Abstract
The Pediatric Heart Network randomized trial of atenolol versus losartan in the Marfan syndrome showed no treatment differences in the rates of aortic-root growth or clinical outcomes. In this report we present treatment effects on aortic stiffness and determine whether baseline aortic stiffness predicts aortic-root growth and clinical outcomes. Echocardiograms at 0, 6, 12, 24 & 36 months from 608 subjects (6 months-25 years) who met original Ghent criteria and had a maximum aortic-root z-score (ARz) >3 were centrally reviewed. Stiffness index (SI) and elastic modulus (EM) were calculated for aortic root and ascending aorta. Data were analyzed using multivariable mixed effects modeling and Cox regression. Heart rate-corrected aortic-root SI over 3 years decreased with atenolol but did not change with losartan (−0.298±0.139 vs. 0.141±0.139/year, p=0.01). In the entire cohort, above-median aortic-root SI (>9.1) and EM (>618 mm Hg) predicted a smaller annual decline in ARz (p≤0.001). Upper-quartile aortic-root EM (>914 mm Hg) predicted the composite outcome of aortic-root surgery, dissection, or death (hazard ratio 2.17, 95% CI 1.02-4.63, p=0.04. Crude 3-year event rates were 10.4% vs. 3.2% for higher versus lower EM groups. In conclusion, atenolol was associated with a fall in aortic-root SI, while losartan was not. Higher baseline aortic-root SI and EM were associated with a smaller decrease in ARz and increased risk for clinical outcomes. These data suggest that non-invasive aortic stiffness measures may identify patients at higher risk for progressive aortic enlargement and adverse clinical outcomes, potentially allowing for closer monitoring and more aggressive therapy.
Keywords: Marfan syndrome, aortic stiffness, stiffness index, elastic modulus, clinical outcome, echocardiography
INTRODUCTION
Progressive aortic-root dilation and dissection are the leading cause of death in the Marfan syndrome.1 The Pediatric Heart Network conducted a randomized trial comparing atenolol and losartan in children and young adults with the Marfan syndrome.2 The primary outcome was the rate of aortic-root enlargement, expressed as the change in the maximum aortic-root diameter z-score indexed to body surface area (ARz) over 3 years. Each drug reduced ARz; however, we found no significant difference in the rate of change in ARz or 3-year rate of a composite outcome of aortic-root surgery, aortic dissection and death by treatment. Aortic wall properties in the Marfan syndrome are abnormal and stiffer compare to unaffected controls, even in patients with normal aortic-root dimensions.3,4,5 Aortic stiffness is associated with rate of aortic root disease progression, with higher stiffness resulting in higher frequency of aortic root replacement in patients with connective tissue disorders.6,7,8 Prior studies on the effect of beta blockade on arterial stiffness in the Marfan syndrome demonstrated variable results,9–12 however, recent small studies have reported improved arterial stiffness on atenolol and losartan therapy.13,14 The purpose of the this report is to estimate the treatment effects of atenolol and losartan on echocardiographic measures of aortic stiffness in a large cohort of children and young adults with the Marfan syndrome, and to determine whether aortic stiffness measured at baseline predicts the rates of aortic-root growth and outcomes such as surgery, dissection, and death.
METHODS
The trial was designed and performed by the Marfan Trial Subcommittee of the Pediatric Heart Network, and the study design has been published.15 The protocol was approved by the institutional review board or ethics committee at each of the 21 study centers. The data were collected by center investigators and analyzed at the data coordinating center (New England Research Institutes).
Subjects enrolled in this trial were individuals aged 6 months to 25 years who met the original Ghent criteria for the Marfan syndrome16 with ARz >3 and absolute aortic-root diameter <5 cm. Patients with prior aortic surgery were excluded. Informed consent and assent were obtained, depending on age, from the patient and a parent or legal guardian before trial enrollment.
All patients on prior prophylactic therapy for aortic-root dilation underwent taper over 14 days, followed by a 14–21 day washout period before baseline assessment and randomization. Atenolol (at an initial dose of 0.5 mg/kg) was increased on the basis of hemodynamic response up to a maximum dose of 4.0 mg per kilogram per day (not to exceed a total daily dose of 250 mg), with a goal of a 20% or greater decrease in the mean heart rate as measured on a 24-hour recording. Losartan (at an initial dose of 0.4 mg per kilogram) was adjusted as tolerated on the basis of body weight up to a maximum dose of 1.4 mg per kilogram per day (not to exceed a total daily dose of 100 mg), which is the maximum dose approved by the FDA for treatment of hypertension.
The detailed echocardiographic technical protocol and core laboratory measurement protocol have been published.15,17 Echocardiograms were performed at baseline, and at 6 months, 1 year, 2 years and 3 years after randomization. The echocardiograms were analyzed in a core echocardiography laboratory.
Using 2-dimensional imaging, aortic diameters were measured inner-edge-to-inner edge at their maximum and minimum dimensions in systole and diastole at the aortic-valve annulus, aortic root at the sinuses of Valsalva, sinotubular junction, and ascending aorta (Figure 1). Z-scores were available for the maximum diameters only. An automated vital sign device (Dinamap) was used to record multiple right brachial blood pressures and heart rates during echocardiographic assessment. The blood pressure and heart rate were recorded after the patient had been in a recumbent position for at least 5–10 minutes, during or immediately after recording of aortic images for calculation of stiffness measures. Four measurements were obtained, the first of which was discarded. The other measurements were averaged. Z-scores for the systolic, diastolic, and mean blood pressure were calculated using the Boston Children’s Hospital normative database (courtesy of Steven D. Colan) since they were determined in the same recumbent fashion using the average of 3 machine-determined measurements, in contrast to the American Academy of Pediatrics-published values which were obtained in the sitting position using sphygmomanometry.
FIGURE 1. Aortic Measurements in Systole and Diastole.
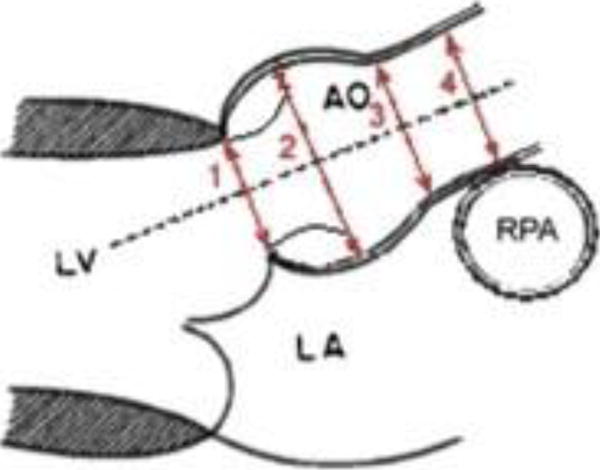
Aortic dimensions were measured by 2D imaging in systole and diastole at the aortic-valve annulus (1), aortic root (2), sinotubular junction (3), and ascending aorta (4). AO: aorta, LA: left atrium, LV: left ventricle; RPA: right pulmonary artery.
Measures of arterial stiffness at the aortic root and ascending aorta used for the purpose of this study were the arterial pressure-strain elastic modulus in mm Hg and the stiffness index:
Elastic modulus = (Systolic blood pressure–Diastolic blood pressure)/[(Aortamax-Aortamin)/Aortamin]18
Stiffness index = [ln (Systolic blood pressure/Diastolic blood pressure)]/[(Aortamax-Aortamin)/Aortamin]).19
These measures were calculated for the aortic root and the ascending aorta where Aortamax and Aortamin are the maximal and minimal diameters of the aortic root at the sinuses of Valsalva or ascending aorta, respectively.
All analyses of treatment effect were performed according to the intention-to-treat principle. Echocardiographic outcomes were modeled with parametric curves longitudinal linear and logistic regressions20, using a compound symmetry covariance structure, which was demonstrated to yield the most parsimonious models based on goodness-of-fit statistics. The baseline-adjusted rates of change in the 2 treatment groups were compared using a test of the treatment-by-time interaction effect. Differential treatment effect according to pre-specified baseline subgroup (adults vs. children, ARz <4.5 vs. ≥4.5, and previous use of a beta blocker) was identified by a test of the subgroup × treatment × time interaction. Age as a continuous variable was also examined using a test of age × treatment × time interaction.
Kaplan-Meier event rate estimation with log-rank test and Cox proportional hazards modeling were used to assess whether baseline measures of aortic stiffness (elastic modulus and stiffness index) predicted time to event defined as the earliest occurring of aortic-root surgery, dissection, or death. Baseline age and ARz were included as covariates in the Cox modeling. Longitudinal regression modeling was used to examine whether baseline measures of aortic stiffness predicted the rate of change in ARz. Older age and higher ARz are known to correlate with both aortic stiffness and the risk of clinical events.8,17 Therefore, the analyses were adjusted for both age and ARz.
A p value ≤0.05 was considered statistically significant. The p values from the tests of treatment difference were not adjusted for the evaluation of multiple echocardiographic outcomes. No imputation was used for missing values. Analyses were conducted using SAS version 9.3 (Statistical Analysis System, SAS Institute, Cary, NC) and R version 3.2.1. Lynn Sleeper, ScD (co-author) had full access to all the data in the study and takes responsibility for its integrity and the data analysis.
The reproducibility of the aortic measurements in our cohort have been published previously using the baseline echocardiograms in this trial (n=608).17 Aortic-root and ascending-aorta measurements by 2D imaging had excellent reproducibility, with inter-observer intra-class correlation coefficients >0.97, and calculated stiffness measures had moderate reproducibility, with ICCs ranging between 0.58 and 0.71.17
RESULTS
A total of 608 subjects (age 11.2±6.3 years; 60% male) were enrolled between February 2007 and February 2011 at 21 clinical centers; 303 participants were randomly assigned to atenolol and 305 to losartan. At baseline, there were no significant differences in clinical and echocardiographic characteristics between the treatment arms.17,21 The withdrawal rate was similar in the 2 groups (11%) with no difference in median time from randomization to withdrawal (2.3 years in the atenolol group and 1.9 years in the losartan group, p= 0.47).
History of prior beta blocker and angiotensin receptor blocker use was similar in subjects randomized to atenolol versus losartan [173 (57%) vs. 170 (56%), p = 0.80 with prior use of beta blockers, and 12 (4%) vs. 22 (7%) with prior use of angiotensin receptor blockers, p = 0.10].
We examined whether the elastic modulus and stiffness index vary with heart rate and R-R interval (60/heart rate). The elastic modulus did not show any association with heart rate in our cohort, whereas stiffness index did. Thus, when stiffness index was used in analysis, we divided it by the square root of the R-R interval, which yielded a measure invariant to heart rate (better correcting for differences in stiffness due to heart rate compared with division by R-R interval), in addition to analysis using the uncorrected measure.
The rates of change in z-scores and absolute diameters for the aortic root and ascending aorta are reported in Table 1.2 As previously reported, the mean absolute dimensions increased over time, in contrast to the mean z-scores, which decreased over time.2 The rates of change in ARz (primary outcome), aortic-root diameter, ascending-aorta diameter z-score, and ascending-aorta diameter did not differ between the atenolol and losartan groups. There was insufficient evidence to declare a differential treatment effect according to any pre-specified subgroup for any of these aortic outcomes.
TABLE 1.
Trial Outcomes By Treatment Assignment for Aortic Measurements
| Baseline | 3 Years | Annual Rate of Change (Slope ± Standard Error) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Variables | n | Atenolol | n | Losartan | p | n | Atenolol | n | Losartan | Atenolol | Losartan | p* |
| Aortic-root diameter z-score | 303 | 4.2 ± 1.2 | 304 | 4.4 ± 1.5 | 0.68 | 268 | 3.7 ± 1.3 | 267 | 3.9 ± 1.5 | −0.139 ± 0.013 | −0.107 ± 0.013 | 0.08 |
| Aortic-root diameter (cm) | 303 | 3.4 ± 0.7 | 304 | 3.4 ± 0.7 | 0.96 | 268 | 3.5 ± 0.7 | 267 | 3.5 ± 0.6 | 0.069 ± 0.004 | 0.075 ± 0.004 | 0.20 |
| Ascending-aorta diameter z-score | 263 | 0.9 ± 0.9 | 280 | 1.1 ± 1.1 | 0.13 | 218 | 0.5 ± 0.8 | 236 | 0.6 ± 1.1 | −0.140 ± 0.013 | −0.114 ± 0.013 | 0.15 |
| Ascending-aorta diameter (cm) | 263 | 2.3 ± 0.5 | 280 | 2.3 ± 0.5 | 0.62 | 218 | 2.4 ± 0.4 | 236 | 2.4 ± 0.4 | 0.039 ± 0.004 | 0.044 ± 0.004 | 0.30 |
| Systolic blood pressure (mm Hg) | 303 | 97 ± 13 | 303 | 98 ± 12 | 0.53 | 268 | 95 ± 12 | 268 | 96 ± 13 | 0.108 ± 0.194 | 0.018 ± 0.195 | 0.73 |
| Diastolic blood pressure (mm Hg) | 303 | 59 ± 10 | 303 | 59 ± 8 | 0.46 | 268 | 54 ± 8 | 268 | 56 ± 8 | −0.919 ± 0.159 | −0.509 ± 0.160 | 0.05 |
| Mean blood pressure (mm Hg) | 298 | 72 ± 11 | 297 | 71 ± 9 | 0.61 | 266 | 68 ± 10 | 268 | 69 ± 9 | −0.574 ± 0.173 | −0.381 ± 0.173 | 0.40 |
| Systolic blood pressure z-score | 303 | −0.7 ±1.0* | 303 | −0.6 ± 1.0* | 0.29 | 268 | −1.1 ± 0.9* | 268 | −1.0 ± 1.0* | −0.070 ± 0.017 | −0.073 ± 0.017 | 0.90 |
| Diastolic blood pressure z-score | 303 | 0.3 ± 1.0* | 303 | 0.3 ± 0.9* | 0.59 | 268 | −0.3 ± 0.9* | 268 | −0.2 ± 0.8* | −0.155 ± 0.017 | −0.107 ± 0.017 | 0.03 |
| Mean blood pressure z-score | 298 | −0.2 ± 1.0* | 297 | −0.3 ± 0.9* | 0.84 | 266 | −0.8 ± 0.9* | 268 | −0.7 ± 0.9* | −0.117 ± 0.017 | −0.092 ± 0.017 | 0.27 |
| Aortic-root elastic modulus (mmHg) | 299 | 730 ± 511 | 299 | 792 ± 548 | 0.15 | 260 | 704 ± 504 | 262 | 774 ± 671 | −0.003 ± 9.745 | 14.1 ± 9.800 | 0.26 |
| Aortic-root stiffness index | 299 | 9.5 ± 6.3 | 299 | 10.3 ± 6.7 | 0.13 | 260 | 9.6 ± 6.5 | 262 | 10.4 ± 8.9 | 0.037 ± 0.128 | 0.219 ± 0.128 | 0.27 |
| Aortic-root stiffness index/√R-R interval | 299 | 10.7 ± 6.8 | 299 | 11.6 ± 7.4 | 0.13 | 260 | 9.8 ± 6.5 | 262 | 11.3 ± 9.3 | 0.298 ± 0.139 | 0.141 ± 0.139 | 0.01 |
| Ascending-aorta elastic modulus (mm Hg) | 237 | 362 ± 195 | 255 | 385 ± 223 | 0.24 | 192 | 382 ± 288 | 205 | 353 ± 192 | 12.584 ± 5.003 | 0.315 ± 4.913 | 0.05† |
| Ascending-aorta stiffness index | 237 | 4.8 ± 2.6 | 255 | 5.1 ± 2.9 | 0.29 | 192 | 5.2 ± 3.9 | 205 | 4.8 ± 2.4 | 0.184 ± 0.068 | 0.017 ± 0.067 | 0.05‡ |
| Ascending-aorta stiffness index/√R-R interval | 237 | 5.5 ± 3.3 | 255 | 5.8 ± 3.4 | 0.33 | 192 | 5.4 ± 4.0 | 205 | 5.2 ± 2.5 | 0.005 ± 0.075 | −0.046 ± 0.074 | 0.57 |
Linear model baseline-adjusted p value comparing slopes under compound symmetry.
Values are mean ± Standard deviation.
0.053
0.047
Table 1 includes the baseline and 3-year absolute values and rates of change of stiffness measures including elastic modulus, stiffness index, and heart rate-corrected stiffness index at the level of the aortic root and ascending aorta for both treatment groups. The rate of change over 3 years in heart rate-corrected aortic-root stiffness index differed by treatment (p=0.01), with a decrease in the atenolol group (p=0.03) and no significant change (p=0.31) in the losartan group (Figure 2). No other treatment differences were observed. There were no treatment differences by pre-specified subgroups. At 3 years, diastolic pressure was slightly lower for atenolol (54±8 mm Hg) vs. losartan (56±8 mm Hg), p=0.04; but there were no significant differences in the systolic or mean blood pressure. As expected, the resting heart rate (64±12 BPM for atenolol vs. 73±13 BPM for losartan, p<0.0001), and average 24-hour heart rate (73±11 BPM for atenolol vs. 82±11 BPM for losartan, p<0.0001) was significantly lower in the atenolol group compared to the losartan group.2
FIGURE 2. Estimated Change in Heart Rate-Corrected Aortic-Root Stiffness, by Assigned Treatment.

Estimated change in heart rate-corrected aortic-root stiffness index with pointwise 95% confidence bands, by assigned treatment: Atenolol in blue, Losartan in red. sqrt = square root.
Data from the full cohort (both treatment groups combined) were used to assess the effects of baseline stiffness on change in aortic-root size over time. Subjects with baseline aortic-root elastic modulus (p<0.001) and heart rate-corrected aortic-root stiffness index (p=0.001) at or below the median had a larger decrease in ARz over time compared with those above the median (Table 2 and Figure 3). The ascending-aorta elastic modulus was a strong predictor of change in ARz; those subjects in the upper quartile had a smaller decrease in ARz over time. However, heart rate-corrected ascending-aorta stiffness index did not predict change in ARz.
TABLE 2.
Estimated Annual Rate of Change in Maximum Aortic-Root Z-Score by Low vs. High Baseline Stiffness Measures
| Threshold Value | Annual Change in Maximum Aortic-Root Z-Score (Slope ± Standard Error) | p value* | ||
|---|---|---|---|---|
| Predictor: Aortic-root elastic modulus (mm Hg) | n = 598 | Number of observations = 2822 | ||
|
| ||||
| Median (Figure 3a) | <0.001 | |||
| ≤618 | −0.163 ± 0.013 | |||
| >618 | −0.081 ± 0.014 | |||
| 75th percentile | <0.001 | |||
| ≤914 | −0.143 ± 0.010 | |||
| >914 | −0.060 ± 0.019 | |||
|
| ||||
| Predictor: Heart Rate-Corrected Aortic-root stiffness index | n = 595 | Number of observations = 2808 | ||
|
| ||||
| Median (Figure 3b) | 0.001 | |||
| ≤9.1 | −0.156 ± 0.014 | |||
| >9.1 | −0.094 ± 0.013 | |||
| 75th percentile | 0.01 | |||
| ≤13.7 | −0.140 ± 0.012 | |||
| >13.7 | −0.089 ± 0.017 | |||
|
| ||||
| Predictor: Ascending-aorta elastic modulus (mm Hg) | n = 492 | Number of observations = 2338 | ||
|
| ||||
| Median | 0.01 | |||
| ≤318 | −0.162 ± 0.014 | |||
| >318 | −0.109 ± 0.015 | |||
| 75th percentile | 0.005 | |||
| ≤447 | −0.152 ± 0.118 | |||
| >447 | −0.085 ± 0.021 | |||
|
| ||||
| Predictor: Heart rate-corrected ascending-aorta stiffness index | n = 490 | Number of observations = 2329 | ||
|
| ||||
| Median | 0.40 | |||
| ≤4.7 | −0.146 ± 0.014 | |||
| >4.7 | −0.129 ± 0.015 | |||
| 75th percentile | 0.17 | |||
| ≤6.6 | −0.145 ± 0.012 | |||
| >6.6 | −0.113 ± 0.021 | |||
The time × group interaction p value denotes whether the 2 slopes for change in maximum aortic root z score over time differ.
FIGURE 3. Estimated Change in Maximum Aortic-Root Z-Score (ARz) By Baseline Aortic-Root Elastic Modulus and Stiffness Index.
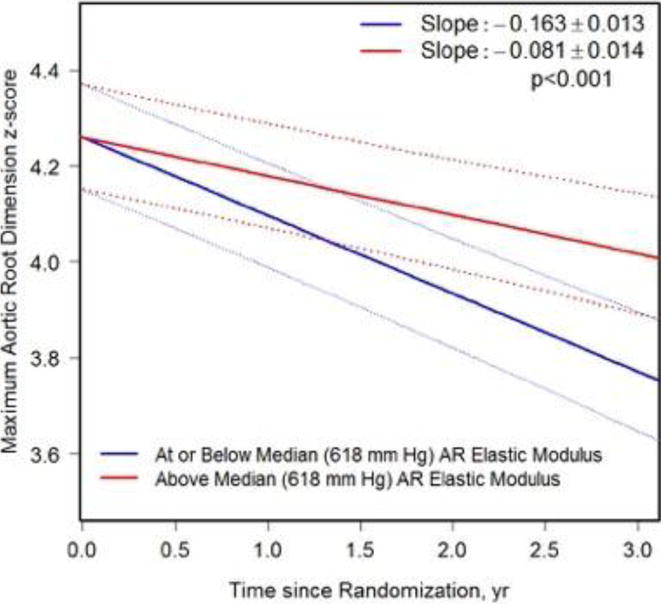

a. Estimated Change in ARz by Baseline Aortic-Root Elastic Modulus Estimated change in maximum aortic-root z-score (ARz) with pointwise 95% confidence bands, for at or below median (blue) vs. above median (red) baseline aortic-root elastic modulus groups. AR = aortic root.
b. Estimated Change in ARz by Baseline Heart Rate-Corrected Aortic-Root Stiffness Index. Estimated change in maximum aortic-root z-score (ARz) with pointwise 95% confidence bands, for at or below median (blue) vs. above median (red) baseline heart rate-corrected aortic-root stiffness index groups. AR = aortic-root; sqrt = square root
There were 29 subjects who met the composite clinical outcome, which included aortic surgery (n=26), aortic dissection and surgery (n=2), or death (from congestive heart failure, n=1). We found no continuous linear association between aortic stiffness and the hazard of a clinical event (data not shown); rather, the associations found were nonlinear, reflected in threshold effects. Table 3 summarizes the analysis of stiffness measures as dichotomous variables, with a median or third-quartile cut point in predicting the composite clinical outcome. After adjustment for baseline age and baseline ARz, continuous stiffness measures (both aortic root and ascending aorta) did not predict clinical outcome. However, aortic-root elastic modulus dichotomized at the third quartile (75th percentile) independently predicted the composite outcome (hazard ratio 2.17, 95% CI 1.02 - 4.63, p = 0.04), controlling for baseline age and ARz. Crude 3-year event rates were 10.4% vs. 3.2% for the higher vs. lower elastic modulus groups, respectively. Interaction analyses (p<0.05) suggested that above-median aortic-root elastic modulus and heart rate-adjusted aortic-root stiffness index (p=0.07 in main effect analysis) were stronger predictors of clinical outcome in patients with a relatively large baseline aortic-root z-score (≥6). The ascending-aorta elastic modulus and heart rate-adjusted stiffness index with a third-quartile cut point had a similar effect size (HR=2.40 for elastic modulus and HR=2.28 for stiffness index), but the p values did not reach significance (p=0.08, p=0.09). Interaction analyses suggested that none of the associations (aortic root or ascending aorta) varied according to age.
TABLE 3.
Association of Baseline Aortic Stiffness with Composite Outcome Adjusted for Age and Maximum Aortic-Root Z-Score
| Baseline Predictors | #Events/Group n | Crude 3-year Event Rate (95% Confidence Interval) | Adjusted Hazard Ratio (95% Confidence Interval) | Main Effect p value | Interaction with Age p value | Interaction with Maximum Aortic Root z score p value |
|---|---|---|---|---|---|---|
| AORTIC ROOT | ||||||
|
| ||||||
| Elastic Modulus | ||||||
| >618 vs. ≤618 mm Hg (median) | 22/299 vs. 7/299 | 7.6% (5.1 to 11.4) vs. 2.4% (1.1 to 5.0) | 2.61 (1.01, 6.76) | 0.05 | 0.77 | 0.04† |
| >914 vs. ≤914 mm Hg (75th percentile) | 15/150 vs. 14/448 | 10.4% (6.4 to 16.7) vs. 3.2% (1.9 to 5.4) | 2.17 (1.02, 4.63) | 0.04 | 0.96 | 0.12 |
| Heart Rate-Adjusted* Stiffness Index | ||||||
| >9.1 vs. ≤9.1 (median) | 20/310 vs. 8/285 | 6.6% (4.3 to 10.0) vs. 2.9% (1.5 to 5.7) | 2.29 (0.95, 5.52) | 0.07 | 0.70 | 0.03§ |
| >13.7 vs. ≤13.7 (75th percentile) | 10/149 vs. 18/446 | 6.9% (3.8 to 12.5) vs. 4.2% (2.6 to 6.5) | 1.40 (0.64, 3.07) | 0.40 | 0.31 | 0.48 |
|
| ||||||
| ASCENDING AORTA | ||||||
|
| ||||||
| Elastic Modulus | ||||||
| >318 vs. ≤318 mm Hg (median) | 17/246 vs. 4/246 | 7.2% (4.5 to 11.2) vs. 1.7% (0.6 to 4.4) | 1.97 (0.62, 6.28) | 0.25 | 0.53 | 0.72 |
| >447 vs. ≤447 mm Hg (75th percentile) | 9/123 vs. 12/369 | 7.5% (4.0 to 13.9) vs. 3.4% (1.9 to 5.8) | 2.40 (0.92, 6.28) | 0.08 | 0.20 | 0.20 |
| Heart Rate-Adjusted* Stiffness Index | ||||||
| >4.7 vs. ≤4.7 (median) | 14/239 vs. 6/251 | 6.0% (3.6 to 9.9) vs. 2.5% (1.1 to 5.4) | 1.61 (0.60, 4.33) | 0.34 | 0.98 | 0.46 |
| >6.6 vs. ≤6.6 (75th percentile) | 8/124 vs. 12/366 | 6.6% (3.3 to 12.7) vs. 3.4% (1.9 to 5.9) | 2.28 (0.88, 5.89) | 0.09 | 0.27 | 0.05 |
Composite Outcome: Aortic-Root Surgery, Dissection, Death
divided by the square root of R-R interval
Interaction p=0.04: Adjusted Hazard Ratios vary with maximum aortic root z score (Max ARz), Hazard Ratio=0.93 at MaxARz=3.0; Hazard Ratio=1.50 at Max ARz=4.5; Hazard Ratio=2.43 at Max ARz=6.0
Interaction p=0.03: Adjusted Hazard Ratios vary with Max ARz, Hazard Ratio=0.81 at MaxARz=3.0; Hazard Ratio=1.38 at Max ARz=4.5; Hazard Ratio=2.35 at Max ARz=6.0
DISCUSSION
In current clinical practice for patients with the Marfan syndrome, aortic-root size is the strongest, albeit imperfect, predictor of adverse clinical outcomes, and timing of prophylactic aortic-root surgery is based largely on aortic dimension. The most important finding of the current study, the largest to date, is the inverse relationship between baseline stiffness measures (aortic-root elastic modulus and heart rate-corrected stiffness index) and decline in ARz. In other words, subjects with higher baseline aortic-root stiffness had a faster rate of growth of the aortic root over a 3-year period of observation. Furthermore, baseline aortic-root elastic modulus independently predicted the composite clinical outcome of aortic-root surgery, dissection, or death, even after adjusting for baseline age and ARz.
We also found that atenolol therapy reduced the heart rate-corrected aortic-root stiffness index over a 3-year follow-up, but losartan did not, despite no significant difference in aortic-root growth rate between the 2 treatment groups. However, the reasons for these results are unclear. It has been speculated that the negative inotropic effects and the reduced peak dp/dt associated with use of beta blockers would result in a lower peak shear stress in the aorta, thereby reducing the stimulus to dilation.22 Losartan does not have this negative inotropic effect and therefore may lack this potential benefit. Furthermore, diastolic blood pressure was slightly lower in the atenolol group, which may have contributed to our findings. There were no treatment differences for aortic-root elastic modulus, ascending-aorta elastic modulus, or heart rate-corrected ascending-aorta stiffness index. Prior studies using a variety of modalities and assessing a number of different measures of aortic stiffness have shown inconsistent response to beta blockers and angiotensin receptor blockers, but these studies suggest that these medications might have unique and complex mechanisms of action in modifying vascular properties in patients with the Marfan syndrome.9–14
Few prior studies have examined the relationship between aortic stiffness and aortic-root growth rate or risk for adverse clinical outcomes in the Marfan syndrome, and their results have been contradictory. In a cohort of 50 adults with the Marfan syndrome, patients with lower aortic stiffness values measured by arterial tonometry (pulse wave velocity and augmentation index) had a significantly lower rate of aortic-root progression; progression was defined as aortic-root dilation >5 mm/year, dissection, or surgery.6 In another study of 78 adults with the Marfan syndrome, descending aortic distensibility (measured by MRI) was an independent predictor of progressive abdominal aortic dilation, defined as a >1 mm/year increase in diameter.7 In contrast, in the study by Pees et al. in 20 pediatric and adolescent patients with the Marfan syndrome treated with losartan, no correlation between elasticity behavior and aortic-root growth rate was observed.23 Recently, Prakash et al. demonstrated in 83 children and young adults with connective tissue disorders including the Marfan syndrome that higher aortic stiffness (decreased aortic strain measured by MRI) is associated with higher rates of aortic-root dilation and surgical aortic replacement.8 Our study and that of Prakash and colleagues provide evidence that aortic stiffness measured by either echocardiography or MRI may identify patients at higher risk for faster aortic-root dilation and adverse clinical outcomes, potentially allowing for closer monitoring and more aggressive therapy for such patients. In addition, those with above-median aortic-root elastic modulus combined with a large aortic root z-score may be at particularly increased risk of adverse outcomes. Stiffness index and elastic modulus are easily measured by echocardiography and could be incorporated feasibly into routine clinical surveillance for patients with the Marfan syndrome. Further studies are needed to confirm that non-invasive aortic stiffness measures can be useful for risk stratification. In our trial, atenolol reduced aortic-root stiffness index favorably over losartan, suggesting that therapy with atenolol (or a combination of atenolol and losartan) may be preferable in patients with higher aortic stiffness.
There are several methods to assess arterial stiffness, including pulse wave velocity, as utilized in some of the investigations described above. Prior investigations support the concept that segmental stiffness in the aorta is clinically important in patients with the Marfan syndrome.24 While pulse wave velocity measures the aortic stiffness along a long segment, for example from the ascending aorta to the femoral artery, aortic-root stiffness measured by 2D echocardiography provides local data on segmental arterial stiffness, for example at the aortic root or ascending aorta.
There are several limitations to this trial. First, study results may not be generalizable to patients with the Marfan syndrome with ARz >3. Second, brachial blood pressure rather than central pressure was used for stiffness analyses. Brachial blood pressure is higher than central blood pressure due to pulse pressure amplification thereby introducing systematic inaccuracy. However, it is noteworthy that beta blockade in non-Marfan populations is inferior to other drug classes, including angiotensin II receptor blockers, in reducing central blood pressure relative to brachial blood pressure.25 Third, aortic wall thickness may influence vascular stiffness but cannot be accurately measured using transthoracic echocardiography and has likewise not been taken into account in previous studies in patients with the Marfan syndrome. Fourth, using 2-dimensional echocardiography, the reproducibility of the stiffness measures was only moderate. While this may in part be due to the fact that derived parameters based on 2 or more measurements are less reproducible than the measurements themselves due to propagation of error26, prior studies using M-mode have reported excellent reproducibility of ascending-aorta stiffness indices5,27, and perhaps M-mode measurement of the aortic diameters may have resulted in better reproducibility at least at the ascending aorta level. Fifth, the number of subjects with clinical events was relatively small (n=29). If the event rate had been higher, we might have had statistical power to declare several clinically significant associations that we observed (hazard ratios >2) as statistically significant. Finally, the non-linear relationship between stress and strain could be a confounder that could limit the validity of the stiffness measures used as predictor of vascular tissue properties.
In conclusion, in the Pediatric Heart Network Marfan Trial, higher baseline aortic-root stiffness index and elastic modulus values were associated with a smaller decrease in ARz (faster aortic-root growth rate), and a higher baseline aortic-root elastic modulus was a risk factor for the composite outcome of aortic-root surgery, dissection, or death. Atenolol was associated with a reduction in heart rate-corrected aortic-root stiffness over 3 years, while losartan was not. These data suggest that non-invasive aortic stiffness measures may identify patients at higher risk for progressive aortic enlargement and adverse clinical outcomes, potentially allowing for closer monitoring and more aggressive therapy for such patients.
Supplementary Material
Acknowledgments
SOURCES OF FUNDING
Supported by U01 grants from the National Heart, Lung, and Blood Institute (HL068269, HL068270, HL068279, HL068281, HL068285, HL068292, HL068290, HL068288, HL085057) and the Food and Drug Administration Office of Orphan Products Development. Additional support provided by The Marfan Foundation, Merck & Co., Inc., and Teva Canada Limited
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
None. The authors do not have any conflict of interest. The views expressed in this article are those of the authors and do not represent the official views of the National Heart, Lung, and Blood Institute (NHLBI) or the National Institutes of Health.
References
- 1.Judge DP, Dietz HC. Marfan’s syndrome. Lancet. 2005;366:1965–1976. doi: 10.1016/S0140-6736(05)67789-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lacro RV, Dietz HC, Sleeper LA, Yetman AT, Bradley TJ, Colan SD. Atenolol versus losartan in children and young adults with Marfan’s syndrome. N Engl J Med. 2014;371:2061–2071. doi: 10.1056/NEJMoa1404731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Savolainen A, Keto P, Hekali P, Nisula L, Kaitila I, Viitasalo M. Aortic distensibility in children with the Marfan syndrome. Am J Cardiol. 1992;70:691–693. doi: 10.1016/0002-9149(92)90215-k. [DOI] [PubMed] [Google Scholar]
- 4.Hirata K, Triposkiadis F, Sparks E, Bowen J, Wooley CF, Boudoulas H. The Marfan syndrome: abnormal aortic elastic properties. J Am Coll Cardiol. 1991;18:57–63. doi: 10.1016/s0735-1097(10)80218-9. [DOI] [PubMed] [Google Scholar]
- 5.Bradley TJ, Potts JE, Potts MT, DeSouza AM, Sandor GG. Echocardiographic Doppler assessment of the biophysical properties of the aorta in pediatric patients with the Marfan syndrome. Am J Cardiol. 2005;96:1317–1321. doi: 10.1016/j.amjcard.2005.06.080. [DOI] [PubMed] [Google Scholar]
- 6.Mortensen K, Aydin MA, Rybczynski M, Baulmann J, Schahidi NA, Kean G. Augmentation index relates to progression of aortic disease in adults with Marfan syndrome. Am J Hypertens. 2009;22:971–979. doi: 10.1038/ajh.2009.115. [DOI] [PubMed] [Google Scholar]
- 7.Nollen GJ, Mulder BJ. What is new in the Marfan syndrome? Int J Cardiol. 2004;97(Suppl 1):103–108. doi: 10.1016/j.ijcard.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 8.Prakash A, Adlakha H, Rabideau N, Hass CJ, Morris SA, Geva T. Segmental Aortic Stiffness in Children and Young Adults With Connective Tissue Disorders: Relationships With Age, Aortic Size, Rate of Dilation, and Surgical Root Replacement. Circulation. 2015;132:595–602. doi: 10.1161/CIRCULATIONAHA.114.014934. [DOI] [PubMed] [Google Scholar]
- 9.Haouzi A, Berglund H, Pelikan PC, Maurer G, Siegel RJ. Heterogeneous aortic response to acute beta-adrenergic blockade in Marfan syndrome. Am Heart J. 1997;133:60–63. doi: 10.1016/s0002-8703(97)70248-5. [DOI] [PubMed] [Google Scholar]
- 10.Groenink M, de Roos A, Mulder BJ, Spaan JA, van der Wall EE. Changes in aortic distensibility and pulse wave velocity assessed with magnetic resonance imaging following beta-blocker therapy in the Marfan syndrome. Am J Cardiol. 1998;82:203–208. doi: 10.1016/s0002-9149(98)00315-4. [DOI] [PubMed] [Google Scholar]
- 11.Rios AS, Silber EN, Bavishi N, Varga P, Burton BK, Clark WA. Effect of long-term beta-blockade on aortic root compliance in patients with Marfan syndrome. Am Heart J. 1999;137:1057–1061. doi: 10.1016/s0002-8703(99)70362-5. [DOI] [PubMed] [Google Scholar]
- 12.Reed CM, Fox ME, Alpert BS. Aortic biomechanical properties in pediatric patients with the Marfan syndrome, and the effects of atenolol. Am J Cardiol. 1993;71:606–608. doi: 10.1016/0002-9149(93)90522-e. [DOI] [PubMed] [Google Scholar]
- 13.Sandor GG, Alghamdi MH, Raffin LA, Potts MT, Williams LD, Potts JE. A randomized, double blind pilot study to assess the effects of losartan vs. atenolol on the biophysical properties of the aorta in patients with Marfan and Loeys-Dietz syndromes. Int J Cardiol. 2015;179:470–475. doi: 10.1016/j.ijcard.2014.11.082. [DOI] [PubMed] [Google Scholar]
- 14.Bhatt AB, Buck JS, Zuflacht JP, Milian J, Kadivar S, Gauvreau K. Distinct effects of losartan and atenolol on vascular stiffness in Marfan syndrome. Vasc Med. 2015;20:317–325. doi: 10.1177/1358863X15569868. [DOI] [PubMed] [Google Scholar]
- 15.Lacro RV, Dietz HC, Wruck LM, Bradley TJ, Colan SD, Devereux RB. Rationale and design of a randomized clinical trial of beta-blocker therapy (atenolol) versus angiotensin II receptor blocker therapy (losartan) in individuals with Marfan syndrome. Am Heart J. 2007;154:624–631. doi: 10.1016/j.ahj.2007.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Paepe A, Devereux RB, Dietz HC, Hennekam RC, Pyeritz RE. Revised diagnostic criteria for the Marfan syndrome. Am J Med Genet. 1996;62:417–426. doi: 10.1002/(SICI)1096-8628(19960424)62:4<417::AID-AJMG15>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 17.Selamet Tierney ES, Levine JC, Chen S, Bradley TJ, Pearson GD, Colan SD. Echocardiographic methods, quality review, and measurement accuracy in a randomized multicenter clinical trial of Marfan syndrome. J Am Soc Echocardiogr. 2013;26:657–666. doi: 10.1016/j.echo.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merillon JP, Motte G, Fruchaud J, Masquet C, Gourgon R. Evaluation of the elasticity and characteristic impedance of the ascending aorta in man. Cardiovasc Res. 1978;12:401–406. doi: 10.1093/cvr/12.7.401. [DOI] [PubMed] [Google Scholar]
- 19.Hirai T, Sasayama S, Kawasaki T, Yagi S. Stiffness of systemic arteries in patients with myocardial infarction. A noninvasive method to predict severity of coronary atherosclerosis. Circulation. 1989;80:78–86. doi: 10.1161/01.cir.80.1.78. [DOI] [PubMed] [Google Scholar]
- 20.Fitzmaurice GM, L NM, Ware JH. Applied Longitudinal Analysis. Hoboken, NJ: John Wiley & Sons, Inc; 2004. [Google Scholar]
- 21.Lacro RV, Guey LT, Dietz HC, Pearson GD, Yetman AT, Gelb BD. Characteristics of children and young adults with Marfan syndrome and aortic root dilation in a randomized trial comparing atenolol and losartan therapy. Am Heart J. 2013;165:828–835 e823. doi: 10.1016/j.ahj.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shores J, Berger KR, Murphy EA, Pyeritz RE. Progression of aortic dilatation and the benefit of long-term beta-adrenergic blockade in Marfan’s syndrome. N Engl J Med. 1994;330:1335–1341. doi: 10.1056/NEJM199405123301902. [DOI] [PubMed] [Google Scholar]
- 23.Pees C, Laccone F, Hagl M, Debrauwer V, Moser E, Michel-Behnke I. Usefulness of losartan on the size of the ascending aorta in an unselected cohort of children, adolescents, and young adults with Marfan syndrome. Am J Cardiol. 2013;112:1477–1483. doi: 10.1016/j.amjcard.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 24.Prakash A, Adlakha H, Rabideau N, Hass CJ, Morris SA, Geva T. Segmental Aortic Stiffness in Children and Young Adults with Connective Tissue Disorders: Relationships with Age, Aortic Size, Rate of Dilation and Surgical Root Replacement. Circulation. 2015 doi: 10.1161/CIRCULATIONAHA.114.014934. [DOI] [PubMed] [Google Scholar]
- 25.McGaughey TJ, Fletcher EA, Shah SA. Impact of Antihypertensive Agents on Central Systolic Blood Pressure and Augmentation Index: A Meta-Analysis. Am J Hypertens. 2016;29:448–457. doi: 10.1093/ajh/hpv134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colan SD, Shirali G, Margossian R, Gallagher D, Altmann K, Canter C. The ventricular volume variability study of the Pediatric Heart Network: study design and impact of beat averaging and variable type on the reproducibility of echocardiographic measurements in children with chronic dilated cardiomyopathy. J Am Soc Echocardiogr. 2012;25:842–854 e846. doi: 10.1016/j.echo.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baumgartner D, Baumgartner C, Matyas G, Steinmann B, Loffler-Ragg J, Schermer E. Diagnostic power of aortic elastic properties in young patients with Marfan syndrome. J Thorac Cardiovasc Surg. 2005;129:730–739. doi: 10.1016/j.jtcvs.2004.07.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


