Abstract
Background
Our objective was to determine whether, compared with control interventions, pharmacologic interventions reduce the severity of fatigue in patients with cancer or recipients of hematopoietic stem-cell transplantation (hsct).
Methods
For a systematic review, we searched medline, embase, the Cochrane Central Register of Controlled Trials, cinahl, and Psychinfo for randomized trials of systemic pharmacologic interventions for the management of fatigue in patients with cancer or recipients of hsct. Two authors independently identified studies and abstracted data. Methodologic quality was assessed using the Cochrane Risk of Bias tool. The primary outcome was fatigue severity measured using various fatigue scales. Data were synthesized using random-effects models.
Results
In the 117 included trials (19,819 patients), the pharmacologic agents used were erythropoietins (n = 31), stimulants (n = 19), l-carnitine (n = 6), corticosteroids (n = 5), antidepressants (n = 5), appetite stimulants (n = 3), and other agents (n = 48). Fatigue was significantly reduced with erythropoietin [standardized mean difference (smd): −0.52; 95% confidence interval (ci): −0.89 to −0.14] and with methylphenidate (smd: −0.36; 95% ci: −0.56 to −0.15); modafinil (or armodafinil) and corticosteroids were not effective.
Conclusions
Erythropoietin and methylphenidate significantly reduced fatigue severity in patients with cancer and in recipients of hsct. Concerns about the safety of those agents might limit their usefulness. Future research should identify effective interventions for fatigue that have minimal adverse effects.
Keywords: Pharmacologic agents, fatigue, meta-analyses, drugs, cancer-related fatigue, erythropoietin, stimulants, corticosteroids
INTRODUCTION
Cancer-related fatigue is increasingly being recognized as one of the most important symptoms in patients with cancer 1,2. It has been described as an unexpected tiredness that is more intense and severe than the fatigue experienced in healthy people 3. Cancer-related fatigue can affect up to 80%–90% of cancer patients, and it can occur before diagnosis, during cancer treatment, and after completion of cancer therapies1,4–9. The origin of cancer-related fatigue is multifactorial: it can be a result of the cancer itself, of cancer treatments, and of comorbid medical and psychological conditions 10,11. Recipients of hematopoietic stem-cell transplantation (hsct) also experience fatigue, likely related to similar underlying mechanisms 12,13.
Interventions including physical activity and psychological and pharmacologic approaches have been investigated for the management of fatigue in cancer patients, and several systematic reviews have been published 14–22. The evaluation of pharmacologic interventions is particularly important, because medications can be associated with adverse effects and high costs. Thus, a good understanding of the benefits and risks are necessary to guide decision-making. However, the systematic reviews of pharmacologic interventions published to date had restrictive inclusion and exclusion criteria, limiting the number of studies included 18,22. The reviews therefore lacked precision in their estimates of treatment effects and had limited power to identify effective interventions.
Our primary objective was to determine whether, compared with control interventions, pharmacologic interventions reduce the severity of fatigue in patients with cancer or in recipients of hsct.
METHODS
We followed the prisma (Preferred Reporting Items for Systematic Reviews and Meta-analyses) statement for the systematic review 23. A search for eligible randomized trials indexed from 1980 to 11 May 2017 was conducted in the medline, medline in-process, embase, Cochrane Central Register of Controlled Trials, cinahl, and Psychinfo electronic databases. The search strategy included mesh terms and text words that identified patients with cancer or recipients of hsct who received an intervention to reduce fatigue. Table i shows the full search strategy.
TABLE I.
Search strategies
| Database | Set | History |
|---|---|---|
| MEDLINE, 1946 to Week 1, May 2017 | ||
| 1 | fatigue/ or (fatigue or fatigued).ti,ab,kf. | |
| 2 | exp neoplasms/ or stem cell transplantation/ or cord blood stem cell transplantation/ or hematopoietic stem cell transplantation/ or mesenchymal stem cell transplantation/ or peripheral blood stem cell transplantation/ or bone marrow transplantation/ or transplantation, autologous/ or exp antineoplastic agents/ or chemotherap*.mp. or exp antineoplastic protocols/ or (cancer* or neoplas* or oncolog* or tumor* or tumour* or transplant* or chemotherap*).mp. | |
| 3 | randomized controlled trial.pt. | |
| 4 | controlled clinical trial.pt. | |
| 5 | randomized.ab. | |
| 6 | randomised.ab. | |
| 7 | randomly.ab. | |
| 8 | (trial or trials).ti,ab. | |
| 9 | or/3–8 | |
| 10 | 1 and 2 and 9 | |
| 11 | limit 10 to yr=“1980 -Current” | |
| 12 | limit 11 to humans | |
| MEDLINE in-process and other non-indexed citations, 10 May 2017 | ||
| 1 | (fatigue or fatigued).ti,ab,kw. | |
| 2 | (neoplasm* or neoplas* or cancer* or oncolog* or tumor* or tumour* or transplant*).mp. | |
| 3 | (hsct or bmt or chemotherap* or (antineoplas* adj2 protocol*) or (antineoplas* adj2 (agent* or drug or treatment*))).mp. | |
| 4 | or/2–3 | |
| 5 | (RCT or RCTS).ti,ab. | |
| 6 | randomized.ab. | |
| 7 | randomised.ab. | |
| 8 | randomly.ab. | |
| 9 | (trial or trials).ti,ab. | |
| 10 | or/5–9 | |
| 11 | 1 and 4 and 10 | |
| EMBASE, 1980 to Week 19, 2017 | ||
| 1 | *fatigue/ or (fatigue or fatigued).ti,ab,kw. | |
| 2 | exp neoplasm/ or exp antineoplastic agent/ or (antineoplas* adj2 protocol*).mp. | |
| 3 | (neoplas* or cancer* or oncolog* or tumor* or tumour* or transplant* or chemotherap*).mp. | |
| 4 | or/2–3 | |
| 5 | 1 and 4 | |
| 6 | cancer fatigue/ or (cancer* adj2 fatigue*).ti,ab,kw. | |
| 7 | 5 or 6 | |
| 8 | limit 7 to (randomized controlled trial or controlled clinical trial) | |
| 9 | (randomized or randomised or randomly).ab. | |
| 10 | (trial or trials).ti,ab. | |
| 11 | or/9–10 | |
| 12 | 8 or (7 and 11) | |
| 13 | limit 12 to conference abstract | |
| 14 | 12 Not 13 | |
| 15 | limit 14 to human | |
| PsycINFO, 1806 to Week 1, May 2017 | ||
| 1 | fatigue/ or (fatigue or fatigued).ti,ab,id. | |
| 2 | exp neoplasms/ or chemotherapy/ or exp antineoplastic drugs/ | |
| 3 | ((“stem cell*” or “stem-cell*” or “cord blood” or “bone marrowor autologous”) adj3 transplant*).mp. | |
| 4 | (cancer* or neoplas* or oncolog* or tumor* or tumour* or transplant* or chemotherap*).mp. | |
| 5 | or/2–4 | |
| 6 | 1 and 5 | |
| 7 | limit 6 to “0300 clinical trial” | |
| 8 | randomized.ab. | |
| 9 | randomised.ab. | |
| 10 | randomly.ab. | |
| 11 | (trial or trials).ti,ab. | |
| 12 | (RCT or CCT).ti,ab. | |
| 13 | clinical trials/ | |
| 14 | or/8–13 | |
| 15 | 7 or (6 and 14) | |
| 16 | limit 15 to yr=“1980 -Current” | |
| Cochrane Central Register of Controlled Trials, Issue 5, 12 May 2017 | ||
| 1 | MeSH descriptor: [Fatigue] this term only | |
| 2 | (fatigue or fatigued):ti,ab | |
| 3 | (or #1-#2) | |
| 4 | MeSH descriptor: [Neoplasms] explode all trees | |
| 5 | MeSH descriptor: [Antineoplastic Agents] explode all trees | |
| 6 | MeSH descriptor: [Antineoplastic Protocols] explode all trees | |
| 7 | (neoplas* or cancer* or oncolog* or tumor* or tumour* or transplant* or chemotherap*):ti,ab | |
| 8 | (or #4-#7) | |
| 9 | #3 and #8 Publication Year from 1980 to 2017 | |
| CINAHL, 1983 to 11 May 2017 | ||
| 1 | (MH “Cancer Fatigue”) OR (MH “Fatigue”) | |
| 2 | TI (fatigue OR fatigued) OR AB (fatigue OR fatigued) | |
| 3 | 1 OR 2 | |
| 4 | (MH “Neoplasms+”) OR (MH “Antineoplastic Agents+”) OR (MH “Antineoplastics, ImmuNosuppressives”) | |
| 5 | TX (antineoplastic N2 protocol*) | |
| 6 | (MH “ImmuNocompromised Host”) | |
| 7 | 4 OR 5 OR 6 | |
| 8 | 3 AND 7 | |
| 9 | (MH “Double-Blind Studies”) OR (MH “Randomized Controlled Trials”) OR (MH “Triple-Blind Studies”) OR (MH “Single-Blind Studies”) | |
| 10 | AB randomized or randomised or randomly or trial or trials | |
| 11 | 9 OR 10 | |
| 12 | 8 AND 11 | |
Study Selection and Data Abstraction
Inclusion and exclusion criteria were defined a priori. Studies were included if participants were adults or children with cancer or recipients of hsct and if the study was a fully published primary randomized or quasi-randomized trial with a parallel-group design that evaluated a pharmacologic intervention for the management of fatigue.
Studies were excluded if fewer than 75% of the participants had cancer or were undergoing hsct, if fatigue was not an endpoint or was reported as an adverse effect, if the intervention was direct cancer treatment, and if fewer than 5 participants were randomized to any study arm. Inclusion was not restricted by language. For the purpose of the analysis, studies were limited to those using a systemically administered pharmacologic agent. Studies using non-systemically administered pharmacologic agents were excluded, as were studies in which only education or advice was provided.
Two reviewers (PDR and SO or LS) independently evaluated the titles and abstracts of publications identified by the search. Any publication considered potentially relevant by at least one reviewer was retrieved in full and assessed for eligibility. Inclusion of studies in this meta-analysis was determined by agreement of two reviewers (PDR and SO or LS). Discrepancies between the two reviewers were resolved by consensus and adjudication by a third reviewer if required (LLD or LS). The kappa statistic was used to evaluate agreement for study inclusion between the two reviewers. Strength of agreement was defined as slight (0.00–0.20), fair (0.21–0.40), moderate (0.41–0.60), substantial (0.61–0.80), or almost perfect (0.81–1.00) 24.
Data were abstracted in duplicate by two reviewers (DT and PDR) and any discrepancies were resolved by consensus. We contacted authors of manuscripts when publications were missing data for the primary fatigue outcome.
Outcomes
The primary outcome was severity of self-reported fatigue using various fatigue scales. Change scores and end-of-intervention scores were both evaluated. For studies that used more than one fatigue scale, we a priori defined a hierarchy, based on prevalence, for the inclusion of scales in the analysis. Table ii shows the prevalence of the scales reported in our systematic review.
TABLE II.
Self-report fatigue assessment scales used in the included trialsa
| Fatigue scale | Studies (n) | Score range | Interpretation of higher score |
|---|---|---|---|
| Functional Assessment of Cancer Therapyb (13-item fatigue subscale) | 55 | 0–52 | Less fatigue |
| EORTC QLQ-C30 (fatigue subscale) | 23 | 0–100 | More fatigue |
| Brief Fatigue Inventoryc | 23 | 0–10 | More fatigue |
| Profile of Mood Statesd (fatigue subscale) | 11 | 0–28 | More fatigue |
| Visual Analog Scale | 8 | 0–10 | More fatigue |
| Number Rating Scale | 7 | 0–10 | More fatigue |
| Edmonton Symptom Assessment System (fatigue subscale) | 4 | 0–10 | More fatigue |
| Multidimensional Fatigue Symptom Inventory–Short Form | 4 | NA | More fatigue |
| Multidimensional Assessment of Fatigue (revised Piper Fatigue Scale) | 3 | 1–50 | More fatigue |
| Multidimensional Fatigue Inventory-20 | 2 | 4–20 | More fatigue |
| Others (used in 1 study each) | 16 | — | — |
Some studies used more than one fatigue scale.
FACIT.org, Elmhurst, IL, U.S.A.
MD Anderson Cancer Center, Houston, TX, U.S.A.
MHS Assessments, Toronto, ON.
EORTC = European Organisation for Research and Treatment of Cancer; QLQ-C30 = 30-question core Quality of Life Questionnaire; NA = not available.
The secondary outcome was the severity of self-reported fatigue using the most common fatigue scale (determined after all scales had been categorized).
Intervention and Control Groups
The intervention was any systemically administered pharmaceutical agent. In studies with more than two arms, the least “active” agent (for example, placebo, usual care, or lowest dose) was used as the control group. Where multiple pharmacologic agents were evaluated, the “intervention group” was the highest dose or the most commonly evaluated intervention (determined after all interventions had been abstracted and categorized).
We categorized the control group type as placebo, usual care, or other pharmacologic intervention.
Study Covariates
Study-level variables included age of the participants (adult or child), cancer diagnosis (breast, lung, other single cancer type, or more than one cancer type), inclusion of hsct patients, timing of the intervention (during cancer treatment, after completion of treatment, or both during and after treatment), exclusive enrolment of palliative care patients (as defined by each study), presence of fatigue as an eligibility criterion for enrolment (as defined by each study), and duration of intervention [<8 weeks, ≥8 weeks, or variable (based on median duration reported by each study)]. We also evaluated the methodologic aspects of the studies.
Risk-of-Bias Assessment
We used the Cochrane Collaboration tool for assessing the risk of bias in randomized trials 25. We evaluated sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, and attrition bias. Because of their potential effect on bias, adequate sequence generation and adequate allocation concealment were prioritized a priori for the stratified analyses 26.
Data Analysis
For this meta-analysis, we combined data at the study level and not at the individual patient level. All synthesized outcomes were continuous. For fatigue scores with missing summary measures, we made these assumptions to facilitate data synthesis: the mean can be approximated by the median; the range contains 6 standard deviations; the 95% confidence interval (ci) contains 4 standard errors; and the interquartile range contains 1.35 standard deviations. Where required, instruments were rescaled such that higher scores reflected more fatigue. We synthesized outcomes when data from at least three studies within a stratum were available.
For the primary outcome of severity of fatigue for all fatigue scales, data were synthesized using the standardized mean difference (smd). For the secondary outcome of the most commonly used fatigue scale, data were synthesized using the weighted mean difference (wmd). A smd or wmd less than 0 indicates that the mean fatigue scores were lower (better) in the intervention group than in the control group. Effect sizes were weighted using the inverse variance method. Given an anticipation of heterogeneity between the studies, a random-effects model was used for all analyses. Statistical heterogeneity between the trials was assessed using the I2 value, which describes the percentage total variation for all studies attributable to heterogeneity rather than to chance.
For the primary analysis, individual pharmacologic intervention groups were compared with all control groups using all fatigue severity scales. Change scores and end-of-intervention scores were both evaluated. Where possible, interventions were also evaluated against placebo. A secondary analysis evaluating the most commonly used fatigue severity scale was similarly conducted.
Potential publication bias was explored by a visual inspection of funnel plots when at least 10 studies were available for synthesis 25. In the event of potential publication bias, the “trim and fill” technique was used to determine the effect of such bias 27. In that technique, outlying studies are deleted, and hypothetical negative studies with equal weight are created.
Meta-analyses were conducted using Review Manager (version 5.2: Cochrane Collaboration, Nordic Cochrane Centre, Copenhagen, Denmark). All tests of significance were two-sided, and statistical significance was defined as p < 0.05.
RESULTS
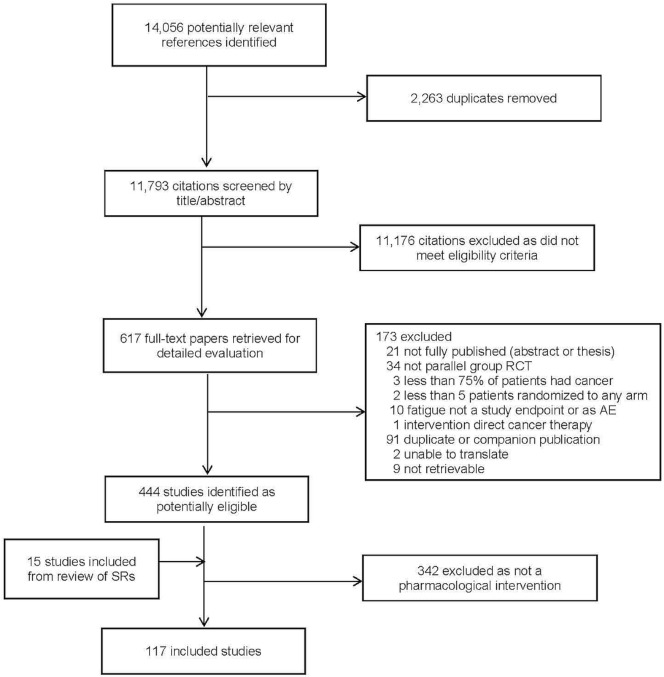
Figure 1 presents the flow diagram of study identification and selection. The search strategy identified 11,793 citations, of which 617 were retrieved for full-text evaluation. Within those 617 citations, 117 studies met the eligibility criteria and were included in the systematic review. Figure 1 indicates the reasons for exclusions. Agreement for study inclusion was almost perfect between the two reviewers (kappa: 0.97; 95% ci: 0.95 to 0.99).
FIGURE 1.
Study identification and selection, and reasons for study exclusion. RCT = randomized controlled trial; AE = adverse event; SRs = systematic reviews.
Tables iii and iv present the characteristics and details of the 117 included studies, which were conducted in more than 30 countries. Most of the studies (69.2%) were published during or after 2007. All were conducted exclusively in adults; no pediatric patients were included in any study. Breast cancer (15.4%) was the most common cancer diagnosis studied. Twenty studies (17.1%) were conducted exclusively in the palliative care setting.
TABLE III.
Characteristics of 117 studies included in the systematic review
| Characteristic | Value [n (%)] |
|---|---|
| Study population | |
| Adults | 117 (100) |
| Children | 0 |
| Cancer diagnosis | |
| Breast | 18 (15.4) |
| Lung | 11 (9.4) |
| Other single cancer type | 25 (21.4) |
| More than one cancer type | 63 (53.8) |
| Included HSCT recipients | 2 (1.7) |
| Timing of intervention | |
| During cancer treatment | 80 (68.4) |
| After treatment completion | 15 (12.8) |
| Both during and after treatment | 18 (15.4) |
| Not reported | 4 (3.4) |
| Palliative care setting only | 20 (17.1) |
| Required fatigue for eligibility | 28 (23.9) |
| Pharmaceutical company sponsor | 42 (35.9) |
| Duration of intervention | |
| <8 Weeks | 43 (36.8) |
| ≥8 Weeks | 57 (48.7) |
| Variable | 17 (14.5) |
| Intervention type | |
| Erythropoietins | 31 (26.5) |
| Stimulants | 19 (16.2) |
| L-Carnitine | 6 (5.1) |
| Corticosteroids | 5 (4.3) |
| Antidepressants | 5 (4.3) |
| Appetite stimulants | 3 (2.6) |
| Other agents | 48 (41.0) |
| Route of administration | |
| Oral | 67 (57.3) |
| Subcutaneous | 36 (30.8) |
| Intravenous | 13 (11.1) |
| Intramuscular | 1 (0.9) |
| Control group type | |
| Placebo | 75 (64.1) |
| Usual care | 26 (22.2) |
| Other pharmacologic | 16 (13.7) |
| Low risk of bias | |
| Adequate sequence generation | 68 (58.1) |
| Adequate allocation concealment | 41 (35.0) |
| Participants and personnel blinded | 44 (37.6) |
| Outcome assessors blinded | 55 (47.0) |
| Lack of attrition bias | 95 (81.2) |
| Free of selective reporting | 106 (90.6) |
HSCT = hematopoietic stem-cell transplantation.
TABLE IV.
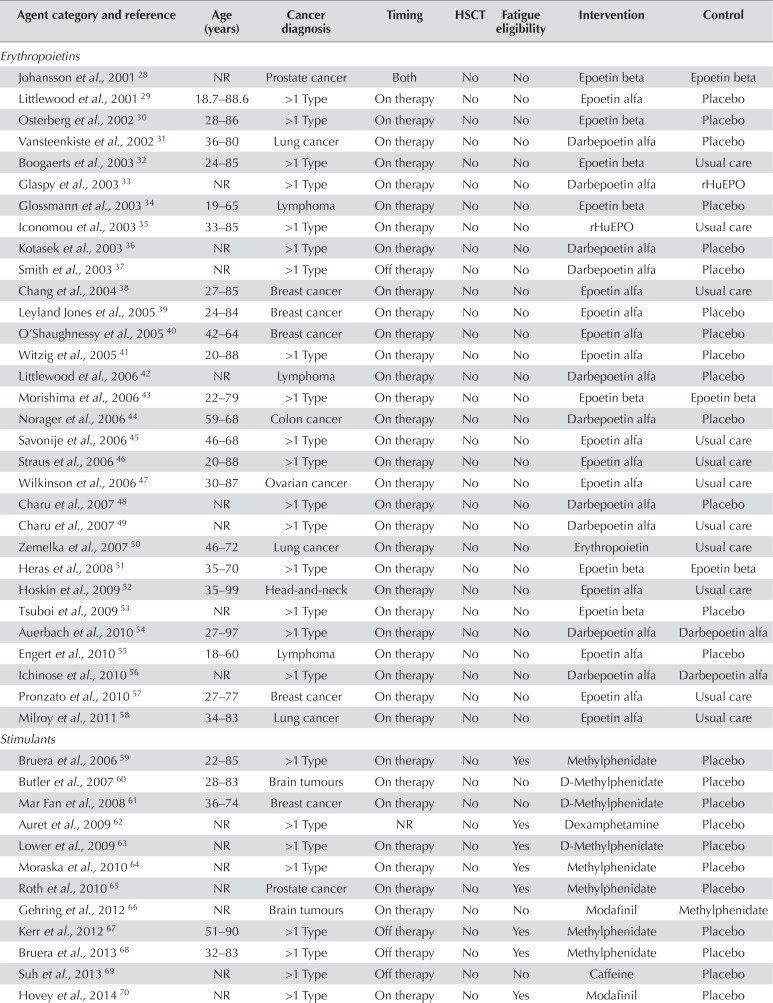
Details of the 117 included studies
| Agent category and reference | Age (years) | Cancer diagnosis | Timing | HSCT | Fatigue eligibility | Intervention | Control |
|---|---|---|---|---|---|---|---|
| Erythropoietins | |||||||
| Johansson et al., 2001 28 | NR | Prostate cancer | Both | No | No | Epoetin beta | Epoetin beta |
| Littlewood et al., 2001 29 | 18.7–88.6 | >1 Type | On therapy | No | No | Epoetin alfa | Placebo |
| Osterberg et al., 2002 30 | 28–86 | >1 Type | On therapy | No | No | Epoetin beta | Placebo |
| Vansteenkiste et al., 2002 31 | 36–80 | Lung cancer | On therapy | No | No | Darbepoetin alfa | Placebo |
| Boogaerts et al., 2003 32 | 24–85 | >1 Type | On therapy | No | No | Epoetin beta | Usual care |
| Glaspy et al., 2003 33 | NR | >1 Type | On therapy | No | No | Darbepoetin alfa | rHuEPO |
| Glossmann et al., 2003 34 | 19–65 | Lymphoma | On therapy | No | No | Epoetin beta | Placebo |
| Iconomou et al., 2003 35 | 33–85 | >1 Type | On therapy | No | No | rHuEPO | Usual care |
| Kotasek et al., 2003 36 | NR | >1 Type | On therapy | No | No | Darbepoetin alfa | Placebo |
| Smith et al., 2003 37 | NR | >1 Type | Off therapy | No | No | Darbepoetin alfa | Placebo |
| Chang et al., 2004 38 | 27–85 | Breast cancer | On therapy | No | No | Epoetin alfa | Usual care |
| Leyland Jones et al., 2005 39 | 24–84 | Breast cancer | On therapy | No | No | Epoetin alfa | Placebo |
| O’Shaughnessy et al., 2005 40 | 42–64 | Breast cancer | On therapy | No | No | Epoetin alfa | Placebo |
| Witzig et al., 2005 41 | 20–88 | >1 Type | On therapy | No | No | Epoetin alfa | Placebo |
| Littlewood et al., 2006 42 | NR | Lymphoma | On therapy | No | No | Darbepoetin alfa | Placebo |
| Morishima et al., 2006 43 | 22–79 | >1 Type | On therapy | No | No | Epoetin beta | Epoetin beta |
| Norager et al., 2006 44 | 59–68 | Colon cancer | On therapy | No | No | Darbepoetin alfa | Placebo |
| Savonije et al., 2006 45 | 46–68 | >1 Type | On therapy | No | No | Epoetin alfa | Usual care |
| Straus et al., 2006 46 | 20–88 | >1 Type | On therapy | No | No | Epoetin alfa | Usual care |
| Wilkinson et al., 2006 47 | 30–87 | Ovarian cancer | On therapy | No | No | Epoetin alfa | Usual care |
| Charu et al., 2007 48 | NR | >1 Type | On therapy | No | No | Darbepoetin alfa | Placebo |
| Charu et al., 2007 49 | NR | >1 Type | On therapy | No | No | Darbepoetin alfa | Usual care |
| Zemelka et al., 2007 50 | 46–72 | Lung cancer | On therapy | No | No | Erythropoietin | Usual care |
| Heras et al., 2008 51 | 35–70 | >1 Type | On therapy | No | No | Epoetin beta | Epoetin beta |
| Hoskin et al., 2009 52 | 35–99 | Head-and-neck | On therapy | No | No | Epoetin alfa | Usual care |
| Tsuboi et al., 2009 53 | NR | >1 Type | On therapy | No | No | Epoetin beta | Placebo |
| Auerbach et al., 2010 54 | 27–97 | >1 Type | On therapy | No | No | Darbepoetin alfa | Darbepoetin alfa |
| Engert et al., 2010 55 | 18–60 | Lymphoma | On therapy | No | No | Epoetin alfa | Placebo |
| Ichinose et al., 2010 56 | NR | >1 Type | On therapy | No | No | Darbepoetin alfa | Darbepoetin alfa |
| Pronzato et al., 2010 57 | 27–77 | Breast cancer | On therapy | No | No | Epoetin alfa | Usual care |
| Milroy et al., 2011 58 | 34–83 | Lung cancer | On therapy | No | No | Epoetin alfa | Usual care |
| Stimulants | |||||||
| Bruera et al., 2006 59 | 22–85 | >1 Type | On therapy | No | Yes | Methylphenidate | Placebo |
| Butler et al., 2007 60 | 28–83 | Brain tumours | On therapy | No | No | D-Methylphenidate | Placebo |
| Mar Fan et al., 2008 61 | 36–74 | Breast cancer | On therapy | No | No | D-Methylphenidate | Placebo |
| Auret et al., 2009 62 | NR | >1 Type | NR | No | Yes | Dexamphetamine | Placebo |
| Lower et al., 2009 63 | NR | >1 Type | On therapy | No | Yes | D-Methylphenidate | Placebo |
| Moraska et al., 2010 64 | NR | >1 Type | On therapy | No | Yes | Methylphenidate | Placebo |
| Roth et al., 2010 65 | NR | Prostate cancer | On therapy | No | Yes | Methylphenidate | Placebo |
| Gehring et al., 2012 66 | NR | Brain tumours | On therapy | No | No | Modafinil | Methylphenidate |
| Kerr et al., 2012 67 | 51–90 | >1 Type | Off therapy | No | Yes | Methylphenidate | Placebo |
| Bruera et al., 2013 68 | 32–83 | >1 Type | Off therapy | No | Yes | Methylphenidate | Placebo |
| Suh et al., 2013 69 | NR | >1 Type | Off therapy | No | No | Caffeine | Placebo |
| Hovey et al., 2014 70 | NR | >1 Type | On therapy | No | Yes | Modafinil | Placebo |
| Spathis et al., 2014 71 | NR | Lung cancer | On therapy | No | Yes | Modafinil | Placebo |
| Berenson et al., 2015 72 | 43–85 | Multiple myeloma | On therapy | No | Yes | Armodafinil | Placebo |
| Page et al., 2015 73 | 20–79 | Brain tumours | On therapy | No | No | Armodafinil | Placebo |
| Richard et al., 2015 74 | NR | Prostate cancer | On therapy | No | Yes | Methylphenidate | Placebo |
| Heckler et al., 2016 75 | NR | >1 Type | Off therapy | No | No | Armodafinil | Placebo |
| Jean-Pierre et al., 2016 76 | 18–90 | >1 Type | Both | No | Yes | Modafinil | Placebo |
| Lee et al., 2016 77 | 19–79 | Brain tumours | On therapy | No | No | Armodafinil | Placebo |
| Corticosteroids | |||||||
| Inoue et al., 2003 78 | 28–78 | >1 Type | On therapy | No | No | Dexamethasone | Placebo |
| Zarger-Shoshtari et al. 2009, 79 | 34–92 | Colorectal cancer | On therapy | No | No | Dexamethasone | Placebo |
| Yennurajalingam et al., 2013 80 | 29–89 | >1 Type | Both | No | Yes | Dexamethasone | Placebo |
| Paulsen et al., 2014 81 | NR | >1 Type | Both | No | Yes | Methylprednisolone | Placebo |
| Eguchi et al., 2015 82 | 46–84 | >1 Type | Off therapy | No | No | Methylprednisolone | Placebo |
| L-Carnitine | |||||||
| Cruciani et al., 2009 83 | 53.7–84.6 | >1 Type | Both | No | Yes | L-Carnitine | Placebo |
| Mantovani et al., 2010 84 | NR | >1 Type | Both | No | No | L-Carnitine | Nutritional supplement |
| Cruciani et al., 2012 85 | NR | >1 Type | Both | No | Yes | L-Carnitine | Placebo |
| Kraft et al., 2012 86 | NR | Pancreatic cancer | Both | No | No | L-Carnitine | Placebo |
| Hershman et al., 2013 87 | 26–80 | Breast cancer | On therapy | No | No | Acetyl-L-carnitine | Placebo |
| Iwase et al., 2016 88 | 22–70 | Breast cancer | Both | No | Yes | L-Carnitine | Usual care |
| Antidepressants | |||||||
| Capuron et al., 2002 89 | 25–74 | Malignant melanoma | On therapy | No | No | Paroxetine | Placebo |
| Morrow et al., 2003 90 | 23–87 | >1 Type | On therapy | No | Yes | Paroxetine | Placebo |
| Roscoe et al., 2005 91 | 31–79 | Breast cancer | On therapy | No | Yes | Paroxetine | Placebo |
| Stockler et al., 2007 92 | NR | >1 Type | On therapy | No | No | Sertraline | Placebo |
| Heras et al., 2013 93 | 32–89 | >1 Type | On therapy | No | Yes | Paroxetine | Placebo |
| Appetite stimulant | |||||||
| Simons et al., 1996 94 | NR | >1 Type | Off therapy | No | No | Medroxyprogesterone acetate | Placebo |
| De Conno et al., 1998 95 | NR | >1 Type | Off therapy | No | No | Megestrol | Placebo |
| Westman et al., 1999 96 | 37–89 | >1 Type | On therapy | No | No | Megestrol acetate | Placebo |
| American ginseng | |||||||
| Barton et al., 2010 97 | NR | >1 Type | On therapy | No | Yes | American ginseng | Placebo |
| Barton et al., 2013 98 | NR | >1 Type | Both | No | Yes | American ginseng | Placebo |
| Adenosine 5′-triphosphate (ATP) | |||||||
| Agteresch et al., 2000 99 | NR | Lung cancer | Off therapy | No | No | ATP | Usual care |
| Beijer et al., 2010 100 | NR | >1 Type | Both | No | No | ATP | Usual care |
| Celecoxib | |||||||
| Cerchietti et al., 2007 101 | 44–90 | Lung cancer | Off therapy | No | No | Celecoxib | Placebo and fish oil |
| Maccio et al., 2012 102 | NR | >1 Type | Both | No | No | Celecoxib, megestrol acetate, L-carnitine, and antioxidants | Megestrol acetate |
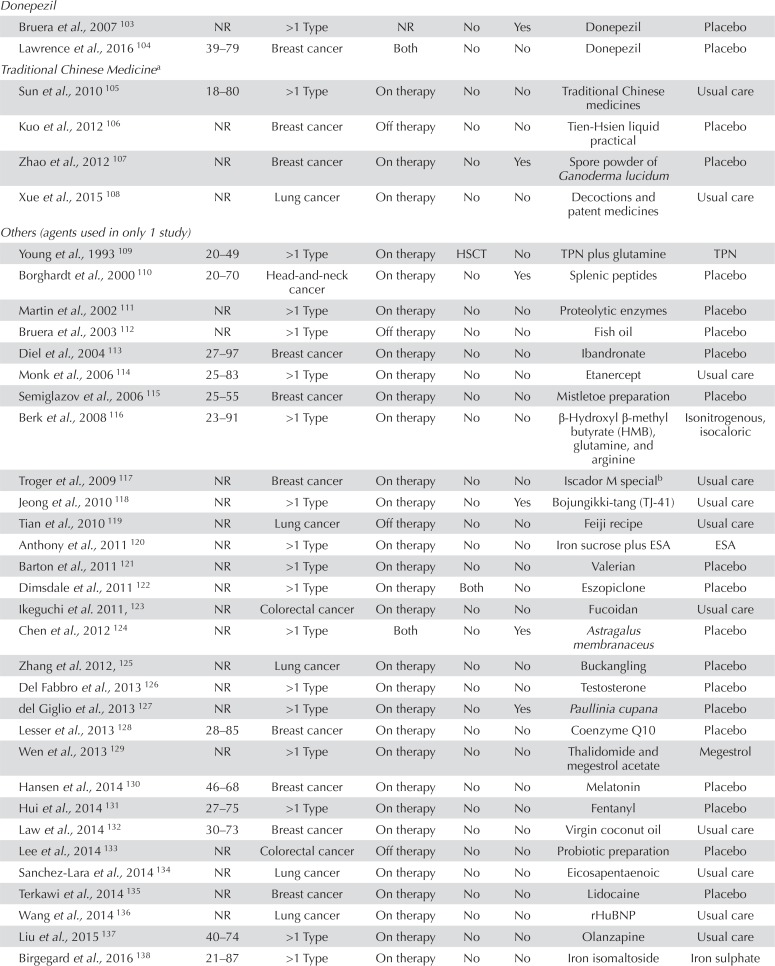
| Donepezil | |||||||
| Bruera et al., 2007 103 | NR | >1 Type | NR | No | Yes | Donepezil | Placebo |
| Lawrence et al., 2016 104 | 39–79 | Breast cancer | Both | No | No | Donepezil | Placebo |
| Traditional Chinese Medicinea | |||||||
| Sun et al., 2010 105 | 18–80 | >1 Type | On therapy | No | No | Traditional Chinese medicines | Usual care |
| Kuo et al., 2012 106 | NR | Breast cancer | Off therapy | No | No | Tien-Hsien liquid practical | Placebo |
| Zhao et al., 2012 107 | NR | Breast cancer | On therapy | No | Yes | Spore powder of Ganoderma lucidum | Placebo |
| Xue et al., 2015 108 | NR | Lung cancer | On therapy | No | No | Decoctions and patent medicines | Usual care |
| Others (agents used in only 1 study) | |||||||
| Young et al., 1993 109 | 20–49 | >1 Type | On therapy | HSCT | No | TPN plus glutamine | TPN |
| Borghardt et al., 2000 110 | 20–70 | Head-and-neck cancer | On therapy | No | Yes | Splenic peptides | Placebo |
| Martin et al., 2002 111 | NR | >1 Type | On therapy | No | No | Proteolytic enzymes | Placebo |
| Bruera et al., 2003 112 | NR | >1 Type | Off therapy | No | No | Fish oil | Placebo |
| Diel et al., 2004 113 | 27–97 | Breast cancer | On therapy | No | No | Ibandronate | Placebo |
| Monk et al., 2006 114 | 25–83 | >1 Type | On therapy | No | No | Etanercept | Usual care |
| Semiglazov et al., 2006 115 | 25–55 | Breast cancer | On therapy | No | No | Mistletoe preparation | Placebo |
| Berk et al., 2008 116 | 23–91 | >1 Type | On therapy | No | No | β-Hydroxyl β-methyl butyrate (HMB), glutamine, and arginine | Isonitrogenous,isocaloric |
| Troger et al., 2009 117 | NR | Breast cancer | On therapy | No | No | Iscador M specialb | Usual care |
| Jeong et al., 2010 118 | NR | >1 Type | On therapy | No | Yes Bojungikki-tang (TJ-41) | Usual care | |
| Tian et al., 2010 119 | NR | Lung cancer | Off therapy | No | No | Feiji recipe | Usual care |
| Anthony et al., 2011 120 | NR | >1 Type | On therapy | No | No | Iron sucrose plus ESA | ESA |
| Barton et al., 2011 121 | NR | >1 Type | On therapy | No | No | Valerian | Placebo |
| Dimsdale et al., 2011 122 | NR | >1 Type | On therapy | Both | No | Eszopiclone | Placebo |
| Ikeguchi et al. 2011, 123 | NR | Colorectal cancer | On therapy | No | No | Fucoidan | Usual care |
| Chen et al., 2012 124 | NR | >1 Type | Both | No | Yes | Astragalus membranaceus | Placebo |
| Zhang et al. 2012, 125 | NR | Lung cancer | On therapy | No | No | Buckangling | Placebo |
| Del Fabbro et al., 2013 126 | NR | >1 Type | On therapy | No | No | Testosterone | Placebo |
| del Giglio et al., 2013 127 | NR | >1 Type | On therapy | No | Yes | Paullinia cupana | Placebo |
| Lesser et al., 2013 128 | 28–85 | Breast cancer | On therapy | No | No | Coenzyme Q10 | Placebo |
| Wen et al., 2013 129 | NR | >1 Type | On therapy | No | No | Thalidomide and megestrol acetate | Megestrol |
| Hansen et al., 2014 130 | 46–68 | Breast cancer | On therapy | No | No | Melatonin | Placebo |
| Hui et al., 2014 131 | 27–75 | >1 Type | On therapy | No | No | Fentanyl | Placebo |
| Law et al., 2014 132 | 30–73 | Breast cancer | On therapy | No | No | Virgin coconut oil | Usual care |
| Lee et al., 2014 133 | NR | Colorectal cancer | Off therapy | No | No | Probiotic preparation | Placebo |
| Sanchez-Lara et al., 2014 134 | NR | Lung cancer | On therapy | No | No | Eicosapentaenoic | Usual care |
| Terkawi et al., 2014 135 | NR | Breast cancer | On therapy | No | No | Lidocaine | Placebo |
| Wang et al., 2014 136 | NR | Lung cancer | On therapy | No | No | rHuBNP | Usual care |
| Liu et al., 2015 137 | 40–74 | >1 Type | On therapy | No | No | Olanzapine | Usual care |
| Birgegard et al., 2016 138 | 21–87 | >1 Type | On therapy | No | No | Iron isomaltoside | Iron sulphate |
| Jeon et al., 2016 139 | NR | Colon cancer | On therapy | No | No | Vitamin C | Placebo |
| Mofid et al. 2016, 140 | NR | >1 Type | On therapy | No | Yes | Royal jelly and honey | Honey |
| Faramarzi et al., 2017 141 | NR | Rectal cancer | On therapy | No | No | Conjugated linoleic acid | Placebo |
| Martins et al., 2017 142 | NR | Head-and-neck cancer | On therapy | No | No | Guarana | Placebo |
| Ribeiro et al., 2017 143 | NR | Colorectal cancer | Both | No | No | Zinc supplement | Placebo |
| Sun et al., 2017 144 | 18–90 | Gastric cancer | Off therapy | No | No | Jinlongshe granule | Placebo |
Studies included differing agents within Traditional Chinese Medicines.
Iscador Ltd., Lörrach, Germany.
HSCT = hematopoietic stem-cell transplantation; NR = not reported; SC = subcutaneous; rHuEPO = recombinant human erythropoietin; PO = oral; IV = intravenous; CTx = chemotherapy; TPN = total parenteral nutrition; ESA = erythropoiesis stimulating agents; IM = intramuscular; CFU = colony-forming units; rHuBNP = recombinant human B-type natriuretic peptide.
The pharmacologic interventions studied were erythropoietins (n = 31, 26.5%), stimulants (n = 19, 16.2%), l-carnitine (n = 6, 5.1%), corticosteroids (n = 5, 4.3%), anti-depressants (n = 5, 4.3%), appetite stimulants (n = 3, 2.6%), and others (n = 48, 41.0%). The comparison groups were placebo (n = 75, 64.1%), usual care (n = 26, 22.2%), and other pharmacologic interventions (n = 16, 13.7%).
Table ii lists all the fatigue assessment scales used in the various studies. The scale most commonly used was the Functional Assessment of Cancer Therapy (fact) 13-item fatigue scale (FACIT.org, Elmhurst, IL, U.S.A.). Of all the studies included in our systematic review, only 35 (29.9%) could be included in any synthesis because of the requirements that an estimate of central tendency (mean or median) and a measure of variability be presented and that at least three studies with such data be included within a stratum. The pharmacologic agents for which synthesizable data were available were erythropoietins, stimulants, and corticosteroids.
Table v shows the effects of the evaluable pharmacologic agents by either change scores or end-of-intervention score. In evaluating erythropoietin, only change scores could be evaluated because too few studies reported end-of-intervention scores for any analysis. Compared with all controls and placebo, erythropoietin significantly improved fatigue. Compared with all controls, its smd was −0.52 (95% ci: −0.89 to −0.14). When the comparison was restricted to studies that reported fatigue using the fact, fatigue was significantly improved in patients receiving erythropoietin compared with all control patients (wmd: −2.98; 95% ci: −4.41 to −1.55).
TABLE V.
Effect of erythropoietins, stimulants, and corticosteroids on fatigue using all fatigue scales and the FACT scalea
| Agent and comparators | Outcome | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Fatigue change score | End-of-intervention fatigue score | |||||||||||
|
|
|
|||||||||||
| Studies (n) | Pts (n) | Effect | 95% CL (%) | I2 | p Value | Studies (n) | Pts (n) | Effect | 95% CL (%) | I2 | p Value | |
| Erythropoietins | ||||||||||||
| All scales | ||||||||||||
| All interventions vs. all controls | 14 | 3,037 | −0.52 SMD | −0.89, −0.14 | 96 | 0.007 | 2 | NSP | ||||
| All interventions vs. placebo | 6 | 1,057 | −0.19 SMD | −0.32, −0.07 | 0 | 0.003 | 1 | NSP | ||||
| FACT scale | ||||||||||||
| All interventions vs. all controls | 12 | 2,587 | −2.98 WMD | −4.41, −1.55 | 79 | <0.001 | 0 | NSP | ||||
| All interventions vs. placebo | 4 | 683 | −2.49 WMD | −4.06, −0.92 | 0 | 0.002 | 0 | NSP | ||||
| Stimulants | ||||||||||||
| All scales | ||||||||||||
| All interventions vs. all controls | 9 | 1,240 | −0.16 SMD | −0.34, 0.02 | 42 | 0.08 | 13 | 1,287 | −0.09 SMD | −0.28, 0.11 | 50 | 0.51 |
| All interventions vs. placebob | 9 | 1,240 | −0.16 SMD | −0.34, 0.02 | 42 | 0.08 | 12 | 1,263 | −0.08 SMD | −0.28, 0.12 | 53 | 0.44 |
| Stratified by agent for all scales | ||||||||||||
| Methylphenidate vs. all controls | 5 | 369 | −0.36 SMD | −0.56, −0.15 | 0 | <0.001 | 6 | 305 | −0.32 SMD | −0.80, 0.17 | 73 | 0.20 |
| Modafinil/armodafinil vs. all controls | 4 | 871 | 0.01 SMD | −0.21, 0.22 | 36 | 0.94 | 5 | 905 | −0.04 SMD | −0.17, 0.09 | 0 | 0.51 |
| FACT scale | ||||||||||||
| All interventions vs. all controls | 7 | 596 | −1.35 WMD | −3.47, 0.78 | 50 | 0.21 | 7 | 424 | 0.80 WMD | −1.57, 3.18 | 0 | 0.51 |
| All interventions vs. placebob | 7 | 596 | −1.35 WMD | −3.47, 0.78 | 50 | 0.21 | 7 | 424 | 0.80 WMD | −1.57, 3.18 | 0 | 0.51 |
| Methylphenidate vs. all controls | 4 | 346 | −2.87 WMD | −4.68, −1.07 | 0 | 0.002 | 3 | 150 | 0.71 WMD | −3.18, 4.59 | 0 | 0.72 |
| Modafinil/armodafinil vs. all controls | 3 | 250 | 1.24 WMD | −2.19, 4.68 | 49 | 0.48 | 4 | 274 | 0.89 WMD | −2.17, 3.94 | 3 | 0.57 |
| Corticosteroids | ||||||||||||
| All interventions vs. all controls | 3 | 165 | −0.43 SMD | −1.00, 0.14 | 67 | 0.14 | 2 | NSP | ||||
| All interventions vs. placebob | 3 | 165 | −0.43 SMD | −1.00, 0.14 | 67 | 0.14 | 2 | NSP | ||||
Outcomes using the FACT (FACIT.org, Elmhurst, IL, U.S.A.) were rescaled (multiplied by −1) such that higher scores reflect more fatigue. One study contributed twice: results were reported separately for the lymphoma and multiple myeloma groups (Littlewood et al., 200129 and Littlewood et al., 200642).
All synthesized studies were placebo-controlled.
FACT = Functional Assessment of Cancer Therapy; Pts = patients; CL = confidence limits; SMD = standardized mean difference; NSP = no synthesis possible (too few studies); WMD = weighted mean difference.
Table v also shows the effect of stimulants compared with all control treatments and with placebo. As a group, stimulants were not effective for improving change or end-of-intervention fatigue scores. However, when stratified by specific agent, methylphenidate was associated with a significant improvement in fatigue (smd: −0.36; 95% ci: −0.56 to −0.15; and wmd: −2.87; 95% ci: −4.68 to −1.07); modafinil (or armodafinil) was not effective in any comparison. Corticosteroids were not associated with improvement in fatigue (Table v).
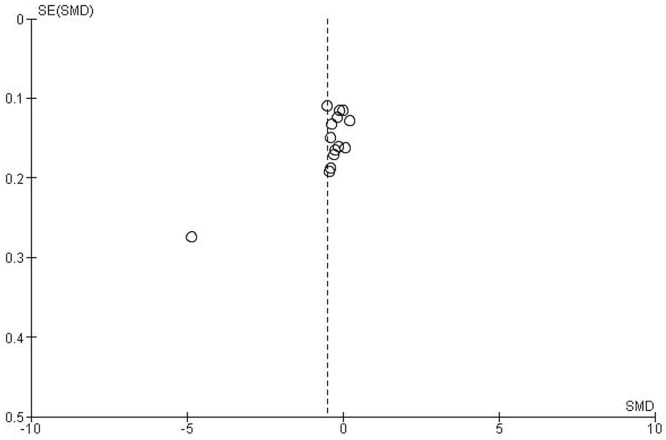
Given the small number of studies having data available for synthesis, stratified analyses could not be conducted for l-carnitine, antidepressants, and appetite stimulants. All other agents were examined in only one or two studies, and thus data synthesis was not possible (see Table iv). Figure 2 presents the funnel plot for erythropoietin compared with all controls; no evidence of publication bias was observed.
FIGURE 2.
Funnel plot comparing erythropoietins with all control medications. SE = standard error; SMD = standardized mean difference.
DISCUSSION
In the present systematic review and meta-analysis, erythropoietin and methylphenidate were found to be associated with significant improvements in fatigue for patients with cancer and for recipients of hsct; modafinil (or armodafinil) and corticosteroids were not found to be effective. Also, despite a very large number of randomized trials, data synthesis was limited. Most interventions were studied only once or twice; and even for agents that were studied more often, the data could not be synthesized because of limited data reporting from many of the studies.
Erythropoietin was found to be effective in reducing fatigue, but the size of the effect—a wmd of 2.49 compared with placebo according to the fact 13-item fatigue subscale—was small. The minimal clinically important difference for the fact 13-item fatigue subscale has been reported to be 3–3.5 145, which suggests that, although statistically significant, the observed effect is not meaningful to patients. Combined with concerns about the tumour protection, venothrombotic events, and worse survival potentially associated with erythropoietin 146,147, that minimal change in outcome suggests that this agent should not routinely be used in clinical practice for fatigue reduction.
The other pharmacologic agent that was found to be effective for fatigue was methylphenidate. However, the wmd of methylphenidate also did not meet the threshold for clinical importance. Further, a Cochrane review of methylphenidate for attention deficit hyperactivity disorder suggested that this agent is associated with an increased risk of non-serious adverse events—sleep problems and decreased appetite being most common 148. Those issues suggest that methylphenidate should not routinely be used to manage fatigue in patients with cancer and in recipients of hsct, but could selectively be used in specific patients for whom the potential benefits outweigh the disadvantages.
None of the studies found during the systematic review of literature included children. That omission is important, because patients with childhood cancer experience severe fatigue 149,150 and are vulnerable to long-term side effects of treatments 151. Pharmacologic interventions might not have been applied in children because dosing considerations and safety concerns add complexity. However, future studies should consider the pediatric population when formulating eligibility criteria.
An interesting observation was that, despite the large number of randomized trials, relatively few studies had data available for meta-analysis. Although the fact 13-item fatigue subscale was used in many of the trials, publications were inconsistent in whether they reported fact change scores or end-of-intervention scores. Additionally, many of the studies did not report a measure of central tendency and a measure of variability for either of the two fatigue outcomes (change or end-of-intervention score). The lack of well-reported fatigue data raises potential concerns about a form of publication bias in which negative endpoints are not reported or the data are not shown. Future randomized studies focused on fatigue reduction should be encouraged to explicitly report data that could be combined for analysis in systematic reviews.
The present systematic review complements two previously published meta-analyses evaluating the effects of pharmacologic agents on fatigue in cancer patients 18,152. Our review adds important insights, given that the review by Mustian et al. 18 reported many types of interventions, citing 14 studies of pharmacologic interventions that were analyzed as a single group. To inform practice, studies must evaluate pharmacologic agents separately. The review by Minton and Stone 152, which analyzed specific pharmacologic interventions, is now outdated, being based on a literature search conducted in 2009.
The strengths of the present review are its broad eligibility criteria, its inclusion of publications in all languages, and its focus on systemically administered pharmacologic agents. However, our meta-analysis was limited because of the data reporting in the primary studies. Furthermore, wide variations in dose and schedule were noted for the individual pharmacologic agents studied, and the limited number of studies available for synthesis meant that stratified analyses were not possible.
CONCLUSIONS
Erythropoietin and methylphenidate significantly reduce fatigue severity in patients with cancer and recipients of hsct; however, the magnitude of the benefit is of questionable clinical significance. Use of those agents is potentially further limited by concerns about safety. Pharmacologic interventions should not routinely be used to reduce fatigue severity. Future meta-analyses should obtain individual data from trials to better understand how pharmacologic interventions affect fatigue. Research is required to identify interventions for fatigue that are effective and have minimal adverse effects.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Weis J. Cancer-related fatigue: prevalence, assessment and treatment strategies. Expert Rev Pharmacoecon Outcomes Res. 2011;11:441–6. doi: 10.1586/erp.11.44. [DOI] [PubMed] [Google Scholar]
- 2.Tomlinson D, Hinds PS, Ethier MC, Ness KK, Zupanec S, Sung L. Psychometric properties of instruments used to measure fatigue in children and adolescents with cancer: a systematic review. J Pain Symptom Manage. 2013;45:83–91. doi: 10.1016/j.jpainsymman.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Wu HS, McSweeney M. Cancer-related fatigue: “It’s so much more than just being tired.”. Eur J Oncol Nurs. 2007;11:117–25. doi: 10.1016/j.ejon.2006.04.037. [DOI] [PubMed] [Google Scholar]
- 4.Henry DH, Viswanathan HN, Elkin EP, Traina S, Wade S, Cella D. Symptoms and treatment burden associated with cancer treatment: results from a cross-sectional national survey in the U.S. Support Care Cancer. 2008;16:791–801. doi: 10.1007/s00520-007-0380-2. [DOI] [PubMed] [Google Scholar]
- 5.Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12(suppl 1):4–10. doi: 10.1634/theoncologist.12-S1-4. [DOI] [PubMed] [Google Scholar]
- 6.Mustian KM, Palesh O, Heckler CE, et al. Cancer-related fatigue interferes with activities of daily living among 753 patients receiving chemotherapy: a urcc ccop study [abstract 9500] J Clin Oncol. 2008;26 doi: 10.1200/jco.2008.26.15_suppl.9500. [Available online at: http://ascopubs.org/doi/abs/10.1200/jco.2008.26.15_suppl.9500; cited 24 March 2018] [DOI] [Google Scholar]
- 7.Wang XS, Zhao F, Fisch MJ, et al. Prevalence and characteristics of moderate to severe fatigue: a multicenter study in cancer patients and survivors. Cancer. 2014;120:425–32. doi: 10.1002/cncr.28434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linder LA. Developmental diversity in symptom research involving children and adolescents with cancer. J Pediatr Nurs. 2008;23:296–309. doi: 10.1016/j.pedn.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Ream E, Gibson F, Edwards J, Seption B, Mulhall A, Richardson A. Experience of fatigue in adolescents living with cancer. Cancer Nurs. 2006;29:317–26. doi: 10.1097/00002820-200607000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Berger AM, Mooney K, Alvarez-Perez A, et al. Cancer-related fatigue, version 2.2015. J Natl Compr Canc Netw. 2015;13:1012–39. doi: 10.6004/jnccn.2015.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wagner LI, Cella D. Fatigue and cancer: causes, prevalence and treatment approaches. Br J Cancer. 2004;91:822–8. doi: 10.1038/sj.bjc.6602012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tonosaki A. The long-term effects after hematopoietic stem cell transplant on leg muscle strength, physical inactivity and fatigue. Eur J Oncol Nurs. 2012;16:475–82. doi: 10.1016/j.ejon.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Graef DM, Phipps S, Parris KR, et al. Sleepiness, fatigue, behavioral functioning, and quality of life in survivors of childhood hematopoietic stem cell transplant. J Pediatr Psychol. 2016;41:600–9. doi: 10.1093/jpepsy/jsw011. [DOI] [PubMed] [Google Scholar]
- 14.Hilfiker R, Meichtry A, Eicher M, et al. Exercise and other non-pharmaceutical interventions for cancer-related fatigue in patients during or after cancer treatment: a systematic review incorporating an indirect-comparisons meta-analysis. Br J Sports Med. 2017 doi: 10.1136/bjsports-2016-096422. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keilani M, Hasenoehrl T, Baumann L, et al. Effects of resistance exercise in prostate cancer patients: a meta-analysis. Support Care Cancer. 2017;25:2953–68. doi: 10.1007/s00520-017-3771-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lipsett A, Barrett S, Haruna F, Mustian K, O’Donovan A. The impact of exercise during adjuvant radiotherapy for breast cancer on fatigue and quality of life: a systematic review and meta-analysis. Breast. 2017;32:144–55. doi: 10.1016/j.breast.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Mishra SI, Scherer RW, Geigle PM, et al. Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst Rev. 2012 doi: 10.1002/14651858.CD007566.pub2. CD007566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mustian KM, Alfano CM, Heckler C, et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: a meta-analysis. JAMA Oncol. 2017;3:961–8. doi: 10.1001/jamaoncol.2016.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pearson EJ, Morris ME, di Stefano M, McKinstry CE. Interventions for cancer-related fatigue: a scoping review. Eur J Cancer Care (Engl) 2018;27:e12516. doi: 10.1111/ecc.12516. [DOI] [PubMed] [Google Scholar]
- 20.Yennurajalingam S, Bruera E. Review of clinical trials of pharmacologic interventions for cancer-related fatigue: focus on psychostimulants and steroids. Cancer J. 2014;20:319–24. doi: 10.1097/PPO.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Q, Zhang L, Yin R, Fu T, Chen H, Shen B. Effectiveness of telephone-based interventions on health-related quality of life and prognostic outcomes in breast cancer patients and survivors—a meta-analysis. Eur J Cancer Care (Engl) 2018;27:e12632. doi: 10.1111/ecc.12632. [DOI] [PubMed] [Google Scholar]
- 22.Minton O, Richardson A, Sharpe M, Hotopf M, Stone P. Drug therapy for the management of cancer-related fatigue. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD006704.pub3. CD006704. [DOI] [PubMed] [Google Scholar]
- 23.Shamseer L, Moher D, Clarke M, et al. on behalf of the prisma-p group Preferred reporting items for systematic review and meta-analysis protocols (prisma-p) 2015: elaboration and explanation. BMJ. 2015;349:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 24.Koch GG, Landis JR, Freeman JL, Freeman DH, Jr, Lehnen RC. A general methodology for the analysis of experiments with repeated measurement of categorical data. Biometrics. 1977;33:133–58. doi: 10.2307/2529309. [DOI] [PubMed] [Google Scholar]
- 25.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. London, UK: The Cochrane Collaboration; 2011. Ver. 5.1.0. [Available online at: http://training.cochrane.org/handbook; cited 15 August 2017] [Google Scholar]
- 26.Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273:408–12. doi: 10.1001/jama.1995.03520290060030. [DOI] [PubMed] [Google Scholar]
- 27.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63. doi: 10.1111/j.0006-341X.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 28.Johansson JE, Wersäll P, Brandberg Y, Andersson SO, Nordström L, on behalf of the epo-Study group Efficacy of epoetin beta on hemoglobin, quality of life, and transfusion needs in patients with anemia due to hormone-refractory prostate cancer—a randomized study. Scand J Urol Nephrol. 2001;35:288–94. doi: 10.1080/003655901750425864. [DOI] [PubMed] [Google Scholar]
- 29.Littlewood TJ, Bajetta E, Nortier JW, Vercammen E, Rapoport B, on behalf of the Epoetin Alfa Study Group Effects of epoetin alfa on hematologic parameters and quality of life in cancer patients receiving nonplatinum chemotherapy: results of a randomized, double-blind, placebo-controlled trial. J Clin Oncol. 2001;19:2865–74. doi: 10.1200/JCO.2001.19.11.2865. [DOI] [PubMed] [Google Scholar]
- 30.Osterborg A, Brandberg Y, Molostova V, et al. Randomized, double-blind, placebo-controlled trial of recombinant human erythropoietin, epoetin beta, in hematologic malignancies. J Clin Oncol. 2002;20:2486–94. doi: 10.1200/JCO.2002.08.131. [DOI] [PubMed] [Google Scholar]
- 31.Vansteenkiste J, Pirker R, Massuti B, et al. on behalf of the Aranesp 980297 study group Double-blind, placebo-controlled, randomized phase iii trial of darbepoetin alfa in lung cancer patients receiving chemotherapy. J Natl Cancer Inst. 2002;94:1211–20. doi: 10.1093/jnci/94.16.1211. [DOI] [PubMed] [Google Scholar]
- 32.Boogaerts M, Coiffier B, Kainz C, on behalf of the Epoetin Beta QOL Working Group Impact of epoetin beta on quality of life in patients with malignant disease. Br J Cancer. 2003;88:988–95. doi: 10.1038/sj.bjc.6600801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glaspy JA, Jadeja JS, Justice G, Fleishman A, Rossi G, Colowick AB. A randomized, active-control, pilot trial of front-loaded dosing regimens of darbepoetin-alfa for the treatment of patients with anemia during chemotherapy for malignant disease. Cancer. 2003;97:1312–20. doi: 10.1002/cncr.11186. [DOI] [PubMed] [Google Scholar]
- 34.Glossmann J, Engert A, Wassmer G, et al. Recombinant human erythropoietin, epoetin beta, in patients with relapsed lymphoma treated with aggressive sequential salvage chemotherapy—results of a randomized trial. Ann Hematol. 2003;82:469–75. doi: 10.1007/s00277-003-0695-0. [DOI] [PubMed] [Google Scholar]
- 35.Iconomou G, Koutras A, Rigopoulos A, Vagenakis AG, Kalofonos HP. Effect of recombinant human erythropoietin on quality of life in cancer patients receiving chemotherapy: results of a randomized, controlled trial. J Pain Symptom Manage. 2003;25:512–18. doi: 10.1016/S0885-3924(03)00070-8. [DOI] [PubMed] [Google Scholar]
- 36.Kotasek D, Steger G, Faught W, et al. on behalf of the Aranesp 980291 study group Darbepoetin alfa administered every 3 weeks alleviates anaemia in patients with solid tumours receiving chemotherapy; results of a double-blind, placebo-controlled, randomised study. Eur J Cancer. 2003;39:2026–34. doi: 10.1016/S0959-8049(03)00456-8. [DOI] [PubMed] [Google Scholar]
- 37.Smith RE, Jr, Tchekmedyian NS, Chan D, et al. A dose- and schedule-finding study of darbepoetin alpha for the treatment of chronic anaemia of cancer. Br J Cancer. 2003;88:1851–8. doi: 10.1038/sj.bjc.6600994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang J, Couture FA, Young SD, Lau CY, Lee McWatters K. Weekly administration of epoetin alfa improves cognition and quality of life in patients with breast cancer receiving chemotherapy. Support Cancer Ther. 2004;2:52–8. doi: 10.3816/SCT.2004.n.023. [DOI] [PubMed] [Google Scholar]
- 39.Leyland-Jones B, Semiglazov V, Pawlicki M, et al. Maintaining normal hemoglobin levels with epoetin alfa in mainly nonanemic patients with metastatic breast cancer receiving first-line chemotherapy: a survival study. J Clin Oncol. 2005;23:5960–72. doi: 10.1200/JCO.2005.06.150. [DOI] [PubMed] [Google Scholar]
- 40.O’Shaughnessy JA, Vukelja SJ, Holmes FA, et al. Feasibility of quantifying the effects of epoetin alfa therapy on cognitive function in women with breast cancer undergoing adjuvant or neoadjuvant chemotherapy. Clin Breast Cancer. 2005;5:439–46. doi: 10.3816/CBC.2005.n.002. [DOI] [PubMed] [Google Scholar]
- 41.Witzig T, Silberstein P, Loprinzi C, et al. Phase iii, randomized, double-blind study of epoetin alfa compared with placebo in anemic patients receiving chemotherapy. J Clin Oncol. 2005;23:2606–17. doi: 10.1200/JCO.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 42.Littlewood TJ, Kallich JD, San Miguel J, Hendricks L, Hedenus M. Efficacy of darbepoetin alfa in alleviating fatigue and the effect of fatigue on quality of life in anemic patients with lymphoproliferative malignancies. J Pain Symptom Manage. 2006;31:317–25. doi: 10.1016/j.jpainsymman.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 43.Morishima Y, Ogura M, Yoneda S, et al. on behalf of the Japan Erythropoietin Study Group Once-weekly epoetin-beta improves hemoglobin levels in cancer patients with chemotherapy-induced anemia: a randomized, double-blind, dose-finding study. Jpn J Clin Oncol. 2006;36:655–61. doi: 10.1093/jjco/hyl097. [DOI] [PubMed] [Google Scholar]
- 44.Norager CB, Jensen MB, Madsen MR, Qvist N, Laurberg S. Effect of darbepoetin alfa on physical function in patients undergoing surgery for colorectal cancer. A randomized, double-blind, placebo-controlled study. Oncology. 2006;71:212–20. doi: 10.1159/000106071. [DOI] [PubMed] [Google Scholar]
- 45.Savonije JH, van Groeningen CJ, Wormhoudt LW, Giaccone G. Early intervention with epoetin alfa during platinum-based chemotherapy: an analysis of the results of a multicenter, randomized, controlled trial based on initial hemoglobin level. Oncologist. 2006;11:206–16. doi: 10.1634/theoncologist.11-2-206. [DOI] [PubMed] [Google Scholar]
- 46.Straus DJ, Testa MA, Sarokhan BJ, et al. Quality-of-life and health benefits of early treatment of mild anemia: a randomized trial of epoetin alfa in patients receiving chemotherapy for hematologic malignancies. Cancer. 2006;107:1909–17. doi: 10.1002/cncr.22221. [DOI] [PubMed] [Google Scholar]
- 47.Wilkinson PM, Antonopoulos M, Lahousen M, Lind M, Kosmidis P, on behalf of the epo-int-45 study group Epoetin alfa in platinum-treated ovarian cancer patients: results of a multinational, multicentre, randomised trial. Br J Cancer. 2006;94:947–54. doi: 10.1038/sj.bjc.6603004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Charu V, Belani CP, Gill AN, et al. Efficacy and safety of every-2-week darbepoetin alfa in patients with anemia of cancer: a controlled, randomized, open-label phase ii trial. Oncologist. 2007;12:727–37. doi: 10.1634/theoncologist.12-6-727. [DOI] [PubMed] [Google Scholar]
- 49.Charu V, Saidman B, Ben-Jacob A, et al. A randomized, open-label, multicenter trial of immediate versus delayed intervention with darbepoetin alfa for chemotherapy-induced anemia. Oncologist. 2007;12:1253–63. doi: 10.1634/theoncologist.12-10-1253. [DOI] [PubMed] [Google Scholar]
- 50.Zemelka T, Rolski J, Ziobro M, Michalczyk A. Opinion on influence of erythropoietin on quality of life and survival in patients with advanced non-small cell lung cancer [Polish] Contemp Oncol (Pozn) 2007;11:37–40. [Google Scholar]
- 51.Heras P, Kritikos K, Hatzopoulos A, Mitsibounas D. Once-weekly epoetin beta therapy in patients with solid tumours and chemotherapy-induced anaemia: a randomized, double-blind, dose-finding study. Eur J Cancer Care (Engl) 2008;17:619–23. doi: 10.1111/j.1365-2354.2007.00892.x. [DOI] [PubMed] [Google Scholar]
- 52.Hoskin PJ, Robinson M, Slevin N, Morgan D, Harrington K, Gaffney C. Effect of epoetin alfa on survival and cancer treatment-related anemia and fatigue in patients receiving radical radiotherapy with curative intent for head and neck cancer. J Clin Oncol. 2009;27:5751–6. doi: 10.1200/JCO.2009.22.3693. [DOI] [PubMed] [Google Scholar]
- 53.Tsuboi M, Ezaki K, Tobinai K, Ohashi Y, Saijo N. Weekly administration of epoetin beta for chemotherapy-induced anemia in cancer patients: results of a multicenter, phase iii, randomized, double-blind, placebo-controlled study. Jpn J Clin Oncol. 2009;39:163–8. doi: 10.1093/jjco/hyn151. [DOI] [PubMed] [Google Scholar]
- 54.Auerbach M, Silberstein PT, Webb RT, et al. Darbepoetin alfa 300 or 500 μg once every 3 weeks with or without intravenous iron in patients with chemotherapy-induced anemia. Am J Hematol. 2010;85:655–63. doi: 10.1002/ajh.21779. [DOI] [PubMed] [Google Scholar]
- 55.Engert A, Josting A, Haverkamp H, et al. Epoetin alfa in patients with advanced-stage Hodgkin’s lymphoma: results of the randomized placebo-controlled ghsg hd15epo trial. J Clin Oncol. 2010;28:2239–45. doi: 10.1200/JCO.2009.25.1835. [DOI] [PubMed] [Google Scholar]
- 56.Ichinose Y, Seto T, Nishiwaki Y, et al. Randomized phase 2 dose-finding study of weekly administration of darbepoetin alpha in anemic patients with lung or ovarian cancer receiving multicycle platinum-containing chemotherapy. Jpn J Clin Oncol. 2010;40:521–9. doi: 10.1093/jjco/hyq017. [DOI] [PubMed] [Google Scholar]
- 57.Pronzato P, Cortesi E, van der Rijt CC, et al. Epoetin alfa improves anemia and anemia-related, patient-reported outcomes in patients with breast cancer receiving myelotoxic chemotherapy: results of a European, multicenter, randomized, controlled trial. Oncologist. 2010;15:935–43. doi: 10.1634/theoncologist.2009-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Milroy R, Bajetta E, van den Berg PM, et al. Effects of epoetin alfa on anemia and patient-reported outcomes in patients with non–small cell lung cancer receiving chemotherapy: results of a European, multicenter, randomized, controlled study. Eur J Clin Med Oncol. 2011;3:49–56. [Google Scholar]
- 59.Bruera E, Valero V, Driver L, et al. Patient-controlled methylphenidate for cancer fatigue: a double-blind, randomized, placebo-controlled trial. J Clin Oncol. 2006;24:2073–8. doi: 10.1200/JCO.2005.02.8506. [DOI] [PubMed] [Google Scholar]
- 60.Butler JM, Jr, Case LD, Atkins J, et al. A phase iii, double-blind, placebo-controlled prospective randomized clinical trial of D-threo-methylphenidate HCl in brain tumor patients receiving radiation therapy. Int J Radiat Oncol Biol Phys. 2007;69:1496–501. doi: 10.1016/j.ijrobp.2007.05.076. [DOI] [PubMed] [Google Scholar]
- 61.Mar Fan HG, Clemons M, Xu W, et al. A randomised, placebo-controlled, double-blind trial of the effects of d-methylphenidate on fatigue and cognitive dysfunction in women undergoing adjuvant chemotherapy for breast cancer. Support Care Cancer. 2008;16:577–83. doi: 10.1007/s00520-007-0341-9. [DOI] [PubMed] [Google Scholar]
- 62.Auret KA, Schug SA, Bremner AP, Bulsara M. A randomized, double-blind, placebo-controlled trial assessing the impact of dexamphetamine on fatigue in patients with advanced cancer. J Pain Symptom Manage. 2009;37:613–21. doi: 10.1016/j.jpainsymman.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 63.Lower EE, Fleishman S, Cooper A, et al. Efficacy of dexmethylphenidate for the treatment of fatigue after cancer chemotherapy: a randomized clinical trial. J Pain Symptom Manage. 2009;38:650–62. doi: 10.1016/j.jpainsymman.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 64.Moraska AR, Sood A, Dakhil SR, et al. Phase iii, randomized, double-blind, placebo-controlled study of long-acting methylphenidate for cancer-related fatigue: North Central Cancer Treatment Group ncctg-n05c7 trial. J Clin Oncol. 2010;28:3673–9. doi: 10.1200/JCO.2010.28.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roth AJ, Nelson C, Rosenfeld B, et al. Methylphenidate for fatigue in ambulatory men with prostate cancer. Cancer. 2010;116:5102–10. doi: 10.1002/cncr.25424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gehring K, Patwardhan S, Collins R, et al. A randomized trial on the efficacy of methylphenidate and modafinil for improving cognitive functioning and symptoms in patients with a primary brain tumor. J Neurooncol. 2012;107:165–74. doi: 10.1007/s11060-011-0723-1. [DOI] [PubMed] [Google Scholar]
- 67.Kerr CW, Drake J, Milch RA, et al. Effects of methylphenidate on fatigue and depression: a randomized, double-blind, placebo-controlled trial. J Pain Symptom Manage. 2012;43:68–77. doi: 10.1016/j.jpainsymman.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 68.Bruera E, Yennurajalingam S, Palmer JL, et al. Methylphenidate and/or a nursing telephone intervention for fatigue in patients with advanced cancer: a randomized, placebo-controlled, phase ii trial. J Clin Oncol. 2013;31:2421–7. doi: 10.1200/JCO.2012.45.3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Suh S, Choi Y, Oh S, et al. Caffeine as an adjuvant therapy to opioids in cancer pain: a randomized, double-blind, placebo-controlled trial. J Pain Symptom Manage. 2013;46:474–82. doi: 10.1016/j.jpainsymman.2012.10.232. [DOI] [PubMed] [Google Scholar]
- 70.Hovey E, de Souza P, Marx G, et al. on behalf of the motif investigators Phase iii, randomized, double-blind, placebo-controlled study of modafinil for fatigue in patients treated with docetaxel-based chemotherapy. Support Care Cancer. 2014;22:1233–42. doi: 10.1007/s00520-013-2076-0. [DOI] [PubMed] [Google Scholar]
- 71.Spathis A, Fife K, Blackhall F, et al. Modafinil for the treatment of fatigue in lung cancer: results of a placebo-controlled, double-blind, randomized trial. J Clin Oncol. 2014;32:1882–8. doi: 10.1200/JCO.2013.54.4346. [DOI] [PubMed] [Google Scholar]
- 72.Berenson JR, Yellin O, Shamasunder HK, et al. A phase 3 trial of armodafinil for the treatment of cancer-related fatigue for patients with multiple myeloma. Support Care Cancer. 2015;23:1503–12. doi: 10.1007/s00520-014-2486-7. [DOI] [PubMed] [Google Scholar]
- 73.Page BR, Shaw EG, Lu L, et al. Phase ii double-blind placebo-controlled randomized study of armodafinil for brain radiation-induced fatigue. Neuro Oncol. 2015;17:1393–401. doi: 10.1093/neuonc/nov084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Richard PO, Fleshner NE, Bhatt JR, Hersey KM, Chahin R, Alibhai SM. Phase ii, randomised, double-blind, placebo-controlled trial of methylphenidate for reduction of fatigue levels in patients with prostate cancer receiving lhrh-agonist therapy. BJU Int. 2015;116:744–52. doi: 10.1111/bju.12755. [DOI] [PubMed] [Google Scholar]
- 75.Heckler CE, Garland SN, Peoples AR, et al. Cognitive behavioral therapy for insomnia, but not armodafinil, improves fatigue in cancer survivors with insomnia: a randomized placebo-controlled trial. Support Care Cancer. 2016;24:2059–66. doi: 10.1007/s00520-015-2996-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jean-Pierre P, Morrow GR, Roscoe JA, et al. A phase 3 randomized, placebo-controlled, double-blind, clinical trial of the effect of modafinil on cancer-related fatigue among 631 patients receiving chemotherapy: a University of Rochester Cancer Center Community Clinical Oncology Program Research Base study. Cancer. 2010;116:3513–20. doi: 10.1002/cncr.25083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee EQ, Muzikansky A, Drappatz J, et al. A randomized, placebo-controlled pilot trial of armodafinil for fatigue in patients with gliomas undergoing radiotherapy. Neuro Oncol. 2016;18:849–54. doi: 10.1093/neuonc/now007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Inoue A, Yamada Y, Matsumura Y, et al. Randomized study of dexamethasone treatment for delayed emesis, anorexia and fatigue induced by irinotecan. Support Care Cancer. 2003;11:528–32. doi: 10.1007/s00520-003-0488-y. [DOI] [PubMed] [Google Scholar]
- 79.Zargar-Shoshtari K, Sammour T, Kahokehr A, Connolly AB, Hill AG. Randomized clinical trial of the effect of glucocorticoids on peritoneal inflammation and postoperative recovery after colectomy. Br J Surg. 2009;96:1253–61. doi: 10.1002/bjs.6744. [DOI] [PubMed] [Google Scholar]
- 80.Yennurajalingam S, Frisbee-Hume S, Palmer JL, et al. Reduction of cancer-related fatigue with dexamethasone: a double-blind, randomized, placebo-controlled trial in patients with advanced cancer. J Clin Oncol. 2013;31:3076–82. doi: 10.1200/JCO.2012.44.4661. [DOI] [PubMed] [Google Scholar]
- 81.Paulsen O, Klepstad P, Rosland JH, et al. Efficacy of methyl-prednisolone on pain, fatigue, and appetite loss in patients with advanced cancer using opioids: a randomized, placebo-controlled, double-blind trial. J Clin Oncol. 2014;32:3221–8. doi: 10.1200/JCO.2013.54.3926. [DOI] [PubMed] [Google Scholar]
- 82.Eguchi K, Honda M, Kataoka T, et al. Efficacy of corticosteroids for cancer-related fatigue: a pilot randomized placebo-controlled trial of advanced cancer patients. Palliat Support Care. 2015;13:1301–8. doi: 10.1017/S1478951514001254. [DOI] [PubMed] [Google Scholar]
- 83.Cruciani RA, Dvorkin E, Homel P, et al. l-Carnitine supplementation in patients with advanced cancer and carnitine deficiency: a double-blind, placebo-controlled study. J Pain Symptom Manage. 2009;37:622–31. doi: 10.1016/j.jpainsymman.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 84.Mantovani G, Maccio A, Madeddu C, et al. Randomized phase iii clinical trial of five different arms of treatment in 332 patients with cancer cachexia. Oncologist. 2010;15:200–11. doi: 10.1634/theoncologist.2009-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cruciani RA, Zhang JJ, Manola J, Cella D, Ansari B, Fisch MJ. l-Carnitine supplementation for the management of fatigue in patients with cancer: an Eastern Cooperative Oncology Group phase iii, randomized, double-blind, placebo-controlled trial. J Clin Oncol. 2012;30:3864–9. doi: 10.1200/JCO.2011.40.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kraft M, Kraft K, Lerch MM. l-Carnitine—more than just a supportive therapy in the treatment of advanced pancreas carcinoma? (carpan trial) [German] Deutsche Zeitschrift für Onkologie. 2012;44:103–8. doi: 10.1055/s-0032-1314696. [DOI] [Google Scholar]
- 87.Hershman D, Unger J, Crew K, et al. Randomized double-blind placebo-controlled trial of acetyl-l-carnitine for the prevention of taxane-induced neuropathy in women undergoing adjuvant breast cancer therapy. J Clin Oncol. 2013;31:2627–33. doi: 10.1200/JCO.2012.44.8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Iwase S, Kawaguchi T, Yotsumoto D, et al. Efficacy and safety of an amino acid jelly containing coenzyme Q10 and l-carnitine in controlling fatigue in breast cancer patients receiving chemotherapy: a multi-institutional, randomized, exploratory trial (jortc-cam01) Support Care Cancer. 2016;24:637–46. doi: 10.1007/s00520-015-2824-4. [DOI] [PubMed] [Google Scholar]
- 89.Capuron L, Gumnick J, Musselman D, et al. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643–52. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- 90.Morrow GR, Hickok JT, Roscoe JA, et al. on behalf of the University of Rochester Cancer Center Community Clinical Oncology Program Differential effects of paroxetine on fatigue and depression: a randomized, double-blind trial from the University of Rochester Cancer Center Community Clinical Oncology Program. J Clin Oncol. 2003;21:4635–41. doi: 10.1200/JCO.2003.04.070. [DOI] [PubMed] [Google Scholar]
- 91.Roscoe JA, Morrow GR, Hickok JT, et al. Effect of paroxetine hydrochloride (Paxil) on fatigue and depression in breast cancer patients receiving chemotherapy. Breast Cancer Res Treat. 2005;89:243–9. doi: 10.1007/s10549-004-2175-1. [DOI] [PubMed] [Google Scholar]
- 92.Stockler M, O’Connell R, Nowak A, et al. on behalf of the Zoloft’s Effects on Symptoms and Survival Time Trial group Effect of sertraline on symptoms and survival in patients with advanced cancer, but without major depression: a placebo-controlled double-blind randomised trial. Lancet Oncol. 2007;8:603–12. doi: 10.1016/S1470-2045(07)70148-1. [DOI] [PubMed] [Google Scholar]
- 93.Heras P, Kritikos K, Hatzopoulos A, Kritikos N, Heras V, Mitsibounas D. The role of paroxetine in fatigue and depression of patients under chemotherapeutic treatment. Am J Ther. 2013;20:254–6. doi: 10.1097/MJT.0b013e318187de2c. [DOI] [PubMed] [Google Scholar]
- 94.Simons JP, Aaronson NK, Vansteenkiste JF, et al. Effects of medroxyprogesterone acetate on appetite, weight, and quality of life in advanced-stage non-hormone-sensitive cancer: a placebo-controlled multicenter study. J Clin Oncol. 1996;14:1077–84. doi: 10.1200/JCO.1996.14.4.1077. [DOI] [PubMed] [Google Scholar]
- 95.De Conno F, Martini C, Zecca E, et al. Megestrol acetate for anorexia in patients with far-advanced cancer: a double-blind controlled clinical trial. Eur J Cancer. 1998;34:1705–9. doi: 10.1016/S0959-8049(98)00219-6. [DOI] [PubMed] [Google Scholar]
- 96.Westman G, Bergman B, Albertsson M, et al. Megestrol acetate in advanced, progressive, hormone-insensitive cancer. Effects on the quality of life: a placebo-controlled, randomised, multicentre trial. Eur J Cancer. 1999;35:586–95. doi: 10.1016/S0959-8049(98)00398-0. [DOI] [PubMed] [Google Scholar]
- 97.Barton DL, Soori GS, Bauer BA, et al. Pilot study of Panax quinquefolius (American ginseng) to improve cancer-related fatigue: a randomized, double-blind, dose-finding evaluation: ncctg trial n03ca. Support Care Cancer. 2010;18:179–87. doi: 10.1007/s00520-009-0642-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Barton DL, Liu H, Dakhil SR, et al. Wisconsin ginseng (Panax quinquefolius) to improve cancer-related fatigue: a randomized, double-blind trial, n07c2. J Natl Cancer Inst. 2013;105:1230–8. doi: 10.1093/jnci/djt181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Agteresch HJ, Dagnelie PC, van der Gaast A, Stijnen T, Wilson JH. Randomized clinical trial of adenosine 5′-triphosphate in patients with advanced non-small-cell lung cancer. J Natl Cancer Inst. 2000;92:321–8. doi: 10.1093/jnci/92.4.321. [DOI] [PubMed] [Google Scholar]
- 100.Beijer S, Hupperets PS, van den Borne BE, et al. Randomized clinical trial on the effects of adenosine 5′-triphosphate infusions on quality of life, functional status, and fatigue in preterminal cancer patients. J Pain Symptom Manage. 2010;40:520–30. doi: 10.1016/j.jpainsymman.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 101.Cerchietti LC, Navigante AH, Castro MA. Effects of eicosapentaenoic and docosahexaenoic n-3 fatty acids from fish oil and preferential Cox-2 inhibition on systemic syndromes in patients with advanced lung cancer. Nutr Cancer. 2007;59:14–20. doi: 10.1080/01635580701365068. [DOI] [PubMed] [Google Scholar]
- 102.Maccio A, Madeddu C, Gramignano G, et al. A randomized phase iii clinical trial of a combined treatment for cachexia in patients with gynecological cancers: evaluating the impact on metabolic and inflammatory profiles and quality of life. Gynecol Oncol. 2012;124:417–25. doi: 10.1016/j.ygyno.2011.12.435. [DOI] [PubMed] [Google Scholar]
- 103.Bruera E, El Osta B, Valero V, et al. Donepezil for cancer fatigue: a double-blind, randomized, placebo-controlled trial. J Clin Oncol. 2007;25:3475–81. doi: 10.1200/JCO.2007.10.9231. [DOI] [PubMed] [Google Scholar]
- 104.Lawrence JA, Griffin L, Balcueva EP, et al. A study of donepezil in female breast cancer survivors with self-reported cognitive dysfunction 1 to 5 years following adjuvant chemotherapy. J Cancer Surviv. 2016;10:176–84. doi: 10.1007/s11764-015-0463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sun H, Li ZD, Wang W, Zhang Y, Li Y, Li P. A randomized controlled trial on Ren-shen-yang-rong decoction for improving fatigue in cancer patients who are receiving chemotherapy [Chinese] Chin J Basic Med in Trad Chin Med. 2010;16:155–7. [Google Scholar]
- 106.Kuo WH, Yao CA, Lin CH, Chang KJ. Safety and efficacy of tien-hsien liquid practical in patients with refractory metastatic breast cancer: a randomized, double-blind, placebo-controlled, parallel-group, phase iia trial. Evid Based Complement Alternat Med. 2012;2012:803239. doi: 10.1155/2012/803239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhao H, Zhang Q, Zhao L, Huang X, Wang J, Kang X. Spore powder of Ganoderma lucidum improves cancer-related fatigue in breast cancer patients undergoing endocrine therapy: a pilot clinical trial. Evid Based Complement Alternat Med. 2012;2012:809614. doi: 10.1155/2012/809614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xue D, Han S, Jiang S, et al. Comprehensive geriatric assessment and traditional Chinese medicine intervention benefit symptom control in elderly patients with advanced non–small cell lung cancer. Med Oncol. 2015;32:114. doi: 10.1007/s12032-015-0563-5. [DOI] [PubMed] [Google Scholar]
- 109.Young L, Bye R, Scheltinga M, Ziegler T, Jacobs D, Wilmore D. Patients receiving glutamine-supplemented intravenous feedings report an improvement in mood. JPEN J Parenter Enteral Nutr. 2012;17:422–7. doi: 10.1177/0148607193017005422. [DOI] [PubMed] [Google Scholar]
- 110.Borghardt J, Rosien B, Gortelmeyer R, Lindemann S, Hartleb M, Klingmuller M. Effects of a spleen peptide preparation as supportive therapy in inoperable head and neck cancer patients. Arzneimittelforschung. 2000;50:178–84. doi: 10.1055/s-0031-1300186. [DOI] [PubMed] [Google Scholar]
- 111.Martin T, Uhder K, Kurek R, et al. Does prophylactic treatment with proteolytic enzymes reduce acute toxicity of adjuvant pelvic irradiation? Results of a double-blind randomized trial. Radiother Oncol. 2002;65:17–22. doi: 10.1016/S0167-8140(02)00192-5. [DOI] [PubMed] [Google Scholar]
- 112.Bruera E, Strasser F, Palmer JL, et al. Effect of fish oil on appetite and other symptoms in patients with advanced cancer and anorexia/cachexia: a double-blind, placebo-controlled study. J Clin Oncol. 2003;21:129–34. doi: 10.1200/JCO.2003.01.101. [DOI] [PubMed] [Google Scholar]
- 113.Diel IJ, Body JJ, Lichinitser MR, et al. on behalf of the MF 4265 study group Improved quality of life after long-term treatment with the bisphosphonate ibandronate in patients with metastatic bone disease due to breast cancer. Eur J Cancer. 2004;40:1704–12. doi: 10.1016/j.ejca.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 114.Monk JP, Phillips G, Waite R, et al. Assessment of tumor necrosis factor alpha blockade as an intervention to improve tolerability of dose-intensive chemotherapy in cancer patients. J Clin Oncol. 2006;24:1852–9. doi: 10.1200/JCO.2005.04.2838. [DOI] [PubMed] [Google Scholar]
- 115.Semiglazov VF, Stepula VV, Dudov A, Schnitker J, Mengs U. Quality of life is improved in breast cancer patients by standardised mistletoe extract PS76A2 during chemotherapy and follow-up: a randomised, placebo-controlled, double-blind, multicentre clinical trial. Anticancer Res. 2006;26:1519–29. [PubMed] [Google Scholar]
- 116.Berk L, James J, Schwartz A, et al. A randomized, double-blind, placebo-controlled trial of a beta-hydroxyl beta-methyl butyrate, glutamine, and arginine mixture for the treatment of cancer cachexia (rtog 0122) Support Care Cancer. 2008;16:1179–88. doi: 10.1007/s00520-008-0403-7. [DOI] [PubMed] [Google Scholar]
- 117.Troger W, Jezdic S, Zdrale Z, Tisma N, Hamre HJ, Matijasevic M. Quality of life and neutropenia in patients with early stage breast cancer: a randomized pilot study comparing additional treatment with mistletoe extract to chemotherapy alone. Breast Cancer (Auckl) 2009;3:35–45. doi: 10.4137/bcbcr.s2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jeong JS, Ryu BH, Kim JS, Park JW, Choi WC, Yoon SW. Bojungikki-tang for cancer-related fatigue: a pilot randomized clinical trial. Integ Cancer Ther. 2010;9:331–8. doi: 10.1177/1534735410383170. [DOI] [PubMed] [Google Scholar]
- 119.Tian JH, Liu LS, Shi ZM, Zhou ZY, Wang L. A randomized controlled pilot trial of “Feiji recipe” on quality of life of non–small cell lung cancer patients. Am J Chin Med. 2010;38:15–25. doi: 10.1142/S0192415X10007646. [DOI] [PubMed] [Google Scholar]
- 120.Anthony LB, Gabrail NY, Ghazal H, et al. IV iron sucrose for cancer and/or chemotherapy-induced anemia in patients treated with erythropoiesis stimulating agents. Community Oncol. 2011;8:270–8. doi: 10.1016/S1548-5315(12)70022-0. [DOI] [Google Scholar]
- 121.Barton DL, Atherton PJ, Bauer BA, et al. The use of Valeriana officinalis (valerian) in improving sleep in patients who are undergoing treatment for cancer: a phase iii randomized, placebo-controlled, double-blind study (ncctg trial, n01c5) J Support Oncol. 2011;9:24–31. doi: 10.1016/j.suponc.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dimsdale JE, Ball ED, Carrier E, et al. Effect of eszopiclone on sleep, fatigue, and pain in patients with mucositis associated with hematologic malignancies. Support Care Cancer. 2011;19:2015–20. doi: 10.1007/s00520-010-1052-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ikeguchi M, Yamamoto M, Arai Y, et al. Fucoidan reduces the toxicities of chemotherapy for patients with unresectable advanced or recurrent colorectal cancer. Oncol Lett. 2011;2:319–22. doi: 10.3892/ol.2011.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen HW, Lin IH, Chen YJ, et al. A novel infusible botanically-derived drug, PG2, for cancer-related fatigue: a phase ii double-blind, randomized placebo-controlled study. Clin Invest Med. 2012;35:E1–11. doi: 10.25011/cim.v35i1.16100. [DOI] [PubMed] [Google Scholar]
- 125.Zhang JL, Yang L, Tan QH. Bukangling combined with chemotherapy improves quality of life in patients with middle-advanced stage non–small cell lung cancer [Chinese] Journal of Practical Oncology. 2012;27:182–4. [Google Scholar]
- 126.Del Fabbro E, Garcia JM, Dev R, et al. Testosterone replacement for fatigue in hypogonadal ambulatory males with advanced cancer: a preliminary double-blind placebo-controlled trial. Support Care Cancer. 2013;21:2599–607. doi: 10.1007/s00520-013-1832-5. [DOI] [PubMed] [Google Scholar]
- 127.del Giglio AB, Cubero Dde I, Lerner TG, et al. Purified dry extract of Paullinia cupana (guarana) (PC-18) for chemotherapy-related fatigue in patients with solid tumors: an early discontinuation study. J Diet Suppl. 2013;10:325–34. doi: 10.3109/19390211.2013.830676. [DOI] [PubMed] [Google Scholar]
- 128.Lesser GJ, Case D, Stark N, et al. on behalf of the Wake Forest University Community Clinical Oncology Program Research Base A randomized, double-blind, placebo-controlled study of oral coenzyme Q10 to relieve self-reported treatment-related fatigue in newly diagnosed patients with breast cancer. J Support Oncol. 2013;11:31–42. doi: 10.1016/j.suponc.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wen HS, Li X, Cao YZ, et al. Clinical studies on the treatment of cancer cachexia with megestrol acetate plus thalidomide. Chemotherapy. 2012;58:461–7. doi: 10.1159/000346446. [DOI] [PubMed] [Google Scholar]
- 130.Hansen MV, Andersen LT, Madsen MT, et al. Effect of melatonin on depressive symptoms and anxiety in patients undergoing breast cancer surgery: a randomized, double-blind, placebo-controlled trial. Breast Cancer Res Treat. 2014;145:683–95. doi: 10.1007/s10549-014-2962-2. [DOI] [PubMed] [Google Scholar]
- 131.Hui D, Xu A, Frisbee-Hume S, et al. Effects of prophylactic subcutaneous fentanyl on exercise-induced breakthrough dyspnea in cancer patients: a preliminary double-blind, randomized, controlled trial. J Pain Symptom Manage. 2014;47:209–17. doi: 10.1016/j.jpainsymman.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Law KS, Azman N, Omar EA, et al. The effects of virgin coconut oil (vco) as supplementation on quality of life (qol) among breast cancer patients. Lipids Health Dis. 2014;13:139. doi: 10.1186/1476-511X-13-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lee JY, Chu SH, Jeon JY, et al. Effects of 12 weeks of probiotic supplementation on quality of life in colorectal cancer survivors: a double-blind, randomized, placebo-controlled trial. Dig Liver Dis. 2014;46:1126–32. doi: 10.1016/j.dld.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 134.Sánchez-Lara K, Turcott J, Juárez-Hernández E, et al. Effects of an oral nutritional supplement containing eicosapentaenoic acid on nutritional and clinical outcomes in patients with advanced non-small cell lung cancer: randomised trial. Clin Nutr. 2014;33:1017–23. doi: 10.1016/j.clnu.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 135.Terkawi AS, Durieux ME, Gottschalk A, Brenin D, Tiouririne M. Effect of intravenous lidocaine on postoperative recovery of patients undergoing mastectomy: a double-blind, placebo-controlled randomized trial. Reg Anesth Pain Med. 2014;39:472–7. doi: 10.1097/AAP.0000000000000140. [DOI] [PubMed] [Google Scholar]
- 136.Wang S, Qu X, Qu Y, Yu Y, Feng W. The effect of B-type brain natriuretic peptide on patients with acute decompensated heart failure coexisting with lung cancer: a randomized controlled clinical trial. Pharmazie. 2014;69:212–16. [PubMed] [Google Scholar]
- 137.Liu J, Tan L, Zhang H, et al. qol evaluation of olanzapine for chemotherapy-induced nausea and vomiting comparing with 5-HT3 receptor antagonist. Eur J Cancer Care (Engl) 2015;24:436–43. doi: 10.1111/ecc.12260. [DOI] [PubMed] [Google Scholar]
- 138.Birgegard G, Henry D, Glaspy J, Chopra R, Thomsen LL, Auerbach M. A randomized noninferiority trial of intravenous iron isomaltoside versus oral iron sulfate in patients with nonmyeloid malignancies and anemia receiving chemotherapy: the profound trial. Pharmacotherapy. 2016;36:402–14. doi: 10.1002/phar.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jeon Y, Park JS, Moon S, Yeo J. Effect of intravenous high dose vitamin C on postoperative pain and morphine use after laparoscopic colectomy: a randomized controlled trial. Pain Res Manag. 2016;2016:9147279. doi: 10.1155/2016/9147279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mofid B, Rezaeizadeh H, Termos A, et al. Effect of processed honey and royal jelly on cancer-related fatigue: a double-blind randomized clinical trial. Electron Physician. 2016;8:2475–82. doi: 10.19082/2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Faramarzi E, Mahdavi R, Mohammad-Zadeh M, Nasirimotlagh B, Sanaie S. Effect of conjugated linoleic acid supplementation on quality of life in rectal cancer patients undergoing preoperative chemoradiotherapy. Pak J Med Sci. 2017;33:383–8. doi: 10.12669/pjms.332.11925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Martins SP, Ferreira CL, Del Giglio A. Placebo-controlled, double-blind, randomized study of a dry guarana extract in patients with head and neck tumors undergoing chemoradiotherapy: effects on fatigue and quality of life. J Diet Suppl. 2017:1–10. doi: 10.1080/19390211.2016.1193081. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 143.Ribeiro SMF, Braga CBM, Peria FM, Martinez EZ, Rocha J, Cunha SFC. Effects of zinc supplementation on fatigue and quality of life in patients with colorectal cancer. Einstein (Sao Paulo) 2017;15:24–8. doi: 10.1590/s1679-45082017ao3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sun D, Jiao J, Zhang X, et al. Therapeutic effect of Jinlongshe granule () on quality of life of stage iv gastric cancer patients using eortc qlq-C30: a double-blind placebo-controlled clinical trial. Chin J Integr Med. 2017;21:579–86. doi: 10.1007/s11655-014-1950-z. [DOI] [PubMed] [Google Scholar]
- 145.Cella D, Eton DT, Lai JS, Peterman AH, Merkel DE. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (fact) anemia and fatigue scales. J Pain Symptom Manage. 2002;24:547–61. doi: 10.1016/S0885-3924(02)00529-8. [DOI] [PubMed] [Google Scholar]
- 146.Hedley BD, Allan AL, Xenocostas A. The role of erythropoietin and erythropoiesis-stimulating agents in tumor progression. Clin Cancer Res. 2011;17:6373–80. doi: 10.1158/1078-0432.CCR-10-2577. [DOI] [PubMed] [Google Scholar]
- 147.Dicato M, Plawny L. Erythropoietin in cancer patients: pros and cons. Curr Opin Oncol. 2010;22:307–11. doi: 10.1097/CCO.0b013e32833aa9de. [DOI] [PubMed] [Google Scholar]
- 148.Storebo OJ, Ramstad E, Krogh HB, et al. Methylphenidate for children and adolescents with attention deficit hyperactivity disorder (adhd) Cochrane Database Syst Rev. 2015 doi: 10.1002/14651858.CD009885.pub2. CD009885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Erickson JM, Beck SL, Christian B, et al. Patterns of fatigue in adolescents receiving chemotherapy. Oncol Nurs Forum. 2010;37:444–55. doi: 10.1188/10.ONF.444-455. [DOI] [PubMed] [Google Scholar]
- 150.Baggott C, Dodd M, Kennedy C, et al. Changes in children’s reports of symptom occurrence and severity during a course of myelosuppressive chemotherapy. J Pediatr Oncol Nurs. 2010;27:307–15. doi: 10.1177/1043454210377619. [DOI] [PubMed] [Google Scholar]
- 151.Geenen MM, Cardous-Ubbink MC, Kremer LC, et al. Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. JAMA. 2007;297:2705–15. doi: 10.1001/jama.297.24.2705. [DOI] [PubMed] [Google Scholar]
- 152.Minton O, Stone P. A systematic review of the scales used for the measurement of cancer-related fatigue (crf) Ann Oncol. 2009;20:17–25. doi: 10.1093/annonc/mdn537. [DOI] [PubMed] [Google Scholar]