Abstract
Juvenile hormone (JH) is a major hormonal regulator in insects. In Aedes aegypti females, JH signals the completion of the ecdysis to the adult stage and initiates reproductive processes. Although the regulation of JH synthesis and titer in Ae. aegypti females has been extensively studied, relatively little is known about changes of JH synthesis and titers in male mosquitoes, as well as on the roles of JH controlling male reproductive biology. A better understanding of male mosquito reproductive biology, including an improved knowledge of the hormonal control of reproduction, could increase the likelihood of success of male-targeting vector control programs. Using a high performance liquid chromatography coupled to electrospray tandem mass spectrometry method, we measured JH biosynthesis and hemolymph levels in male mosquitoes during pupal and adult stages. Our results revealed tightly concomitant changes in JH biosynthesis and JH hemolymph titers. Synthesis of JH III was very low in late pupae, significantly increased during the first 24 h after adult eclosion, and then remained relatively constant during the first six days after adult eclosion. Feeding high sugar diets resulted in an increase of JH synthesis and titers, and starvation significantly decreased JH synthesis, but this effect could be reversed by changing the males back to a high sugar diet. JH synthesis rates were similar in virgin and mated males, but hemolymph JH levels were different in well-nourished virgin and mated males. Starvation resulted in a significant reduction in insemination rates; with well-nourished males inseminating 2 times more females than water-fed. Giving a 20% sugar meal for 24 h to those mosquitoes that were previously starved for 6 days, caused a significant rise in insemination rates, restoring them to levels similar to those recorded for 20% fed males. These results suggest that nutrition plays a role on male fecundity, and this effect might be mediated by JH.
Keywords: Juvenile hormone, mosquito, male, hemolymph, biosynthesis, corpora allata
Graphical abstract
1. Introduction
Female Aedes aegypti mosquitoes are important vectors of viral diseases such as Yellow fever, Dengue, Zika and Chikungunya (Chen and Vasilakis, 2011; Burt et al., 2012). Unlike females, male mosquitoes do not feed on blood and thus do not transmit diseases. Although the hormonal control of female reproductive biology has been extensively studied, relatively little is known about the topic for male mosquitoes. New control strategies have been recently implemented based on the massive release of male mosquitoes. These strategies include the release of sterile males (Bourtzis et al., 2016), Wolbachia-infected males to induce sexual sterility (Hoffman et al., 2011) and genetically modified mosquitoes that produce non-viable offspring (Carvalho et al., 2015). A better understanding of male biology, including an improved knowledge of the hormonal control of reproductive biology, could increase the likelihood of success of such male-targeting vector control programs.
Juvenile hormones (JHs) play key roles in insect development and reproduction (Riddiford, 2012; Goodman and Cusson, 2012). JHs are synthesized by the corpora allata (CA), a pair of endocrine glands with neural connections to the brain (Tobe and Stay, 1985). In Ae. aegypti females the CA is inactive for most of the duration of the pupal stage (Nouzova et al., 2011; Rivera-Perez et al, 2014). As the anti-metamorphic role of JH comes to an end, the CA of the late pupa (or pharate adult) is reactivated and begins to synthesize JH, which would now play an essential role orchestrating reproductive maturation (Klowden, 1997; Zhu and Noriega, 2016).
Less is known about JH-regulated processes in male mosquitoes. It has been reported that JH controls the secretory process of the male accessory glands (MAG) of Ae. aegypti. Topical application of a juvenile hormone analogue (JHA) induced precocious and enhanced MAG secretions (Ramalingam and Craig, 1977). Treatment with the JHA also increased the potency of males, resulting in the insemination of a larger number of females, and also stimulated secretion in fully depleted MAGs. Males whose accessory glands were depleted due to multiple matings, and were later treated with JHA also displayed higher inseminating capacity. Cauterization of the CA impaired MAG secretory functions (Ramalingam and Craig, 1977).
In the present studies, using high performance liquid chromatography coupled to electrospray tandem mass spectrometry (HPLC-MS/MS), we measured changes in JH biosynthesis and levels in the hemolymph of male mosquitoes during the pupal and adult stages. Our results revealed tightly concomitant changes in JH biosynthesis and JH hemolymph titers, confirming that JH titer in mosquitoes is fundamentally determined by the rate of biosynthesis in the CA. Feeding high sugar diets resulted in an increase of JH titers, and mating also modified JH titers in hemolymph.
2. Materials and methods
2.1. Insects
Ae. aegypti of the Rockefeller strain were reared at 28°C and 80% humidity as previously described (Nouzova et al., 2011). Male pupae were isolated, and virgin males were kept separately from females since adult emergence. Mated males were obtained by mixing them with females immediately after emergence in a 1 male: 3 female ratio. Adult mosquitos were offered a cotton pad soaked in water (0%), 3% or 20% sucrose solutions as previously described (Nouzova et al., 2011).
2.2. Hemolymph collection
Hemolymph of adult mosquitoes was obtained by perfusion (Hernandez et al., 1999). Fine needles were made from 100-µl micro-glass capillary tubes using a pipette puller P-30 (Sutter Instrument, Novato, CA), and mounted in a pipette pump (Drummond, Broomall, PA). Needles were inserted manually through the neck membrane into the thoracic cavity, and insects were perfused with 20 µl of a “bleeding solution” of phosphate-buffered saline (PBS, pH 7.2) containing a protease inhibitor cocktail (1 mM phenylmethylsulfonyl fluoride, 1 mM ethylenediaminetetraacetic acid, 0.2 mM Na-p-tosyl-L-lysine chloromethyl ketone and,1 mM leupeptine). The hemolymph was obtained from a small tear made laterally on the intersegmentary membrane of the last abdominal segment. The first drop of perfused hemolymph was collected directly on a glass silanized tube (Thermo Scientific) placed on ice. Pupae were briefly washed with 70% ethanol and air-dried, and hemolymph was obtained by capillary action using a 10 µl micropipette tip containing 2 µl of bleeding solution that was introduced into a small tear made laterally on the intersegmentary membrane between the 4th and 5th abdominal segments (Hernandez-Martinez et al., 2015). For each data point at least four independent samples of hemolymph were collected from pools of five insects each.
2.3. JH quantification
After hemolymph extraction, 10 µL of 6.25 ppb of JH III-D3 in acetonitrile were added to each sample, followed by 600 µL of hexane. Samples were vortexed for 1 minute, and spun for 5 minutes at 4°C and 2000 g. The organic phase was transferred to a new silanized vial and dried under nitrogen flow and stored at −20°C. Dried extracts were re-suspended in 50 µl of acetonitrile, vortexed 1 minute, transferred to a new silanized vial with a fused 250 µL insert. JH quantifications by high performance liquid chromatography coupled to electrospray tandem mass spectrometry (HPLC-MS/MS) were done as previously described by Ramirez et al. (2016). Briefly, we employed a HPLC-MS/MS workflow based on multiple reaction monitoring (MRM) using the two most abundant fragmentation transitions: 267->235 (primary) and 267->147 (secondary). In order to accurately quantify the amount of JH III present in the hemolymph or produced by the BR-CA-CC complexes, the heavy deuterated JH III analog (JH III-D3) was utilized as an internal standard to normalize recoveries during the sample preparation, extraction and analysis steps. An extraction recovery of near 55% was routinely observed regardless of the analyte concentration (Ramirez et al., 2016).
2.4. Dissections of corpora allata complexes and JH biosynthesis assay
Adult mosquitos were cold-anesthetized and brain-corpora allata–corpora cardiaca complexes (BR-CA-CC) were dissected and incubated at 32°C for 4 h in 150 µl of tissue culture media M-199 (Gibco, Grand Island, NY, USA) containing 2% Ficoll, 25 mM HEPES (pH 6.5) and methionine (100 µM) (Nouzova et al., 2011). Biosynthesized JH was analyzed by HPLC-MS/MS as previously described (Ramirez et al., 2016). Stimulation of JH synthesis was performed with 200 µM (E, E)-farnesoic acid (Echelon; Salt Lake City, UT).
2.5. Insemination rates measurements
Six days virgin male mosquitoes fed either water or a 20% sugar diet were individually isolated in small 250 ml containers with three virgin females. Mosquitoes were left undisturbed for 45 min to allow mating to occur. Female mosquitoes were anesthetized by chilling, spermathecae were dissected and the presence of sperm was assessed under the microscope.
2.6. Statistical analysis
Statistical analyses were performed using the GraphPad Prism Software (San Diego, CA, USA). The results are expressed as means ± S.E.M. Significant differences (p< 0.01) were determined with a one tailed students t-test performed in a pair wise manner or by one-way ANOVA followed by Tukey’s test.
3. Results
3.1 JH biosynthesis and hemolymph titers in pupae and adult male Ae. aegypti
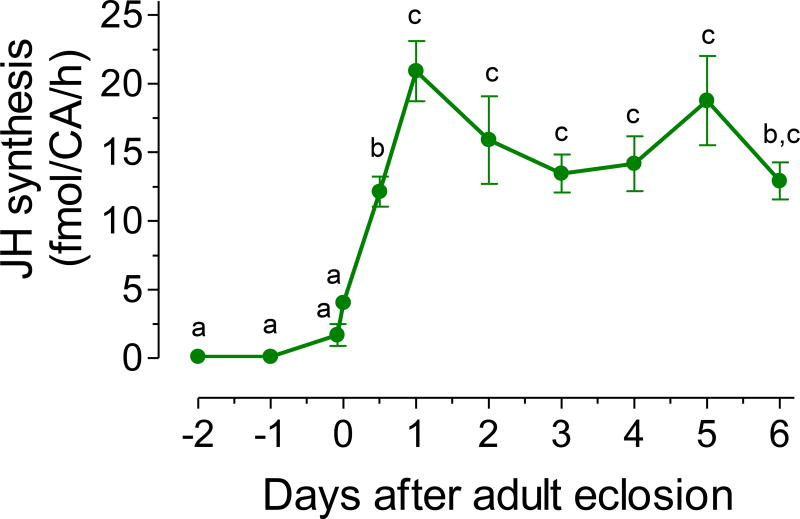
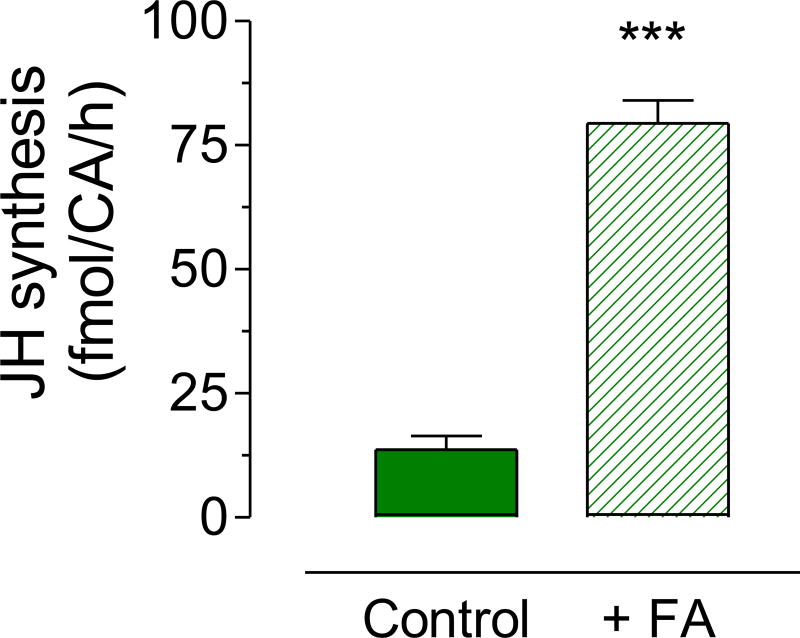
We analyzed the amount of JH III synthesized in vitro by brain-corpora allata-corpora cardiaca complexes (BR-CA-CC), dissected from virgin males fed a 3% sugar meal. Synthesis of JH III was very low in late pupae, significantly increased during the first 24 h after adult eclosion, and then remained relatively constant with values around 15–20 fmol/CA/h during the first six days after adult eclosion (Fig. 1). The addition of 200 µM of exogenous farnesoic acid (a JH III precursor) to the incubation media resulted in a 6-fold increase on JH synthesis (Fig. 2).
Fig. 1. JH biosynthesis.
JH biosynthesis by CA dissected from pupa, and 3% sugar-fed virgin adult males. Y axis: JH biosynthesis expressed as fmol/CA/h. Points represent the means ± SEM of 4 to 15 independent replicates of groups of 4 CA. Different letters above the points indicate significant differences among treatments (one way ANOVA p < 0.05, with Tukey’s test of multiple comparisons).
Fig. 2. Stimulation of JH synthesis by exogenous addition of farnesoic acid.
JH biosynthesis was evaluated in CA dissected 3 days after eclosion from virgin males fed a 3% sugar diet. CA were incubated in vitro in culture medium alone (control) or with the addition of 200 µM of farnesoic acid (+FA). Bars represent the means ± S.E.M. of 4 independent replicates of incubations of groups of 4 CA. Asterisk denotes significant difference (unpaired t-test; *** P ≤ 0.001).
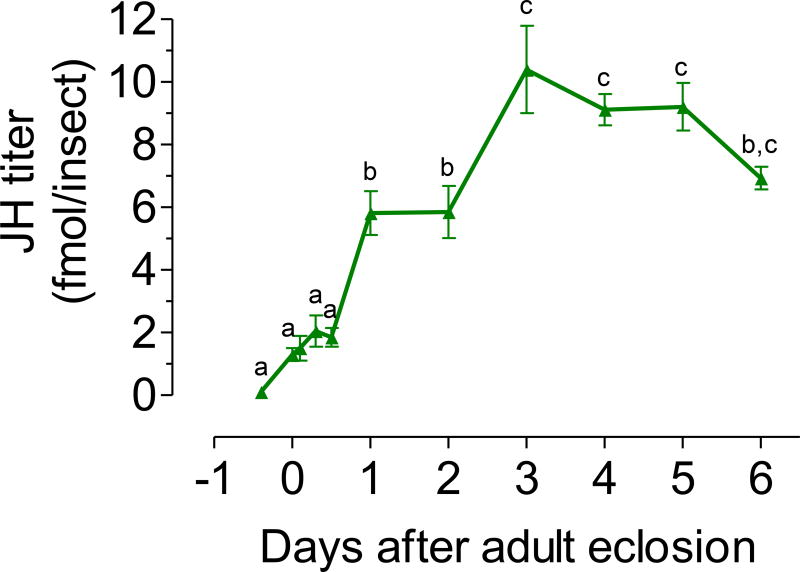
In addition, we used a simple and efficient protocol to collect adult mosquito hemolymph; this method assured the reproducible recovery of single drops of similar volumes (Hernandez-Martinez et al., 2015). The changes in the titers of JH detected in hemolymph extracts of virgin males fed a 3% sugar meal for the first 6 days after adult emergence are presented in Fig. 3. Titers significantly increased during the first 3 days after adult eclosion; remained relatively constant with titers around 10 fmol/male until day 5, and slightly decreased by day 6.
Fig. 3. JH hemolymph titers.
JH hemolymph titers in virgin males fed a 3% sugar meal. X axis: represent days before (pupa) and after adult eclosion. JH titers are expressed as fmol/insect. Each data point represents the mean ± SEM of 4 to 20 independent replicates of groups of 5 males. Different letters above the points indicate significant differences among treatments (one way ANOVA p < 0.05, with Tukey’s test of multiple comparisons).
We observed a significant positive correlation between changes in JH biosynthesis rates and changes in JH hemolymph titers (Supplemental Fig. 1); underscoring again the correlation between biosynthetic activity of the CA and titers of JH in hemolymph previously reported for female mosquitoes (Hernandez-Martinez et al., 2015).
3.2. Effect of nutrients on JH biosynthesis and hemolymph titers
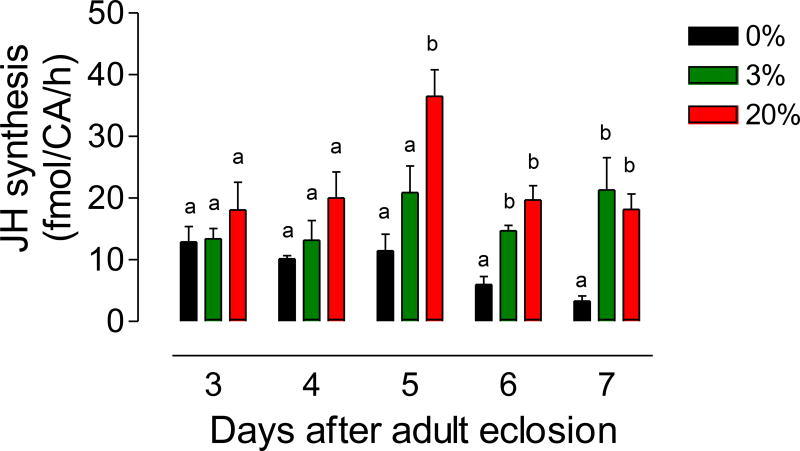
We tested the effect of high and low sugar diets on JH synthesis and hemolymph titers. Virgin males were fed 0%, 3% or 20% sucrose solutions, and JH biosynthesis was evaluated at different days after adult eclosion. Biosynthesis in sugar-fed mosquitoes remained high during the first 7 days, but starvation (0% sugar) significantly decreased JH synthesis (Fig. 4). By day 6 there was over a 5-fold reduction of JH synthesis in starved males, but giving 20% sugar for 24h to those mosquitoes that were previously starved for 6 days, resulted in a very significant increase of JH synthesis by day 7 (Fig. 5). Hemolymph titers in sugar-fed mosquitoes, remained high during the first 6 days, but starvation (0% sugar) significantly decreased JH titers in hemolymph; surprisingly, after day 3 virgin males fed a 3% sugar meal had consistently significantly higher JH titers than males fed a 20% sugar diet (Fig. 6).
Fig. 4. Effect of sugar feeding on JH biosynthesis.
Virgin males were fed a 0%, 3% or 20% sucrose solution. JH synthesis was evaluated at different days after emergence. JH synthesis is expressed as fmol/CA/h. Bars represent the means ± SEM of 4 to 16 independent replicates of groups of 4 CA. Different letters above the columns indicate significant differences among treatments (one way ANOVA p < 0.05, with Tukey’s test of multiple comparisons).
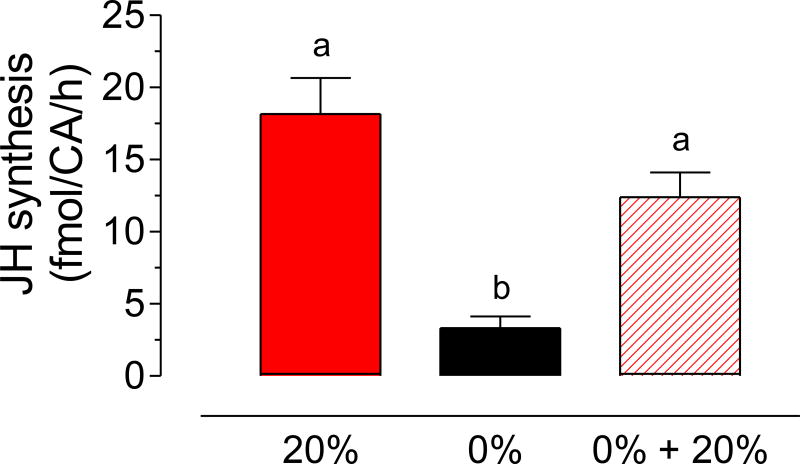
Fig. 5. Effect of sugar feeding on JH biosynthesis by males that were previously starved.
Three groups of virgin males were fed either a 20% sucrose solution for 7 days (20%), water for 7 days (0%) or water for 6 days and then given 20% sucrose for a day (0% + 20%). JH synthesis was evaluated at 7 days for the three groups. JH synthesis is expressed as fmol/CA/h. Bars represent the means ± SEM of 4 to 16 independent replicates of groups of 4 CA. Different letters above the columns indicate significant differences among treatments (one way ANOVA p < 0.05, with Tukey’s test of multiple comparisons).
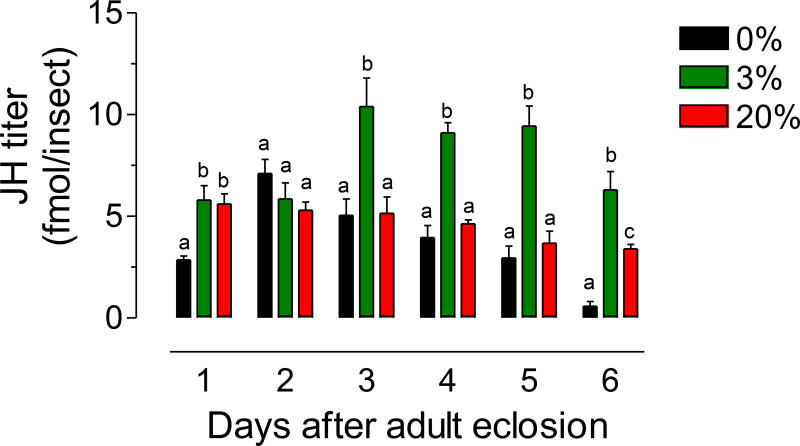
Fig. 6. Effect of sugar feeding on JH hemolymph titers.
Virgin males were fed a 0%, 3% or a 20% sucrose solution, and JH titers in hemolymph were evaluated at different days after emergence. JH titers are expressed as fmol/insect. Bars represent the means ± SEM of 4 to 16 independent replicates of groups of 5 insects. Different letters above the columns indicate significant differences among treatments (one way ANOVA p < 0.05, with Tukey’s test of multiple comparisons).
3.3. Effect of mating on JH biosynthesis and hemolymph titers
We tested the effect of mating on JH synthesis and titers. Virgin and mated males were fed 0%, 3% or 20% sucrose solutions, and JH biosynthesis was evaluated at six days after adult eclosion. There were no significant differences in JH synthesis rates by CA from virgin or mated males raised in the three different sugar-diets (Fig. 7). JH hemolymph titers were similar in six-day old virgin and mated males raised on water or a 3% sugar diet; but mating significantly increased JH hemolymph levels in males fed a 20% sugar diet (Fig. 8). Similar increases in hemolymph titers in mated males fed a 20% sugar diet were observed in two to five day’s old males (Supplemental Fig. 2).
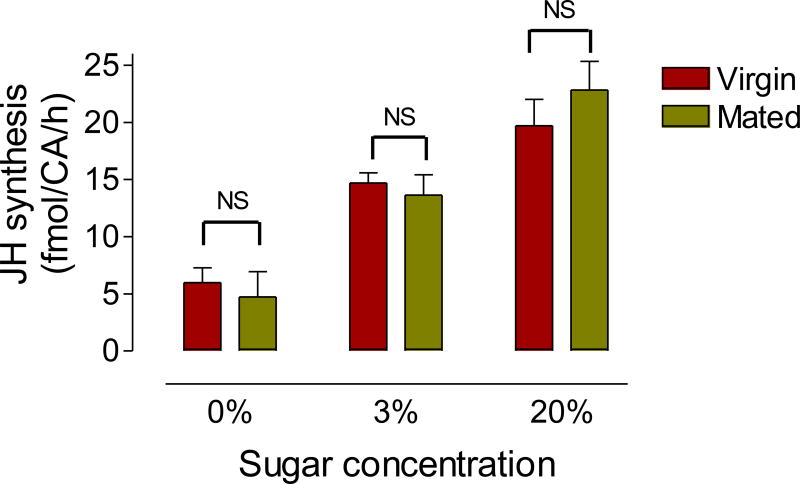
Fig. 7. Effect of mating on JH biosynthesis.
Virgin and mated males were fed a 0%, 3% or 20% sucrose solution, and JH synthesis was evaluated 6 days after emergence. JH synthesis is expressed as fmol/CA/h. Bars represent the means ± SEM of 4 to 16 independent replicates of groups of 4 CA. NS denotes no significant difference (unpaired t-test).
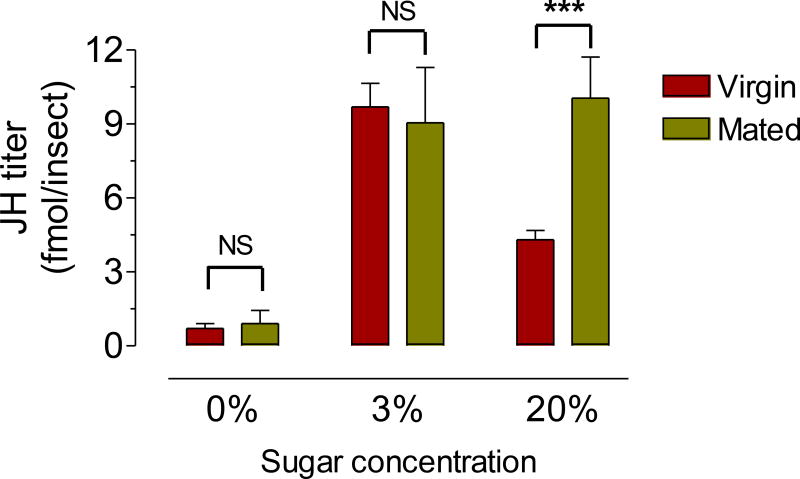
Fig. 8. Effect of mating on JH hemolymph titers.
Virgin and mated males were fed a 0%, 3% or 20% sucrose solution, and JH titers in hemolymph were evaluated 6 days after emergence. JH titers are expressed as fmol/insect. Bars represent the means ± SEM of 4 independent replicates of groups of 5 insects. Asterisk denotes significant difference (unpaired t-test; * P ≤ 0.05). NS denotes no significant difference (unpaired t-test).
3.4. Effect of nutrients on insemination rates
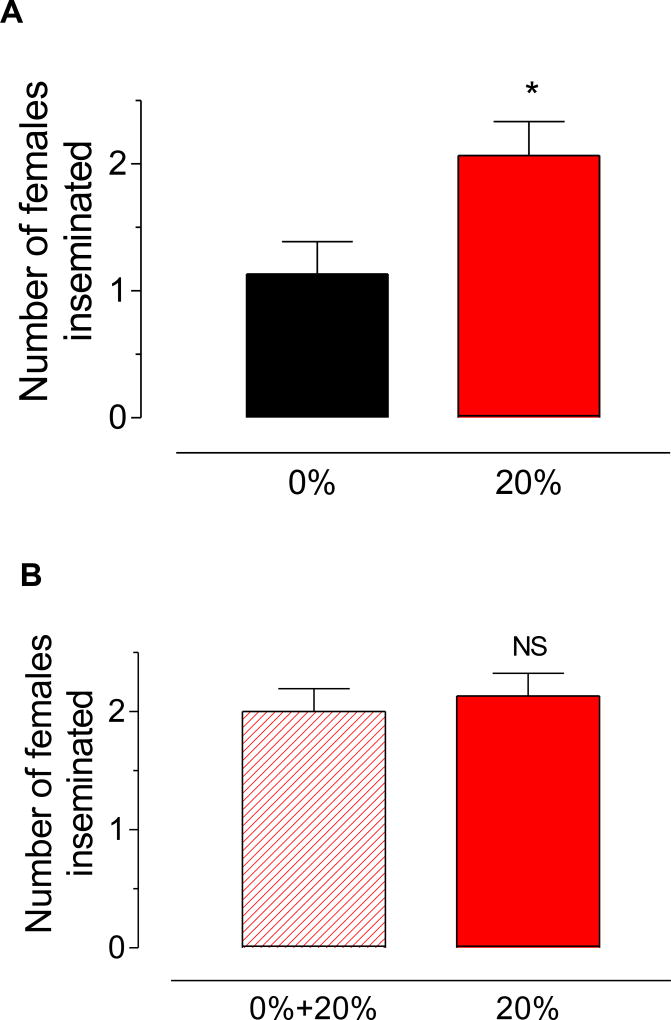
We tested the effect of high and low sugar diets on the ability of virgin males to inseminate virgin females. Isolated virgin 6-day old males were left undisturbed for 45 min with three 6-day old virgin females to allow mating to occur; female spermathecae were dissected and the presence of sperm was evaluated. Starvation resulted in a significant reduction in insemination rates; with well-nourished males inseminating 2 times more females than water-fed (Fig. 9A). Giving a 20% sugar meal for 24 h to those mosquitoes that were previously starved for 6 days, caused a significant rise in insemination rates, restoring them to levels similar to those recorded for 20% fed males (Fig. 9B).
Fig. 9. Effect of sugar feeding on insemination rates.
A) Virgin males were fed a 0% or a 20% sucrose solution for 6 days. Individual males were left undisturbed for 45 min with three virgin females to allow mating to occur. Fifteen males were evaluated for each treatment. B) Virgin males were fed a 0% or a 20% sucrose solution for 6 days. After that, starved males were given 20% sugar for 24 h, and insemination rates were determined at 7 days for both groups. Fifteen males were evaluated for each treatment. Positive insemination rates were determined by dissecting female spermathecae and evaluating the presence of sperm. Bars represent the means ± SEM of the number of females (0 to 3) inseminated by individual males (n = 15). Asterisk denotes significant difference (unpaired t-test; * P ≤ 0.05). NS denotes no significant difference (unpaired t-test).
4. Discussion
4.1. Coordinated changes in JH biosynthesis and JH hemolymph titers in male mosquitoes
JH titer is regulated by the balance between biosynthesis and release of the hormone from the CA, and its degradation and clearance from the hemolymph by tissue uptake and excretion (Feyereisen, 1985; Goodman and Couson, 2012). Numerous studies indicated that JH biosynthesis is a major regulator of JH titer. It is also widely accepted that JH is not stored in the CA and therefore, the amount of JH “released” to the incubation medium or hemolymph represents the amount of JH synthesized (Feyereisen, 1985). JH biosynthesis and titer has been comprehensively studied in female Ae. aegypti (reviewed in Zhu and Noriega, 2016). These previous studies underscored the dynamic character of circulating JH titers, which are tightly linked to the reproductive physiology of the female mosquito. During a gonotrophic cycle, JH hemolymph levels fluctuated sharply between females of different conditions. At adult eclosion JH hemolymph was 20 fmol/female, 1-day old insects fed a 3% sugar diet had 100 fmol/female and 0.5 fmol/female was found in blood fed individuals (Hernandez-Martinez et al., 2015). In male mosquitoes, overall synthetic rates and titers were significantly lower than those observed in females, however the concentration of JH as seen by target cells might be comparable, as males are smaller in size and hemolymph volumes are reduced when compared with females.
JH synthesis and hemolymphatic titers were low in male pupae and increased significantly during the first 2–3 days of adult life. These initial post-eclosion increases were similar in 3% sugar-fed and starved males, suggesting that teneral reserves were sufficient to stimulate CA activity. The amount of teneral reserves accurately reflects the body size of eclosing male mosquitoes (Briegel, 1990). The male mosquitoes used in our studies were raised as larvae in optimal conditions, and emerged with an average wing length of 2.42 mm. The high teneral reserves masked any potential differences on JH synthesis and titers due to sugar concentrations in the first days after adult eclosion. It is only after 3 days that starvation starts decreasing JH synthesis and titer. Our laboratory colony is raised on a 3% sugar diet. This sugar concentration is enough to allow females to complete successfully their gonotrophic cycle after getting a blood meal, as well as enough to allow males to normally inseminate females. Females raised on a high sugar diet (20%) synthesize more JH that females fed a low sugar diet (3%) (Hernandez-Martinez et al., 2015). Feeding high sugar diets (20%) results in a very significant increase in JH titers in both virgin and mated females (2–3-fold) (Hernandez-Martinez et al., 2015), therefore the effects of a 20% sugar meal on male JH synthesis and hemolymph titers were also explored.
Although Ae. aegypti males are smaller than females, total lipids are 2–4 times higher in males than in females of equal size (Briegel, 1990). Adult females will consume most of these lipid reserves during the first 2 days to mature their previtellogenic ovaries, and JH titers play a key role controlling this nutrient allocation (Noriega, 2014). Male mosquitoes, in contrast, will use these teneral lipid reserves over the course of several days to flight, find females and mate.
After this initial post-emergence increase, JH synthesis and titers profiles in virgin males were less variable than those observed in females, remaining quite constant for at least 6–7 days after adult eclosion. The addition of farnesoic acid resulted in a 6 fold induction of JH synthesis by CA dissected from 3% sugar fed virgin males. The rate of JH synthesis from CA stimulated with the precursor was markedly higher that the spontaneous rate, suggesting that the last 2 enzymes in the JH pathway (juvenile hormone acid methyl transferase and epoxidase) are “not limiting”, and as described in the female, the spontaneous biosynthetic rates are lower because of a reduced supply of precursors (Nouzova et al., 2011; 2015).
4.2. JH hemolymph titers and reproductive biology in adult male mosquitoes
Male mosquito reproductive maturation involves the development of secondary reproductive characters, accessory gland components and sperm, as well as changes in sexual behavior (Oliva et al., 2014). Any of these processes could be potentially regulated by hormones such as JH or 20-OH ecdysone, which play critical roles in female mosquito reproductive maturation (Zhu and Noriega, 2016).
Sperm maturation starts during larval life in Ae. aegypti, with the testes growing in size, and germ cells greatly increasing in numbers around the time of each larval ecdysis (Jones, 1967). Fully differentiated spermatozoa are never observed in larvae and differentiation of spermatids begins shortly before pupation (Jones 1967). Maturation of spermatocysts is preeminently a pupal event. Sperm is never observed in the sperm ducts during the pupal stage in Ae. aegypti (Jones, 1967). In pharate adults there are around 6500 spermatozoa within each testis. Sperm begin to fill the post gonadal system shortly after adult emerge, this process is not completed until the second day of adult life (Jones, 1967). There is no experimental evidence that any of the processes involved in sperm maturation could be under JH control.
After adult eclosion, male mosquitoes must complete the maturation of two secondary reproductive characters before becoming sexually active. During the first day after emergence of Aedes males, the fibrillar hairs of the antennae become fully erect (Roth, 1948; Foster and Lea, 1975; Nijhout, 1977). The erection of the fibrillae are critical to respond to female flight sounds during mating (Roth, 1948). In addition, males undergo a permanent 180° rotation of the terminalia part of the genitalia during the first 24 hours of adult life. As a result of this rotation the claspers become oriented ventrally and the aedaegus lies above the anus. These modifications are critical for proper mating. Sub lethal doses of methoprene applied in the pupal stage slow this rotation and prevents successful mating with females (O'Donnell and Klowden, 1997); although this is a classical morphogenetic effects of JHA at the onset of metamorphosis, when JH is cleared from the pupae (Wilson, 2005), and treatment with methoprene after adult emergence, decapitation, or ventral nerve cord transection had no significant effect on postemergence terminalia rotation stage (O'Donnell and Klowden, 1997). Likewise, there is no experimental evidence that any of the processes involved in the maturation of secondary reproductive structures in the adult could be under JH control.
On the other hand, there are several reports in insects stating that JH controls the secretory processes of the male accessory glands (MAG). JH stimulate the synthesis of accessory gland proteins in Drosophila (Herndon et al., 1997). Juvenile hormone analogue (JHA) treatment resulted in an increase in size of MAG, as well as an increase in total RNA and protein content of MAG in the red flour beetle, Tribolium castaneum (Parthasarathy et al., 2009). JHA treatment also increased the expression of accessory protein (Acp) genes in the beetle MAG. RNAi-mediated knock-down in the expression of juvenile hormone acid methyl transferase gene (JHAMT, a critical JH biosynthetic enzyme) decreased the size of MAGs and expression of Acps. JH deficiency influenced beetle male reproductive fitness as evidenced by less vigour in mating behaviour, poor sperm transfer and low egg production by females mated with JH deficient males (Parthasarathy et al., 2009). In Ae. aegypti, topical application of JHA also induced precocious and enhanced MAG secretions, as well as insemination of a larger number of females (Ramalingam and Craig, 1977). These data suggest a critical role for JH in the regulation of mosquito male reproduction especially through MAG secretions.
4.3. Effect of nutrients and mating on JH hemolymph titers and insemination rates in male mosquitoes
Male Aedes are polygynous (they mate with many females), a condition favored by their abundant supply of sperm, rapid copulation, and the high probability of encountering numerous females in high concentrations at feeding, resting, or oviposition sites (Thornhill and Alcock, 2001).
Mating is an energetically costly behavior in mosquitoes. The metabolic expenditure of mating in Anopheles gambiae was around 50% of the male’s sugar and glycogen reserves (Maiga et al., 2014). The energy budget for male mating behavior in Aedes albopictus is also elevated, as it includes the costs of finding a mate, courtship, copulation, semen transfer, and male–male competition (Oliva et al., 2012).
Several studies reported good correlations in male mosquitoes between 1) nutritional status (sugar feeding access) and insemination rates, 2) MAG contents and insemination rates, 3) Nutrients and MAG contents, and 4) JH and MAG contents. Sugar feeding increases MAG contents and insemination rates, while frequent mating decreases MAG contents and insemination rates. Administration of JH has similar positive effects on MAG contents and insemination rates, while sugar feeding results in an increase of JH synthesis (Gary et al., 2009; Stone et al., 2009; Bellini et al., 2014; Lees et al, 2014; Jones 1967; Helinksy and Harrington, 2011; Chadee et al., 2014; Ramalingam and Craig, 1977).
Limited access to sugar feeding negatively correlates with mating success, and a frequent acquisition of sugar meals is significantly associated with reproductive output in male mosquitoes (Gary et al., 2009; Stone et al., 2009). Sugar meals increase survival and mating performance of Ae. albopictus males (Bellini et al., 2014), and are associated with greater insemination rates in Ae. aegypti (Lees et al, 2014). After inseminating 6 females, Ae. aegypti males quickly use up nearly all of the sperm and seminal fluid stored. When such males are isolated from females, they replenish the sperm in their reproductive system in two to three days (Jones 1967). Small males experience more rapid seminal depletion than large males (Helinksy and Harrington, 2011). Laboratory studies on the effects of sugar feeding on the insemination rates of Ae. aegypti females showed similar inseminations rates among sugar and water fed males, but after 4 days all water fed males died while the sugar fed males continued to survive and inseminate females (Chadee et al., 2014). Males whose accessory glands were depleted due to multiple mating, and were later treated with JHA also displayed higher inseminating capacity (Ramalingam and Craig, 1977).
In our studies, starvation decreased both JH titers and insemination rates; while refeeding high sugar diets reactivated JH synthesis and restored insemination rates to normal high levels. It is difficult to establish a cause-effect relationship between both events. Was the increase in JH responsible for the increase of insemination rates? The starved mosquitoes that received a new sugar meal also replenished their energetic reserves, which provided fuel to start inseminating females again. In summary, sugar level affected both JH and insemination, but these could be independent effects.
Aedes males produce in their single pair of male accessory glands (MAGs) a complex mixture of seminal substances that is transferred to the female during mating. Secretions of the reproductive glands of male Ae. aegypti have previously been shown to induce post-mating changes in female reproductive and feeding behavior (Sirot et al., 2014). Mating enhances egg development (Klowden and Chambers, 1991), and part of this effect is mediated through the transfer of JH III during copulation (Clifton et al., 2014).
In our studies, mating did not modify JH synthesis rates in males raised in any of the different sugar diets, but surprisingly hemolymph JH levels were different in well-nourished virgin and mated males. We have previously reported that mating decreases the amount of JH III in the male accessory gland and increases the amount of JH III found in the bursa copulatrix-spermathecae complex of female mosquitoes (Clifton et al, 2014). We do not know if JH is synthetized in the MAG or is taken up from the hemolymph.
The male nutritional status is also an important determinant of reproductive output. Starved males had less protein in their accessory glands, transferred less protein to females during copulation and exerted a smaller influence on female host-seeking behavior (Fernandez and Briegel, 1995). Male mosquito fed 20% sucrose had more JH III in the MAG, and transferred more JH III to females during mating (Clifton et al, 2014). Females mated with well-nourished mosquitoes, resorbed fewer follicles, increased the lipid content of the ovary, and laid more eggs (Clifton et al., 2014). Why virgin well-nourished males had less JH in the hemolymph than mated insects, if the synthetic rates are similar. As previously discussed, two key determinants of JH titer are biosynthesis and tissue uptake. Lower hemolymph titers indicates that either MAG from virgin males are accumulating more JH, or the higher titers in the hemolymph of mated males are related with the process of replenishment of MAG contents after mating.
In summary, our results revealed tightly correlated changes in JH biosynthesis and JH hemolymph titers in male mosquitoes. Changes in the physiological state of the mosquitoes caused by sugar-feeding and mating modified JH titers in hemolymph. Understanding in detail the complex interactions between modulatory factors that control JH biosynthesis and titer, such as diet and mating, is a topic of future research.
Supplementary Material
JH III levels were measured in the hemolymph of pupae and adult male mosquitoes.
There were coordinated changes in JH biosynthesis and JH hemolymph titers
Feeding high sugar diets resulted in an increase of JH titers.
Mating also modified JH titers in hemolymph.
Feeding high sugar diets resulted a significant rise in insemination rates.
Acknowledgments
We thank Mark Clifton and Sassan Asgari for critical reading of the manuscript. This work was supported by NIH Grant No AI 45545 to F.G.N.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bellini R, Puggioli A, Balestrino F, Brunelli P, Medici A, Urbanelli S, Carrieri M. Sugar administration to newly emerged Aedes albopictus males increases their survival probability and mating performance. Acta Trop. 2014;132:S116–123. doi: 10.1016/j.actatropica.2013.11.022. [DOI] [PubMed] [Google Scholar]
- Briegel H. Metabolic relationship between female body size, reserves, and fecundity of Aedes aegypti. J. Insect Physiol. 1990;36:165–172. [Google Scholar]
- Bourtzis K, Lees RS, Hendrichs J, Vreysen MJB. More than one rabbit out of the hat: Radiation, transgenic and symbiont-based approaches for sustainable management of mosquito and tsetse fly populations. Acta Tropica. 2016;157:115–130. doi: 10.1016/j.actatropica.2016.01.009. [DOI] [PubMed] [Google Scholar]
- Burt FJ, Rolph MS, Rulli NE, Mahalingam S, Heise MT. Chikungunya: a reemerging virus. Lancet. 2012;379:662–671. doi: 10.1016/S0140-6736(11)60281-X. [DOI] [PubMed] [Google Scholar]
- Chadee DD, Sutherland JM, Gilles JR. Diel sugar feeding and reproductive behaviours of Aedes aegypti mosquitoes in Trinidad: with implications for mass release of sterile mosquitoes. Acta Trop. 2014;132:S86–90. doi: 10.1016/j.actatropica.2013.09.019. [DOI] [PubMed] [Google Scholar]
- Chen R, Vasilakis N. Dengue - Quo tu et quo vadis? Viruses. 2011;3:1562–1608. doi: 10.3390/v3091562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton ME, Correa S, Rivera-Perez C, Nouzova M, Noriega FG. Male Aedes aegypti mosquitoes use JH III transferred during copulation to influence previtellogenic ovary physiology and affect the reproductive output of female mosquitoes. J Insect Physiol. 2014;64:40–47. doi: 10.1016/j.jinsphys.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho DO, McKemey AR, Garziera L, Lacroix R, Donnelly CA, Alphey A, Malavasi A, Capurro ML. Suppression of a Field Population of Aedes aegypti in Brazil by Sustained Release of Transgenic Male mosquitoes. PLOS Neglected Tropical Diseases. 2015 doi: 10.1371/journal.pntd.0003864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes L, Briegel H. Reproductive physiology of Anopheles gambiae. J Vector Ecol. 2005;30:11–26. [PubMed] [Google Scholar]
- Feyereisen R. Regulation of juvenile hormone titer: synthesis. In: Kerkut GA, Gilbert LI, editors. Comprehensive Insect Physiology Biochemistry and Pharmacology. Vol. 7. Oxford: Pergamon Press; 1985. pp. 391–430. [Google Scholar]
- Foster WA, Lea AO. Renewable fecundity of male Aedes aegypti following replenishment of seminal vesicles and accessory glands. J. Insect Physiol. 1975;21:1085–90. doi: 10.1016/0022-1910(75)90120-1. [DOI] [PubMed] [Google Scholar]
- Gary RE, Cannon JW, Foster WA. Effect of sugar on male Anopheles gambiae mating performance, as modified by temperature, space, and body size. Parasites Vectors. 2009;2:19. doi: 10.1186/1756-3305-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman WG, Cusson M. The Juvenile Hormones. In: Gilbert LI, editor. Insect Endocrinology. San Diego: Academic Press; 2012. pp. 310–365. [Google Scholar]
- Helinski ME, Harrington L. Male mating history and body size influence female fecundity and longevity of the dengue vector Aedes aegypti. J Med Entomol. 2011;48:202–211. doi: 10.1603/me10071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Martinez S, Lanz H, Rodriguez MH, Torres JA, Martinez-Palomo A, Tsutsumi V. Morphological and cytochemical characterization of female Anopheles albimanus (Diptera: Culicidae) hemocytes. J. Med. Entomol. 1999;36:426–434. doi: 10.1093/jmedent/36.4.426. [DOI] [PubMed] [Google Scholar]
- Hernandez-Martinez S, Rivera-Perez C, Nouzova M, Noriega FG. Coordinated changes in JH biosynthesis and JH hemolymph titers in Aedes aegypti mosquitoes. J. Insect Physiology. 2015;72:22–27. doi: 10.1016/j.jinsphys.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon LA, Chapman T, Kalb JM, Lewin S, Partridge L, Wolfner MF. Mating and hormonal triggers regulate accessory gland gene expression in male Drosophila. J. Insect Physiol. 1997;43:1117–1123. doi: 10.1016/s0022-1910(97)00062-0. [DOI] [PubMed] [Google Scholar]
- Hoffmann AA, Montgomery BL, Popovici J, Iturbe-Ormaetxe I, Johnson PH, Muzzi F, Greenfield M, Durkan M, Leong YS, Dong Y, Cook H, Axford J, Callahan AG, Kenny N, Omodei C, McGraw EA, Ryan PA, SARitchie SA, Turelli M, O’Neill SL. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 2011;476:454–457. doi: 10.1038/nature10356. [DOI] [PubMed] [Google Scholar]
- Jones JC. Spermatocysts in Aedes aegypti (Linnaeus) Biological Bulletin. 1967;132:23–33. doi: 10.2307/1539874. [DOI] [PubMed] [Google Scholar]
- Klowden MJ, Chambers GM. Male accessory gland substances activate egg development in nutritionally stressed Aedes aegypti mosquitoes. J. Insect Physiol. 1991;37:721–726. [Google Scholar]
- Klowden MJ. Endocrine aspects of mosquito reproduction. Arch. Insect Biochem. Physiol. 1997;35:491–512. [Google Scholar]
- Maïga H, Niang A, Sawadogo SP, Dabiré RK, Lees RS, Gilles JR, Tripet F, Diabaté A. Anopheles gambiae males mating success. Acta Tropica. 2014;132S:S102–S107. doi: 10.1016/j.actatropica.2013.08.018. [DOI] [PubMed] [Google Scholar]
- Nijhout FH. Control of antennal hair erection in male mosquitoes. Biol. Bull. 1977;153:591–603. [Google Scholar]
- Noriega FG. Juvenile hormone biosynthesis in insects: What is new, what do we know, what questions remain? ISRN. 2014 doi: 10.1155/2014/967361. doi.org/10.1155/2014/967361. [DOI] [PMC free article] [PubMed]
- Nouzova M, Edwards MJ, Mayoral JG, Noriega FG. A coordinated expression of biosynthetic enzymes controls the flux of juvenile hormone precursors in the corpora allata of mosquitoes. Insect Biochem. Mol. Biol. 2011;41:660–669. doi: 10.1016/j.ibmb.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouzova M, Rivera-Perez C, Noriega FG. Allatostatin-C reversibly blocks the transport of citrate out of the mitochondria and inhibits juvenile hormone synthesis in mosquitoes. Insect Biochem. Mol. Biol. 2015;57:20–26. doi: 10.1016/j.ibmb.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva CF, Damiens D, Vreysen MJB, Lempérière G, Gilles J. Reproductive strategies of Aedes albopictus (Diptera: Culicidae) and implications for the sterile insect technique. PLoS ONE. 2013;8:e78884. doi: 10.1371/journal.pone.0078884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva CF, Damiens D, Benedict MQ. Male reproductive biology of Aedes mosquitoes. Acta Tropica. 2014;132S:S12–S19. doi: 10.1016/j.actatropica.2013.11.021. [DOI] [PubMed] [Google Scholar]
- Parthasarathy R, Tan A, Sun Z, Chen Z, Rankin M, Palli SR. Juvenile hormone regulation of male accessory gland activity in the red flour beetle, Tribolium castaneum. Mech Dev. 2009;126:563–579. doi: 10.1016/j.mod.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalingam S, Craig GB. The effects of a JH mimic and cauterization of the corpus allatum complex on the male accessory glands of Aedes aegypti (Diptera: Culicidae) Canadian Entomol. 1977;109:897–906. [Google Scholar]
- Ramirez CE, Nouzova M, Benigni P, Quirke JM, Noriega FG, Fernandez-Lima F. Fast, ultra-trace detection of juvenile hormone III from mosquitoes using mass spectrometry. Talanta. 2016;159:371–8. doi: 10.1016/j.talanta.2016.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddiford LM. How does juvenile hormone control insect metamorphosis and reproduction? Gen. Comp. Endocrinol. 2012;179:477–484. doi: 10.1016/j.ygcen.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Rivera-Perez C, Nouzova M, Lamboglia I, Noriega FG. Metabolic analysis reveals changes in the mevalonate and juvenile hormone synthesis pathways linked to the mosquito reproductive physiology. Insect Biochem. Mol. Biol. 2014;51:1–9. doi: 10.1016/j.ibmb.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth LM. A study of mosquito behavior. An experimental laboratory study of the sexual behavior of Aedes aegypti (Linnaeus) American Midland Naturalist. 1948;40:265–352. [Google Scholar]
- Sirot LK, Wong A, Chapman T, Wolfner MF. Sexual conflict and seminal fluid proteins: a dynamic landscape of sexual interactions. Cold Spring Harb Perspect Biol 2015. 2014;7:a017533. doi: 10.1101/cshperspect.a017533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone CM, Taylor RM, Roitberg BD, Foster WA. Sugar deprivation reduces insemination of Anopheles gambiae (Diptera: Culicidae), despite daily recruitment of adults, and predicts decline in model populations. J. Med. Entomol. 2009;46:1327–1337. doi: 10.1603/033.046.0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobe SS, Stay B. Structure and regulation of the corpus allatum. Adv. Insect Physiol. 1985;18:305–431. [Google Scholar]
- Thornhill R, Alcock J. The Evolution of Insect Mating Systems. iUniverse.com,Inc.; Lincoln, USA: 2001. [Google Scholar]
- Zhu J, Noriega FG. The role of juvenile hormone in mosquito development and reproduction. Adv. Insect Physiol. Progress in Mosquito Research. Editor. Alex Raikhel. 2016;51:93–113. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.