Abstract
We investigated whether intra-pancreatic coagulation, with deposition of the fibrinogen-γ dimer (Fib-γD) and hypoxia, affect the severity of acute pancreatitis (AP) in mice. Pancreata of mice with AP induced by administration of cerulein or by L-arginine, or from patients with AP, had increased deposition of Fib-γD compared to control pancreata. Heparin administration protected mice from cerulein-induced AP and prevented Fib-γD formation. Cerulein administration resulted in activation and stabilization of HIF1-alpha in pancreata of ODD-luc HIF1-alpha reporter mice. Cerulein also led to induction of genes regulated by HIF1-alpha, including VEGFA and ERO1A, before evidence of Fib-γD deposition or histologic features of AP. Expression of tissue factor, which is regulated by VEGF, also increased following cerulein administration. Mice with acinar cell-specific disruption of Hif1a (Hif1aAc−/−) developed spontaneous endoplasmic reticulum stress and less severe AP, but did not accumulate Fib-γD following administration of cerulein. Feeding mice increased pancreatic expression of HIF1-alpha, indicating a physiologic role in the exocrine pancreas. Therefore, HIF1-alpha has bifunctional roles, in exocrine pancreas homeostasis and progression of AP that is promoted by intra-pancreatic coagulation.
Keywords: mouse model, fibrinogen, blood clotting, factor VIII
Systemic alterations in coagulation are associated with complications from acute pancreatitis (AP), and are one of the reasons for the high mortality rate of AP1,2. Fibrinogen, a major coagulation protein, is composed of a dimer of three polypeptide chains (α,β,γ), of which γ-chains form protruded structures and contain sites allowing interaction with other factors such as clotting factors and cytokines, including vascular endothelial growth factor (VEGF) and fibroblast growth factor-23,4. Notably, insoluble fibrinogen-γ dimers (Fib-γD) deposit in liver during acute liver injury in mice and humans and are an early marker of tissue damage5, however, no other tissues were assessed and the underlying mechanism is poorly understood.
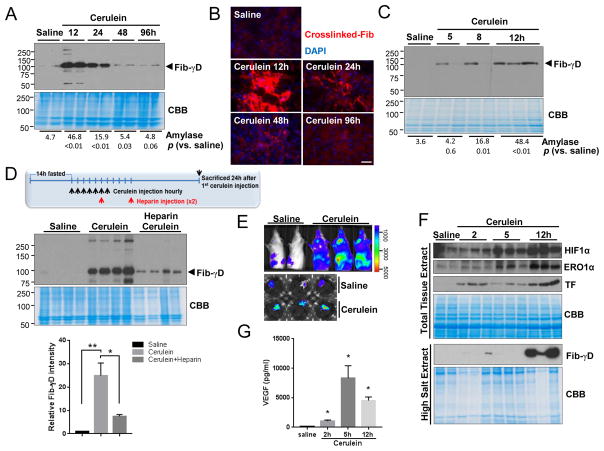
To investigate whether intra-parenchymal coagulation occurs during AP, pancreatitis was induced in mice by cerulein administration. As expected, histologic and serologic changes were noted including interstitial edema, intracellular vacuoles and inflammatory infiltration, and elevated serum amylase (Fig. S1A). Notably, Fib-γD was readily detectable in the insoluble protein fractions from the pancreata, and crosslinked fibrin was dramatically increased without changes in serum D-dimers, in parallel with severity of the AP (Fig. 1A,B; Fig. S1B). Examination of early time points after cerulein administration showed that Fib-γD begins to accumulate in the early stage of AP (Fig. 1C; Fig. S1C). Another AP mouse model, induced by L-arginine, also showed elevated Fib-γD and crosslinked fibrin (Fig. S2). The Fib-γD forms at the earliest stages when serum amylase is either normal or just beginning to increase in both AP models, before obvious histopathologic alterations. Importantly, Fib-γD was observed in human surgical pancreata samples from patients with pancreatitis (Fig. S3). Unlike the cerulein or L-arginine models, choline-deficient ethionine-supplemented (CDE) diet-induced AP did not lead to Fib-γD formation or fibrin crosslinking despite significant pancreatic injury (Fig. S4A–C). This is likely because the CDE-diet-induced injury also causes prominent liver damage with hemorrhage earlier than development of pancreatitis (Fig. S4D,E). Notably, administration of heparin as a potential therapy after initiation of cerulein-medicated injury alleviated the extent of pancreatic injury and prevented Fib-γD formation and fibrin crosslink-formation (Fig. 1D; Fig. S5), supporting a beneficial effect of heparin in improving the resolution of AP.
Fig. 1. Intra-pancreatic coagulation and HIF1α induction in pancreata of mice during pancreatitis.
(A–C) Fibrinogen-γ immunoblotting of insoluble proteins isolated from pancreata of mice treated with saline or cerulean, and immunofluorescence (B) of crosslinked-fibrin (red) and DAPI-counterstaining (blue) in pancreata (bar=50μm). Serum amylase (U/ml, 3 mice/group) and p-values are included. (D) Heparin was administered 5h and 10h after the first cerulein injection as shown schematically. Insoluble proteins were blotted with antibodies to fibrinogen-γ. CBB, Coomassie stain to show protein loading (bottom panel: Fib-γD band intensity compared to CBB). (E) Bio-luminescence of ODD-luc mice (whole body and isolated pancreata) 12h after initial saline/cerulein injections. (F) Total lysates or insoluble fractions from pancreata of mice injected with saline/cerulein were blotted with antibodies to the indicated antigens. (G) VEGF levels in pancreata were measured using enzyme-linked immunosorbent assay. Mean±SEM (*p<0.05).
Coagulation is accomplished by activation of the intrinsic and extrinsic pathways. As an essential and terminal blood-clotting factor in the intrinsic coagulation pathway, the effect of factor VIII (FVIII) on Fib-γD formation was evaluated. During cerulein-induced AP, FVIII activity was elevated but FVIII-deficient mice had similar levels of Fib-γD deposition during AP (Fig. S6), indicating that Fib-γD formation is not directly related to the intrinsic coagulation pathway.
We hypothesized that the enhanced intra-pancreatic coagulation during AP causes hypoxia. Indeed, cerulein administration resulted in activation of hypoxia-inducible factor HIF1α in pancreata of ODD-luc HIF1α reporter mice, and promoted HIF1α stabilization with induction of HIF1α transcriptional targets such as Vegfa and Ero1a within 2h of cerulein administration and before evidence of Fib-γD deposition or histologic AP (Fig. 1E–G; Fig. S7). Importantly, the HIF1α target, VEGF, is a well-known factor that binds to fibrinogen and regulates cell proliferation6,7. Consistent with this, mRNA and protein levels of the extrinsic initiator of coagulation, tissue factor (TF), increased (Fig. 1F,G), consistent with previously-known TF induction by VEGF8,9. These findings suggest a feed-forward cycle, in which the HIF1α-VEGF-TF cascade not only induces intra-pancreatic coagulation but this clotting, in turn, further enhances HIF1α signaling during AP.
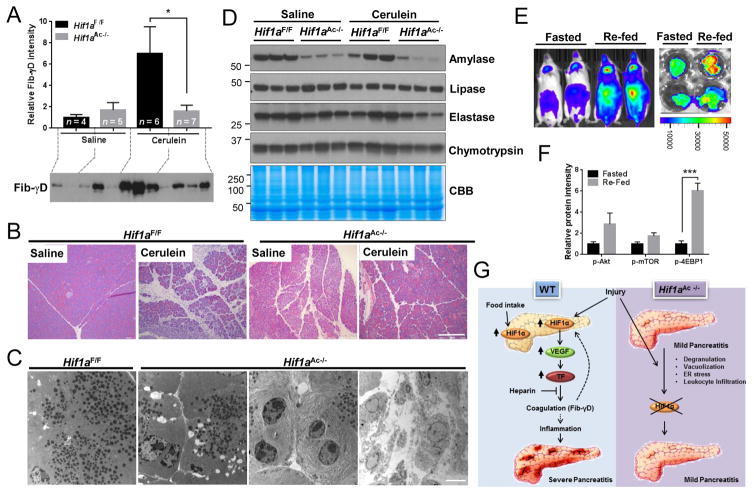
The observation of early activation of HIF1α signaling, before Fib-γD formation, led us to hypothesize that HIF1α signaling contributes directly to Fib-γD formation rather than being an output of coagulation. Indeed, acinar cell-specific HIF1α deficiency (Hif1aAc−/−) prevented cerulein-induced Fib-γD accumulation and ameliorated the histopathologic abnormalities and amylase release (Fig. 2A,B; Fig. S8), thereby suggesting an upstream regulatory tissue hemostasis role of HIF1α during AP. Pancreatic HIF1α deficiency led to several pancreatic alterations including increased vacuolization, degranulation and ER dilation (Fig. 2B,C; Fig. S8; Fig. S9A), induction of ER stress proteins including GRP78 and CHOP, and alterations in autophagy-related proteins (p62,ATGs) (Fig. S9B). Pancreatic infiltration of leukocytes and cell death were also elevated in Hif1aAc−/− mice without a significant change in fibrosis (Fig. S9C–F). Also, isolated acini from Hif1aAc−/− mice were susceptible to cerulein despite the basally damaged pancreas (Fig. S9G,H), suggesting that HIF1α deficiency alleviates cerulein-induced severe AP at least in part through preventing coagulation, rather than basal damage preventing traditional pancreatitis responses. The decrease in amylase was observed in Hif1aAc−/− pancreata without alterations in other pancreatic enzymes, while gene expressions of amylase, lipase and elastase were decreased implying potential direct or indirect regulation by HIF1α (Fig. 2D; Fig. S10). Additional evidence for the importance of HIF1α in normal pancreas function is the finding that re-feeding ODD-luc mice after fasting triggers marked upregulation of HIF1α, possibly through activation of Akt-mTOR signaling (Fig. 2E,F; Fig. S11).
Fig. 2. HIF1α regulates pancreatic physiologic and pathophysiologic functions.
(A) Fib-γD immunoblot (and relative intensity) of the insoluble protein fraction isolated from pancreata after saline or cerulein administration. (B) Histology (bar=200μm), and (C) Transmission electron microscopy (bar=5μm) images of pancreata from Hif1aF/F or Hif1aAc−/− mice. (D) Total pancreas lysates were blotted with antibodies to the indicated antigens. (E) Bioluminescence of ODD-luc mice and pancreata after 14h-fasting or re-feeding. (F) Relative protein levels of immunoblots of pancreata lysates (see Fig. 11S for blots). (G) Summary of findings. HIF1α contributes to the post-prandial function of the pancreas. In addition, HIF1α is inducible by AP-causing triggers, followed by upregulation of downstream targets thereby promoting intra-pancreatic coagulation and tissue injury. In contrast, acinar cell-specific HIF1α deficiency prevents coagulation and provides protection from cerulein-induced pancreatitis.
Several prior findings lend support for our observations. For example, spontaneous pancreatitis and decreased tissue amylase have been reported in several acinar cell-specific ATGs null mice10,11. Similarly, Elastase-Cre mediated Atg5−/− mice showed normal morphology during basal conditions but protection from cerulein-induced injury12. In addition, the Human Protein Atlas database shows moderate expression of HIF1α in normal pancreas, particularly in exocrine cells13, thereby suggesting a fundamental role of HIF1α during normal pancreatic exocrine function (Fig. 2G). Although a link between HIF1α and amylase regulation remains to be investigated, there is strong evidence for the involvement of HIF1α in insulin secretion in β-cells and the regulation of glucose metabolism genes including Glut2, G6pi, aldoB and Hnf4a, in a hypoxia-independent manner14.
Coagulation abnormalities during AP lead to severe complications in some patients ranging from localized intravascular thrombosis to disseminated intravascular coagulation15. The findings herein demonstrate that Fib-γD formation is an early event during AP and is observed in mouse and human pancreata. Our findings show novel functions of HIF1α in promoting coagulation during AP through HIF1α-VEGF-TF cascade, and in the response of the exocrine pancreas to normal physiologic stimulation (Fig. 2G).
Supplementary Material
Supplementary Figure 1. Histologic assessment of cerulein-induced pancreatitis and measurement of serum D-dimer levels.
(A) Hematoxylin and eosin staining of pancreata from mice administered saline or cerulein (Cer). Pancreata were harvested at the indicated time points (3 mice/time point) after injection of Cer (12, 24, 48, 96 h) or saline (96 h). Scale bar, 100 μm. Representative images from of at least three independent experiments are shown.
(B) Serum D-dimer levels (pg/ml) of mice administered saline or cerulein. n=3 per group. There was no statistically-significant difference in the D-dimer levels.
(C) Hematoxylin and eosin staining of pancreata from mice administered saline or cerulein. Pancreata were harvested at the indicated time points (3 mice/time point) after injection of Cer (5, 8, 12 h) or saline (12 h). Scale bar, 200 μm. Representative images out of at least three independent experiments are shown. Similar results were obtained from a repeat experiment.
Supplementary Figure 2. Histologic assessment of L-arginine-induced pancreatitis and measurement of fibrinogen-γ dimers.
(A) Hematoxylin and eosin stain of pancreata from mice administered saline or L-arginine (Arg). Pancreata were harvested at the indicated time points (3 mice/time point) after injection of L-Arg (24, 48, 72, 96 h) or saline (96 h). Scale bar, 200 μm. Similar results were obtained from a repeat experiment.
(B) Insoluble proteins extracted from pancreata of mice administered L-Arg were analyzed by blotting using antibodies to fibrinogen-γ. CBB, Coomassie brilliant blue stain to show the insoluble protein loaded. Serum amylase (U/ml) was measured from 3 mice per group with the mean and p values (versus the saline-injected mice) included. Similar results were obtained in another independent experiment.
(C) Frozen sections of pancreata from L-Arg treated mice were incubated with fibrinogen-γ antibodies (red) and counterstained using DAPI to visualize nuclei (blue). Scale bar, 50 μm.
Supplementary Figure 3. Fibrinogen-γ dimers formation during human pancreatitis.
Fragments of pancreas obtained during surgical resection from patients with chronic pancreatitis were solubilized followed by collection of the insoluble fraction then analysis by blotting using fibrinogen-γ antibodies. CBB, Coomassie brilliant blue staining is included to show the insoluble protein loaded.
Supplementary Figure 4. Fib-γD deposition in the liver of mice with AP induced by CDE-diet.
(A) Insoluble proteins extracted from pancreata of female mice fed control or CDE diets were analyzed by blotting using antibodies to fibrinogen-γ. An insoluble protein fraction from the pancreas of a cerulein-injected mouse was also included in the same gel (far right lane) for comparison. A Coomassie brilliant blue stain (CBB) of the analyzed insoluble protein fractions is included to show protein loading. Each lane represents a separate pancreas extract. Also shown are the averages of serum amylase (U/ml) measured from 3 mice per condition, with p values that compare control diet vs. CDE-fed mice. Results were similar in three independent experiments.
(B) Frozen sections were fixed then stained with antibody to crosslinked fibrinogen-γ (red) with counterstaining using DAPI to visualize nuclei (blue). Scale bar, 50 μm. Note the very faint staining that is consistent with the immunoblot data (as compared with staining after cerulein treatment shown in Fig. 1B).
(C) Hematoxylin and eosin stain of pancreata of female mice fed control diet or CDE-diet. Pancreata were harvested at the indicated time points (3 mice/time point) after feeding CDE (48, 72, 96 h) or control (96 h) diets. Scale bar, 200 μm. Similar results were obtained in two additional independent experiments.
(D) Hematoxylin and eosin stain of livers obtained from the mice described in Panel C. Scale bar, 100 μm.
(E) Insoluble proteins were extracted from the livers of mice fed control or CDE diets. Each lane represents a separate liver extract. The arrowhead highlights the Fib-γD. CBB, Coomassie brilliant blue staining is included to show the insoluble protein loaded.
Supplementary Figure 5. Heparin administration diminishes intra-pancreatic coagulation and ameliorates AP.
FVB/N mice were fasted for 14 h, then injected with cerulein hourly (7 times) with mice having access to food ad lib. Heparin was then administered at 5 and 10 h after the first cerulein injection, and sacrificed at 24 h. Saline (n=6 mice), Saline+heparin (n=6 mice), Cerulein (n=8 mice), Cerulein+heparin (n=12 mice).
(A) Hematoxylin and eosin stains of pancreata. Scale bar, 200 μm.
(B) Histological scores for interstitial edema, intracellular vacuoles, inflammatory infiltration, parenchyma necrosis and hemorrhage were counted in a blind manner. p values of the group with both cerulein and heparin versus the group with cerulein are indicated.
(C) Pancreatic edema was assessed by weight measurement of dried versus wet pancreas. Collected intact pancreata were weighed (wet weight), then dried (95°C, 48 h) then weighed again (dry weight). Saline (n=5), Cerulein (n=5), Cerulein plus heparin (n=5).
(D) Serum amylase levels. Saline (n=7), Cerulein (n=10), Cerulein plus heparin (n=10).
(E) Pancreatic myeloperoxidase (MPO) activity. Saline (n=4), Cerulein (n=5), Cerulein plus heparin (n=5).
(F) Lung myeloperoxidase activity. Saline (n=7), Cerulein (n=10), Cerulein plus heparin (n=10). Data show mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
(G) Frozen sections of the mice pancreata were fixed with 2% acetic acid in 10% NBF, and incubated with antibodies to fibrinogen-γ (red) and CD31 (green) for immunofluorescence analysis, followed by counterstaining using DAPI to visualize nuclei (blue). Scale bar, 100 μm.
Supplementary Figure 6. Fib-γD formation during acute pancreatitis is factor VIII-independent.
(A) Schematic of the intrinsic and extrinsic coagulation system cascades. F, factor; TF, tissue factor.
(B) Factor VIII activity was measured from sera of mice injected with saline (n=5) or cerulein (n=5 for 12 h, n=4 for 48 h). *p < 0. 05
(C) Insoluble proteins were extracted from pancreata of wild type (WT) or factor VIII-null (FVIII KO) mice that were intraperitoneally (ip) administered saline (−) or cerulein (+). The extracts were analyzed by immunoblotting using anti-fibrinogen-γ antibody. CBB, Coomassie brilliant blue staining is included to show the insoluble protein loaded.
Supplementary Figure 7. HIF1α is induced in mouse pancreata during cerulein-mediated pancreatitis.
(A) Immunofluorescence analysis of frozen sections of pancreata obtained from saline or cerulein administered mice (the pancreata were from those used in Figure 1A). The sections were incubated with antibodies directed to: HIF1, CD31 and fibrinogen-γ. After staining, the slides were mounted in anti-fade reagent with DAPI (to visualize nuclei). Scale bar, 50 μm.
(B) Analysis of the relative gene transcription in pancreata of mice injected ip with saline or cerulein (up to 7 hourly injections) after the indicated times. n=3 per group. Data show mean ± SEM. *p < 0.05. F3 and Krt18, genes for tissue factor and keratin18, respectively.
Supplementary Figure 8. Acinar cell-specific HIF1α absence attenuates cerulein-induced pancreatitis.
(A) Serum amylase levels of Hif1aF/F or Hif1aAc−/− mice injected with saline or cerulein. Hif1aF/F Saline (n=6), Hif1aF/F Cerulein 12 h (n=6), Hif1aF/F Cerulein 36 h (n=3), Hif1aAc−/− Saline (n=3), Hif1aAc−/− Cerulein 12 h (n=3), Hif1aAc−/− Cerulein 36 h (n=7). Data show mean ± SEM. *p < 0.05 (vs. Hif1aF/F with saline).
(B) Histological scores for interstitial edema, intracellular vacuoles, inflammatory infiltration, parenchyma necrosis and hemorrhage were counted in a blind manner. Hif1aF/F Saline (n=8), Hif1aAc−/− Saline (n=8), Hif1aF/F Cerulein (n=8), Hif1aAc−/− Cerulein (n=11). *p<0.05 (Hif1aF/F Cerulein vs. Hif1aAc−/− Cerulein. n.s., not significant.
Supplementary Figure 9. Acinar cell-specific HIF1α absence leads to acinar cell damage.
(A) Toluidine Blue staining of pancreata from Hif1aF/F or Hif1aAc−/− mice shows a dramatic decrease in zymogen granules, consistent with the ultrastructural findings shown in Fig. 2C. Bar=20 μm.
(B) Total pancreas lysates from Hif1aF/F or Hif1aAc−/− mice were subjected to SDS-PAGE then blotted with antibodies directed to: GRP78, CHOP, p62, ATG-7, ATG-3, ATG-5, and actin.
(C–F) Pancreata from Hif1aF/F or Hif1aAc−/− mice after three days (3 d) or thirty days (30 d) post tamoxifen administration were subjected to CD45 immunofluorescence staining, TUNEL staining, alpha-SMA immunofluorescence staining, or PicroSirius Red staining [(C, D) Numbers of CD45- or TUNEL-positive cells per high power field (HFP); (E) Alpha-SMA fluorescence intensity per area; SMA, smooth muscle actin; (F) Stained Sirius red color intensity per area]. Hif1aF/F (n=3 mice), Hif1aAc−/− tamoxifen 3 d (n=5 mice), Hif1aAc−/− tamoxifen 30 d (n=5 mice).
(G, H) Acini were isolated from Hif1aF/F or Hif1aAc−/− mice (see top of panel G for their images) then incubated with cerulein for 30 min in a dose-dependent manner. The culture media were subjected to (G) measurement of amylase activity, or (H) SDS-PAGE, followed by immunoblotting with antibodies to amylase or lactate dehydrogenase (LDH). CBB, Coomassie brilliant blue staining is included to show the level of culture media loaded protein.
Supplementary Figure 10. Pancreatic amylase is decreased in pancreata of acinar cell-specific HIF1α deficient mice.
(A) Amylase (red) and CD31 (green) immunofluorescence staining of pancreata obtained from Hif1aF/F or Hif1aAc−/− mice. Note the near-complete absence of amylase staining (red) in the pancreas of HIF1α-null acinar cells.
(B) Total pancreas lysates from Hif1aF/F or Hif1aAc−/− mice administrated saline or cerulein were subjected to immunoblotting with anti-amylase antibody. The histogram summarizes the relative band intensity of amylase from five sets of experiments. Hif1aF/F Saline (n=16), Hif1aAc−/− Saline (n=26), Hif1aF/F Cerulein 12 h (n=7), Hif1aAc−/− Cerulein 12 h (n=9).
(C) Analysis of the relative gene transcription in pancreata of Hif1aF/F or Hif1aAc−/− mice. n=3 per group. Amy2, Pnlip, and Cela2a; genes for pancreatic amylase, lipase, and elastase, respectively. Data show mean ± SEM. *p < 0.05 (vs. Hif1aF/F).
Supplementary Figure 11. Akt/mTOR/4E-BP1 signaling is up-regulated in pancreata of mice after feeding.
FVB/N mice were fasted for 14 h, then, administered water (n=5) or regular liquid diet (n=5) via oral gavage. After 10 minutes, the mice were sacrificed and the pancreas lysates were analyzed by immunoblotting using antibodies to: p(phospho)-Akt (S473), total Akt, p-mTOR (S2448), total mTOR, p-4E-BP1 (S65), and pan-actin.
Human specimen used in this study
* PanIN; Pancreatic Intraepithelial Neoplasia
Antibodies used in this study
IF, immunofluorescence; WB, Western blot
Primers used in this study
F, forward; R, reverse
Acknowledgments
This work was supported by the National Institutes of Health (NIH) grants R01 DK47918 and the Department of Veterans Affairs (M.B.O.); Clinical and Translational Science Award grant UL1TR000433 (M.J.P.); American Gastroenterological Association Research Scholar Award (X.X.); the Goerlich Foundation (S.W.P.); and NIH grant P30 DK34933 to the University of Michigan. We thank Bradley Nelson and Maria-Dolors Sans-Gili, for excellent technical support in the electron microscopy experiments and in acini isolation, respectively.
Abbreviations
- AP
acute pancreatitis
- Fib-γD
fibrinogen-γ dimers
- CDE
choline-deficient ethionine-supplemented
- HIF1α
hypoxia-inducible factor-1α
- VEGF
vascular endothelial growth factor
- ODD
oxygen-dependent degradation domain
- FVIII
factor VIII
- TF
tissue factor
- ATGs
autophagy-related proteins
Footnotes
Author contributions MJP, SI, JAW, YMS and MBO designed the experiments; MJP, SI, XX, JBC, DM and SG performed the experiments; MJP, SI, XX, JBC, SG, DM, SWP, JAW, DMS, YMS and MBO analyzed the data; MJP and MBO wrote the manuscript. All authors read and approved the manuscript.
Conflict of interest The authors declare that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
* Author names in bold designate shared co-first authorship.
- 1.Kakafika A, et al. Coagulation, platelets, and acute pancreatitis. Pancreas. 2007;34:15–20. doi: 10.1097/01.mpa.0000240617.66215.d2. [DOI] [PubMed] [Google Scholar]
- 2.Lisman T, Porte RJ. Activation and regulation of hemostasis in acute liver failure and acute pancreatitis. Semin Thromb Hemost. 2010;36:437–43. doi: 10.1055/s-0030-1254052. [DOI] [PubMed] [Google Scholar]
- 3.Mosesson MW. Fibrinogen gamma chain functions. J Thromb Haemost. 2003;1:231–8. doi: 10.1046/j.1538-7836.2003.00063.x. [DOI] [PubMed] [Google Scholar]
- 4.Farrell DH. Pathophysiologic roles of the fibrinogen gamma chain. Curr Opin Hematol. 2004;11:151–5. doi: 10.1097/01.moh.0000131440.02397.a4. [DOI] [PubMed] [Google Scholar]
- 5.Weerasinghe SV, et al. Fibrinogen-gamma proteolysis and solubility dynamics during apoptotic mouse liver injury: heparin prevents and treats liver damage. Hepatology. 2011;53:1323–32. doi: 10.1002/hep.24203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sahni A, Francis CW. Vascular endothelial growth factor binds to fibrinogen and fibrin and stimulates endothelial cell proliferation. Blood. 2000;96:3772–8. [PubMed] [Google Scholar]
- 7.Verheul HM, et al. The relationship of vascular endothelial growth factor and coagulation factor (fibrin and fibrinogen) expression in clear cell renal cell carcinoma. Urology. 2010;75:608–14. doi: 10.1016/j.urology.2009.05.075. [DOI] [PubMed] [Google Scholar]
- 8.Mechtcheriakova D, et al. Vascular endothelial cell growth factor-induced tissue factor expression in endothelial cells is mediated by EGR-1. Blood. 1999;93:3811–23. [PubMed] [Google Scholar]
- 9.Zucker S, et al. Vascular endothelial growth factor induces tissue factor and matrix metalloproteinase production in endothelial cells: conversion of prothrombin to thrombin results in progelatinase A activation and cell proliferation. Int J Cancer. 1998;75:780–6. doi: 10.1002/(sici)1097-0215(19980302)75:5<780::aid-ijc19>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 10.Antonucci L, Fagman JB, et al. Basal autophagy maintains pancreatic acinar cell homeostasis and protein synthesis and prevents ER stress. Proc Natl Acad Sci U S A. 2015;112:E6166–74. doi: 10.1073/pnas.1519384112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diakopoulos KN, et al. Impaired autophagy induces chronic atrophic pancreatitis in mice via sex- and nutrition-dependent processes. Gastroenterology. 2015;148:626–638e17. doi: 10.1053/j.gastro.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto D, Ohmuraya M, et al. Involvement of autophagy in trypsinogen activation within the pancreatic acinar cells. J Cell Biol. 2008;181:1065–1072. doi: 10.1083/jcb.200712156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uhlen M, et al. Proteomics. Tissue-based map of the human proteome Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 14.Cheng K, et al. Hypoxia-inducible factor-1alpha regulates beta cell function in mouse and human islets. J Clin Invest. 2010;120:2171–2183. doi: 10.1172/JCI35846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maeda K, et al. Applicability of disseminated intravascular coagulation parameters in the assessment of the severity of acute pancreatitis. Pancreas. 2006;32:87–92. doi: 10.1097/01.mpa.0000186248.89081.44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Histologic assessment of cerulein-induced pancreatitis and measurement of serum D-dimer levels.
(A) Hematoxylin and eosin staining of pancreata from mice administered saline or cerulein (Cer). Pancreata were harvested at the indicated time points (3 mice/time point) after injection of Cer (12, 24, 48, 96 h) or saline (96 h). Scale bar, 100 μm. Representative images from of at least three independent experiments are shown.
(B) Serum D-dimer levels (pg/ml) of mice administered saline or cerulein. n=3 per group. There was no statistically-significant difference in the D-dimer levels.
(C) Hematoxylin and eosin staining of pancreata from mice administered saline or cerulein. Pancreata were harvested at the indicated time points (3 mice/time point) after injection of Cer (5, 8, 12 h) or saline (12 h). Scale bar, 200 μm. Representative images out of at least three independent experiments are shown. Similar results were obtained from a repeat experiment.
Supplementary Figure 2. Histologic assessment of L-arginine-induced pancreatitis and measurement of fibrinogen-γ dimers.
(A) Hematoxylin and eosin stain of pancreata from mice administered saline or L-arginine (Arg). Pancreata were harvested at the indicated time points (3 mice/time point) after injection of L-Arg (24, 48, 72, 96 h) or saline (96 h). Scale bar, 200 μm. Similar results were obtained from a repeat experiment.
(B) Insoluble proteins extracted from pancreata of mice administered L-Arg were analyzed by blotting using antibodies to fibrinogen-γ. CBB, Coomassie brilliant blue stain to show the insoluble protein loaded. Serum amylase (U/ml) was measured from 3 mice per group with the mean and p values (versus the saline-injected mice) included. Similar results were obtained in another independent experiment.
(C) Frozen sections of pancreata from L-Arg treated mice were incubated with fibrinogen-γ antibodies (red) and counterstained using DAPI to visualize nuclei (blue). Scale bar, 50 μm.
Supplementary Figure 3. Fibrinogen-γ dimers formation during human pancreatitis.
Fragments of pancreas obtained during surgical resection from patients with chronic pancreatitis were solubilized followed by collection of the insoluble fraction then analysis by blotting using fibrinogen-γ antibodies. CBB, Coomassie brilliant blue staining is included to show the insoluble protein loaded.
Supplementary Figure 4. Fib-γD deposition in the liver of mice with AP induced by CDE-diet.
(A) Insoluble proteins extracted from pancreata of female mice fed control or CDE diets were analyzed by blotting using antibodies to fibrinogen-γ. An insoluble protein fraction from the pancreas of a cerulein-injected mouse was also included in the same gel (far right lane) for comparison. A Coomassie brilliant blue stain (CBB) of the analyzed insoluble protein fractions is included to show protein loading. Each lane represents a separate pancreas extract. Also shown are the averages of serum amylase (U/ml) measured from 3 mice per condition, with p values that compare control diet vs. CDE-fed mice. Results were similar in three independent experiments.
(B) Frozen sections were fixed then stained with antibody to crosslinked fibrinogen-γ (red) with counterstaining using DAPI to visualize nuclei (blue). Scale bar, 50 μm. Note the very faint staining that is consistent with the immunoblot data (as compared with staining after cerulein treatment shown in Fig. 1B).
(C) Hematoxylin and eosin stain of pancreata of female mice fed control diet or CDE-diet. Pancreata were harvested at the indicated time points (3 mice/time point) after feeding CDE (48, 72, 96 h) or control (96 h) diets. Scale bar, 200 μm. Similar results were obtained in two additional independent experiments.
(D) Hematoxylin and eosin stain of livers obtained from the mice described in Panel C. Scale bar, 100 μm.
(E) Insoluble proteins were extracted from the livers of mice fed control or CDE diets. Each lane represents a separate liver extract. The arrowhead highlights the Fib-γD. CBB, Coomassie brilliant blue staining is included to show the insoluble protein loaded.
Supplementary Figure 5. Heparin administration diminishes intra-pancreatic coagulation and ameliorates AP.
FVB/N mice were fasted for 14 h, then injected with cerulein hourly (7 times) with mice having access to food ad lib. Heparin was then administered at 5 and 10 h after the first cerulein injection, and sacrificed at 24 h. Saline (n=6 mice), Saline+heparin (n=6 mice), Cerulein (n=8 mice), Cerulein+heparin (n=12 mice).
(A) Hematoxylin and eosin stains of pancreata. Scale bar, 200 μm.
(B) Histological scores for interstitial edema, intracellular vacuoles, inflammatory infiltration, parenchyma necrosis and hemorrhage were counted in a blind manner. p values of the group with both cerulein and heparin versus the group with cerulein are indicated.
(C) Pancreatic edema was assessed by weight measurement of dried versus wet pancreas. Collected intact pancreata were weighed (wet weight), then dried (95°C, 48 h) then weighed again (dry weight). Saline (n=5), Cerulein (n=5), Cerulein plus heparin (n=5).
(D) Serum amylase levels. Saline (n=7), Cerulein (n=10), Cerulein plus heparin (n=10).
(E) Pancreatic myeloperoxidase (MPO) activity. Saline (n=4), Cerulein (n=5), Cerulein plus heparin (n=5).
(F) Lung myeloperoxidase activity. Saline (n=7), Cerulein (n=10), Cerulein plus heparin (n=10). Data show mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
(G) Frozen sections of the mice pancreata were fixed with 2% acetic acid in 10% NBF, and incubated with antibodies to fibrinogen-γ (red) and CD31 (green) for immunofluorescence analysis, followed by counterstaining using DAPI to visualize nuclei (blue). Scale bar, 100 μm.
Supplementary Figure 6. Fib-γD formation during acute pancreatitis is factor VIII-independent.
(A) Schematic of the intrinsic and extrinsic coagulation system cascades. F, factor; TF, tissue factor.
(B) Factor VIII activity was measured from sera of mice injected with saline (n=5) or cerulein (n=5 for 12 h, n=4 for 48 h). *p < 0. 05
(C) Insoluble proteins were extracted from pancreata of wild type (WT) or factor VIII-null (FVIII KO) mice that were intraperitoneally (ip) administered saline (−) or cerulein (+). The extracts were analyzed by immunoblotting using anti-fibrinogen-γ antibody. CBB, Coomassie brilliant blue staining is included to show the insoluble protein loaded.
Supplementary Figure 7. HIF1α is induced in mouse pancreata during cerulein-mediated pancreatitis.
(A) Immunofluorescence analysis of frozen sections of pancreata obtained from saline or cerulein administered mice (the pancreata were from those used in Figure 1A). The sections were incubated with antibodies directed to: HIF1, CD31 and fibrinogen-γ. After staining, the slides were mounted in anti-fade reagent with DAPI (to visualize nuclei). Scale bar, 50 μm.
(B) Analysis of the relative gene transcription in pancreata of mice injected ip with saline or cerulein (up to 7 hourly injections) after the indicated times. n=3 per group. Data show mean ± SEM. *p < 0.05. F3 and Krt18, genes for tissue factor and keratin18, respectively.
Supplementary Figure 8. Acinar cell-specific HIF1α absence attenuates cerulein-induced pancreatitis.
(A) Serum amylase levels of Hif1aF/F or Hif1aAc−/− mice injected with saline or cerulein. Hif1aF/F Saline (n=6), Hif1aF/F Cerulein 12 h (n=6), Hif1aF/F Cerulein 36 h (n=3), Hif1aAc−/− Saline (n=3), Hif1aAc−/− Cerulein 12 h (n=3), Hif1aAc−/− Cerulein 36 h (n=7). Data show mean ± SEM. *p < 0.05 (vs. Hif1aF/F with saline).
(B) Histological scores for interstitial edema, intracellular vacuoles, inflammatory infiltration, parenchyma necrosis and hemorrhage were counted in a blind manner. Hif1aF/F Saline (n=8), Hif1aAc−/− Saline (n=8), Hif1aF/F Cerulein (n=8), Hif1aAc−/− Cerulein (n=11). *p<0.05 (Hif1aF/F Cerulein vs. Hif1aAc−/− Cerulein. n.s., not significant.
Supplementary Figure 9. Acinar cell-specific HIF1α absence leads to acinar cell damage.
(A) Toluidine Blue staining of pancreata from Hif1aF/F or Hif1aAc−/− mice shows a dramatic decrease in zymogen granules, consistent with the ultrastructural findings shown in Fig. 2C. Bar=20 μm.
(B) Total pancreas lysates from Hif1aF/F or Hif1aAc−/− mice were subjected to SDS-PAGE then blotted with antibodies directed to: GRP78, CHOP, p62, ATG-7, ATG-3, ATG-5, and actin.
(C–F) Pancreata from Hif1aF/F or Hif1aAc−/− mice after three days (3 d) or thirty days (30 d) post tamoxifen administration were subjected to CD45 immunofluorescence staining, TUNEL staining, alpha-SMA immunofluorescence staining, or PicroSirius Red staining [(C, D) Numbers of CD45- or TUNEL-positive cells per high power field (HFP); (E) Alpha-SMA fluorescence intensity per area; SMA, smooth muscle actin; (F) Stained Sirius red color intensity per area]. Hif1aF/F (n=3 mice), Hif1aAc−/− tamoxifen 3 d (n=5 mice), Hif1aAc−/− tamoxifen 30 d (n=5 mice).
(G, H) Acini were isolated from Hif1aF/F or Hif1aAc−/− mice (see top of panel G for their images) then incubated with cerulein for 30 min in a dose-dependent manner. The culture media were subjected to (G) measurement of amylase activity, or (H) SDS-PAGE, followed by immunoblotting with antibodies to amylase or lactate dehydrogenase (LDH). CBB, Coomassie brilliant blue staining is included to show the level of culture media loaded protein.
Supplementary Figure 10. Pancreatic amylase is decreased in pancreata of acinar cell-specific HIF1α deficient mice.
(A) Amylase (red) and CD31 (green) immunofluorescence staining of pancreata obtained from Hif1aF/F or Hif1aAc−/− mice. Note the near-complete absence of amylase staining (red) in the pancreas of HIF1α-null acinar cells.
(B) Total pancreas lysates from Hif1aF/F or Hif1aAc−/− mice administrated saline or cerulein were subjected to immunoblotting with anti-amylase antibody. The histogram summarizes the relative band intensity of amylase from five sets of experiments. Hif1aF/F Saline (n=16), Hif1aAc−/− Saline (n=26), Hif1aF/F Cerulein 12 h (n=7), Hif1aAc−/− Cerulein 12 h (n=9).
(C) Analysis of the relative gene transcription in pancreata of Hif1aF/F or Hif1aAc−/− mice. n=3 per group. Amy2, Pnlip, and Cela2a; genes for pancreatic amylase, lipase, and elastase, respectively. Data show mean ± SEM. *p < 0.05 (vs. Hif1aF/F).
Supplementary Figure 11. Akt/mTOR/4E-BP1 signaling is up-regulated in pancreata of mice after feeding.
FVB/N mice were fasted for 14 h, then, administered water (n=5) or regular liquid diet (n=5) via oral gavage. After 10 minutes, the mice were sacrificed and the pancreas lysates were analyzed by immunoblotting using antibodies to: p(phospho)-Akt (S473), total Akt, p-mTOR (S2448), total mTOR, p-4E-BP1 (S65), and pan-actin.
Human specimen used in this study
* PanIN; Pancreatic Intraepithelial Neoplasia
Antibodies used in this study
IF, immunofluorescence; WB, Western blot
Primers used in this study
F, forward; R, reverse