Abstract
Chronic unresolvable stress leads to the development of depression and cardiovascular disease (CVD). There is a high prevalence of depression with the metabolic syndrome (MetS), however, to what extent the MetS concurrent with psychological stress affects cerebrovascular function is unknown. We investigated the differential effect of MetS on cerebrovascular structure/function in rats (16–17-wk-old) following 8 wk of unpredictable chronic mild stress (UCMS), and whether exercise training could limit any cerebrovascular dysfunction. In healthy lean rats (LZR), UCMS decreased (28%, p<0.05) ex-vivo middle cerebral artery (MCA) endothelium-dependent dilation (EDD), however, changes in MCA remodeling and stiffness were not evident but cerebral microvessel density decreased (MVD; 30%, p<0.05). The presence of UCMS and MetS (OZR; obese Zucker rats) decreased MCA EDD (35%, p<0.05), and dilation to sodium nitroprusside (20%, p<0.05), while MCA stiffness increased, and cerebral MVD decreased (31%, p<0.05), which were linked to a reduced nitric oxide and increased oxidative levels. Aerobic exercise prevented UCMS impairments in MCA function and MVD in LZR, and partly restored MCA function, stiffness and MVD in OZR. Our data suggests that the benefits of exercise with UCMS was due to a reduction in oxidative stress, and increased production of nitric oxide in the cerebral vessels. In conclusion, UCMS significantly impaired MCA structure and function, but the effects of UCMS were more substantial in OZR vs. LZR. Importantly, aerobic exercise when combined with UCMS prevented the MCA dysfunction through subtle shifts in nitric oxide and oxidative stress in the cerebral microvasculature.
Keywords: Depression, Exercise, Metabolic Syndrome, Stress, Vascular
Introduction
Over 350 million people worldwide suffer from depressive disorders, establishing depression as a leading cause of disease burden (Kessler et al., 1993; CDC.gov, 2010). Chronic exposure to stressful conditions and circumstances play a major role in the development of depression (Mah et al., 2016). Importantly, both stress and depression are linked to cardiovascular disease (CVD) (Musselman et al., 1998; Rottenberg et al., 2014), and clinical depression or depressive symptoms are an independent risk factor for stroke (Everson-Rose et al., 2014). The metabolic syndrome (MetS) affects ~20–25% of adults worldwide, and has a strong association with depression (Pratt & Brody, 2014; Kahl et al., 2015). However, the events linking psychological stress to accelerated CVD/stroke risk, especially in the presence of multiple CV risk factors such as MetS, are not fully clarified.
Chronic exposure to stress up-regulates pro-inflammatory cytokines, and reactive oxygen species (ROS) (Kemeny & Schedlowski, 2007; Miller et al., 2008), which are thought to contribute to depression associated CVD (Goshen et al., 2008). Indeed, patients suffering from depressive episodes, with no prior history of CVD, exhibit impaired endothelial-dependent dilation (EDD) (Chrapko et al., 2004; Chen et al., 2013). We have previously shown decreased aortic EDD in healthy mice after 8 weeks of unpredictable chronic mild stress (UCMS) (Stanley et al., 2014), a reliable and sensitive model for studying depressive disorders in rodents (Willner, 2005). In addition, the normal development of MetS is characterized by chronic low-grade inflammation and ROS that negatively affects multiple vascular beds, including in the brain (Phillips et al., 2005; Brooks et al., 2015). Thus, there is a general agreement that the endothelium plays a key role in the adverse effects of stress and MetS on the CV system. However, to what extent the cerebral vasculature is affected by psychological stress is not fully known, especially during the simultaneous development of MetS and chronic stress. Thus, the first aim of the study was to determine the impact of concurrent MetS and UCMS on cerebrovascular function and structure.
Regular exercise is an effective therapy for improving depressive symptoms and preventing recurrent depressive episodes, and the pathological changes associated with MetS (Frisbee et al., 2006; Donley et al., 2014). In the vasculature, exercise has anti-oxidant benefits, and is known to improve EDD (Frisbee et al., 2006). Thus, the second aim of this study was to identify the prophylactic benefits of exercise training on cerebrovascular function in MetS undergoing UCMS. We hypothesize: 1) UCMS impairs cerebrovascular EDD, with a worse response in the obese zucker (OZR) vs. lean zucker (LZR) rat; and 2) that exercise training prevents any further decrease in cerebrovascular EDD during the combination of MetS and UCMS.
MATERIALS AND METHODS
Ethical Approval
Zucker rats from Envigo Laboratories were used to conduct the experiments reported in this manuscript on an approved protocol by the West Virginia University Health Science Center (WVUHSC) Animal Care and Use Committee, which meets the NIH guidelines for care and use of laboratory animals and complies with the animal use ethics checklist set forth by the Journal of Experimental Physiology.
Animals
Male lean (LZR) and obese (OZR) Zucker rats were housed in the animal care facility at the WVU HSC and fed standard chow/drinking water ab libitum. Animals undergoing exercise training and/or chronic stress treatment were handled and inspected daily, with regular supervision of the veterinary support staff. At 8–9 weeks of age, LZR and OZR were divided (8 per strain per group; total of 64 rats) into 4 groups: 1) age-matched controls; 2) UCMS; 3) treadmill exercise (Ex); or 4) UCMS with treadmill exercise (UCMS+Ex).
1) Control group
All control animals were singly housed and remained in their home cages throughout the 8-week period. Animals were exposed to daily handling to normalize for the interaction with the staff.
2) UCMS Protocol
Previous investigators developed the UCMS model to induce depression-like behaviors in rodents (Mineur et al., 2003). The UCMS model is considered to be the most appropriate rodent model for human clinical depression, based on its ability to reproduce the development of many human clinical depressive symptoms, including anhedonia and learned helplessness (Willner, 2005).
All rats were singly housed. In UCMS groups, rats were exposed to the following mild environmental stressors in randomly chosen sequences for 8 hours each day, 5 days/week, over the course of 8 weeks:
Damp bedding – 10 oz. of water was added to each standard cage
Bath – all bedding was removed and ~0.5 inches of water was added to empty cage. Water temperature was room temperature, ~24°C
Cage Tilt - cage was tilted to 45 degrees without bedding
Social stress – each rat was switched into a cage of a neighboring rat
No bedding
Alteration of light/dark cycles – turning lights off/on in random increments for scheduled period.
3) Exercise Training group
LZR and OZR underwent 8 weeks of treadmill running. Animals ran 5 days/week on a motor driven treadmill set at a 5% grade. During the first week, animals were acclimatized to the treadmill by running for 20 minutes, then increasing by 10 minutes/day until sustainable duration of 60 minutes daily was achieved. A maximum speed test was performed on each animal and target running speed was set for 60–70% of that maximum, which represented 10–12 m/min and 18–20 m/min for OZR and LZR at a 5% incline, respectively. After the acclimatization week, the first 15 minutes of the total 60 minutes consisted of a gradual increase until reaching target running speed. Rats ran at this speed for the remaining 45 minutes. There was a 48-hour wash out period between the last Ex bout and the terminal surgery at the end of 8-week treatment.
4) UCMS+Exercise group
Following the above protocols for UCMS and exercise training, LZR and OZR underwent 8 weeks of treadmill running concurrently with UCMS. Of note, on each day exercise training was performed first followed by the UCMS protocol.
Coat Status
The condition of the animal’s coat was evaluated throughout the duration of the study. Each week, rats were weighed and inspected for grooming habits. The total cumulative score was computed by giving an individual score of 0 (clean) or 1 (dirty) to eight body parts (head, neck, back, stomach, tail, forelimbs, hindlimbs, genitals). Within LZR and OZR groups, the individual assessing the animal’s coat score was blinded to the group allocation.
Collection of Middle Cerebral Arteries
After the 8-week treatment period, rats were deeply anesthetized by IP injection of sodium pentobarbital (50 mg·kg-1 i.p.) and a carotid artery was cannulated for determination of mean arterial pressure. Animals were then euthanized via severing of the diaphragm and subsequent removal of aorta and skull. The brain was removed from the skull and placed in cold physiological salt solution (PSS; 4ºC). Both middle cerebral arteries (MCA), which supplies ≈50% of the cerebral blood flow (Harper et al., 1984), were dissected from their origin at the Circle of Willis and placed into an isolated microvessel chamber filled with PSS. Each MCA was subsequently doubly cannulated within a heated chamber (37°C) that allowed the lumen and exterior of the vessel to be perfused and superfused, respectively, with PSS from separate reservoirs. The PSS was equilibrated with a 21% O2, 5% CO2, and 74% N2 gas mixture and had the following composition (mM): 119 NaCl, 4.7 KCl, 1.17 MgSO4, 1.6 CaCl2, 1.18 NaH2PO4, 24 NaHCO3, 0.026 EDTA, and 5.5 glucose. Any side branches were ligated using a single strand teased from 6-0 suture. MCA diameter was measured using television microscopy and an on-screen video micrometer.
Measurements of Vascular Reactivity in Isolated MCA
Following cannulation, MCAs were extended to their in-situ length and were equilibrated at 80% of the animal’s mean arterial pressure to approximate in vivo perfusion pressure. Any vessel that did not demonstrate significant active tone (>25% for all groups) at the equilibration pressure was discarded. Active tone at the equilibration pressure was calculated as (ΔD/Dmax) × 100, where ΔD is the diameter increase from rest in response to Ca2+-free PSS, and Dmax is the maximum diameter measured at the equilibration pressure in Ca2+-free PSS.
Following equilibration, the MCA dilator reactivity was assessed in response to increasing concentrations of an endothelial dependent dilator (acetylcholine; ACh, 10−9M – 10−5M), endothelial independent dilator (sodium nitroprusside; SNP 10−9M – 10−5M), and a potent cerebrovascular constrictor (serotonin; 5-hydroxytryptamine, 5-HT 10−10M – 10−7M). MCA responses to ACh were also measured following acute incubation (45–60 minutes) with L-NAME (10−4M) and TEMPOL (10−4M), to assess the contributions of NO and oxidative stress, respectively, in modulating MCA reactivity.
Following completion of all procedures, the perfusate and superfusate were replaced with Ca2+-free PSS and the passive diameter of the fully relaxed MCA was determined over an intralumenal pressure range of 5–120 mmHg at 20-mmHg pressure increments. After 7 min at each intraluminal pressure, the inner and outer diameters of the passive MCA were determined. Media thickness, lumen and outer diameters, and vessel cross-sectional areas (used as indicators of structural alterations to the individual microvessel) were determined as follows: Media thickness (WT, μm) = Outer diameter – lumen diameter (i.e., OD – LD); media-to-lumen (M:L) ratio = MT/LD; Cross-sectional area (μm2) = Outer vessel area – lumen area.
Determination of Cerebral Microvessel Density
Cerebral microvessel and capillary density was evaluated by fluorescence immunohistochemistry using purified rat anti-mouse CD31 antibody (BD Biosciences, San Diego, CA; endothelial marker). Briefly, brain sections were fixed with 10% formalin, permeabilized with 0.2% Triton X-100, and blocked with blocking solution (5% BSA in PBS) and incubated with anti-CD31 (1:100) overnight in a humidified chamber at 4°C. Following three washes with PBS, sections were incubated with AlexaFluor 555-conjugated anti-mouse IgG (1:1000; Invitrogen) and AlexaFluor 488-conjugated anti-rabbit IgG (1:1000; Invitrogen) for 2h at room temperature in a humidified chamber. After a final wash with PBS, cover glass was mounted with hard-set DAPI medium (Vector Laboratories, Burlingame, CA). Negative control was performed with the same procedure without primary antibody. Images were obtained with an epifluorescence microscope (EVOS, ThermoFisher Scientific) using a 40x objective. Semiquantitative microvessel density analysis was performed by counting the number of positive CD31+ cells intersecting the horizontal and vertical lines of a 45 μm × 45 μm grid using ImageJ. A total of 6 regions of interest (3 in cortex and 3 in striatum) in the MCA territory were evaluated and mean values from each animal were used for further analysis as described previously (Zechariah et al., 2013).
Measurement of Vascular NO Bioavailability
Cerebral NO production was measured by fluorescence microscopy with the NO-specific dye 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FM-DA, Invitrogen) according to manufacturer’s instructions (Rathel et al., 2003). The posterior cerebral arteries (PCA) were removed from the brain and washed in cooled PSS. The PCAs were then bathed in 200μl of HEPES buffer containing 5μM DAF-FM and 100μM L-Arginine, in a 96 well plate. The PCAs were incubated at 37°C for 30 minutes to load the cells with the DAF-FM. PCAs were then washed in HEPES buffer for 15 minutes to clear excess DAF-FM. Following the wash, vessels were placed in a fresh well containing 5μM DAF-FM and 100μM L-arginine and stimulated with Methacholine (10−6 M) for 5 minutes at 37°C. PCAs were then immediately washed in HEPES, placed in optimal cutting temperature (O.C.T) media (Fisher Scientific, Pittsburgh, PA) and frozen in liquid nitrogen–cooled isopentane and stored at −80°C. DAF-FM blocks were then cut into 8μm slices using a cryostat at −22°C and transferred to charged slides (Fisherbrand® Superfrost® plus microscope slides) and stained/mounted with DAPI mounting media. Slides were imaged with an EVOS fluorescent microscope, 3 sections per vessel-treatment, and analyzed in ImageJ (NIH) as fluorescent density per area (DAF-FM), the mean of the 3 images were used as the mean for each animal. However, due to technical issues DAF-FM was not performed on the Ex groups.
Dihydroethidium Staining for ROS
Dihydroethidium (DHE) staining was performed on unfixed vessel sections to evaluate in situ ROS in cerebral arteries (Katakam et al., 2012). The basilar artery was removed from the brain and placed in PSS to clean and cut into 3 sections. Sections were placed in individual wells of a 96 well plate-containing 200μl of HEPES buffer with the following treatments. Sections were incubated for 30 minutes at 37°C with the respective treatment. Then 2μl of stock DHE solution was added to each well to a concentration of 10μM and incubated at 37°C for another 30 minutes in a dark humid chamber. Following completion of DHE incubation, vessel sections were washed in HEPES buffer, placed in O.C.T media (Fisher Scientific, Pittsburgh, PA) and frozen in liquid nitrogen–cooled isopentane and stored at −80°C. DHE O.C.T blocks were then cut into 8μm slices using a cryostat at −22°C and transferred to charged slides (Fisherbrand® Superfrost® plus microscope slides) and stained/mounted with DAPI mounting media. Slides were imaged with an EVOS fluorescent microscope, 3 sections/vessel-treatment, and analyzed (ImageJ, NIH) as fluorescent density/nucleus, the mean of the 3 images were used for each animal.
Plasma Clinical Markers
Fasting blood was drawn intravenously from anesthetized rats into lithium-heparin coated blood tubes and transported immediately to the laboratory for analysis. Levels of triglycerides (TG), total cholesterol (TC), high-density lipoprotein (HDL) were measured by the clinical laboratory service at Ruby Memorial Hospital (Morgantown, WV). Blood glucose was measured using a commercially available glucometer (FreeStyle, Abbott), and insulin was measured using a rat ELISA kit (Cayman Chemical, item #589501).
Circulating Cortisol
Corticosterone is a glucocorticoid produced by the adrenal cortex in response to ACTH (corticotropic hormone) and is the precursor to aldosterone. This is the main glucocorticoid in rodents as cortisol is in humans. The production of glucocorticoids is increased by stress. Using a commercially available ELISA Kit (Cayman Chemical, Item #501320) serum corticosterone levels were measured in duplicate accordingly to the manufacturer’s instructions
Data and Statistical Analyses
All calculations of passive arteriolar wall mechanics (used as indicators of structural alterations to the individual microvessel) are based on those used previously (Baumbach & Hajdu, 1993), with minor modification. Vessel wall thickness was calculated as:
where WT represents wall thickness (μm) and OD and ID represent arteriolar outer and inner diameter, respectively (μm).
For the calculation of circumferential stress, intraluminal pressure (PIL) was converted from mmHg to N/m2, where 1 mmHg=1.334×102 N/m2. Circumferential stress (σ) was then calculated as:
Circumferential strain (ε) was calculated as:
where ID5 represents the internal arteriolar diameter at the lowest intralumenal pressure (i.e., 5 mmHg). The stress versus strain relationship from each vessel was fit (ordinary least squares analyses, r2>0.85) with the following exponential equation:
where σ5 represents circumferential stress at ID5 and β is the slope coefficient describing arterial stiffness. Higher levels of β are indicative of increasing arterial stiffness (i.e., requiring a greater degree of distending pressure to achieve a given level of wall deformation).
All data are presented as mean±SD. Normality was evaluated by the Kolmogorov–Smirnov test. The vascular reactivity in the MCA was analyzed by repeated-measures two-way analysis of variance (ANOVA), with a Tukey post-hoc test to determine differences between doses of ACh, 5-HT, or SNP. Differences in passive mechanical characteristics, MVD and descriptive characteristics between LZR and OZR groups were assessed using a multifactorial analysis of variance (ANOVA) [i.e., strain (i.e., LZR vs. OZR), and experimental condition (i.e., Control, UCMS, Ex, and UCMS+Ex)] with an interaction term (strain-by-group), and a Tukey post-hoc test was performed to determine differences between groups, as appropriate. DHE, and DAF-FM were examined using Kruskal Wallis test, within group comparisons were examined using the Mann-Whitney test. The β–slope of the stress-strain relationships between groups were compared with a one-way ANOVA with Tukey post-hoc test, as appropriate. In all cases, p≤0.05 was taken to reflect statistical significance.
Results
Table 1 presents the characteristics of the animal groups. At the completion of the study, a small decrease in body mass was evident in all experimental groups compared to controls, with no significant differences between UCMS, Ex, or UCMS+Ex groups. No differences were noted between experimental groups (within LZR and OZR) for MAP, and TG. A significant group by strain interaction was identified for TC, indicating that the response of TC differed between LZR and OZRs (higher in OZRs) and between intervention groups (lower with exercise in OZRs). Glucose and insulin levels were higher (27% and 75%, p<0.05) in the LZR UCMS vs. LZR controls, respectively; whereas, Ex and UCMS+Ex did not significantly affect glucose and insulin levels (Table 1). In OZR UCMS, both glucose and insulin levels were higher (25% and 18%, p<0.05) vs. OZR control. The OZR Ex group had a lower glucose and insulin levels vs. OZR control (p<0.05), whereas UCMS+Ex prevented the increase in glucose and insulin levels noted in the OZR UCMS group (Table 1).
Table 1.
Clinical Characteristics of Animal Groups
| LZR | OZR | |||||||
|---|---|---|---|---|---|---|---|---|
| Con | UCMS | Ex | UCMS+Ex | Con | UCMS | Ex | UCMS+Ex | |
| BM, g | 400±20 | 361±12* | 358±15* | 343±12* | 641±29 | 585±20* | 599±27* | 567±25* |
| MAP, mmHg | 112±5 | 122±7 | 120±7 | 120±7 | 139±10 | 142±10 | 140±7 | 132±7 |
| Glucose, mg/dl | 98±15 | 124±17* | 101±20^ | 115±15 | 184±29 | 230±34* | 154±29*^ | 219±37*+ |
| Insulin plasma, ng/ml | 1.2±0.5 | 2.1±1.0 | 1.4±0.7 | 1.8±1.0 | 6.6±2.7 | 7.8±3.7* | 4.6±2.4* | 6.2±2.4 |
| TG, mg/dl | 25±7 | 54±39 | 31±7 | 36±7 | 124±20 | 114±34 | 82±37 | 103±39 |
| TC, mg/dl† | 86±10 | 87±12 | 95±15 | 88±5 | 256±64 | 213±42 | 181±20* | 187±27* |
| Corticosterone, ng/ml | 6.97±0.4 | 8.78±1.3* | 9.44±2.6 | 10.67±3.6* | 13.82±1.4 | 17.30±3.6* | 10.60±2.4* | 15.78±3.6+ |
| Coat scores, AU | 0.8±0.5 | 1.8±0.5* | 0.9±0.5 | 2.0±0.7* | 2.7 ± 0.5 | 5.1 ± 0.5* | 3.4 ± 0.7 | 4.8 ± 0.5*+ |
MAP, mean arterial pressure; TG, triglycerides, TC, total cholesterol.
p<0.05 vs. con group within the same strain (i.e., LZR or OZR);
p<0.05 vs. UCMS group within strain;
p<0.05 vs. Ex group;
p<0.05 signifies group by strain interaction, i.e., a significant group-by-strain interaction was identified for TC, indicating that the response of TC differed between LZR and OZRs (higher in OZRs) and between intervention groups (lower with exercise in OZRs). Data expressed as means ± SD. N=6–8/group
Serum levels of corticosterone were significantly increased in the UCMS, Ex, and UCMS+Ex groups compared to LZR and OZR controls (Table 1). Similarly, throughout the 8 week UCMS protocol, the coat status in rats undergoing UCMS was consistently poorer compared with control animals. However, an increase in coat score was also noted in the LZR UCMS+Ex group, OZR Ex, and the UCMS+Ex groups compared to their respective controls (Table 1). We also measured adrenal weights and noted that LZR and OZR UCMS groups had a significant (p<0.05) increase in adrenal weights compared to LZR (16.8±1.2 vs. 25.2±2.9 g) and OZR (27.2±2.2 vs. 32.7±3.9 g) controls. Unfortunately, the adrenal weights were not collected for the Ex groups; however, in the LZR UCMS+Ex group, the adrenal weights did not differ from LZR control weights (21.4±1.9vs. 16.8±1.2 g). In contrast, the adrenal weights in the OZR UCMS+Ex group remained elevated compared to the OZR control (32.0±2.9 vs. 27.2±2.2 g, p<0.05) and was similar to the OZR UCMS group (32.0±2.9 vs. 32.7±3.9 g). These data support the corticosterone and coat scores data suggesting that UCMS induced significant stress.
The average work performed during the treadmill running was slightly lower (p<0.05) in the LZR UCMS+Ex (330±49 J) vs. LZR Ex (402±39 J), and slightly higher (p<0.05) in the OZR UCMS+Ex (502±56 J) vs. OZR UCMS (421±49 J).
MCA Reactivity
Endothelial-Dependent Dilation
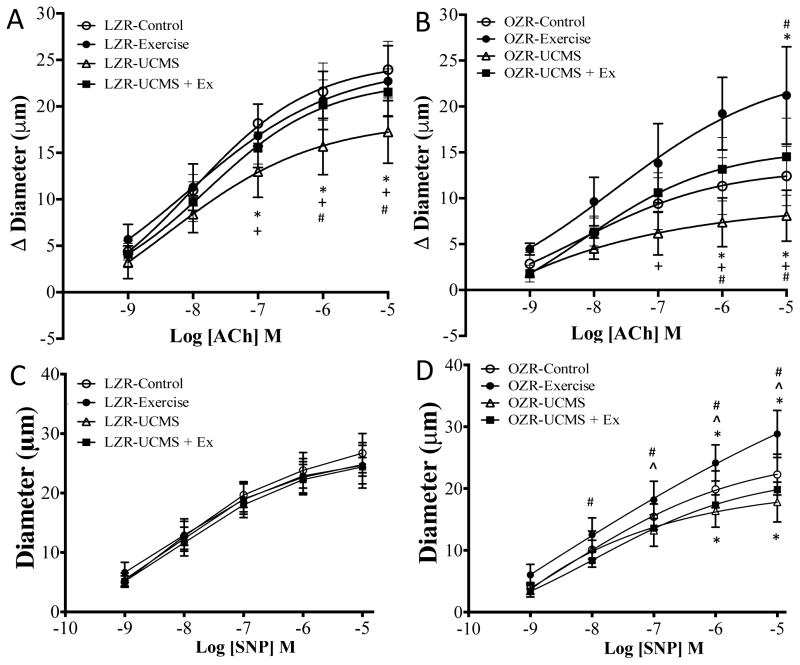
The dilator responses of the MCA in response to increasing concentrations of ACh are summarized in Fig 1. UCMS reduced (28%) maximum MCA EDD (ACh 10−5 M) in LZR compared to LZR controls (Fig 1.) Acutely incubation of the MCA with TEMPOL rescued most of the vasodilation to ACh in LZR UCMS, while incubation with L-NAME eliminated ~73% of vasodilation (p<0.05) (Table 2). The MCA dilator response to ACh did not differ between LZR control and Ex groups (Fig 1A). In contrast, when Ex was combined with UCMS, the LZR MCA dilator response (10−5 M) was 31% higher (p<0.05) compared to LZR UCMS, and similar to LZR controls (Fig 1A). As before, acute incubation with L-NAME blunted most of the dilatory response to ACh (p<0.05), and that this response did not differ in Ex, or UCMS+Ex compared to LZR controls (Table 2). Similarly, TEMPOL incubation had no additional benefit on ACh dilation in LZR Ex, or LZR UCMS+Ex.
Figure 1.
The effects of stress and exercise on middle cerebral artery dilator capacity. Endothelial dependent and independent dilatory responses of the middle cerebral artery to increasing concentrations of acetylcholine (ACh) and sodium nitroprusside (SNP) from LZR (A and C) and OZR (B and D) under control conditions (open circles), and following 8 weeks of UCMS (open triangle), Ex (closed circle), or UCMS+Ex (closed square). Data represented as MEAN±SD. n=6–8/group. *p<0.05 vs. control rats within strain), ^p<0.05 vs. UCMS group (within strain), +p<0.05 vs. Ex group (within strain), #p<0.05 vs. UCMS+Ex group (within strain). Please see text for details.
Table 2.
The Change in Middle Cerebral Artery Maximal Dilation to Ach in the Presence of TEMPOL or L-NAME
| MCA Endothelial dependent dilation | |||
|---|---|---|---|
| No treatment (μm) | L-NAME (μm) | TEMPOL (μm) | |
| LZR Con | 24.0±2.9 | 6.0±2.9‡ | 27.5±5.9// |
| LZR UCMS | 17.3±3.7 | 5.7±2.9‡ | 21.6±3.9‡// |
| LZR Ex | 22.7±4.2 | 4.7±2.9‡ | 24.3±5.1// |
| LZR UCMS+Ex | 21.5±2.7 | 5.2±2.4‡ | 22.1±2.2// |
| OZR Con | 12.4±3.4 | 3.2±3.9‡ | 18.1±2.0‡// |
| OZR UCMS | 8.1±6.1 | −1.3±5.6‡ | 13.9±4.9‡// |
| OZR Ex | 22.4±6.1 | 11.0±3.2‡ | 26.6±3.7‡// |
| OZR UCMS+Ex | 15.8±3.7 | −1.4±9.6‡ | 16.8±2.7// |
MCA dilation to maximal dose of ACh (10−6M).
p<0.05 vs. no-treatment within group;
p<0.05 vs. L-NAME treatment within group.
MEANS ± SD; n=5–8/group
In OZR, the MCA dilator response to ACh was 35% lower (p<0.05) compared to OZR controls (Fig 1), and TEMPOL incubation restored MCA dilation to OZR control levels, while L-NAME incubation completely eliminated all MCA reactivity to ACh (p<0.05) (Table 2). In OZRs, Ex alone improved MCA dilation (10−5 M) 2-fold (p<0.05) vs. OZR controls. Further, the OZR UCMS+Ex group had a 95% higher (p<0.05) MCA dilation (10−5 M) compared to OZR UCMS, and a slightly higher (27%, p=NS) MCA dilation compared to OZR controls (Fig 3B). In OZR, acute incubation with L-NAME reduced MCA dilation to ACh (p<0.05), however a significant dilator response was still evident on OZR Ex suggesting other non-NO dependent pathways were upregulated (Table 2). In OZR UCMS+Ex, L-NAME completely removed the MCA dilation to ACh (p<0.05). TEMPOL incubation had little to no additional benefit on ACh dilation in OZR Ex, or OZR UCMS+Ex.
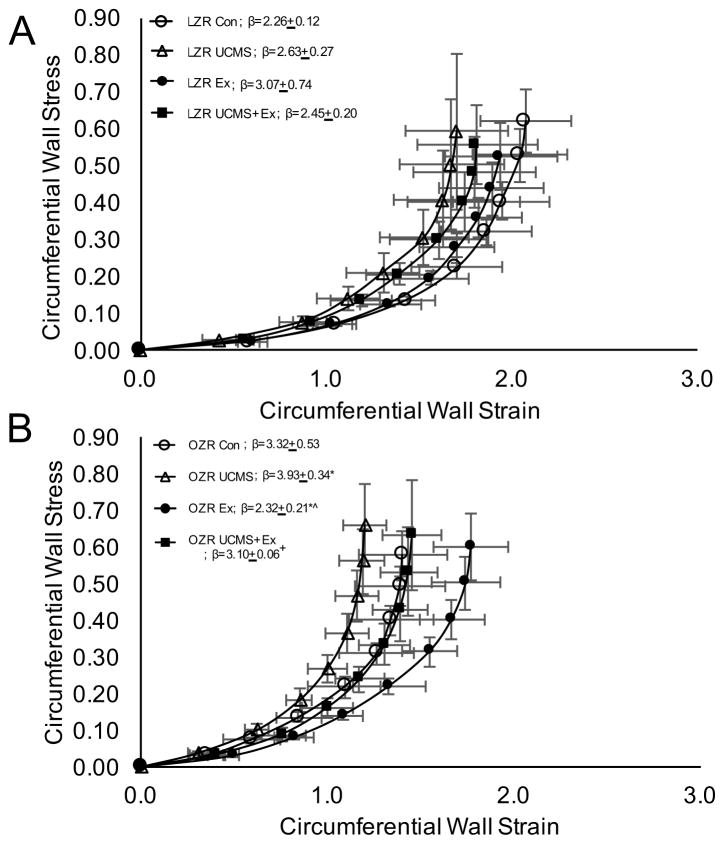
Figure 3.
The effects of stress and exercise on middle cerebral artery stiffness. The circumferential stress vs. strain relationship in the middle cerebral artery from LZR (A) and OZR (B) under control conditions (open circles), and following 8 weeks of UCMS (open triangle), Ex (closed circle), or UCMS+Ex (closed square). Data represented as MEAN±SD. n=6–8/group. The beta slope represents the steepness of the slope for the strain vs stress relationship. *p<0.05 vs. control (within strain), ^p<0.05 vs. UCMS group (within strain). +p<0.05 vs. Ex group (within strain). Please see text for details.
Endothelial-Independent Dilation
Figure 1C and D present the change in endothelial-independent change in MCA reactivity to SNP. In LZR, the MCA dilation to SNP was not affected by UCMS, Ex, or the combination of UCMS+Ex.
In OZR, a 20% lower (p<0.05) MCA dilation to SNP (10−5 M) was noted in UCMS vs. OZR controls (Fig 1). In addition, in OZR, Ex improved MCA dilation to SNP by 29%, vs. OZR controls (p<0.05), and UCMS+Ex prevented the reduction in MCA dilation to SNP in the OZR UCMS group (p<0.05) (Fig 1D).
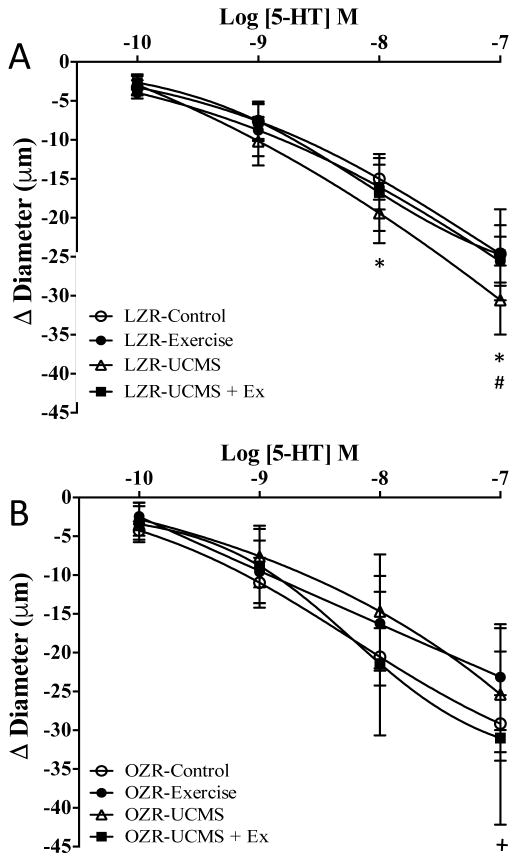
Constrictor Responses
The response of the MCA to 5-HT, a potent vasoconstrictor, is presented in Figure 2. In LZR, UCMS increased the MCA constriction to 5-HT by 24% (p<0.05) compared to LZR controls. Pretreatment of the MCA with L-NAME further increased the constriction response in healthy LZR controls, but this response was not evident in the LZR UCMS group (Table 3) suggesting that the UCMS group had reduced NO bioavailability. The MCA constriction response to 5-HT in LZR Ex was similar to that of LZR controls (Table 3); however, Ex prevented the increased MCA constrictor response to 5-HT in the LZR UCMS+Ex vs. LZR UCMS groups. Pre-incubating the MCA with either L-NAME or TEMPOL did not affect the MCA response to 5-HT in any LZR group.
Figure 2.
The effects of stress and exercise on middle cerebral artery constrictor response. Constrictor responses of the middle cerebral artery to increasing concentrations of serotonin (5-HT) from LZR (A) and OZR (B) under control conditions (open circles), and following 8 weeks of UCMS (open triangle), Ex (closed circle), or UCMS+Ex (closed square). Data represented as MEAN±SD. n=6–8/group. *p<0.05 vs. control LZR or OZR’s, ^p<0.05 vs. UCMS group (within strain), +p<0.05 vs. Ex group (within strain), #p<0.05 vs. UCMS+Ex group (within strain). Please see text for details.
Table 3.
The Change in Middle Cerebral Artery Maximal Constriction to 5-HT in the Presence of TEMPOL or L-NAME
| MCA Constriction | |||
|---|---|---|---|
| No treatment (μm) | L-NAME (μm) | TEMPOL (μm) | |
| LZR Con | −24.6±3.2 | −27.8±5.6 | −23.4±5.1 |
| LZR UCMS | −30.6±3.9 | −29.7±6.6 | −29.2±3.4 |
| LZR Ex | −25.6±2.7 | −28.8±5.4 | −22.9±4.7 |
| LZR UCMS+Ex | −24.7±5.1 | −25.7±7.3 | −25.4±6.4 |
| OZR Con | −29.2±3.2 | −28.7±5.4 | −27.8±2.4 |
| OZR UCMS | −25.4±7.3 | −28.9±4.7 | −22.5±4.2 |
| OZR Ex | −23.2±5.9 | −24.6±12.2 | −20.7±4.2 |
| OZR UCMS+Ex | −31.0±9.0 | −32.3±12.0 | −27.8±9.8 |
MCA reactivity to maximal dose of 5-HT (10−6M). MEANS ± SD; n=5–8/group
In OZR, the MCA constriction response was 16% lower (p<0.05) in UCMS vs. OZR controls (Fig 2B). In OZR controls and UCMS, pretreatment of the MCA with L-NAME or TMEPOL had no effect on the response to 5-HT (Table 3). Further, the MCA constrictor response to 5-HT was slightly, albeit not significantly lower in OZR Ex vs. OZR controls (Fig 2B). The MCA constrictor response was similar between UCMS+Ex vs. UCMS OZR groups (Fig 2B). The only significant difference was noted between Ex vs. UCMS+Ex groups, with a greater constriction response in the UCMS+Ex. Pre-incubating the OZR MCA with either L-NAME or TEMPOL did not affect the MCA response to 5-HT in either the UCMS, Ex, or UCMS+Ex groups.
MCA Wall Diameters, Stiffness, and Vessel Density
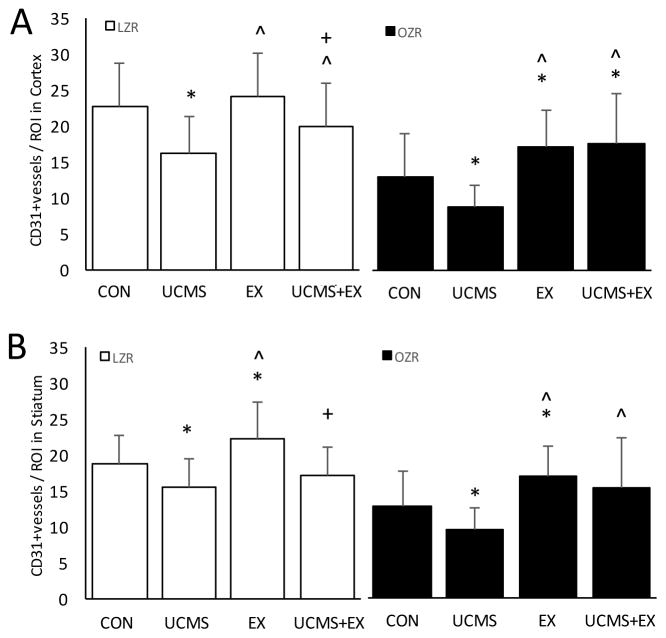
We next examined the extent to which UCMS affected the stiffness, and remodeling of the MCA, and MVD. In LZR, there were no significant differences in the MCA stress-strain relationship (or β-slope), diameters, and WT between LZR controls and UCMS (Table 4 and Fig 3A). However, MVD (cortex and striatum) was reduced (p<0.05) in LZR UCMS vs. LZR controls (Fig. 4). In LZR, MCA wall diameters, WT and stiffness did not differ between UCMS, Ex and UCMS+Ex groups (Table 4 and Fig 3A). Importantly, cortical MVD in the LZR was 25% higher (p<0.05) in the UCMS+Ex vs. UCMS group, however no differences in MVD were noted in the striatum (p=0.12). When compared to the Ex alone group, MVD (both cortex and striatum) was lower (p<0.05) in the LZR UCMS+Ex group (Fig. 4).
Table 4.
Middle Cerebral Artery Remodeling
| LZR | OZR | |||||||
|---|---|---|---|---|---|---|---|---|
| Con | UCMS | Ex | UCMS+Ex | Con | UCMS | Ex | UCMS+Ex | |
| ID, μm | 226±17 | 230±25 | 219±20 | 227±15 | 209±22 | 208±7 | 221±12 | 224±20 |
| OD, μm | 320±22 | 334±22 | 325±29 | 330±15 | 300±25 | 286±10 | 316±17^ | 315±17^ |
| WT, μm | 47±5 | 52±10 | 53±7 | 51±7 | 45±5 | 39±5* | 48±7 | 47±12 |
| W-L-R | 0.42±0.05 | 0.46±0.12 | 0.49±0.07 | 0.46±0.10 | 0.43±0.05 | 0.37±0.07 | 0.43±0.07 | 0.42±0.15 |
ID, inner MCA diameter; OD, outer MCA diameter; WT, wall thickness; W-L-R, wall to lumen ratio.
p<0.05 vs. con group (within strain, i.e., LZR or OZR);
p<0.05 vs. UCMS group (within strain).
MEAN ± SD; n=5–8/group
Figure 4.
The effects of stress and exercise on cerebral microvessel density. Cerebral microvessel density in the cortex (A) and striatum (B) were measured in LZR (open bars) and OZRs (closed bars) under either control, UCMS, Ex, or UCMS+Ex conditions. Data represented as MEAN±SD. n=5–6/group. *p<0.05 vs. control (within strain), ^p<0.05 vs. UCMS group (within strain). +p<0.05 vs. Ex group (within strain). Please see text for details.
In OZR, UCMS resulted in a slight decrease in MCA outer diameter, a reduced WT (p<0.05), and reduced wall to lumen ratio (p=0.08) compared to OZR controls (Table 4). Further, there was a significant (p<0.05) leftward shift in the stress-strain relationship and an increased β-slope, suggesting increased MCA stiffness in the OZR UCMS vs. OZR control group (Fig 3B). In addition, MVD (cortex and striatum) was reduced (p<0.05) in OZR UCMS vs. OZR controls (Fig. 4). In OZR, 8 weeks of Ex generally increased inner and outer MCA wall diameters compared to control and UCMS groups. This response was also evident in the UCMS+Ex group compared to OZR control and OZR UCMS (Table 4). Due to the similar general increase in inner and outer MCA wall diameters no differences were noted in WT or wall to lumen ratio in Ex or UCMS+Ex vs. OZR control, or OZR UCMS (Table 4). Importantly, Ex in the OZR group shifted the stress-strain relationship to the right (reduced stiffness) compared to OZR control with a reduced β-slope (p<0.05). Further, UCMS+Ex prevented the leftward shift in the stress-strain relationship noted in the OZR UCMS group (Fig 3B). Similar beneficial effects were noted with MVD. MVD (both cortex and striatum) was higher (p<0.05) in the OZR Ex vs. control group (Fig. 4). Further, the combination of UCMS+Ex resulted in a higher (p<0.05) MVD (both cortex and striatum) compared to the OZR UCMS group (Fig. 4). No differences were noted in MVD between OZR Ex and OZR UCMS+Ex groups in both cortex or striatum.
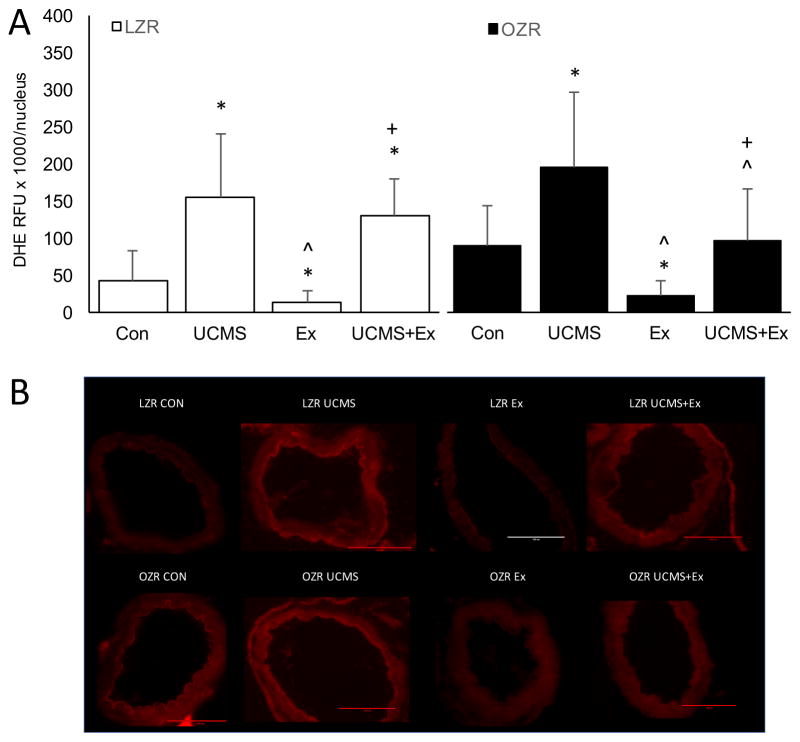
Oxidative Stress and NO
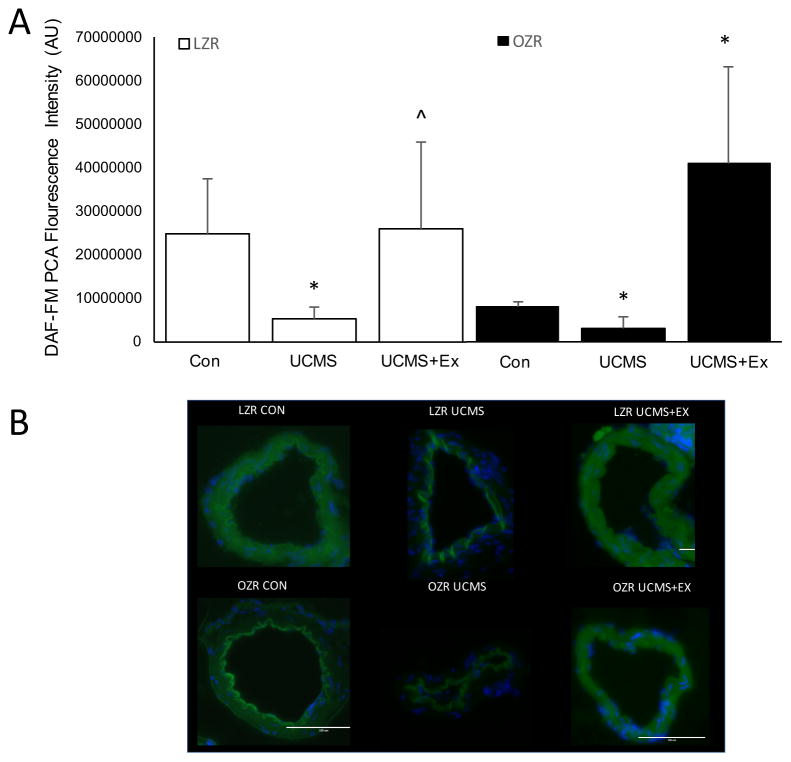
Because both MCA’s were used for ex-vivo reactivity data, we isolated the basilar and posterior cerebral arteries (PCA) and measured the levels of ROS (DHE) and NO (DAF-FM), respectively. In LZR, the basilar artery from UCMS rats demonstrated a greater relative increase (p<0.05) in the fluorescent signal elicited by DHE compared to LZR controls (Fig 5). Further, DAF fluorescence was significantly reduced in the PCA from LZR UCMS compared to controls, indicating that NO production was diminished with UCMS (Fig 6). Compared to LZR controls, the DHE fluorescence signal was significantly reduced after 8 weeks of Ex (Fig 5), suggesting lower levels of ROS. The ROS levels in the PCA was similar in the LZR UCMS vs. LZR UCMS+Ex (Fig 5). However, the DAF fluorescence signal was higher in the UCMS+Ex vs. LZR UCMS (Fig 6).
Figure 5.
The effects of stress and exercise on oxidative stress production. The production of reactive oxygen species in the basilar artery measured by dihydroethidium (A) in LZR (open bars) and OZR (closed bars) under either control, UCMS, Ex, or UCMS+Ex conditions. B), representative images of the DHE. Data represented as MEAN±SD. n=4–6/group. *p<0.05 vs. control (within strain), ^p<0.05 vs. UCMS group (within strain). +p<0.05 vs. Ex group (within strain). Please see text for details.
Figure 6.
The effects of stress and exercise on nitric oxide production. The production of nitric oxide in the posterior cerebral artery (PCA) measured by 4-Amino-5-methylamino-2′,7′-difluorofluorescein diacetate in LZR (open bars) and OZR (closed bars) under either control, UCMS, Ex, or UCMS+Ex conditions. B), Representative images of the DAF-FM. Data represented as MEAN±SD. n=4–6/group. *p<0.05 vs. control (within strain), ^p<0.05 vs. UCMS group (within strain). Please see text for details.
In OZR, UCMS rats had a higher (p<0.05) fluorescent signal elicited by DHE compared to OZR controls (Fig 5). Further, DAF fluorescence was significantly reduced in the PCA from OZR UCMS rats compared to OZR controls (Fig 6). Compared to OZR controls, the DHE fluorescence signal was significantly reduced after 8 weeks of Ex (Fig 5). Whereas, in OZR UCMS+Ex the DHE fluorescence signal was reduced compared to UCMS alone, albeit not to the same level as in the OZR Ex group (Fig 5). Further, the OZR UCMS+Ex group had a significantly higher DAF fluorescence signal compared to the OZR UCMS group (Fig 6).
Discussion
We present novel findings showing cerebrovascular dysfunction following 8 weeks of UCMS, and how aerobic exercise affected this response. The main findings were: 1) UCMS significantly decreased MCA EDD in LZR and OZR; 2) UCMS significantly decreased endothelial-independent dilation but only in OZR; 3) UCMS resulted in hypotrophic remodeling with an increase in MCA stiffness, but this was only evident in the OZR, however UCMS in both LZR and OZR decrease MVD; 4) aerobic exercise concurrent with UCMS prevented the impaired EDD noted in the UCMS groups; 5) aerobic exercise concurrent with UCMS prevented or limited the impaired endothelial-independent dilation, MCA remodeling and MVD rarefaction; and 6) the actions of UCMS and Ex, were in part reflective of shifts in the levels of ROS and NO. These results suggest that compared with healthy lean controls, rats with MetS have additional vasculopathies in association with chronic stress.
Role of UCMS
Stressful circumstances activate the hypothalamic-pituitary-adrenal (HPA) axis and the autonomic nervous system to release stress hormones. Chronic exposure to stressful conditions also leads to an allostatic overload, resulting in maladaptive responses to various organs; and as our data would suggest, initiates patho-physiological adaptations to the cerebrovasculature. Indeed, corticosterone levels were elevated in the UCMS groups, coupled with an increase in adrenal weights, and reduced grooming response, suggesting significant activation of the HPA axis. We have previously shown, in a lean healthy mouse model, that UCMS leads to a pre-atherosclerotic phenotype in the aorta (Stanley et al., 2014). We now show that chronic stress in a health lean rat leads to significant impairment in MCA EDD and an exaggerated MCA constriction response, coupled with an increase in ROS and reduced NO bioavailability in the cerebral vessels. Interestingly, these cerebrovascular adaptions to UCMS in LZR reflect what we see in a OZR control (without UCMS) i.e., 8 weeks of UCMS transformed the LZR cerebrovasculature to one with similar dysfunction seen in the OZR. However, as seen in OZR UCMS group, stress combined with development of MetS resulted in additional pathological cerebrovascular adaptations. Of note, given the lack of change in blood pressure in the UCMS groups would suggest that other mechanisms were involved in the development of the cerebrovascular dysfunction noted with UCMS.
The blunted EDD in the MCA with UCMS likely reflects the ROS impairment of NO bioavailability in the cerebral vessels as shown by the increased DHE and reduced DAF fluorescence signal in both LZR and OZR UCMS vessels. Whereas, acute treatment of the MCA with L-NAME further reduced EDD in LZR UCMS, some dilatory response to ACh remained (i.e., prostanoid dilators and endothelium-derived hyperpolarization factors) (Fig 1C). In contrast, acute treatment of the MCA with L-NAME wiped out all EDD in the OZR UCMS group (Fig 1D), suggesting that in addition to a loss of NO bioavailability, the ancillary dilatory pathways were also affected. Indeed, pro-oxidative conditions, such as UCMS, can alter the balance between constricting and dilating metabolites, such as shifting arachidonic acid metabolism by COX to the production of vasoconstricting metabolites (e.g., thromboxane) that can compete against the vasodilatory stimulus from NO, prostacyclin, or other vasodilators (Isingrini et al., 2011). We have previously shown such a response in the mouse aorta after UCMS (Stanley et al., 2014). There is also ample evidence supporting a role for cortisol in the UCMS induced EDD dysfunction. Increased cortisol levels have also been shown to decrease NO synthesis or bioavailability by inhibiting endothelial NO synthase directly (Liu et al., 2009), and indirectly via the cortisol actions on increasing ROS production (Iuchi et al., 2003).
It is also interesting to note that unlike the LZR UCMS group, UCMS in OZR negatively altered smooth muscle dilation (Fig 2B). This impairment in smooth muscle dilation may reflect issues at the level of the secondary messengers and receptors (i.e., soluble guanylyl cyclase, cyclic guanosine monophosphate, protein kinase G, cyclic adenosine monophosphate, etc). Previous studies have shown that increased superoxide and peroxynitrite levels can directly inhibit soluble guanylyl cyclase and affect protein kinase G (Weber et al., 2001; Burgoyne et al., 2007). Further, an increase in cortisol has been shown to decrease cyclic adenosine monophosphate expression (Borski et al., 2002). Thus, the combined actions of MetS and UCMS may have affected these secondary messengers and receptors. The reduced NO bioavailability in the MCA of the LZR UCMS may also play a role in the hyperconstriction response to 5-HT i.e., less NO equals less of a brake to prevent the hyperconstriction response to 5-HT.
In addition to the functional changes, UCMS reduced cerebral MVD in LZR and OZR, with additional structural adaptations (hypotrophic remodeling and increased MCA stiffness) noted only in OZR. It is possible that the progressive loss of vascular wall distensibility may have contributed to the reduction of dilator reactivity (noted above) between OZR controls and OZR UCMS. However, the extent to which wall distensibility contributed to impaired reactivity is difficult to assess owing to the concurrent impairment of the mechanisms of dilator reactivity (i.e., loss of NO bioavailability). Hypertension is known to decrease the MCA inner diameter (Baumbach & Heistad, 1989; Izzard et al., 2006), however unlike our study, hypertension also increases wall thickness to normalize the increased wall stress and protect the downstream arterioles from the increased pressure (Baumbach & Heistad, 1989). In our study, the lack of change in wall thickness with UCMS likely reflects the minor blood pressure changes. The hypotrophic remodeling noted in the OZR UCMS group might reflect chronic decreases in blood flow, which is in agreement with previous studies in resistance arteries (Pourageaud & De Mey, 1997; Buus et al., 2001). Vessel diameter, and functional hyperemic responses play a role in maintaining adequate blood flow to each region of the brain, and must be able to respond accordingly to accommodate increases in flow during periods of higher neural activity. Impairments in any/all of these processes may have significant impact on neuronal metabolism and activity.
The effect of chronic stress on cerebral MVD has not been thoroughly studied. The present study showed that CD31+ cells were decreased with UCMS in LZR and OZR. Our previous efforts into the physiological mechanisms contributing to microvascular rarefaction in the brain (Chantler et al., 2015) and skeletal muscle (Frisbee et al., 2014), has implicated the balance between oxidant stress and endothelial function (e.g., NO bioavailability, altered arachidonic acid metabolism) as a key contributor to the progression and severity of microvascular rarefaction. With the degree of loss in NO bioavailability reflecting the severity of the rarefaction that followed (Chantler et al., 2015). As such the reduction in NO bioavailability, with a corresponding higher ROS with UCMS might have directly contributed to the loss in cerebral MVD. This reduction in the cerebral MVD with UCMS could have clinical consequences. Microvascular rarefaction affects spatial hemodynamics and induces a non-uniform blood flow distribution, and has been implicated in reducing the capillary transport of small solutes (Henrich et al., 1988). Such pathological adaptations could also damage cerebral auto-regulation and cerebral blood flow reserve, favoring the occurrence of cognitive impairment, and ischemic stroke (Brown et al., 2007; van Dijk et al., 2008).
Role of Exercise
For interventions aimed to treat or alleviate the effects of chronic stress on the cerebrovascular to be successful, it is essential to understand the pathways that might explain the association between psycho-social stress and CVD. Given that the proposed pathways by which chronic stress affects are extensive, we deployed an intervention that is known to have extensive actions on multiple pathways. It is well known that exercise has been shown to have beneficial effects on CV function (Sattelmair et al., 2011) and mental health outcomes (Herring et al., 2010). Evidence also suggests that physiological adaptations to regular exercise lead not only to improved physiological control during exercise but also in response to psychological stressors, reflecting a “cross-stressor adaptation” (Sothmann et al., 1996).
Exercise training did not improve MCA function or MVD in healthy lean rats, this was likely due to the optimal functional status of the MCA (i.e., low ROS, and good NO bioavailability). In contrast, exercise in the OZR group was able to prevent MCA dysfunction, MCA stiffness, and maintain MVD to levels similar to LZR controls. These data show that exercise training can help to preserve MCA endothelial function with MetS possibly by limiting the development of ROS. Importantly, when UCMS was concurrent with exercise training, the negative actions of UCMS on MCA function, stiffness, and microvascular rarefaction were prevented. Thus, our data supports the idea that the benefits of exercise reach beyond the vascular beds of just the exercising muscles, in this case to maintain vascular healthy in the brain.
The mechanisms by which regular exercise induces beneficial effects in the cerebral vasculature are not fully known. But it seems likely that the repeated exposure to increases in blood flow and shear stress in specific regions of the brain during exercise will play a role (Endres et al., 2003). Exercise training has been shown to stimulate brain activity, increase cerebral blood flow (van Hall et al., 2009), induce eNOS expression, reduce ROS production, and upregulate angiogenic proteins in the cerebral arterioles with a corresponding improvement in cognitive function (van Praag et al., 1999; Mayhan et al., 2004; Mayhan et al., 2011; Cechetti et al., 2012). In our study, exercise may have limited the deleterious effects of UCMS on the cerebrovasculature, in part, by increasing NO bioavailability, and reducing ROS (Fig 5). This improved NO bioavailability was also supported by the L-NAME data whereby in the OZR UCMS+Ex group, L-NAME completely removed all EDD to ACh suggesting that the increased EDD to ACh was reflective of an increase in NO rather than the additional dilator pathways. However, the increased NO in the LZR UCMS+Ex group compared to LZR UCMS seemed to occur without major changes in ROS. There are two possibilities for this: first, the bioavailability of NO in the LZR Ex group may have exceeded the levels noted in the LZR UCMS+Ex, and thus despite elevated ROS, NO production exceeded what was being converted to peroxynitrite. Unfortunately, we do not have the NO-levels in the LZR exercise group to verify this scenario. Second, exercise might prevent eNOS uncoupling. In an aged model, exercise was shown to increase tetrahydrobiopterin (an important co-factor for NO production) levels (Sindler et al., 2009). Furthermore, exercise may prevent the co-localization of NADPH oxidase with eNOS by caveolin-1, known to occur upon stimulation with stress hormones. In this scenario, ROS production could stay elevated but compartmentalized away from eNOS allowing eNOS to remain coupled and reduce the interaction of NO and superoxide, thus improving EDD. Irrespective of the mechanism, our data supports numerous previous studies demonstrating that a chronic exercise regimen is effective in increasing vascular NO bioavailability (Frisbee et al., 2006; Durrant et al., 2009).
In addition to the improved EDD, exercise improved endothelial dilation to SNP in OZR. However, this beneficial response was lost when exercise was combined with UCMS in OZR. To what extent chronic exercise training affects the secondary messengers and receptors involved in smooth muscle relaxation are relatively unknown. In adult male rats, 6 weeks of exercise training increased brain tissue levels of soluble guanylyl cyclase, a key enzyme and receptor in the NO/sGC/cGMP pathway (Chalimoniuk et al., 2015). It is possible that exercise training was able to increase the sensitivity of the MCA smooth muscle to secondary messengers, restoring SNP dilation in the OZRs.
Our study also demonstrated that exercise shifted the stress-strain relationship to the right with a reduced β-slope in the OZR Ex group, suggesting reduced MCA stiffness in the OZR with exercise. Importantly, the combination of UCMS+Ex, although not as effective as Ex alone, prevented the leftward shift in the stress-strain relationship noted in OZR UCMS. Exercise training is well known to improve arterial stiffness of various arterial beds (Laughlin et al., 2012; Donley et al., 2014). However, there is little information on how exercise affects cerebrovascular stiffness and remodeling. In the small mesenteric and coronary arteries, exercise training improved arterial stiffness in hypertensive rats (Roque et al., 2013). Collagen and elastin, and their organization at the internal elastic lamina are central elements in arterial stiffness (Briones et al., 2003). Thus, the reduced MCA stiffness with exercise may reflect a normalization of collagen disposition as previously shown (Roque et al., 2013). However, the reduced MCA stiffness with exercise may also be due to a reduction in ROS, which can increase arterial stiffness via its effects on the inactivation of NO, regulation of cell growth and differentiation, modulation of synthesis and degradation of extracellular matrix proteins and activation of many kinases (Lee & Griendling, 2008). Indeed, a reduction in ROS production correlates with the normalization of the increased arterial stiffness in the mesenteric artery (Briones et al., 2009) and MCA (Brooks et al., 2015), likely via improved NO bioavailability. Thus, the improved NO-ROS environment in the cerebrovasculature could play an important role in maintaining cerebral MVD with exercise training, especially when combined with UCMS. We have previously shown that interventions aimed at improving vascular NO bioavailability and reducing oxidant stress (e.g., tempol, captopril, metformin, rosiglitazone) were the most effective at blunting the severity of the cortical microvascular rarefaction (Chantler et al., 2015). Thus, our current data further supports the argument that loss of NO bioavailability and increased ROS are driving the pathological changes in the MVD, and to the MCA morphological changes noted above in the OZR.
Exercise can affect both the adrenergic and corticosteroid systems involved in stress response, thus it is not surprising that other studies have demonstrated an increase in stress hormones with exercise, especially forced treadmill running (Svensson et al., 2015). Indeed, forced running exercise in rodents can lead to anxiety and increase the levels of stress hormones (Leasure & Jones, 2008; Svensson et al., 2016). The current study also showed higher levels of corticosterone in the LZR and OZR, albeit significance was only obtained in the OZR. Despite the elevated stressed levels in the exercise groups, beneficial effects on the cerebrovasculature were evident especially in the OZR. However, the overall positive effect of exercise in experimental models could potentially be masked by the stress response. Given the sedentary nature of the OZR it was important to deploy a forced treadmill running protocol as opposed to voluntary wheel running, which in itself can cause stress, to ensure all rats were subjected to an exercise stimulus.
Clinical Significance
Early recognition of stress/depression-associated vascular impairments, especially in individuals with MetS, may provide a valuable time window to treat individuals to protect their cerebral vasculature. The current study deployed the Ex protocol each day prior to the UCMS protocol to limit the likelihood of poor Ex training post-UCMS. Further, the Ex protocol was deployed during rather than after the development of stress and depression. To what extent the positive changes in cerebrovascular function would have been affected if the UCMS exposure was performed before the Ex protocol, or whether Ex training began after the development of chronic stress and depression is beyond the scope of the present study. However, it certainly has clinical relevance given that over 350 million people worldwide suffer from depressive disorders. Numerous studies have shown Ex to be beneficial in improving depressive symptoms in already depressed individuals (Mather et al., 2002) (McNeil et al., 1991; Dunn et al., 2001; Nabkasorn et al., 2006). In addition, it has also been shown that reducing physical activity leads to increased symptoms of depression (Lampinen et al., 2000) and that depressive symptoms decrease when physical activity is resumed (Farmer et al., 1988). These data would suggest that as long as the individual can or wishes to Ex, improvements in depressive symptoms will occur. Thus, we speculate and expect to see some improvements in cerebrovascular if Ex was performed after the UCMS or after the development of depression in our rodents. However, in individuals with depression who are unwilling or unable to exercise daily could be prophylactically treated with drugs that have pleiotropic benefits such as atorvastatin (Pretnar-Oblak et al., 2006; Brooks et al., 2015; Chantler et al., 2015) that may limit the deleterious impact of chronic stress on neurological and cerebrovascular health in individuals with MetS, potentially conferring the anti-depressant and vasculo-protective benefits of exercise.
Limitations
A limitation of the study reflects the MCA remodeling which were determined ex-vivo during the passive mechanics experiments. Future research should directly examine the compositional changes (collagen, elastin etc.) in the cerebral vessels to gain further insights into the role of UCMS and Ex on MCA remodeling. Further, due to the use of the MCA’s for ex-vivo pressure myograph, we needed to examine other cranial arteries (PCA and basilar) for the DHE and DAF-FM procedures. We also realize the limitation associated with measuring tissue superoxide levels by DHE. While our excitation/emission gating does detect signals produced by oxidation products that may originate from reactions independent of superoxides, the spectral overlap does not exclude considerable contributions from 2-hydroxyethidium, the superoxides-specific oxidation product of hydroethidine (Zielonka & Kalyanaraman, 2010). With this in mind, and considering our data demonstrates an inverse relationship between DHE-detectable superoxides and NO, we believe these data strongly support the hypothesis that the rapid, diffusion-controlled reaction between NO and superoxides forming peroxynitrite. Another limitation of the study is that we did not collect data on muscle citrate synthase activity to determine improvements in exercise capacity. However, we have previously deployed a similar exercise protocol which resulted in significant improvements in muscle citrate synthase activity (30–50%) in LZR and OZR (Frisbee et al., 2006), but we are unable to determine whether the Ex vs. UCMS+Ex groups resulted in a similar improvement in exercise capacity. A limitation to the exercise training was that we exercised the rats at approximately the same relatively intensity (60–70% max running speed), which resulted in slight differences in absolute work performed. Whereby, the average workload performed during each treadmill session was similar between LZR- and OZR-Ex groups, but lower in the LZR UCMS+Ex group (p<0.05) compared LZR and OZR Ex groups, and higher in the OZR UCMS+Ex group (p<0.05) compared to the other groups. As such, the differences in absolute work performed may have affected the results of the study; however, the UCMS+Ex groups demonstrated important cerebral vascular irrespective of differences in absolute workload performed. Other limitations include the single blood pressure measurement during the terminal procedures and our data were collected in only male rats. Our collaborators, have previously shown that compared to healthy males, healthy female mice were more susceptible to chronic stress in terms of the severity of depressive behaviors, but that the subsequent development of vasculopathy was blunted (Stanley et al., 2014). Thus, future research should examine how UCMS and Ex affect LZR and OZR female rats, and that blood pressure should be tracked during the development of stress and depression.
Conclusion
Our data shows that chronic exposure to stressful conditions leads to pathological adaptations to the cerebrovasculature via an ROS-NO pathway. Further, we have shown that MetS (OZR) has an additive effect on the cerebral vascular dysfunction noted with UCMS compared to lean healthy rats. Our results also provide initial insight into the mechanisms that may contribute to the influence of UCMS and the role of exercise on preventing the negative actions of UCMS on cerebrovascular function and structure.
New Findings.
What is the central question of this study?
How chronic stress impacts cerebrovascular function, and whether metabolic syndrome accelerates the cerebrovascular adaptations to stress? What role does exercise training have in preventing cerebrovascular changes to stress and metabolic syndrome?
What is the main finding and its importance?
We show stressful conditions leads to pathological adaptations to the cerebrovasculature via an oxidative-nitric oxide pathway, and that the presence of metabolic syndrome has a greater susceptibility to stress-induced cerebrovascular dysfunction than lean healthy rats. Our results also provide insight into the mechanisms that may contribute to the influence of stress and the role of exercise on preventing the negative actions of stress on cerebrovascular function and structure.
Acknowledgments
Funding: This study was supported by the AHA pre-doctoral fellowship 14PRE 20380386, and the National Institute of General Medical Sciences of the National Institutes of Health under Award Numbers U54GM104942, and 5P20GM109098.
The authors would like to thank WVU Department of Exercise Physiology for arranging extensive undergraduate student support for the stress protocol and exercise training protocol used in this paper. The authors would also like to thank the WVU Animal Care facilities for excellent technical support. Additionally, we also acknowledge the support provided through Center for Cardiovascular and Respiratory Sciences, Clinical and Translational Sciences Institute, and the Center for Basic and Translational Stroke Research at the West Virginia University Health Sciences Center.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
Author contributions
S.B., K.B., I.M.O J.C.F., and P.D.C conception and design of research; S.B., K.B., E.D., R.S., K.L., W.S., C.R.P., and S.A., performed experiments; S.B., K.B., E.D., and P.D.C analyzed data; S.B., K.B., E.D., J.C.F., and P.D.C. interpreted results of experiments; S.B., K.B., and P.D.C drafted manuscript; I.M.O, R.B., J.C.W., and P.D.C. edited and revised manuscript; All authors approved final version of manuscript
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Baumbach GL, Hajdu MA. Mechanics and composition of cerebral arterioles in renal and spontaneously hypertensive rats. Hypertension. 1993;21:816–826. doi: 10.1161/01.hyp.21.6.816. [DOI] [PubMed] [Google Scholar]
- Baumbach GL, Heistad DD. Remodeling of cerebral arterioles in chronic hypertension. Hypertension. 1989;13:968–972. doi: 10.1161/01.hyp.13.6.968. [DOI] [PubMed] [Google Scholar]
- Borski RJ, Hyde GN, Fruchtman S. Signal transduction mechanisms mediating rapid, nongenomic effects of cortisol on prolactin release. Steroids. 2002;67:539–548. doi: 10.1016/s0039-128x(01)00197-0. [DOI] [PubMed] [Google Scholar]
- Briones AM, Gonzalez JM, Somoza B, Giraldo J, Daly CJ, Vila E, … Arribas SM. Role of elastin in spontaneously hypertensive rat small mesenteric artery remodelling. J Physiol. 2003;552:185–195. doi: 10.1113/jphysiol.2003.046904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briones AM, Rodriguez-Criado N, Hernanz R, Garcia-Redondo AB, Rodrigues-Diez RR, Alonso MJ, … Salaices M. Atorvastatin prevents angiotensin II-induced vascular remodeling and oxidative stress. Hypertension. 2009;54:142–149. doi: 10.1161/HYPERTENSIONAHA.109.133710. [DOI] [PubMed] [Google Scholar]
- Brooks SD, DeVallance E, d’Audiffret AC, Frisbee SJ, Tabone LE, Shrader CD, … Chantler PD. Metabolic Syndrome Impairs Reactivity and Wall Mechanics of Cerebral Resistance Arteries in Obese Zucker Rats. Am J Physiol Heart Circ Physiol. 2015;309:H1846–1859. doi: 10.1152/ajpheart.00691.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown WR, Blair RM, Moody DM, Thore CR, Ahmed S, Robbins ME, Wheeler KT. Capillary loss precedes the cognitive impairment induced by fractionated whole-brain irradiation: a potential rat model of vascular dementia. J Neurol Sci. 2007;257:67–71. doi: 10.1016/j.jns.2007.01.014. [DOI] [PubMed] [Google Scholar]
- Burgoyne JR, Madhani M, Cuello F, Charles RL, Brennan JP, Schroder E, … Eaton P. Cysteine redox sensor in PKGIa enables oxidant-induced activation. Science. 2007;317:1393–1397. doi: 10.1126/science.1144318. [DOI] [PubMed] [Google Scholar]
- Buus CL, Pourageaud F, Fazzi GE, Janssen G, Mulvany MJ, De Mey JG. Smooth muscle cell changes during flow-related remodeling of rat mesenteric resistance arteries. Circ Res. 2001;89:180–186. doi: 10.1161/hh1401.093575. [DOI] [PubMed] [Google Scholar]
- CDC.gov. Current Depression Among Adults - United States, 2006 and 2008. MMWR. 2010;59:1229–1235. [PubMed] [Google Scholar]
- Cechetti F, Worm PV, Elsner VR, Bertoldi K, Sanches E, Ben J, … Netto CA. Forced treadmill exercise prevents oxidative stress and memory deficits following chronic cerebral hypoperfusion in the rat. Neurobiol Learn Mem. 2012;97:90–96. doi: 10.1016/j.nlm.2011.09.008. [DOI] [PubMed] [Google Scholar]
- Chalimoniuk M, Chrapusta SJ, Lukacova N, Langfort J. Endurance training upregulates the nitric oxide/soluble guanylyl cyclase/cyclic guanosine 3′,5′-monophosphate pathway in the striatum, midbrain and cerebellum of male rats. Brain Res. 2015;1618:29–40. doi: 10.1016/j.brainres.2015.05.020. [DOI] [PubMed] [Google Scholar]
- Chantler P, Shrader C, Tabone L, d’Audiffret A, Huseynova K, Brooks S, … Frisbee J. Cerebral cortical microvascular rarefaction in metabolic syndrome is dependent on insulin resistance and loss of nitric oxide bioavailability. Microcirulation. 2015;22:435–445. doi: 10.1111/micc.12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Zhang L, Zhang M, Song X, Zhang H, Liu Y, Lv S. Relationship of depression, stress and endothelial function in stable angina patients. Physiol Behav. 2013;118:152–158. doi: 10.1016/j.physbeh.2013.05.024. [DOI] [PubMed] [Google Scholar]
- Chrapko WE, Jurasz P, Radomski MW, Lara N, Archer SL, Le Melledo JM. Decreased platelet nitric oxide synthase activity and plasma nitric oxide metabolites in major depressive disorder. Biol Psychiatry. 2004;56:129–134. doi: 10.1016/j.biopsych.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Donley DA, Fournier SB, Reger BL, DeVallance E, Bonner DE, Olfert IM, … Chantler PD. Aerobic exercise training reduces arterial stiffness in metabolic syndrome. J Appl Physiol (1985) 2014;116:1396–1404. doi: 10.1152/japplphysiol.00151.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AL, Trivedi MH, O’Neal HA. Physical activity dose-response effects on outcomes of depression and anxiety. Med Sci Sports Exerc. 2001;33:S587–597. doi: 10.1097/00005768-200106001-00027. discussion 609–510. [DOI] [PubMed] [Google Scholar]
- Durrant JR, Seals DR, Connell ML, Russell MJ, Lawson BR, Folian BJ, … Lesniewski LA. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol. 2009;587:3271–3285. doi: 10.1113/jphysiol.2009.169771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres M, Gertz K, Lindauer U, Katchanov J, Schultze J, Schrock H, … Laufs U. Mechanisms of stroke protection by physical activity. Ann Neurol. 2003;54:582–590. doi: 10.1002/ana.10722. [DOI] [PubMed] [Google Scholar]
- Everson-Rose SA, Roetker NS, Lutsey PL, Kershaw KN, Longstreth WT, Jr, Sacco RL, … Alonso DA. Chronic stress, depressive symptoms, anger, hostility, and risk of stroke and transient ischemic attack in the multi-ethnic study of atherosclerosis. Stroke. 2014;45:2318–2323. doi: 10.1161/STROKEAHA.114.004815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer ME, Locke BZ, Moscicki EK, Dannenberg AL, Larson DB, Radloff LS. Physical activity and depressive symptoms: the NHANES I Epidemiologic Follow-up Study. Am J Epidemiol. 1988;128:1340–1351. doi: 10.1093/oxfordjournals.aje.a115087. [DOI] [PubMed] [Google Scholar]
- Frisbee JC, Goodwill AG, Frisbee SJ, Butcher JT, Brock RW, Olfert IM, … Chantler PD. Distinct temporal phases of microvascular rarefaction in skeletal muscle of obese Zucker rats. Am J Physiol Heart Circ Physiol. 2014;307:H1714–1728. doi: 10.1152/ajpheart.00605.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisbee JC, Samora JB, Peterson J, Bryner R. Exercise training blunts microvascular rarefaction in the metabolic syndrome. Am J Physiol Heart Circ Physiol. 2006;291:H2483–2492. doi: 10.1152/ajpheart.00566.2006. [DOI] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T, Yirmiya R. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiatry. 2008;13:717–728. doi: 10.1038/sj.mp.4002055. [DOI] [PubMed] [Google Scholar]
- Harper SL, Bohlen HG, Rubin MJ. Arterial and microvascular contributions to cerebral cortical autoregulation in rats. Am J Physiol. 1984;246:H17–24. doi: 10.1152/ajpheart.1984.246.1.H17. [DOI] [PubMed] [Google Scholar]
- Henrich HA, Romen W, Heimgartner W, Hartung E, Baumer F. Capillary rarefaction characteristic of the skeletal muscle of hypertensive patients. Klin Wochenschr. 1988;66:54–60. doi: 10.1007/BF01713011. [DOI] [PubMed] [Google Scholar]
- Herring MP, O’Connor PJ, Dishman RK. The effect of exercise training on anxiety symptoms among patients: a systematic review. Arch Intern Med. 2010;170:321–331. doi: 10.1001/archinternmed.2009.530. [DOI] [PubMed] [Google Scholar]
- Isingrini E, Surget A, Belzung C, Freslon JL, Frisbee J, O’Donnell J, Camus V, d’Audiffret A. Altered aortic vascular reactivity in the unpredictable chronic mild stress model of depression in mice: UCMS causes relaxation impairment to ACh. Physiol Behav. 2011;103:540–546. doi: 10.1016/j.physbeh.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Iuchi T, Akaike M, Mitsui T, Ohshima Y, Shintani Y, Azuma H, Matsumoto T. Glucocorticoid excess induces superoxide production in vascular endothelial cells and elicits vascular endothelial dysfunction. Circ Res. 2003;92:81–87. doi: 10.1161/01.res.0000050588.35034.3c. [DOI] [PubMed] [Google Scholar]
- Izzard AS, Horton S, Heerkens EH, Shaw L, Heagerty AM. Middle cerebral artery structure and distensibility during developing and established phases of hypertension in the spontaneously hypertensive rat. J Hypertens. 2006;24:875–880. doi: 10.1097/01.hjh.0000222757.54111.06. [DOI] [PubMed] [Google Scholar]
- Kahl KG, Schweiger U, Correll C, Muller C, Busch ML, Bauer M, Schwarz P. Depression, anxiety disorders, and metabolic syndrome in a population at risk for type 2 diabetes mellitus. Brain Behav. 2015;5:e00306. doi: 10.1002/brb3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katakam PV, Snipes JA, Steed MM, Busija DW. Insulin-induced generation of reactive oxygen species and uncoupling of nitric oxide synthase underlie the cerebrovascular insulin resistance in obese rats. J Cereb Blood Flow Metab. 2012;32:792–804. doi: 10.1038/jcbfm.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemeny ME, Schedlowski M. Understanding the interaction between psychosocial stress and immune-related diseases: a stepwise progression. Brain Behav Immun. 2007;21:1009–1018. doi: 10.1016/j.bbi.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- Lampinen P, Heikkinen RL, Ruoppila I. Changes in intensity of physical exercise as predictors of depressive symptoms among older adults: an eight-year follow-up. Prev Med. 2000;30:371–380. doi: 10.1006/pmed.2000.0641. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Bowles DK, Duncker DJ. The coronary circulation in exercise training. Am J Physiol Heart Circ Physiol. 2012;302:H10–23. doi: 10.1152/ajpheart.00574.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leasure JL, Jones M. Forced and voluntary exercise differentially affect brain and behavior. Neuroscience. 2008;156:456–465. doi: 10.1016/j.neuroscience.2008.07.041. [DOI] [PubMed] [Google Scholar]
- Lee MY, Griendling KK. Redox signaling, vascular function, and hypertension. Antioxid Redox Signal. 2008;10:1045–1059. doi: 10.1089/ars.2007.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Mladinov D, Pietrusz JL, Usa K, Liang M. Glucocorticoid response elements and 11 beta-hydroxysteroid dehydrogenases in the regulation of endothelial nitric oxide synthase expression. Cardiovasc Res. 2009;81:140–147. doi: 10.1093/cvr/cvn231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah L, Szabuniewicz C, Fiocco AJ. Can anxiety damage the brain? Curr Opin Psychiatry. 2016;29:56–63. doi: 10.1097/YCO.0000000000000223. [DOI] [PubMed] [Google Scholar]
- Mather AS, Rodriguez C, Guthrie MF, McHarg AM, Reid IC, McMurdo ME. Effects of exercise on depressive symptoms in older adults with poorly responsive depressive disorder: randomised controlled trial. Br J Psychiatry. 2002;180:411–415. doi: 10.1192/bjp.180.5.411. [DOI] [PubMed] [Google Scholar]
- Mayhan WG, Arrick DM, Patel KP, Sun H. Exercise training normalizes impaired NOS-dependent responses of cerebral arterioles in type 1 diabetic rats. Am J Physiol Heart Circ Physiol. 2011;300:H1013–1020. doi: 10.1152/ajpheart.00873.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhan WG, Sun H, Mayhan JF, Patel KP. Influence of exercise on dilatation of the basilar artery during diabetes mellitus. J Appl Physiol (1985) 2004;96:1730–1737. doi: 10.1152/japplphysiol.01185.2003. [DOI] [PubMed] [Google Scholar]
- McNeil JK, LeBlanc EM, Joyner M. The effect of exercise on depressive symptoms in the moderately depressed elderly. Psychol Aging. 1991;6:487–488. doi: 10.1037//0882-7974.6.3.487. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Sze J, Marin T, Arevalo JM, Doll R, Ma R, Cole SW. A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-kappaB signaling. Biol Psychiatry. 2008;64:266–272. doi: 10.1016/j.biopsych.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Prasol DJ, Belzung C, Crusio WE. Agonistic behavior and unpredictable chronic mild stress in mice. Behav Genet. 2003;33:513–519. doi: 10.1023/a:1025770616068. [DOI] [PubMed] [Google Scholar]
- Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Arch Gen Psychiatry. 1998;55:580–592. doi: 10.1001/archpsyc.55.7.580. [DOI] [PubMed] [Google Scholar]
- Nabkasorn C, Miyai N, Sootmongkol A, Junprasert S, Yamamoto H, Arita M, Miyashita K. Effects of physical exercise on depression, neuroendocrine stress hormones and physiological fitness in adolescent females with depressive symptoms. Eur J Public Health. 2006;16:179–184. doi: 10.1093/eurpub/cki159. [DOI] [PubMed] [Google Scholar]
- Phillips SA, Sylvester FA, Frisbee JC. Oxidant stress and constrictor reactivity impair cerebral artery dilation in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R522–530. doi: 10.1152/ajpregu.00655.2004. [DOI] [PubMed] [Google Scholar]
- Pourageaud F, De Mey JG. Structural properties of rat mesenteric small arteries after 4-wk exposure to elevated or reduced blood flow. Am J Physiol. 1997;273:H1699–1706. doi: 10.1152/ajpheart.1997.273.4.H1699. [DOI] [PubMed] [Google Scholar]
- Pratt LA, Brody DJ. Depression and Obesity in the U.S. Adult Household Population, 2005–2010. National Health and Nutrition Examination Survey data. NCHS Data Brief No. 167. 2014 https://www.cdc.gov/nchs/products/databriefs/db167.htm. [PubMed]
- Pretnar-Oblak J, Sabovic M, Sebestjen M, Pogacnik T, Zaletel M. Influence of atorvastatin treatment on L-arginine cerebrovascular reactivity and flow-mediated dilatation in patients with lacunar infarctions. Stroke. 2006;37:2540–2545. doi: 10.1161/01.STR.0000239659.99112.fb. [DOI] [PubMed] [Google Scholar]
- Rathel TR, Leikert JJ, Vollmar AM, Dirsch VM. Application of 4,5-diaminofluorescein to reliably measure nitric oxide released from endothelial cells in vitro. Biol Proced Online. 2003;5:136–142. doi: 10.1251/bpo55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roque FR, Briones AM, Garcia-Redondo AB, Galan M, Martinez-Revelles S, Avendano MS, … Salaices M. Aerobic exercise reduces oxidative stress and improves vascular changes of small mesenteric and coronary arteries in hypertension. Br J Pharmacol. 2013;168:686–703. doi: 10.1111/j.1476-5381.2012.02224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg J, Yaroslavsky I, Carney RM, Freedland KE, George CJ, Baji I, … Kovacs M. The association between major depressive disorder in childhood and risk factors for cardiovascular disease in adolescence. Psychosom Med. 2014;76:122–127. doi: 10.1097/PSY.0000000000000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattelmair J, Pertman J, Ding EL, Kohl HW, 3rd, Haskell W, Lee IM. Dose response between physical activity and risk of coronary heart disease: a meta-analysis. Circulation. 2011;124:789–795. doi: 10.1161/CIRCULATIONAHA.110.010710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindler AL, Delp MD, Reyes R, Wu G, Muller-Delp JM. Effects of ageing and exercise training on eNOS uncoupling in skeletal muscle resistance arterioles. J Physiol. 2009;587:3885–3897. doi: 10.1113/jphysiol.2009.172221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sothmann MS, Buckworth J, Claytor RP, Cox RH, White-Welkley JE, Dishman RK. Exercise training and the cross-stressor adaptation hypothesis. Exerc Sport Sci Rev. 1996;24:267–287. [PubMed] [Google Scholar]
- Stanley SC, Brooks SD, Butcher JT, d’Audiffret AC, Frisbee SJ, Frisbee JC. Protective effect of sex on chronic stress- and depressive behavior-induced vascular dysfunction in BALB/cJ mice. J Appl Physiol (1985) 2014;117:959–970. doi: 10.1152/japplphysiol.00537.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson M, Lexell J, Deierborg T. Effects of Physical Exercise on Neuroinflammation, Neuroplasticity, Neurodegeneration, and Behavior: What We Can Learn From Animal Models in Clinical Settings. Neurorehabil Neural Repair. 2015;29:577–589. doi: 10.1177/1545968314562108. [DOI] [PubMed] [Google Scholar]
- Svensson M, Rosvall P, Boza-Serrano A, Andersson E, Lexell J, Deierborg T. Forced treadmill exercise can induce stress and increase neuronal damage in a mouse model of global cerebral ischemia. Neurobiol Stress. 2016;5:8–18. doi: 10.1016/j.ynstr.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk EJ, Prins ND, Vrooman HA, Hofman A, Koudstaal PJ, Breteler MM. Progression of cerebral small vessel disease in relation to risk factors and cognitive consequences: Rotterdam Scan study. Stroke. 2008;39:2712–2719. doi: 10.1161/STROKEAHA.107.513176. [DOI] [PubMed] [Google Scholar]
- van Hall G, Stromstad M, Rasmussen P, Jans O, Zaar M, Gam C, … Nielsen HB. Blood lactate is an important energy source for the human brain. J Cereb Blood Flow Metab. 2009;29:1121–1129. doi: 10.1038/jcbfm.2009.35. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M, Lauer N, Mulsch A, Kojda G. The effect of peroxynitrite on the catalytic activity of soluble guanylyl cyclase. Free Radic Biol Med. 2001;31:1360–1367. doi: 10.1016/s0891-5849(01)00706-7. [DOI] [PubMed] [Google Scholar]
- Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- Zechariah A, ElAli A, Doeppner TR, Jin F, Hasan MR, Helfrich I, Mies G, Hermann DM. Vascular endothelial growth factor promotes pericyte coverage of brain capillaries, improves cerebral blood flow during subsequent focal cerebral ischemia, and preserves the metabolic penumbra. Stroke. 2013;44:1690–1697. doi: 10.1161/STROKEAHA.111.000240. [DOI] [PubMed] [Google Scholar]
- Zielonka J, Kalyanaraman B. Hydroethidine- and MitoSOX-derived red fluorescence is not a reliable indicator of intracellular superoxide formation: another inconvenient truth. Free Radic Biol Med. 2010;48:983–1001. doi: 10.1016/j.freeradbiomed.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]