Abstract
Background
The neutrophil–lymphocyte ratio (nlr) has been reported to correlate with patient outcome in several cancers, including breast cancer. We evaluated whether the nlr can be a predictive factor for pathologic complete response (pcr) after neoadjuvant chemotherapy (nac) in patients with triple-negative breast cancer (tnbc).
Methods
We analyzed the correlation between response to nac and various factors, including the nlr, in 87 patients with tnbc who underwent nac. In addition, we analyzed the association between the nlr and recurrence-free survival (rfs) in patients with tnbc.
Results
Of the 87 patients, 25 (28.7%) achieved a pcr. A high Ki-67 index and a low nlr were significantly associated with pcr. The pcr rate was higher in patients having a high Ki-67 index (≥15%) than in those having a low Ki-67 index (35.7% vs. 0%, p = 0.002) and higher in patients having a low nlr (≤1.7) than in those having a high nlr (42.1% vs. 18.4%, p = 0.018). In multiple logistic analysis, a low nlr remained the only predictive factor for pcr (odds ratio: 4.274; p = 0.008). In the survival analysis, the rfs was significantly higher in the low nlr group than in the high nlr group (5-year rfs rate: 83.7% vs. 66.9%; log-rank p = 0.016).
Conclusions
Our findings that the nlr is a predictor of pcr to nac and also a prognosticator of recurrence suggest an association between response to chemotherapy and inflammation in patients with tnbc. The pretreatment nlr can be a useful predictive and prognostic marker in patients with tnbc scheduled for nac.
Keywords: Triple-negative breast cancer, neutrophil–lymphocyte ratio, neoadjuvant chemotherapy, pathologic complete response, recurrence-free survival
INTRODUCTION
A dna microarray analysis can classify breast cancer into several subtypes, which are associated with different prognoses. Those subtypes include hormone receptor–positive (such as luminal A and B), her2 (human epidermal growth factor receptor 2)–enriched, basal-like, and normal breast-like1,2. The basal-like subtype is characterized by high expression of the basal gene cluster and proliferation signature, and is commonly negative for the estrogen receptor (er), progesterone receptor (pgr), and her2 expression (“triple negative”). Triple-negative breast cancer (tnbc) accounts for approximately 11%–20% of breast cancers and is associated with poor outcomes owing to limited therapeutic targets. Hormone or her2-targeted therapies are ineffective in tnbc; chemotherapy is the only therapeutic option3,4.
Neoadjuvant chemotherapy (nac) has become the standard treatment in locally advanced breast cancer. It can render surgically inoperable tumours operable or can downstage a primary tumour, allowing for breast-conserving surgery in patients who might have needed a mastectomy at initial diagnosis5,6. Several randomized clinical trials have demonstrated no difference in long-term outcome between patients who receive nac and those who receive adjuvant chemotherapy7,8. Additionally, unlike adjuvant chemotherapy, nac is associated with an in vivo response of the tumour to chemotherapy.
More importantly, response to nac can predict patient outcome, with improved survival associated with a pathologic complete response (pcr). The correlation between pathologic response and long-term outcome is strongest for tnbc9. Although tnbc is the most chemoresponsive subtype of breast cancer3,10, the magnitude of the response to nac varies, in practice, among patients with tnbc. Some experience an excellent response to nac; others experience little response. However, the predictive factors determining the sensitivity of tnbc to chemotherapy have not been sufficiently elucidated. Identifying the factors predicting response to nac in patients with tnbc is important for predicting patient outcome and planning the optimal treatment strategy.
The prognosis of cancer patients and the chemoresponsiveness of the tumour are determined by patient-related factors as well as by intrinsic tumour characteristics11,12. Cancer-related inflammation plays a critical role in cancer development and progression, and could be responsible for treatment response. The systemic inflammatory response has been regarded as an independent prognostic factor in patients with malignancy. Neutrophils can facilitate tumour proliferation, invasion, and distant metastasis by secreting factors that promote tumour growth13. In contrast, lymphocytes, particularly cytotoxic T cells, play a crucial role in the antitumour immune response by promoting apoptosis and suppressing tumour growth14. Accordingly, the neutrophil–lymphocyte ratio (nlr), a cost-effective and simple inflammatory parameter, might correlate both with patient outcome and with response to chemo therapy15. An elevated nlr has been described to correlate with poor outcome in a variety of cancers, including breast cancer16–19.
In the present study, we assessed the clinical value of the nlr in patients with tnbc who underwent nac. We evaluated whether the nlr can be a predictor for pcr and a prognosticator for recurrence in patients with tnbc.
METHODS
Study Cohort
Patients with tnbc who underwent nac and subsequent breast surgery from October 2004 to August 2012 at Seoul National University Bundang Hospital were identified. Exclusion criteria included previous treatment for contralateral breast cancer, distant metastasis at initial diagnosis, and a diagnosis of ductal carcinoma in situ. Figure 1 summarizes the identification of the study population. Clinicopathologic characteristics (age, menstrual status, histologic type, clinical stage before initiation of nac, histologic grade, chemotherapy regimen, nlr, and molecular biomarkers including er, pgr, her2, Ki-67, p53, and epidermal growth factor receptor) were extracted from electronic medical records. The study protocol was approved by the Institutional Review Board at Seoul National University Bundang Hospital (B-1602/334-109). For this type of study, formal patient consent is not required.
FIGURE 1.
Study population selection. TNBC = triple-negative breast cancer.
NAC and Response Assessment
The nac regimen consisted of 3–4 cycles of doxorubicin (60 mg/m2) and cyclophosphamide (600 mg/m2), followed by 2–4 cycles of docetaxel (75 mg/m2). Some patients received doxorubicin (50 mg/m2 or 60 mg/m2) plus docetaxel (75 mg/m2) or doxorubicin (50 mg/m2 or 60 mg/m2) plus cyclophosphamide (600 mg/m2) by intravenous infusion every 3 weeks for 2–6 cycles.
After completion of nac, pathologic response was evaluated by microscopic examination of resected specimens. A pcr was defined as an absence of invasive tumour in the breast and nodes. Patients with residual ductal carcinoma in situ were also considered to have achieved a pcr.
Immunohistochemical Analysis and NLR
Expression by immunohistochemistry of er, pgr, her2, and Ki-67 was analyzed. Tumours with fewer than 10% stained cells were considered to be negative for er and pgr. Immunohistochemistry results of 0 or 1+ were considered negative for her2; 3+ was considered positive. A result of 2+ was considered equivocal and had to be confirmed by fluorescence or silver in situ hybridization. A Ki-67 value of 15% or greater was considered positive.
Blood samples were examined before the initiation of nac. The nlr was defined as the number of neutrophils divided by the number of lymphocytes from the complete blood count. A receiver operating characteristic curve analysis was performed to determine the optimal cut-off value for the nlr. An nlr value of 1.7, which represented the highest sum for sensitivity and specificity, was used as the cut-off value to discriminate between high and low nlr in our analysis.
Statistical Analysis
The statistical analysis was performed using the SPSS statistical software application (version 21.0: IBM, Armonk, NY, U.S.A.). We evaluated correlations between clinicopathologic variables, including biomarkers and the nlr, and the response to nac was assessed using the chi-square and Fisher exact tests. A binary logistic regression model was used for a multiple analysis of pcr. The Firth penalized likelihood approach was used to reduce small-sample bias. Odds ratios estimated from logistic regression were reported with corresponding 95% confidence intervals. The Kaplan–Meier method and log-rank test were performed to evaluate and compare recurrence-free survival (rfs) by nlr and response to nac. A p value less than 0.05 was considered statistically significant.
RESULTS
The 87 patients eligible for the study had a mean age at the time of treatment of 45.8 years, and their median follow-up was 57 months. The pre-nac clinical T stage was T0–2 in 64 patients (73.6%) and T3–4 in 23 patients (26.4%). One case was classified as cT0 (occult breast cancer with biopsy-proven axillary metastasis). In 71 patients (81.6%), lymph node involvement was clinically detected or metastasis was assumed on pathology assessment of fine-needle aspiration specimens. Table i shows the baseline characteristics of the study patients.
TABLE I.
Characteristics of 87 patients with triple-negative breast cancer
| Variable | Value |
|---|---|
| Mean age (years) | 45.8±11.2 |
| Age group [n (%)] | |
| ≤50 Years | 63 (72.4) |
| >50 Years | 24 (27.6) |
| Menopausal status [n (%)] | |
| Pre-menopause | 57 (65.5) |
| Post-menopause | 24 (27.6) |
| Unknown | 6 (6.9) |
| Histology [n (%)] | |
| Invasive ductal carcinoma | 79 (90.8) |
| Others | 8 (9.2) |
| T Stage before NAC [n (%)] | |
| cT0–2 | 64 (73.6) |
| cT3–4 | 23 (26.4) |
| Nodal status before NAC [n (%)] | |
| Negative | 16 (18.4) |
| Positive | 71 (81.6) |
| Grade [n (%)] | |
| 1/2 | 27 (31.0) |
| 3 | 58 (66.7) |
| Unknown | 2 (2.3) |
| Ki-67 status [n (%)] | |
| <15% | 17 (19.5) |
| ≥15% | 70 (80.5) |
| TP53 [n (%)] | |
| Negative | 44 (50.6) |
| Positive | 43 (49.4) |
| EGFR status [n (%)] | |
| Negative | 24 (27.6) |
| Positive | 41 (47.1) |
| Unknown | 22 (25.3) |
| HER2 status | |
| − or 1+ | 24 (27.6) |
| 2+ | 63 (72.4) |
| Neutrophil–lymphocyte ratio [n (%)] | |
| ≤1.7 | 38 (43.7) |
| >1.7 | 49 (56.3) |
| NAC regimen [n (%)] | |
| AC | 26 (29.9) |
| DA | 25 (28.7) |
| AC→T | 36 (41.4) |
| pCR with NAC [n (%)] | |
| Yes | 25 (28.7) |
| No | 62 (71.3) |
NAC = neoadjuvant chemotherapy; EGFR = epidermal growth factor receptor; HER2 = human epidermal growth factor receptor 2; AC = doxorubicin–cyclophosphamide; DA = docetaxel–doxorubicin; T = paclitaxel; pCR = pathologic complete response.
After completion of nac, pcr was observed in 25 patients (28.7%), including in 1 patient with residual ductal carcinoma in situ. Table ii summarizes the baseline characteristics of the 87 patients by experience of pcr. The nlr and expression of Ki-67 were significantly different in the pcr and non-pcr groups. Compared with patients having a low Ki-67 index, those with a high Ki-67 index experienced a higher pcr rate after nac (35.7% vs. 0%, p = 0.002). The mean nlr was 2.18 (range: 0.74–7.91), with 38 patients (44%) being classified into the low nlr group (≤1.7), and 49 (56%), into the high nlr group (>1.7). Compared with the high nlr group, the low nlr group had a significantly higher rate of pcr (42.1% vs. 18.4%, p = 0.018). In multiple logistic analysis, a low nlr remained the only significant predictive factor for pcr (odds ratio: 4.274; p = 0.008; Table iii).
TABLE II.
Correlations between response to neoadjuvant chemo-therapy and clinicopathologic parameters
| Variable | Achieved pCR | p Value | |
|---|---|---|---|
|
| |||
| Yes | No | ||
| Patients (n) | 25 | 62 | |
| Age group [n (%)] | 0.956 | ||
| ≤50 Years | 18 (28.6) | 45 (71.4) | |
| >50 Years | 7 (29.2) | 17 (70.8) | |
| Histology [n (%)] | 0.099 | ||
| Invasive ductal carcinoma | 25 (31.6) | 54 (68.4) | |
| Others | 0 (0) | 8 (100) | |
| T Stage before NAC [n (%)] | 0.743 | ||
| cT0–2 | 19 (29.7) | 45 (70.3) | |
| cT3–4 | 6 (26.1) | 17 (73.9) | |
| Nodal status before NAC [n (%)] | 0.541 | ||
| Negative | 3 (18.8) | 13 (81.2) | |
| Positive | 22 (31) | 49 (69) | |
| Grade [n (%)] | 0.061 | ||
| 1/2 | 4 (14.8) | 23 (85.2) | |
| 3 | 20 (34.5) | 38 (65.5) | |
| Unknown | 1 (50) | 1 (50) | |
| Ki-67 status [n (%)] | 0.002 | ||
| <15% | 0 (0) | 17 (100) | |
| ≥15% | 25 (35.7) | 45 (64.3) | |
| TP53 status [n (%)] | 0.520 | ||
| Negative | 14 (31.8) | 30 (68.2) | |
| Positive | 11 (25.6) | 32 (74.4) | |
| EGFR status [n (%)] | 0.368 | ||
| Negative | 9 (37.5) | 15 (62.5) | |
| Positive | 11 (26.8) | 30 (73.2) | |
| Unknown | 5 (22.7) | 17 (77.3) | |
| HER2 status [n (%)] | 0.635 | ||
| − or 1+ | 6 (25) | 18 (75) | |
| 2+ | 19 (30.2) | 44 (69.8) | |
| Neutrophil–lymphocyte ratio [n (%)] | 0.018 | ||
| ≤1.7 | 16 (42.1) | 22 (57.9) | |
| >1.7 | 9 (18.4) | 40 (81.6) | |
pCR = pathologic complete response; NAC = neoadjuvant chemotherapy; EGFR = epidermal growth factor receptor; HER2 = human epidermal growth factor receptor.
TABLE III.
Multivariate logistic analysis of factors affecting pathologic complete response to neoadjuvant chemotherapy in triple-negative breast cancer
| Variable | OR | 95% CI | p Value |
|---|---|---|---|
| Histology (IDC vs. others) | 5.405 | 0.191 to 142.857 | 0.322 |
| Grade (3 vs. 1/2) | 1.816 | 0.445 to 7.413 | 0.406 |
| Ki-67 (>15% vs. ≤15%) | 12.748 | 0.621 to 261.550 | 0.099 |
| NLR (≤1.7 vs. >1.7) | 4.274 | 1.451 to 12.658 | 0.008 |
OR = odds ratio; CI = confidence interval; IDC = invasive ductal carcinoma; NLR = neutrophil–lymphocyte ratio.
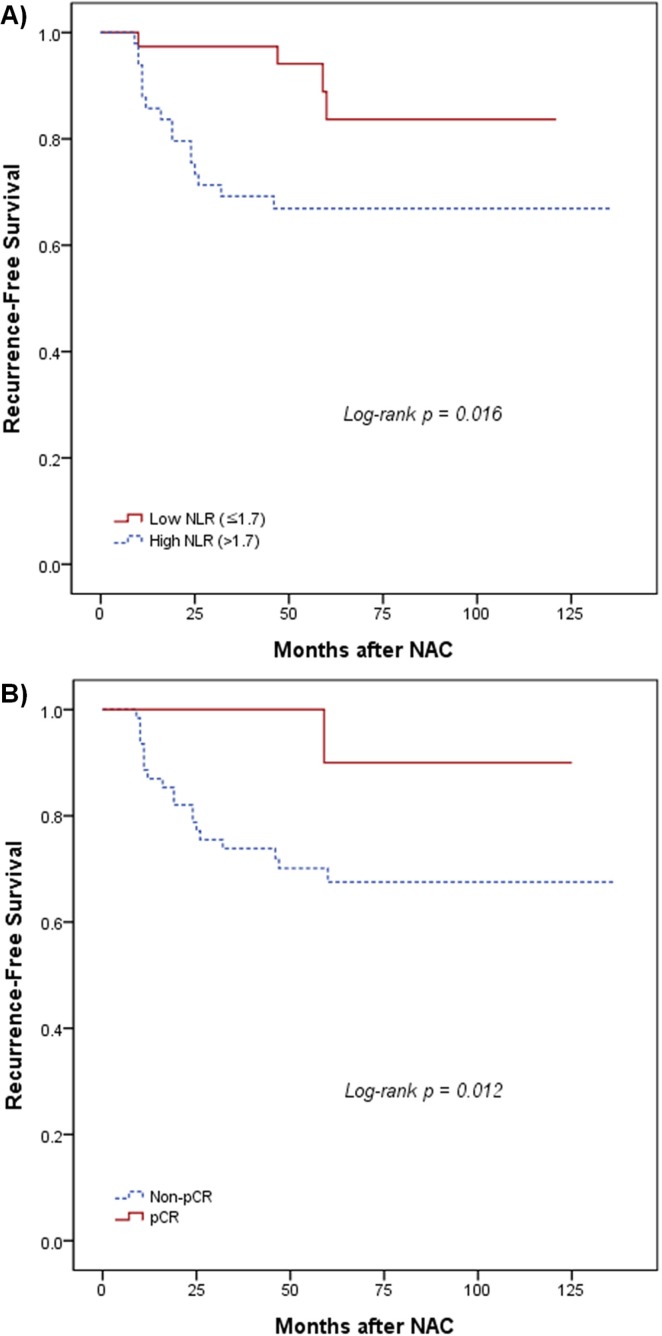
In the survival analysis, the rfs rate was significantly better for patients with a low nlr than for those with a high nlr: the 5-year rfs rate was 83.7% in the low nlr group and 66.9% in the high nlr group [p = 0.016, Figure 2(A)]. Mean survival duration was 109.2 months in the low nlr group and 97.4 months in the high nlr group. As expected, the rfs rate was also significantly different between the pcr and non-pcr groups. Compared with patients who did not experience a pcr, those who did had a significantly better 5-year rfs [90% vs. 67.5%, p = 0.012, Figure 2(B)].
FIGURE 2.
Recurrence-free survival by (A) neutrophil–lymphocyte ratio (NLR) and by (B) pathologic complete response (pCR). NAC = neoadjuvant chemotherapy.
DISCUSSION
In patients with breast cancer, the degree of response to nac is regarded as a prognostic marker. Earlier studies into nac demonstrated improved pcr rates in patients with tnbc than in those with other breast cancer subtypes, and an excellent prognosis for those who achieve a pcr9,20,21. Meanwhile, compared with patients having other breast cancer subtypes, those with tnbc who do not reach a pcr after nac experience particularly poor survival. With regard to patient outcome and therapeutic strategy, it is important to investigate predictive markers for efficacy of nac in patients with tnbc.
In the present study, we assessed 87 patients with tnbc who underwent nac and found that the pre-nac nlr predicted response to nac and disease recurrence. Moreover, compared with patients having a high pre-nac nlr, those having a low pre-nac nlr experienced a significantly higher rate of pcr and better rfs.
Recent studies have reported a correlation between systemic inflammation and cancer, suggesting that the immune response is crucial for cancer growth, progression, and treatment resistance, and that it is even associated with relapse and metastasis22,23. Several studies have suggested that, compared with other subtypes of breast cancer, tnbc might be more strongly affected by inflammatory cells. Matsumoto et al.24 demonstrated that inflammatory cells are related to sensitivity to chemotherapy and prognosis in patients with tnbc, and Loi25 found that the immune response positively affected progression-free survival, therapy response, and overall survival, especially in patients with tnbc. Retsky et al.26 demonstrated that perioperative use of the nonsteroidal anti-inflammatory drug ketorolac suppresses early breast cancer relapse, especially in patients with tnbc. Ono et al.27 reported that levels of tumour-infiltrating lymphocytes are significantly higher in patients with tnbc and that high levels of tumour infiltrating lymphocytes are related to improved survival and pcr in the neoadjuvant setting.
Since the early 2000s, various systemic inflammatory markers have been examined for their potential to predict response to therapy and patient outcome in cancer. The nlr is an available systemic inflammatory marker, and it can be calculated from the complete blood count that is routinely assessed in cancer patients28. The prognostic value of the nlr has been examined in various cancers—esophageal, gastric, colorectal, hepatic, pancreatic, pulmonary, urologic, and gynecologic28–31. In those studies, a high nlr was associated with poor survival probability. In breast cancer, the role of the nlr as a prognosticator has been evaluated in various settings17,19,32,33. Azab et al.17 were the first to report that the nlr is an independent predictor for both short- and long-term mortality, with a higher nlr being associated with worse outcomes. In their analysis, patients were divided into four quartiles, and compared with the patients having the lowest nlr (<1.8), those with the highest nlr (>3.3) experienced higher 1-year and 5-year mortality rates. Noh et al.19 also demonstrated that, compared with breast cancer patients having a lower nlr (<2.5), patients with higher nlr (≥2.5) experienced poorer disease-specific survival—a result that was most evident for the luminal A subtype.
The mechanisms of the relationship between the nlr and prognosis in cancer patients are not yet well understood. Neutrophils are effector cells involved in innate and adaptive immunity; they play an important role in the pathogenesis of various diseases, including inflammation and cancer. Neutrophils are known to be related to pro-tumour activity, such as promoting tumour cell proliferation, producing proangiogenic factors, and enhancing neoplastic cell invasiveness34–36. Neutrophilia, often observed in cancer, might be caused by cancer-related cytokines such as interleukin 6 and tumour necrosis factor α37,38. Furthermore, neutrophilia inhibits the cytotoxic activity of lymphocytes such as T cells and natural killer cells and therefore counteracts the antitumour immune response39,40. On the other hand, the lymphocyte response is an important component of immune surveillance and control of progression in cancer41. The importance of lymphocytes in mounting an antitumour response has been emphasized in several studies, in which the presence of lymphocytes in tumours was implicated in the killing of tumour cells and increased chemoresponsiveness and better outcome14. By contrast, lymphocytopenia and low intratumoural T lymphocytic activity indicate impairment in the cell-mediated immune system—conditions that correlate with tumour progression and poorer outcome42.
Although several studies have shown a correlation between the nlr and patient outcome in breast cancer, few studies have assessed the relationship between the nlr and outcome after nac. Recently, Asano et al.43 evaluated 177 patients with breast cancer who received nac and subsequent surgery, reporting that, compared with the high nlr group, the lower nlr group (<3) experienced a higher pcr rate (p < 0.001). Their study included 61 patients with tnbc, and 72.2% of the tnbc patients with a low nlr (26 of 36) experienced a pcr after nac. In contrast to our study and previous reports, no difference in survival was observed between the low- and high-nlr groups, whether those groups encompassed all patients and only those with tnbc. However, among the tnbc patients who achieved a pcr in our study, disease-free survival and overall survival were significantly longer for the low-nlr group than for the high-nlr group (p = 0.006 and p < 0.001 respectively).
As previously described, the correlation between outcome after nac and patient survival is strongest in patients with tnbc, and it has been suggested that, compared with other breast cancer subtypes, tnbc is more strongly affected by systemic inflammation. Furthermore, nac is much more frequently administered to patients with tnbc than to those with the luminal subtypes of breast cancer. For those reasons, we focused on tnbc rather than on the other subtypes, and we tried to identify whether the nlr could be not only a predictive marker of pcr to nac but also a prognostic marker of disease recurrence. We also evaluated prognosis by achievement of pcr to determine whether pcr was acting as a prognostic factor in our cohort, as is already known. As expected, our study showed that the pre-nac nlr is an independent predictor of pcr to nac and a prognostic marker of outcome in patients with tnbc. Our result could be very useful in clinical practice, because the nlr can be obtained without difficulty—and without additional examination or cost—from the complete blood count that is routinely performed in cancer patients.
In general, an nlr value of 3 is considered the optimal threshold for survival. However, after a receiver operating characteristic curve analysis, our study defined a high nlr as 1.7 or greater, because 1.7 was the cut-off providing the best predictive value for pcr. It is thought that the nlr might have different optimal cut-offs depending on the ethnic group and the endpoint being considered. Patients of Asian ethnicity, compared with white patients, are reported to have lower neutrophil counts and higher lymphocyte counts44. Additionally, although survival (disease-free or overall) has been used in other studies, our study used pcr to nac as the endpoint to evaluate the predictive value of the nlr, which could have contributed to the lower optimal nlr value established here. As in the present study, many other studies have reported that the nlr might have prognostic value in breast cancer. However, the role of the nlr as a predictive and prognostic marker has not always been clear. Several studies reported no association between the nlr and prognosis45,46. However, those negative reports included many patients with er-positive breast cancers (approximately 50%) known to be insusceptible to nac. The composition of the patient cohorts might have been responsible for the negative results obtained in those studies.
Our study has some limitations. It is a retrospective single-centre analysis with a small sample size. Furthermore, the nlr can be influenced by certain conditions (infection, and hepatic or renal dysfunction) and lifestyle habits (smoking and alcohol consumption). Most of our patients did not have those conditions, and we did not consider those conditions in the analysis.
Chemoresistance is a crucial clinical issue in the treatment of tnbc. For successful chemotherapy, markers to more specifically predict response and to help in the selection of patients likely to respond to chemotherapy have to be investigated. Furthermore, it is important to develop novel targeted agents that will enhance chemosensitivity in selected patients. Our study suggests that the relationship between inflammation and chemoresistance could be exploited, allowing the pre-nac nlr to be used as a factor predictive of chemoresistance and a new therapeutic target for improving chemosensitivity in selected patients. In rectal cancer, the administration of aspirin during preoperative chemoradiation was demonstrated to be associated with a higher rate of tumour downstaging, good pathologic response, and higher rate of pcr47. Determination of the mechanism by which systemic inflammation affects chemoresponsiveness will help in the selection of patients who will be most likely to benefit from nac.
CONCLUSIONS
Our finding that the pre-nac nlr is a predictor of pcr to nac and a prognosticator of recurrence suggests an association between response to chemotherapy and the patient’s inflammatory status. The preoperative nlr can be a useful predictive and prognostic marker in tnbc patients who undergo nac. Further prospective studies are warranted to support our results and to confirm the role of the nlr in patients with tnbc who require nac.
ACKNOWLEDGMENTS
We thank the Division of Statistics in the Medical Research Collaborating Centre at the Seoul National University Bundang Hospital for statistical analyses.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–74. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sotiriou C, Neo SY, McShane LM, et al. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci U S A. 2003;100:10393–8. doi: 10.1073/pnas.1732912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dawson SJ, Provenzano E, Caldas C. Triple negative breast cancers: clinical and prognostic implications. Eur j Cancer. 2009;45(suppl 1):27–40. doi: 10.1016/S0959-8049(09)70013-9. [DOI] [PubMed] [Google Scholar]
- 4.Gluz O, Liedtke C, Gottschalk N, Pusztai L, Nitz U, Harbeck N. Triple-negative breast cancer—current status and future directions. Ann Oncol. 2009;20:1913–27. doi: 10.1093/annonc/mdp492. [DOI] [PubMed] [Google Scholar]
- 5.Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001;30:96–102. doi: 10.1093/oxfordjournals.jncimonographs.a003469. [DOI] [PubMed] [Google Scholar]
- 6.Sachelarie I, Grossbard ML, Chadha M, Feldman S, Ghesani M, Blum RH. Primary systemic therapy of breast cancer. Oncologist. 2006;11:574–89. doi: 10.1634/theoncologist.11-6-574. [DOI] [PubMed] [Google Scholar]
- 7.Fisher B, Brown A, Mamounas E, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997;15:2483–93. doi: 10.1200/JCO.1997.15.7.2483. [DOI] [PubMed] [Google Scholar]
- 8.van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol. 2001;19:4224–37. doi: 10.1200/JCO.2001.19.22.4224. [DOI] [PubMed] [Google Scholar]
- 9.Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26:1275–81. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 10.Colleoni M, Cole BF, Viale G, et al. Classical cyclophosphamide, methotrexate, and fluorouracil chemotherapy is more effective in triple-negative, node-negative breast cancer: results from two randomized trials of adjuvant chemoendocrine therapy for node-negative breast cancer. J Clin Oncol. 2010;28:2966–73. doi: 10.1200/JCO.2009.25.9549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prat A, Fan C, Fernandez A, et al. Response and survival of breast cancer intrinsic subtypes following multi-agent neo-adjuvant chemotherapy. BMC Med. 2015;13:303. doi: 10.1186/s12916-015-0540-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796–804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 13.McCourt M, Wang JH, Sookhai S, Redmond HP. Activated human neutrophils release hepatocyte growth factor/scatter factor. Eur J Surg Oncol. 2001;27:396–403. doi: 10.1053/ejso.2001.1133. [DOI] [PubMed] [Google Scholar]
- 14.Mahmoud SM, Paish EC, Powe DG, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29:1949–55. doi: 10.1200/JCO.2010.30.5037. [DOI] [PubMed] [Google Scholar]
- 15.Rossi L, Santoni M, Crabb SJ, et al. High neutrophil-to-lymphocyte ratio persistent during first-line chemotherapy predicts poor clinical outcome in patients with advanced urothelial cancer. Ann Surg Oncol. 2015;22:1377–84. doi: 10.1245/s10434-014-4097-4. [DOI] [PubMed] [Google Scholar]
- 16.Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 17.Azab B, Bhatt VR, Phookan J, et al. Usefulness of the neutrophil-to-lymphocyte ratio in predicting short- and long-term mortality in breast cancer patients. Ann Surg Oncol. 2012;19:217–24. doi: 10.1245/s10434-011-1814-0. [DOI] [PubMed] [Google Scholar]
- 18.Koh CH, Bhoo-Pathy N, Ng KL, et al. Utility of pre-treatment neutrophil–lymphocyte ratio and platelet–lymphocyte ratio as prognostic factors in breast cancer. Br J Cancer. 2015;113:150–8. doi: 10.1038/bjc.2015.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noh H, Eomm M, Han A. Usefulness of pretreatment neutrophil to lymphocyte ratio in predicting disease-specific survival in breast cancer patients. J Breast Cancer. 2013;16:55–9. doi: 10.4048/jbc.2013.16.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fisher CS, Ma CX, Gillanders WE, et al. Neoadjuvant chemotherapy is associated with improved survival compared with adjuvant chemotherapy in patients with triple-negative breast cancer only after complete pathologic response. Ann Surg Oncol. 2012;19:253–8. doi: 10.1245/s10434-011-1877-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carey LA, Dees EC, Sawyer L, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13:2329–34. doi: 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- 22.Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13:759–71. doi: 10.1038/nrc3611. [DOI] [PubMed] [Google Scholar]
- 23.Chanrion M, Negre V, Fontaine H, et al. A gene expression signature that can predict the recurrence of tamoxifen-treated primary breast cancer. Clin Cancer Res. 2008;14:1744–52. doi: 10.1158/1078-0432.CCR-07-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsumoto H, Koo SL, Dent R, Tan PH, Iqbal J. Role of inflammatory infiltrates in triple negative breast cancer. J Clin Pathol. 2015;68:506–10. doi: 10.1136/jclinpath-2015-202944. [DOI] [PubMed] [Google Scholar]
- 25.Loi S. Host antitumor immunity plays a role in the survival of patients with newly diagnosed triple-negative breast cancer. J Clin Oncol. 2014;32:2935–7. doi: 10.1200/JCO.2014.56.7677. [DOI] [PubMed] [Google Scholar]
- 26.Retsky M, Rogers R, Demicheli R, et al. nsaid analgesic ketorolac used perioperatively may suppress early breast cancer relapse: particular relevance to triple negative subgroup. Breast Cancer Res Treat. 2012;134:881–8. doi: 10.1007/s10549-012-2094-5. [DOI] [PubMed] [Google Scholar]
- 27.Ono M, Tsuda H, Shimizu C, et al. Tumor-infiltrating lymphocytes are correlated with response to neoadjuvant chemotherapy in triple-negative breast cancer. Breast Cancer Res Treat. 2012;132:793–805. doi: 10.1007/s10549-011-1554-7. [DOI] [PubMed] [Google Scholar]
- 28.Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil–lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88:218–30. doi: 10.1016/j.critrevonc.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 29.Yamanaka T, Matsumoto S, Teramukai S, Ishiwata R, Nagai Y, Fukushima M. The baseline ratio of neutrophils to lymphocytes is associated with patient prognosis in advanced gastric cancer. Oncology. 2007;73:215–20. doi: 10.1159/000127412. [DOI] [PubMed] [Google Scholar]
- 30.Wang DS, Luo HY, Qiu MZ, et al. Comparison of the prognostic values of various inflammation based factors in patients with pancreatic cancer. Med Oncol. 2012;29:3092–100. doi: 10.1007/s12032-012-0226-8. [DOI] [PubMed] [Google Scholar]
- 31.Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophil–lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91:181–4. doi: 10.1002/jso.20329. [DOI] [PubMed] [Google Scholar]
- 32.Pistelli M, De Lisa M, Ballatore Z, et al. Pre-treatment neutrophil to lymphocyte ratio may be a useful tool in predicting survival in early triple negative breast cancer patients. BMC Cancer. 2015;15:195. doi: 10.1186/s12885-015-1204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hong J, Mao Y, Chen X, et al. Elevated preoperative neutrophil-to-lymphocyte ratio predicts poor disease-free survival in Chinese women with breast cancer. Tumour Biol. 2016;37:4135–42. doi: 10.1007/s13277-015-4233-1. [DOI] [PubMed] [Google Scholar]
- 34.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeNardo DG, Coussens LM. Inflammation and breast cancer. Balancing immune response: crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res. 2007;9:212. doi: 10.1186/bcr1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuang DM, Zhao Q, Wu Y, et al. Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma. J Hepatol. 2011;54:948–55. doi: 10.1016/j.jhep.2010.08.041. [DOI] [PubMed] [Google Scholar]
- 37.Ulich TR, del Castillo J, Keys M, Granger GA, Ni RX. Kinetics and mechanisms of recombinant human interleukin 1 and tumor necrosis factor-alpha–induced changes in circulating numbers of neutrophils and lymphocytes. J Immunol. 1987;139:3406–15. [PubMed] [Google Scholar]
- 38.Teramukai S, Kitano T, Kishida Y, et al. Pretreatment neutrophil count as an independent prognostic factor in advanced non-small-cell lung cancer: an analysis of Japan Multinational Trial Organisation LC00-03. Eur J Cancer. 2009;45:1950–8. doi: 10.1016/j.ejca.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 39.el-Hag A, Clark RA. Immunosuppression by activated human neutrophils. Dependence on the myeloperoxidase system. J Immunol. 1987;139:2406–13. [PubMed] [Google Scholar]
- 40.Rotondo R, Bertolotto M, Barisione G, et al. Exocytosis of azurophil and arginase 1–containing granules by activated polymorphonuclear neutrophils is required to inhibit T lymphocyte proliferation. J Leukoc Biol. 2011;89:721–7. doi: 10.1189/jlb.1109737. [DOI] [PubMed] [Google Scholar]
- 41.Shankaran V, Ikeda H, Bruce AT, et al. ifngamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–11. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 42.Mallappa S, Sinha A, Gupta S, Chadwick SJ. Preoperative neutrophil to lymphocyte ratio > 5 is a prognostic factor for recurrent colorectal cancer. Colorectal Dis. 2013;15:323–8. doi: 10.1111/codi.12008. [DOI] [PubMed] [Google Scholar]
- 43.Asano Y, Kashiwagi S, Onoda N, et al. Predictive value of neutrophil/lymphocyte ratio for efficacy of preoperative chemotherapy in triple-negative breast cancer. Ann Surg Oncol. 2016;23:1104–10. doi: 10.1245/s10434-015-4934-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bain B, Seed M, Godsland I. Normal values for peripheral blood white cell counts in women of four different ethnic origins. J Clin Pathol. 1984;37:188–93. doi: 10.1136/jcp.37.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cihan YB, Arslan A, Cetindag MF, Mutlu H. Lack of prognostic value of blood parameters in patients receiving adjuvant radiotherapy for breast cancer. Asian Pac J Cancer Prev. 2014;15:4225–31. doi: 10.7314/APJCP.2014.15.10.4225. [DOI] [PubMed] [Google Scholar]
- 46.Suppan C, Bjelic-Radisic V, La Garde M, et al. Neutrophil/lymphocyte ratio has no predictive or prognostic value in breast cancer patients undergoing preoperative systemic therapy. BMC Cancer. 2015;15:1027. doi: 10.1186/s12885-015-2005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Restivo A, Cocco IM, Casula G, et al. Aspirin as a neoadjuvant agent during preoperative chemoradiation for rectal cancer. Br J Cancer. 2015;113:1133–9. doi: 10.1038/bjc.2015.336. [DOI] [PMC free article] [PubMed] [Google Scholar]