Abstract
The immune system provides defense against tumors and pathogens. Here, we propose that by elucidating the shared principles of immunity that underlie cancer and infectious disease, oncologists and microbiologists can learn from each other and achieve the deeper mechanistic understanding critical the development of therapeutic approaches.
INTRODUCTION
From their earliest days, the fields of microbiology and immunology have been inextricably linked. Collaborations between microbiologists, immunologists, and infectious disease specialists led to vaccines that have saved millions of lives by virtually or entirely eliminating many of humankind’s major scourges, including diphtheria, polio, and smallpox. Although certain infectious diseases have resisted vaccine development, such as malaria, tuberculosis (TB), and AIDS, vaccines remain among the most effective measures to combat infectious disease.
In contrast to infectious diseases, which have been severely curtailed in the developed world, cancer remains a major cause of morbidity in both the developed and developing world. Unlike microbiology and immunology, the fields of infectious diseases and cancer have not been extensively linked, although there are a few exceptions. For example, up to 20% of cancers are caused by infectious agents, including Helicobacter pylori, Kaposi’s sarcoma-associated herpesvirus (KSHV), hepatitis C virus, and Rous sarcoma virus (RSV) (1). Indeed, studies of RSV were instrumental to the discovery of oncogenes (2). Although the importance of the immune system in combating infectious disease is indisputable, for many years there was controversy on the role of the immune system in naturally combating cancer and on whether it would be possible to exploit the immune system to treat cancer. With the recent successes of cancer immunotherapy, however, it is clear that therapeutic and prophylactic manipulations of the immune system are key approaches to treating and/or preventing both infectious diseases and cancer.
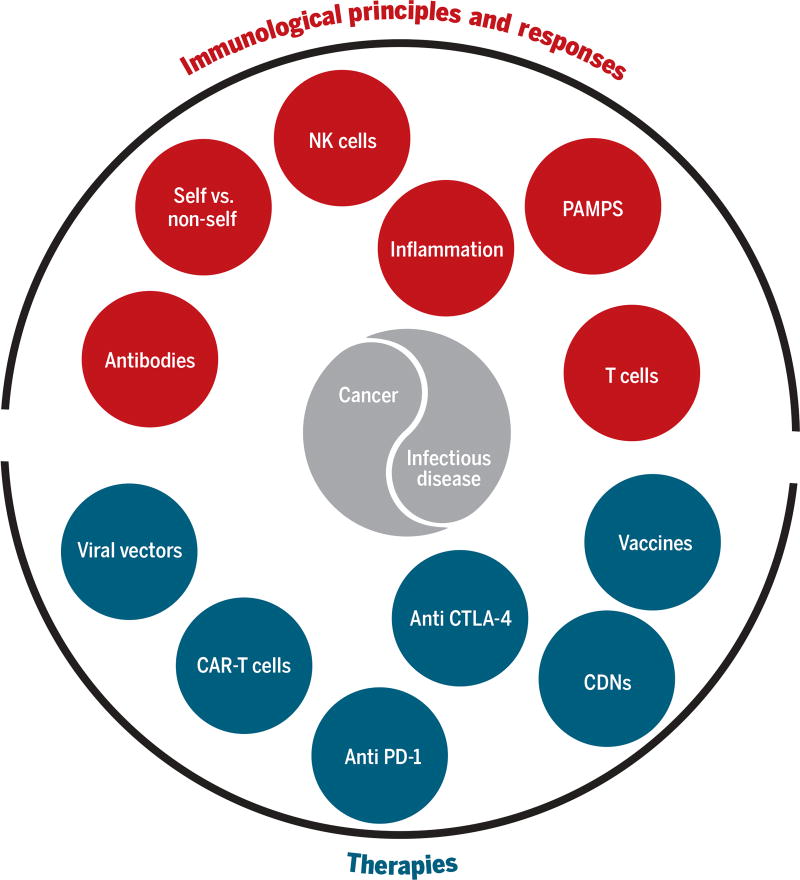
Here, we ask how the study of infectious diseases has and may continue to influence cancer immunotherapy, and likewise, we ask how the success of cancer immunotherapy might reciprocally lead to approaches with which to attack infectious diseases that have remained intractable to vaccine development (Fig. 1).
Fig. 1. Shared immunological principles, responses, and potential therapies for infectious disease and cancer.
Although cancers and infections exhibit considerable diversity and encompass many unique aspects, the figure emphasizes how shared immunological principles, responses, and therapies potentially play important roles in both types of disease.
Do similar immunological principles apply to infections and tumors?
Our understanding of the immune response has largely been derived from studies of infections and model antigens. It is therefore reasonable to ask to what extent this understanding applies to the immune response to tumors. The diversity of both infectious agents and cancers makes generalizations difficult. Indeed, the principles of immunity derived from one infectious agent do not necessarily apply even to another infectious agent, let alone a tumor. Nevertheless, we propose that there exist shared immunological mechanisms that underlie infectious diseases and cancer pathologies, and thus, cross-fertilization of ideas between these fields has been and will likely continue to be fruitful.
One important principle of immunity is that immune responses are usually initiated upon detection of conserved microbe-specific molecules called pathogen-associated molecular patterns (PAMPs) that are recognized by germline-encoded pattern recognition receptors (PRRs) (3). PAMPs are expected to be absent from tumors, which are (with a few exceptions mentioned below) self-derived, implying that the PAMP concept might not have relevance to cancer immunology. However, not all PAMPs are microbe-specific. For example, double-stranded DNA (dsDNA), found in all cells, is a PAMP that is recognized by at least three distinct PRRs (TLR9, AIM2, and cGAS). The ability of the innate immune system to distinguish self from foreign dsDNA relies on detecting dsDNA of different origins in distinct subcellular compartments (4). Self dsDNA is generally nuclear, whereas TLR9 recognizes (foreign) dsDNA in endosomes, and AIM2 and cGAS recognize cytosolic dsDNA. It is plausible that the cell death and genomic instability that occur in tumors might lead to aberrant localization of DNA. Indeed, there is growing evidence that the cGAS-mediated dsDNA detection pathway plays a role in initiation of immune responses to tumors [(5); discussed below]. In addition, despite the centrality of PAMPs to innate immunity, it has long been appreciated that non-PAMP-based mechanisms can also initiate immune responses. These mechanisms are diverse but are unified by the common idea that disruptions of normal cellular physiology can be detected by the immune system (6–8). For example, natural killer (NK) cells recognize cells that have aberrantly downregulated expression of MHC class I (9), a characteristic often exhibited by both transformed and viral ly infected cells. The DNA damage response, often activated in transformed cells and precancerous lesions, is also now appreciated to stimulate a diverse set of immune responses (10). Thus, it is likely that diverse innate immune mechanisms that evolved primarily to defend against infections might also have utility in detecting tumors.
Another hallmark of immunity is self/non-self discrimination, a principle that is most clearly exemplified by the specific recognition of pathogen-encoded epitopes by the antigen receptors of B and T lymphocytes. It is not immediately obvious that this central concept would apply to tumors, because again, tumors are essentially “self” cells. Indeed, although immune responses can be raised against arbitrary non-natural chemical structures, it is generally understood that the immune system evolved in large part to recognize “non-self” in the form of pathogenic microbes. However, numerous recent cancer exome sequencing projects have revealed that tumors can carry a large spectrum of mutated proteins that can be detected as non-self (11). In addition, there is evidence that unmutated self proteins can also be the subject of “autoimmune” recognition in the context of cancer (12).
Self/non-self discrimination is not the only immunological concept with relevance to both infectious disease and cancer: Phagocytosis, cell death, cell-cell communication, cell migration, cell extravasation, development of immune microenvironments, and immune evasion and suppression are all immunological processes with direct relevance to cancer (13). However, the most prominent immunological process relevant to infections and tumors is almost certainly inflammation, a complex constellation of physiological states that arises during immune responses. Inflammation, as is often pointed out, is a double-edged sword, with obvious benefits but also important negative consequences for the host. During acute infections, inflammation is critical to recruit anti-microbial immune cells to the site of infection. This results in tissue destruction, but ideally, the damage is localized. Systemic effects of inflammation, such as fever, are also ideally transient. Indeed, a key component of the inflammatory cascade is the initiation of pro-resolution tissue repair and healing responses (14). Thus, when it functions well during an acute response, inflammation can eliminate infections and tumors and restore homeostasis. Problems arise when inflammation does not resolve appropriately or is inappropriately regulated; this scenario is relevant to both chronic infections and cancer. It is now well-appreciated that chronic inflammation is an important cause of cellular damage, enhanced cellular proliferation, angiogenesis, and immunosuppression that can promote tumorigenesis (1, 13). Indeed, the chronic inflammation associated with certain persistent infections, for example, those caused by H. pylori or human papilloma virus (HPV), is believed to be an important cause of the gastric and cervical cancers associated with these agents. In addition to causing cancer, inflammation also appears to contribute to the morbidity and mortality of cancer. The multi-organ dysfunction and systemic metabolic pathology, such as cachexia (wasting), which ultimately underlie many cancer deaths, likely arise at least in part due to chronic inflammation (15). Interestingly, similar physiological effects are observed in chronic infectious diseases such as AIDS or TB. Indeed, an interesting way to think of cancer is as a chronic infectious disease—the consequence of the unresolved presence and growth of a “foreign” body. For example, many concepts relevant to the treatment of chronic infections, including multi-drug resistance, are also relevant to the treatment of tumors. The close conceptual similarity between chronic infections and cancers is underlined most dramatically by several examples of cancers that are literally infectious agents, for example, the transmissible tumors of Tasmanian devils (16) and bivalves (17).
How have studies of infection led to cancer immunotherapies?
Given the close conceptual similarities between infections and cancer, it is interesting to reflect on how infection studies have affected approaches for immunotherapy of cancer ever since William Coley pioneered the notion in the late 19th century. Bacille Calmette–Guérin (BCG), injected intravesically, was the first effective immunotherapy for cancer. BCG was shown to be effective in treating high-risk non-muscle invasive bladder cancer in 1976 and is still standard care for this disease (18). Animal studies suggest that BCG injections mobilize CD4 and CD8 T cells as well as NK cells against tumors (19).
Checkpoint immunotherapies also owe a major debt to infection studies. Although the concept of CTLA-4 blockade arose from studies of T cell costimulation (20), the role of PD-1 in T cell exhaustion emerged most directly in studies of T cell dysfunction in chronic viral infections in mice (21). Evidence emerged from those studies that blockade of the PD-1 interaction with its ligand PD-L1 restored the activity of exhausted CD8 T cells and reduced viral loads, vitalizing efforts to apply PD-1 blockade for immunotherapy of cancer.
Chimeric antigen receptor (CAR) therapy, which has proven successful against hematological malignancies, was first conceived in a proof-of-principle format, with a test antigen, trinitrophenyl, as a target (22). However, the first attempt to engineer disease-specific T cells involved targeting HIV-infected cells and not cancer (23). In fact, targeting pathogen infections with CAR T cells remains a very active field of research (24).
An obvious area of overlap of infectious disease and cancer immunology is in the design and application of vaccines. Most vaccines for infectious disease agents are prophylactic (for example, healthy people are vaccinated to prevent infections), whereas most efforts in cancer immunology are therapeutic (patients are treated after diagnosis). To date, prophylactic vaccines to prevent cancer are limited to instances where tumors are caused by pathogens, and the vaccine targets the pathogen. Important examples are the HPV and hepatitis B virus vaccines, which strongly reduce the risk of cervical cancer and hepatocellular carcinoma, respectively (25, 26). Prophylactic vaccines against cancers that are not pathogen-induced remain an ambitious goal (27). On the other hand, therapeutic vaccination for cancer is an area of intense investigation (28). Therapeutic vaccination is unlikely to be effective for most acute infections due to the rapidity of pathogen growth but has been applied for rabies and may be a valuable future approach for other chronic or slowly developing infections (29).
The basis of most, if not all, successful infectious disease vaccines is the induction of antibodies that effectively neutralize microbes or their toxins. Indeed, the first Nobel Prize was awarded to Emil von Behring for his discovery that diphtheria anti-toxin prevented disease. Since the emergence of antibiotics and vaccines, passive serotherapy is no longer commonly applied in infectious disease. In contrast, anti-tumor monoclonal (for example, rituximab) and bispecific (such as catumaxomab) antibodies are used clinically for cancer with variable success (30). However, many current immunotherapy approaches seek to stimulate another arm of the immune system, cell-mediated immunity, which involves cytolytic lymphocytes that are able to recognize and kill tumor cells. Cancer vaccines have typically used tumor antigens mixed with antigen-presenting cells and adjuvant costimuli to amplify the immunogenicity of the antigen-presenting cells. Such cancer vaccines, for example Provenge, have so far been modestly successful. Vaccines targeting T cell responses may be limited in potency because the antigen-presenting cells are not adequately activated and are therefore not sufficiently immunostimulatory. Platforms to drive stronger CD8 T cell responses are therefore of great interest. Interestingly, many of the important infectious diseases for which we have failed to develop protective vaccines (such as, AIDS, TB, and malaria) are caused by intracellular pathogens (31), and successful immune responses to intracellular pathogens also generally involve cell-mediated immunity. Thus, strategies to enhance cell-mediated immunity are likely of interest to the fields of both immuno-oncology and infectious disease. New types of attenuated vaccine platforms have been developed based on the understanding of the infectious life cycle of intracellular pathogens such as Listeria monocytogenes (32) or viruses of various categories (33). These vaccines deliver antigens to the cytoplasm of infected cells and provide adjuvant effects as well as sustained availability of the target antigen and antigens for T cell help, all conducive to generating strong CD8 T cell responses. Such vaccine strains may be especially useful for emerging cancer vaccines that are based on the post-diagnosis identification of “neoantigens” specific to a patient’s tumor (11), followed by generation and application of vaccines to amplify tumor-specific T cells against those antigens.
Therapeutic targeting of PRRs represents another very important bridge from infection studies to cancer therapies. PAMPs that engage Toll-like receptors (TLR), including CpG and poly(I:C) (double-stranded RNA), have been tested in clinical trials (34), and one such drug that activates TLR7, imiquimod, is approved for treating superficial basal cell carcinomas (35). Combining such ligands with vaccines or conventional therapies is also being extensively investigated.
A new PRR-based approach with great promise for cancer immunotherapy has emerged from the findings that bacteria secrete immune-stimulating cyclic dinucleotides (CDNs), consisting of cyclized dimers of guanosine and/or adenosine nucleotides. Bacterial CDNs are potent inducers of type I interferon (IFN) responses by immune cells (36–38). A key finding was that CDNs bind and activate the endoplasmic reticulum–membrane resident signaling protein STING (39), which is also essential for the IFN response of cells that accumulate intracytoplasmic DNA (40). Mammalian cells contain a cytoplasmic enzyme, cGAS, which is activated by cytoplasmic DNA to synthesize a specific CDN isoform called cGAMP (41). The STING-cGAS pathway is now known to be necessary for protective responses against certain viruses (42). A role for this pathway in immune-oncology emerged when it was found that the STING pathway is essential for strong anti-tumor immune responses against transferred tumors (43). With respect to therapy, CDNs, injected directly into tumors, stimulate potent immunemediated anti-tumor responses, expansions of cytotoxic T cells, and tumor regressions (43). Activity against distant metastases was also observed. Based on these promising findings, CDNs are now entering clinical trials in multiple types of cancer.
How might the success of cancer immunotherapy be applied to infectious disease?
The success of cancer immunotherapy is leading to new insights and renewed efforts to enhance and modulate the immune system’s activity toward infectious diseases. For example, “checkpoint” therapies that block inhibitory immunoreceptors are being advanced in chronic infections to overcome T cell exhaustion. PD-1 blockade in the SIV macaque model demonstrated rapid expansion of virus-specific CD8 T cells with improved functional quality, leading to reduced viral loads and prolonged survival (44). Blocking of PD-1 in a chimpanzee HCV model similarly showed an ability to restore anti-viral CD4 and CD8 T cell responses and control of viral replication (45). The importance of PD-1 as well as other co-inhibitory receptors, such as CTLA-4, TIM-3, and LAG-3, has been confirmed in multiple viral, bacterial, and parasitic infections (46), suggesting broader application of this approach to managing infections.
The emphasis on innate immune stimulation, generating cytotoxic T cell responses, and identifying effective T cell antigens for cancer immunotherapies are destined, in turn, to give infectious disease vaccinology a boost. Computational and “-omics” approaches to identify and validate immunostimulatory antigens and epitopes are critical for both cancer and infectious disease vaccine design (47, 48). Applications of these approaches to infectious disease challenges, such as creating a universal vaccine for influenza, have shown promising results (49). Adjuvants that promote cellular immunity will be essential for targeting intracellular pathogens and viral infections. Studies applying STING pathway agonists as adjuvants or therapies in a variety of infections are underway (50). Vector systems that selectively elicit strong CD8 T cell responses such as L. monocytogenes, as well as poxviruses and adenoviruses, are seeing application in infections such as HIV, hepatitis C, TB, malaria, and leishmaniasis (51). Exciting approaches to enhancing NK cell responses, which target both cancers and infectious diseases, are emerging (52). As with vaccine technology, advances in CAR T cell therapy for hematological malignancies may lead to applications in infectious diseases. Methods for receptor design and delivery have advanced rapidly (53) and are being reapplied to CAR T cell therapies for HIV and other chronic infections (24).
CONCLUSION: FUTURE OPPORTUNITIES
Despite many notable therapeutic successes, infectious diseases and cancer remain as major causes of global morbidity and mortality. A better understanding of how the immune system successfully responds to pathogens may lead to the design and implementation of strategies to elicit similar responses to tumors. Conversely, therapeutic approaches proven to work in the context of cancer may have application to infectious diseases, particularly chronic infections and/or those caused by intracellular pathogens. Thus, we suspect that immuno-oncologists and infectious disease immunologists still have a lot to learn from each other (Table 1).
Table 1. Questions for further thought.
There are still numerous outstanding questions to be addressed to better understand the similarities and differences between immune responses to infections and tumors.
| Questions for further thought |
| • What innate immune pathways are important for immune recognition of tumors? |
| • In what respects is the immune response to a tumor similar to the immune response to a chronic infection? |
| • Why are therapeutic vaccines rarely used to treat infections? Given this, why is it believed that they may be useful in treating cancer? |
| • Can prophylactic vaccines be developed for cancer? |
| • Could passive monoclonal or bispecific antibody therapies used to treat cancer also be applied to infections? |
| • Can checkpoint blockade provide an effective therapeutic strategy for infections? |
| • How can cytotoxic T cell responses best be elicited in a vaccine? Would such vaccines be of benefit for immunization against tumors and intracellular pathogens for which we currently lack effective vaccines? |
Acknowledgments
Funding: Research in R.E.V.’s laboratory is funded by the HHMI and NIH (AI063302 and AI075039). Research in D.A.P.’s laboratory is funded by the NIH (AI063302 and AI027655). Research in D.H.R.’s laboratory is funded by the NIH (R01CA093678, R01AI039642, and R01AI113041).
Footnotes
Competing interests: D.H.R. is a co-founder of Dragonfly Therapeutics, serves on the Scientific Advisory Boards of Innate Pharma and Aduro Biotech, and receives research funding from both Innate Pharma and Aduro Biotech. D.A.P. has a consulting relationship with and a financial interest in Aduro Biotech and also receives research funding from them, and both he and the company stand to benefit from commercialization of the results of his research.
References
- 1.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 2.Vogt PK. Retroviral oncogenes: A historical primer. Nat. Rev. Cancer. 2012;12:639–648. doi: 10.1038/nrc3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 1989;54(Pt. 1):1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Barton GM, Kagan JC. A cell biological view of Toll-like receptor function: Regulation through compartmentalization. Nat. Rev. Immunol. 2009;9:535–542. doi: 10.1038/nri2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corrales L, McWhirter SM, Dubensky TW, Jr, Gajewski TF. The host STING pathway at the interface of cancer and immunity. J. Clin. Invest. 2016;126:2404–2411. doi: 10.1172/JCI86892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuchs EJ, Matzinger P. Is cancer dangerous to the immune system? Semin. Immunol. 1996;8:271–280. doi: 10.1006/smim.1996.0035. [DOI] [PubMed] [Google Scholar]
- 7.Matzinger P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 8.Vance RE, Isberg RR, Portnoy DA. Patterns of pathogenesis: Discrimination of pathogenic and nonpathogenic microbes by the innate immune system. Cell Host Microbe. 2009;6:10–21. doi: 10.1016/j.chom.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kärre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H–2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319:675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 10.Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436:1186–1190. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 12.Savage PA, Vosseller K, Kang C, Larimore K, Riedel E, Wojnoonski K, Jungbluth AA, Allison JP. Recognition of a ubiquitous self antigen by prostate cancer-infiltrating CD8+ T lymphocytes. Science. 2008;319:215–220. doi: 10.1126/science.1148886. [DOI] [PubMed] [Google Scholar]
- 13.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 15.Petruzzelli M, Wagner EF. Mechanisms of metabolic dysfunction in cancer-associated cachexia. Genes Dev. 2016;30:489–501. doi: 10.1101/gad.276733.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pearse A-M, Swift K. Allograft theory: Transmission of devil facial-tumour disease. Nature. 2006;439:549. doi: 10.1038/439549a. [DOI] [PubMed] [Google Scholar]
- 17.Metzger MJ, Villalba A, Carballal MJ, Iglesias D, Sherry J, Reinisch C, Muttray AF, Baldwin SA, Goff SP. Widespread transmission of independent cancer lineages within multiple bivalve species. Nature. 2016;534:705–709. doi: 10.1038/nature18599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J. Urol. 1976;116:180–183. doi: 10.1016/s0022-5347(17)58737-6. [DOI] [PubMed] [Google Scholar]
- 19.Redelman-Sidi G, Glickman MS, Bochner BH. The mechanism of action of BCG therapy for bladder cancer—A current perspective. Nat. Rev. Urol. 2014;11:153–162. doi: 10.1038/nrurol.2014.15. [DOI] [PubMed] [Google Scholar]
- 20.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 21.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 22.Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc. Natl. Acad. Sci. U.S.A. 1993;90:720–724. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts MR, Qin L, Zhang D, Smith DH, Tran A-C, Dull TJ, Groopman JE, Capon DJ, Byrn RA, Finer MH. Targeting of human immunodeficiency virus-infected cells by CD8+ T lymphocytes armed with universal T-cell receptors. Blood. 1994;84:2878–2889. [PubMed] [Google Scholar]
- 24.Parida SK, Poiret T, Zhenjiang L, Meng Q, Heyckendorf J, Lange C, Ambati AS, Rao MV, Valentini D, Ferrara G, Rangelova E, Dodoo E, Zumla A, Maeurer M. T-cell therapy: Options for infectious diseases. Clin. Infect. Dis. 2015;61(Suppl. 3):S217–S224. doi: 10.1093/cid/civ615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang M-H, You S-L, Chen C-J, Liu C-J, Lee C-M, Lin S-M, Chu H-C, Wu T-C, Yang S-S, Kuo H-S, Chen D-S Taiwan Hepatoma Study Group. Decreased incidence of hepatocellular carcinoma in hepatitis B vaccinees: A 20-year follow-up study. J. Natl. Cancer Inst. 2009;101:1348–1355. doi: 10.1093/jnci/djp288. [DOI] [PubMed] [Google Scholar]
- 26.Joura EA, Giuliano AR, Iversen O-E, Bouchard C, Mao C, Mehlsen J, Moreira ED, Jr, Ngan Y, Kjeld Petersen L, Lazcano-Ponce E, Pitisuttithum P, Alberto Restrepo J, Stuart G, Woelber L, Cheng Yang Y, Cuzick J, Garland SM, Huh W, Kjaer SK, Bautista OM, Chan ISF, Chen J, Gesser R, Moeller E, Ritter M, Vuocolo S, Luxembourg A. Broad Spectrum HPV Vaccine Study, A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N. Engl. J. Med. 2015;372:711–723. doi: 10.1056/NEJMoa1405044. [DOI] [PubMed] [Google Scholar]
- 27.Lollini P-L, Cavallo F, Nanni P, Quaglino E. The promise of preventive cancer vaccines. Vaccines. 2015;3:467–489. doi: 10.3390/vaccines3020467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melief CJ, van Hall T, Arens R, Ossendorp F, van der Burg SH. Therapeutic cancer vaccines. J. Clin. Invest. 2015;125:3401–3412. doi: 10.1172/JCI80009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gröschel MI, Prabowo SA, Cardona PJ, Stanford JL, van der Werf TS. Therapeutic vaccines for tuberculosis—A systematic review. Vaccine. 2014;32:3162–3168. doi: 10.1016/j.vaccine.2014.03.047. [DOI] [PubMed] [Google Scholar]
- 30.Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat. Rev. Cancer. 2012;12:278–287. doi: 10.1038/nrc3236. [DOI] [PubMed] [Google Scholar]
- 31.Seder RA, Hill AVS. Vaccines against intracellular infections requiring cellular immunity. Nature. 2000;406:793–798. doi: 10.1038/35021239. [DOI] [PubMed] [Google Scholar]
- 32.Brockstedt DG, Giedlin MA, Leong ML, Bahjat KS, Gao Y, Luckett W, Liu W, Cook DN, Portnoy DA, Dubensky TW., Jr Listeria-based cancer vaccines that segregate immunogenicity from toxicity. Proc. Natl. Acad. Sci. U.S.A. 2004;101:13832–13837. doi: 10.1073/pnas.0406035101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Draper SJ, Heeney JL. Viruses as vaccine vectors for infectious diseases and cancer. Nat. Rev. Microbiol. 2010;8:62–73. doi: 10.1038/nrmicro2240. [DOI] [PubMed] [Google Scholar]
- 34.Rakoff-Nahoum S, Medzhitov R. Toll-like receptors and cancer. Nat. Rev. Cancer. 2009;9:57–63. doi: 10.1038/nrc2541. [DOI] [PubMed] [Google Scholar]
- 35.Bath-Hextall F, Ozolins M, Armstrong SJ, Colver GB, Perkins W, Miller PSJ, Williams HC Surgery versus Imiquimod for Nodular Superficial basal cell carcinoma (SINS) study group. Surgical excision versus imiquimod 5% cream for nodular and superficial basal-cell carcinoma (SINS): A multicentre, non-inferiority, randomised controlled trial. Lancet Oncol. 2014;15:96–105. doi: 10.1016/S1470-2045(13)70530-8. [DOI] [PubMed] [Google Scholar]
- 36.Karaolis DK, Means TK, Yang D, Takahashi M, Yoshimura T, Muraille E, Philpott D, Schroeder JT, Hyodo M, Hayakawa Y, Talbot BG, Brouillette E, Malouin F. Bacterial c-di-GMP is an immunostimulatory molecule. J. Immunol. 2007;178:2171–2181. doi: 10.4049/jimmunol.178.4.2171. [DOI] [PubMed] [Google Scholar]
- 37.McWhirter SM, Barbalat R, Monroe KM, Fontana MF, Hyodo M, Joncker NT, Ishii KJ, Akira S, Colonna M, Chen ZJ, Fitzgerald KA, Hayakawa Y, Vance RE. A host type I interferon response is induced by cytosolic sensing of the bacterial second messenger cyclic-di-GMP. J. Exp. Med. 2009;206:1899–1911. doi: 10.1084/jem.20082874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woodward JJ, Iavarone AT, Portnoy DA. c-di-AMP secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science. 2010;328:1703–1705. doi: 10.1126/science.1189801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burdette DL, Monroe KM, Sotelo-Troha K, Iwig JS, Eckert B, Hyodo M, Hayakawa Y, Vance RE. STING is a direct innate immune sensor of cyclic di-GMP. Nature. 2011;478:515–518. doi: 10.1038/nature10429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461:788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li X-D, Wu J, Gao D, Wang H, Sun L, Chen ZJ. Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science. 2013;341:1390–1394. doi: 10.1126/science.1244040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corrales L, Hix Glickman L, McWhirter SM, Kanne DB, Sivick KE, Katibah GE, Woo S-R, Lemmens E, Banda T, Leong JJ, Metchette K, Dubensky TW, Jr, Gajewski TF. Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell Rep. 2015;11:1018–1030. doi: 10.1016/j.celrep.2015.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Velu V, Titanji K, Zhu B, Husain S, Pladevega A, Lai L, Vanderford TH, Chennareddi L, Silvestri G, Freeman GJ, Ahmed R, Rao Amara R. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458:206–210. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fuller MJ, Callendret B, Zhu B, Freeman GJ, Hasselschwert DL, Satterfield W, Sharpe AH, Dustin LB, Rice CM, Grakoui A, Ahmed R, Walker CM. Immunotherapy of chronic hepatitis C virus infection with antibodies against programmed cell death-1 (PD-1) Proc. Natl. Acad. Sci. U.S.A. 2013;110:15001–15006. doi: 10.1073/pnas.1312772110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Attanasio J, Wherry EJ. Costimulatory and coinhibitory receptor pathways in infectious disease. Immunity. 2016;44:1052–1068. doi: 10.1016/j.immuni.2016.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Circelli L, Petrizzo A, Tagliamonte M, Tornesello ML, Buonaguro FM, Buonaguro L. Systems biology approach for cancer vaccine development and evaluation. Vaccines. 2015;3:544–555. doi: 10.3390/vaccines3030544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao L, Zhang M, Cong H. Advances in the study of HLA-restricted epitope vaccines. Hum. Vaccin. Immunother. 2013;9:2566–2577. doi: 10.4161/hv.26088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gottlieb T, Ben-Yedidia T. Epitope-based approaches to a universal influenza vaccine. J. Autoimmun. 2014;54:15–20. doi: 10.1016/j.jaut.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 50.Dubensky TW, Jr, Kanne DB, Leong ML. Rationale, progress and development of vaccines utilizing STING-activating cyclic dinucleotide adjuvants. Ther. Adv. Vaccines. 2013;1:131–143. doi: 10.1177/2051013613501988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Griffiths KL, Khader SA. Novel vaccine approaches for protection against intracellular pathogens. Curr. Opin. Immunol. 2014;28:58–63. doi: 10.1016/j.coi.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guillerey C, Huntington ND, Smyth MJ. Targeting natural killer cells in cancer immunotherapy. Nat. Immunol. 2016;17:1025–1036. doi: 10.1038/ni.3518. [DOI] [PubMed] [Google Scholar]
- 53.Figueroa JA, Reidy A, Mirandola L, Trotter K, Suvorava N, Figueroa A, Konala V, Aulakh A, Littlefield L, Grizzi F, Layeequr Rahman R, Jenkins MR, Musgrove B, Radhi S, D’Cunha N, D’Cunha LN, Hermonat PL, Cobos E, Chiriva-Internati M. Chimeric antigen receptor engineering: A right step in the evolution of adoptive cellular immunotherapy. Int. Rev. Immunol. 2015;34:154–187. doi: 10.3109/08830185.2015.1018419. [DOI] [PubMed] [Google Scholar]