agreement was a clinical trial alert system to draw attention to new trials. One study noted that “a centralised system of trial information available to all clinicians would potentially overcome some barriers to recruitment”7. Clinical or academic work credits for clinical trial participation also received strong support. Overall, it seems that the most effective strategies would be academic or work credits for physician participation and aids that facilitate physician awareness of trials and recruitment of patients through organizations such as the Canadian Cancer Clinical Trials Network or the cctg. Indeed, “organizations that provided support for physicians to consent and enroll patients, offered incentives for enrollment, and mandated expectations for enrollment ... increased physician enrollment”14.
The foregoing strategies were echoed in the ideas about motivating interventions that respondents provided on their own in question 6 of the survey. Additional suggestions given by survey respondents included reduced paperwork and regulatory burden, a commitment from administration to see research as a priority, increased support staff and nurse or coordinator availability, and improvements in resources and funding. As well as streamlining contracts and ethics, a more unified system would motivate participation. Ancillary services that make trials easier to conduct would also be worthwhile. Revamping the structure of disease site groups, both locally at their centre and at the cctg, was recommended. Lastly, respondents indicated that trials raising clinically relevant questions with a clear benefit to patients should be a priority. A 2009 study found that “the most prominent factors that encouraged research participation were interest in the research question, intellectual curiosity and potential benefit to patients (including access to treatments or drugs, and closer monitoring)”15.
With regard to limitations in the present study, a major limitation is that survey respondents were clinicians who are probably more likely to be interested and engaged in oncology trials to begin with, and so our survey results are likely have an inherent selection bias. We might not be adequately capturing data from oncologists who are not motivated to participate in trials in the first place. Another limitation is that our survey questions covered only a subset of the relevant factors that might affect trial participation, although in an attempt to address that limitation, the last part of the survey invited oncologists to provide their own motivating interventions.
CONCLUSIONS
The results of the present study reveal a willingness on the part of Canadian oncologists to participate in clinical research, but they also demonstrate several significant barriers to trial participation that hopefully can be mitigated through implementation of motivational interventions proposed here and suggested by Canadian physicians in oncology practice.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Williams RJ, Tse T, DiPiazza K, Zarin DA. Terminated trials in the ClinicalTrials.gov results database: evaluation of availability of primary outcome data and reasons for termination. PLoS One. 2015;10:e0127242. doi: 10.1371/journal.pone.0127242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sherber NS, Powe NR, Braunstein JB. Personal physicians as study investigators: impact on patients’ willingness to participate in clinical trials. Contemp Clin Trials. 2009;30:227–32. doi: 10.1016/j.cct.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Butryn T, Cornejo K, Wojda TR, et al. Keys to success in clinical trials: a practical review. Int J Acad Med. 2016;2:203–16. [Google Scholar]
- 4.Mills EJ, Seely D, Rachlis B, et al. Barriers to participation in clinical trials of cancer: a meta-analysis and systematic review of patient-reported factors. Lancet Oncol. 2006;7:141–48. doi: 10.1016/S1470-2045(06)70576-9. [DOI] [PubMed] [Google Scholar]
- 5.Klabunde CN, Keating NL, Potosky AL, et al. A population-based assessment of specialty physician involvement in cancer clinical trials. J Natl Cancer Inst. 2011;103:384–97. doi: 10.1093/jnci/djq549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canadian Medical Association (CMA). Number of physicians by specialty and age, Canada, 2016. Ottawa, ON: CMA; 2016. [Current version available online at: https://www.cma.ca/Assets/assets-library/document/en/advocacy/02-physicians-by-specialty-age-e.pdf; cited 12 December 2016] [Google Scholar]
- 7.Ford E, Jenkins V, Fallowfield L, Stuart N, Farewell D, Farewell V. Clinicians’ attitudes towards clinical trials of cancer therapy. Br J Cancer. 2011;104:1535–43. doi: 10.1038/bjc.2011.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Somkin CP, Altschuler A, Ackerson L, et al. Organizational barriers to physician participation in cancer clinical trials. Am J Manag Care. 2005;11:413–21. [PubMed] [Google Scholar]
- 9.Hudson SV, Momperousse A, Leventhal H. Physician perspectives on cancer clinical trials and barriers to minority recruitment. Cancer Control. 2005;12(suppl 2):93–6. doi: 10.1177/1073274805012004S14. [DOI] [PubMed] [Google Scholar]
- 10.Tournoux C, Katsahian S, Chevret S, Levy V. Factors influencing inclusion of patients with malignancies in clinical trials. Cancer. 2006;106:258–70. doi: 10.1002/cncr.21613. [DOI] [PubMed] [Google Scholar]
- 11.American Society of Clinical Oncology. The state of cancer care in America, 2014: a report by the American Society of Clinical Oncology. J Oncol Pract. 2014;10:119–42. doi: 10.1200/JOP.2014.001386. [DOI] [PubMed] [Google Scholar]
- 12.Brigden ML, Lam W, Silvana S, El-Maraghi R, Whitlock P, Champion P. Update on community oncology practice in Canada: a view from the trenches. Oncology Exchange. 2015;14:12–16. [Google Scholar]
- 13.Schumi J, Wittes JT. Through the looking glass: understanding non-inferiority. Trials. 2011;12:106. doi: 10.1186/1745-6215-12-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobs SR, Weiner BJ, Reeve BB, Weinberger M, Minasian LM, Good MJ. Organizational and physician factors associated with patient enrollment in cancer clinical trials. Clin Trials. 2014;11:565–75. doi: 10.1177/1740774514536000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raftery J, Kerr C, Hawker S, Powell J. Paying clinicians to join clinical trials: a review of guidelines and interview study of trialists. Trials. 2009;10:15. doi: 10.1186/1745-6215-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]