Abstract
Background & Aims
Clostridium difficile induces intestinal inflammation by releasing toxins A and B. The antimicrobial compound cationic steroid antimicrobial 13 (CSA13) has been developed for treating gastrointestinal infections. The CSA13-Eudragit formulation can be given orally and releases CSA13 in the terminal ileum and colon. We investigated whether this form of CSA13 reduces C difficile infection (CDI) in mice.
Methods
C57BL/6J mice were infected with C difficile on day 0, followed by subcutaneous administration of pure CSA13 or oral administration of CSA13-Eudragit (10 mg/kg/day for 10 days). Some mice were given intraperitoneal vancomycin (50 mg/kg daily) days 0–4 and relapse was measured after antibiotic withdrawal. The mice were monitored until day 20; colon and fecal samples were collected on day 3 for analysis. Blood samples were collected for flow cytometry analyses. Fecal pellets were collected each day from mice injected with CSA13 and analyzed by high-performance liquid chromatography or 16S sequencing; feces were also homogenized in phosphate-buffered saline and fed to mice with CDI via gavage.
Results
CDI of mice caused 60% mortality, significant body weight loss, and colonic damage 3 days after infection; these events were prevented by subcutaneous injection of CSA13 or oral administration CSA13-Eudragit. There was reduced relapse of CDI after administration of CSA13 was stopped. Levels of CSA13 in feces from mice given CSA13-Eudragit were significantly higher than those of mice given subcutaneous CSA13. Subcutaneous and oral CSA13 each significantly increased the abundance of Peptostreptococcaceae bacteria and reduced the abundance of C difficile in fecal samples of mice. When feces from mice with CDI and given CSA13 were fed to mice with CDI that had not received CSA13, the recipient mice had significantly increased rates of survival. CSA13 reduced fecal levels of inflammatory metabolites (endocannabinoids) and increased fecal levels of 4 protective metabolites (citrulline, 3-aminoisobutyric acid, retinol, and ursodeoxycholic acid) in mice with CDI. Oral administration of these CSA13-dependent protective metabolites reduced the severity of CDI.
Conclusions
In studies of mice, we found the CSA13-Eudragit formulation to be effective in eradicating CDI, by modulating the intestinal microbiota and metabolites.
Keywords: bacteria, intestine, metabolomics, colitis
Introduction
C. difficile infection (CDI) is common nosocomial infection following antibiotic treatment. Toxigenic C. difficile bacteria produce toxins A and B, and the more recently discovered binary toxin 1. Toxins A and B both mediate C. difficile pathogenicity, and induce intestinal inflammation and tissue damage. Patients with C. difficile infection experience watery diarrhea, bloody stool, abdominal pain, fever, and weight loss. Antibiotic therapies for CDI such as vancomycin, metronidazole, and fidaxomicin are efficacious. However, some cases are refractory to conventional therapy and are associated with multiple relapses, which may eventually require surgical resection of the involved tissue - adversely affecting the patients’ quality of life 2. The high cost of fidaxomicin has limited its clinical use, despite its effectiveness against relapsing CDI 3. New medications against CDI are needed.
Disruption of intestinal microflora due to antibiotic treatment is often associated with a high risk of C. difficile recurrence. Fidaxomicin diminishes the risk of CDI relapse due to reduced disruption of intestinal microbiota 4. Recently, fecal microbiota transplantation (FMT) has been shown to cure CDI, in particular, relapsing infection with up to 90% success rates 5. These findings suggest that modulation of intestinal microbiota may be crucial to prevention and treatment of CDI.
CSA13 is a non-peptide antimicrobial compound which is stable at room temperature, resistant to proteolysis, and inexpensive to synthesize 6, 7. It effectively suppresses peritoneal infection in mice 8. We believe that CSA13 may be useful for treating gastrointestinal infections. However, the efficacy of CSA13 against CDI has not been evaluated. To make CSA13 convenient for clinical use, it was combined with Eudragit polymer and methylcellulose to produce an orally active formulation that slowly releases CSA13 at alkaline pH, i.e. the terminal ileum and colon. The pH-specific release and favorable safety profile of Eudragit have been demonstrated in clinical trials 9. Daily administration of oral CSA13-Eudragit up to 200mg/kg for seven days did not cause mortality and liver or kidney toxicity in rats (confidential data of N8 Medical, Inc.).
We have evaluated the performance of CSA13 for inhibition of C. difficile primary infection and relapse. We hypothesize that CSA13 suppresses CDI via changes to the intestinal microbiota and metabolic pathways. This study includes microbiome and metabolomic analysis of mouse fecal samples and functional studies to elucidate the protective mechanism of CSA13.
Materials and Methods
Production of CSA13-Eudragit
CSA13 was coated with Eudragit FS30D polymer. This pH-responsive polymer is insoluble in acid but dissolves in a mildly alkaline environment (i.e., pH 7 or above), which is optimal for colonic delivery. CSA13-Eudragit was packaged into microparticles using an SWRI-patented spinning disk atomization technology. This packaging prevented leakage of CSA13 in acidic, aqueous solution. The CSA13-Eudragit microparticles were dried. The resulting powder was suspended in 0.5% methylcellulose in water.
C. difficile infection
Eight-week-old male and female C56BL/6J mice were provided with an antibiotic cocktail added in their drinking water (from day −6 to day −3) as previously described 10. Mice were then switched to regular drinking water. On day −1, mice were injected intraperitoneally with clindamycin (30mg/kg) followed by a C. difficile VPI 10463 inoculation (105 spores) by oral gavage. Mice were administered with either subcutaneous CSA13, oral CSA13-Eudragit, or oral metabolites from day 0 to day 10 followed by observation until day 20. Other groups received intracolonic administration of CSA13 and CSA44 from day 0 to day 3. Mouse colonic and fecal samples were collected on day 3 for further analyses.
Blood samples were collected for immunophenotyping using flow cytometry and stained for CD3, CD4, CD8a, CD19, CD138, CD69, CD86, CD11b, CD161, CD11c, MHC II, Ly6G, Ly6C with antibodies from BioLegend, BD, eBioscience, and Milteny Biotech. Dead cells were excluded with eFluor 520 Fix Viability dye (Miltenyi Biotech).
Vancomycin-dependent relapse model
Vancomycin treatment suppresses initial C. difficile-mediated colitis, but causes relapse after withdrawal 11. To determine whether CSA13 or oral metabolites prevented C. difficile relapse after vancomycin treatment, the C. difficile-infected mice were treated with vancomycin (50mg/kg daily) intraperitoneally from day 0 to day 4. Treatments started 8 hours after inoculation. Mice were concurrently administered with either subcutaneous CSA13, oral CSA13-Eudragit, or oral metabolites from day 0 to day 4 followed by observation until day 10.
Quantitation of CSA13 by combined liquid chromatography/tandem mass spectrometry-multiple reaction monitoring
Fecal samples were weighed (10–20 mg each), homogenized in water (300 μL) after the addition of the internal standard (CSA 44, a structural analogue of CSA 13; 1 nmol), centrifuged, (16,000 x g, 5 min, room temperature), and the supernatant was treated with acetic anhydride (100μL, 60°C, 60 min). The reaction mixture was then dried in a vacuum centrifuge, HPLC injection solvent added (water/acetonitrile/formic acid, 50/50/0.1, 100μL), and after centrifugation (as above) aliquots (typically 10μL) were injected onto a reverse phase HPLC column that was eluted with an increasing concentration of acetonitrile. The effluent from the column was passed into an electrospray ionization source connected to a triple quadrupole mass spectrometer (Agilent 6460) scanning in previously optimized conditions for the parent-fragment ion transitions for triacetyl derivatives of CSA13 and CSA44. Peak areas for the corresponding signals were computed with instrument manufacturer-supplied software and interpolated from a standard curve simultaneously prepared with each batch of samples using the same amount of internal standard and increasing amounts of CSA13 (0, 0.5, 1, 2 and 4 nmol, each in duplicate). The assay has a Limit of Detection of around 1 pmol injected.
Fecal microbiota transplantation and sterile fecal filtrate transplantation
Fecal pellets from s.c. CSA13-treated C. difficile-infected donor mice were collected daily. These were immediately homogenized in ice-cold PBS (1g/ml), followed by low-speed centrifugation for one minute. Via oral gavage, aliquots of the supernatant (200μL) were transferred to a C. difficile-infected recipient mice without CSA13 treatment. The supernatant from one pellet of fecal material from one mouse was transferred to a recipient animal daily from day 0 to day 10. In the control group, the fecal microbiota of CSA13-treated uninfected mice was transferred to untreated uninfected mice. For sterile fecal filtrate preparation, the fecal supernatant was sterilized by 0.22μm filtration. The sterile fecal filtrate (200μL/mouse) was transferred to C. difficile-infected recipient mice without CSA13 treatment via oral gavage.
Toxin A-mediated enteritis
Eight-week-old male and female C56BL/6J mice were anesthetized with isoflurane. Ileal loops (2cm long) were formed using surgical sutures. Toxin A (10μg/loop) and/or CSA13 (3μM) in normal saline (total volume 200μL per loop) was injected into the ileal loops as previously described 10, 12. Four hours later, mice were euthanized and ileal tissues were obtained for further analyses.
Animal experiment statement
All animal studies were approved by the UCLA Institutional Animal Research Committee (#2007-116). All experiments adhered to standards of ARRIVE guidelines. C57BL/6J mice (stock #000664) were purchased from Jackson Laboratories and maintained at the UCLA animal facility under standard environmental conditions with a 12-hour light period and a 12-hour dark period per day; 25°C room temperature. They were housed in disposable plastic cages with HEPA-filtered air circulation, autoclaved bedding, regular animal chow (6% fat, #7013 Harlan Laboratories), and sterile water ad libitum. All interventions were performed during the light cycle. The severity of toxin A-mediated enteritis and CDI colitis was scored as previously published 13.
Antimicrobial activity assays
C. difficile strain VPI 10463 (105) spores were incubated with various concentrations of CSA13 or metabolites in 1mL of BHIS+0.1% taurocholate liquid media for 24 hours at 37°C. The bacterial viability was measured by absorbance at 600nm. For the determination of minimum inhibitory concentrations (MICs), the same overnight culture (100μL) was inoculated to the BHIS-taurocholate-agar plates. The plates were incubated for 24 hours at 37°C, followed by observation of the formation of C. difficile colonies.
Fecal microbiome and metabolomic analysis
Fecal samples were collected for 16S sequencing. DNA extraction, amplification of the V4 region of the 16S ribosomal RNA gene, and 2×150bp sequencing on an Illumina HiSeq 2500 were performed as previously described 14. Observable taxonomic units were picked against the May 2013 version of the Greengenes database, pre-filtered at 97% identity, in QIIME v1.9.1 15. Sequence depth ranged from 166,423 to 406,785. Alpha diversity metrics (i.e. bacterial diversity within a sample) and beta diversity (differences in composition across samples) were calculated using data rarefied to 166,423 sequences. Alpha diversity metrics included Faith’s phylogenetic diversity metric, Chao1, and Shannon index. Beta diversity was calculated using unweighted UniFrac and visualized by principal coordinates analysis. Adonis, a permutational analysis of variance, was performed using 100,000 permutations to test for differences in beta diversity across the groups 16. Fecal samples collected on day 3 post-infection were also used for metabolomic analysis (Metabolon, Inc.).
Quantitative real-time RT-PCR and PCR arrays
Quantitative real-time RT-PCR was performed as previously described 10. For PCR arrays, RNA was converted to cDNA with RT2 First Strand kit (#330401, Qiagen). Mouse antibacterial response PCR arrays (PAMM-148Z) were performed with the RT2 SYBR Green PCR master mix (#330501, Qiagen) in Bio-Rad CFX384 PCR machine. Results were expressed as relative fold difference.
Cytotoxicity tests
Mouse 3T3 fibroblasts were serum starved overnight and then incubated with serum free DMEM media containing toxin A (0.1μg/ml) and metabolites (1mg/ml) for 2 hours. 3-Aminoisobutyric acid (3AIBA) and citrulline were dissolved in water. Retinyl acetate was used as a water-soluble form of retinol. Ursodeoxycholic acid (UDCA) was suspended in 2% BSA solution. Microphotographs to observe cell rounding were taken in a blinded manner.
One gram of mouse fecal material was suspended in 1ml of ice-cold PBS, followed by low-speed centrifugation for one minute. The fecal supernatant was sterilized by 0.22μm filtration. Human colonic CCD-18Co fibroblasts were serum starved overnight and then incubated with serum-free MEM media containing toxin A, CSA13, and sterile fecal filtrate for 4 hours as previously described 12, 17. Ten microliters of the sterile fecal filtrate was added to the 1ml of fibroblast cell culture. Microphotographs to observe cell rounding were taken in a blinded manner. The quantitative difference in cell rounding was observed and scored as previously described 18. The relative cytotoxicity of the sterile fecal filtrates was based on the cell rounding severity scores and a standard curve using known toxin A concentrations.
Cell culture conditions in the cytoskeletal staining study was the same as the cell rounding study. Cells were stained with ActinGreen reagent and observed as previously described 18.
Intestinal organoid rupture tests
Mouse intestinal organoids were prepared following the protocol described by Stemcell Technologies. The intestinal organoids in Matrigel (#356231, Corning) were incubated with IntestiCult Organoid Growth Medium (#06005, Stemcell Technologies) for 7–10 days until the 3D spherical structure was fully developed. The spherical organoids were incubated with toxin A and CSA13 for 4 hours. In a blinded manner microphotographs were taken to record organoid rupture.
TNFα ELISA and inflammasome activity assay
The mouse RAW264.7 macrophages were serum starved overnight and then incubated with toxin A and CSA13 for 4 hours as previously described 10. The TNFα levels in cell-conditioned media were measured with mouse TNFα ELISA (DY410, R&D Systems) as previously described 10.
Inflammasome activity was determined with Caspase-Glo 1 inflammasome assay (G9951, Promega). The macrophages were incubated with C. difficile-conditioned supernatant and CSA13 for 2 hours. The cell-conditioned media were mixed with Caspase-Glo 1 reagent. The luminescence was measured by a Promega GloMax luminometer.
Cell viability assay
Serum-starved human colonic epithelial HT29 cells were seeded in 96-well plates (1 million cells/plate) and were exposed to CSA13, CSA44, or water (vehicle control) for 24 hours. Twenty microliters of CellTiter AQeuous One solution (MTS tetrazolium compound, Promega) was added to each well and incubated at 37°C for 30 minutes. Absorbance at 490 nm (indicating cell viability) was measured using a 96-well plate reader as described previously 19.
Western blot analyses
Western blotting was performed as previously described 10. Proteins were labeled with antibodies (phosphorylated p65 #3032 and GAPDH #2188, Cell Signaling Technologies) and signals were detected by chemiluminescence.
Power analysis and statistical analysis
We included 8 mice per group to achieve statistical significance of body weight difference of the uninfected and C. difficile-infected mice (98% vs. 74%) with SD=12, alpha=0.05, and power=0.8. All experiments were repeated 2–3 times to ensure reproducibility.
Quantitative results were expressed as mean+/−standard error of the mean. Results were analyzed using Prism professional statistics software program (GraphPad, San Diego, CA). Student’s t-tests with Mann-Whitney post tests were used for intergroup comparisons.
Results
Subcutaneous CSA13 and oral CSA13-Eudragit protected mice from primary CDI and relapse
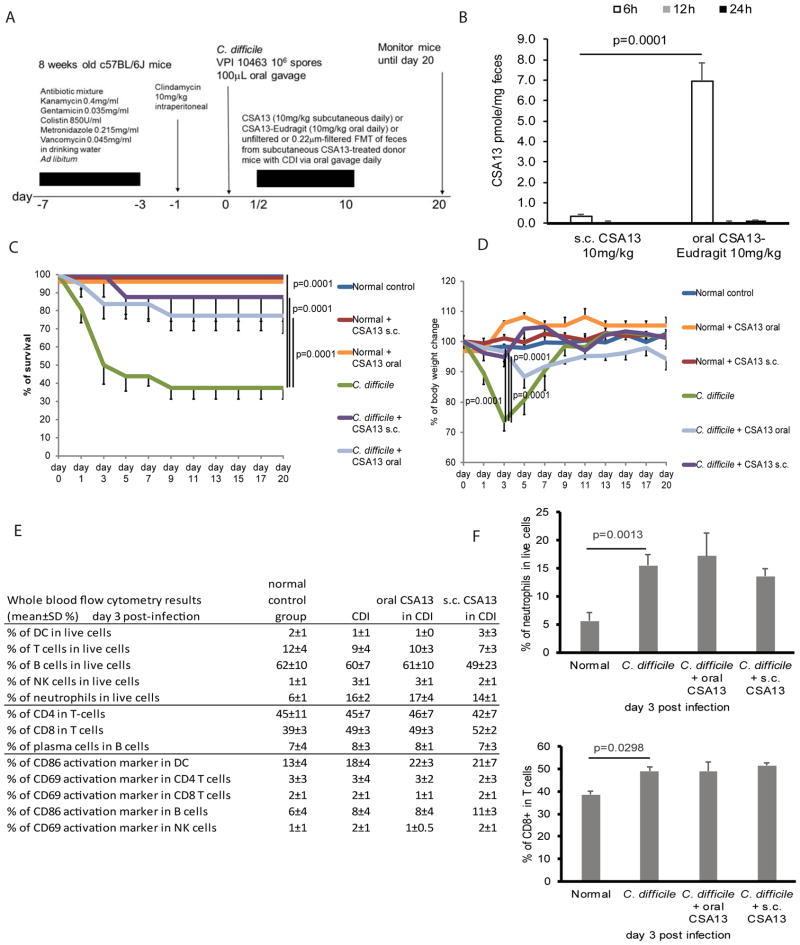
To determine whether CSA13 provides protection against CDI, infected mice were treated with either pure CSA13 subcutaneously or CSA13-Eudragit orally (Figure 1A). Treatments started 8 hours after inoculation as CDI symptoms emerged. Combined liquid chromatography coupled to tandem mass spectrometry with multiple reaction monitoring (MRM) was used to measure CSA13 fecal levels (Supplementary Figure 1). Oral CSA13-Eudragit administration resulted in significantly higher fecal CSA13 levels than subcutaneous CSA13 administration at 6 hours, and then returned to low levels at 12 and 24 hours (Figure 1B). CDI resulted in 60% mortality (day 20) and significant (26%) body weight loss (day 3) among the infected mice (Figure 1C–D). Both subcutaneous and oral administration of CSA13 (day 0–10) significantly reduced mortality (12.5% s.c. CSA13 and 23% oral CSA13-Eudragit) and body weight loss (5% s.c. CSA13 and 3% oral CSA13-Eudragit on day 3) of mice with CDI, suggesting therapeutic effects. After the withdrawal of CSA13 administration, relapse did not occur in the treated mice through day 20. Besides protection against primary CDI, CSA13 was also effective at preventing relapse after vancomycin treatment (data not shown).
Figure 1. Subcutaneous and oral CSA13 administration inhibited mortality and body weight loss caused by CDI.
(A) Experimental plan of C. difficile infection. (B) Fecal levels of CSA13. The fecal levels of CSA13 at 12 and 24 hours were around 0.1 pmole/mg feces. (C) Survival curve. Survival curves of normal groups overlap since all of them had 100% survival. (D) Body weight of mice. (E) Flow cytometry based immunophenotyping of circulating blood cells in mice with CDI collected on day 3. (F) Percentages of neutrophils and CD8 T-cells in blood. Each group consisted of 8 mice.
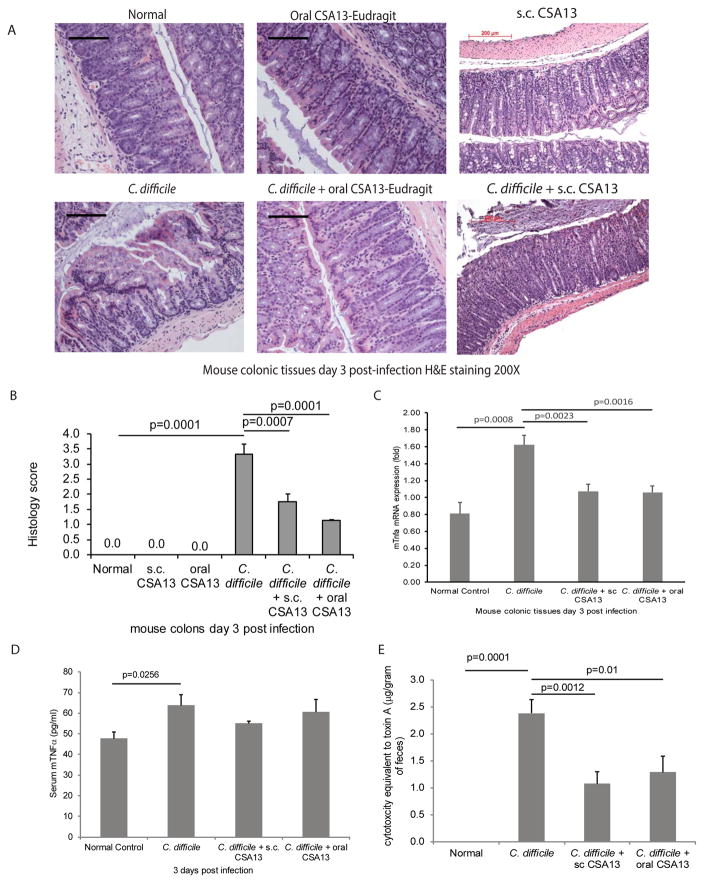
The most severe CDI-mediated colitis appeared on day 3 post-infection. Phenotyping of immune cells indicated that C. difficile increased the proportion of neutrophils and CD8+ cytotoxic T cells in the circulation (Figure 1E–F). The increase of neutrophils and CD8+ T cells was not affected by route of CSA13 administration. CDI caused colonic damages characterized by altered epithelial layer and neutrophil infiltration that was significantly reduced by both subcutaneous CSA13 and oral CSA-Eudragit administration (Figure 2A–B). C. difficile toxin A induces TNFα expression in immune cells and intestinal tissues 10, 20. CSA13 significantly reduced colonic TNFα mRNA expression without affecting serum TNFα levels in mice with CDI (Figure 2C–D). CSA13 modulated colonic, but not systemic, inflammatory responses.
Figure 2. Subcutaneous and oral CSA13 administration reduced colonic damages in CDI.
(A) H&E staining of colonic tissues. Black bars indicate 200μm. (B) Histology score. (C) Colonic tissue TNFα mRNA expression (fold). (D) Serum TNFα levels. (E) Cytotoxicity of toxin in the sterile fecal filtrate. Each group consisted of 8 mice.
As C. difficile produces toxins in the intestine, the sterile fecal filtrates contain C. difficile toxins that can cause cell rounding in fibroblasts 12, 18. Cell rounding was observed in fibroblasts exposed to sterile fecal filtrates of the C. difficile-infected mice (Figure 2E). Reduced cell rounding was observed in fibroblasts exposed to sterile fecal filtrates of CSA13-treated mice, compared to fibroblasts exposed to those of untreated mice (Figure 2E).
CSA13 inhibited C. difficile via modulation of the intestinal microbiome
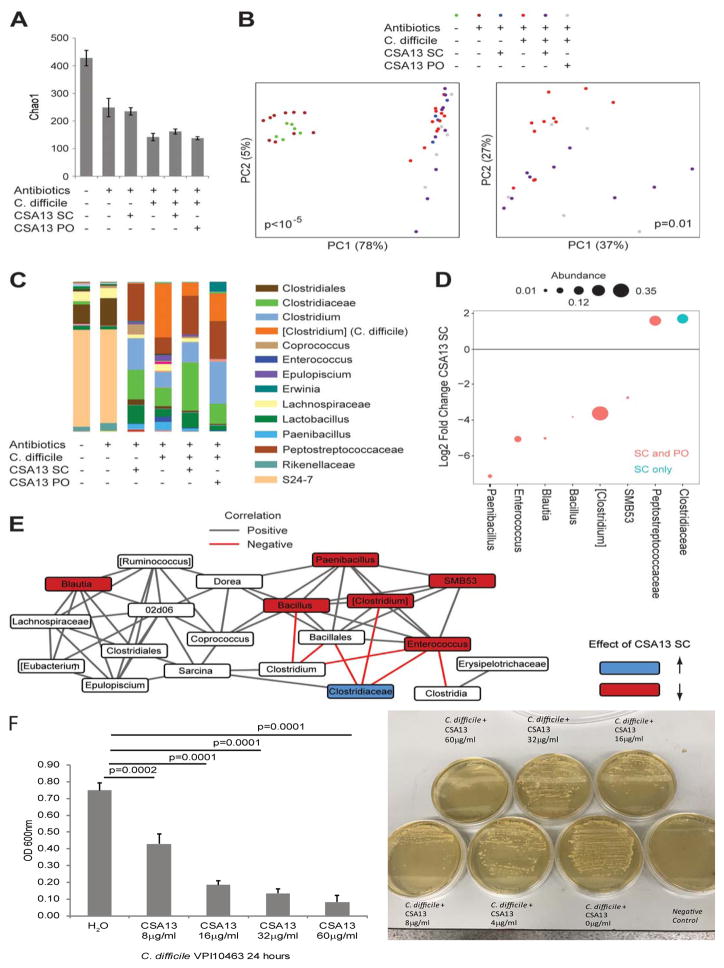
Alpha diversity (Chao1) indicated that microbial richness and evenness of fecal microbiome was reduced by antibiotics, CDI, and CSA13 treatment (Figure 3A). Mice with CDI had significantly altered microbial composition compared to uninfected mice (Figure 3B left panel). The microbial composition of subcutaneous CSA13-treated mice and oral CSA13-Eudragit treated mice were significantly different from the untreated C. difficile-infected mice (Figure 3B right panel). Both subcutaneous CSA13 and oral CSA13-Eudragit administration significantly increased the abundance of Peptostreptococcaceae family while reduced C. difficile abundance (Figure 3C–D). Subcutaneous CSA13 treatment also significantly promoted the abundance of Clostridiaceae family (Figure 3C–D). The effect of subcutaneous CSA13 treatment on the co-occurrence of bacterial genera is shown in Figure 3E. The Clostridiaceae family was inversely correlated with C. difficile. Enterococcus genus was inversely correlated with Clostridia class and Clostridium genus. However, the analyses failed to identify individual bacteria species that mediated the inhibition of CDI.
Figure 3. Subcutaneous and oral CSA13 administration reduced C. difficile abundance in mice with CDI.
(A) Alpha diversity (richness as measured by Chao1) is shown for fecal samples from untreated mice (n=6), antibiotic-pretreated mice (n=10), antibiotic-pretreated mice administered s.c. CSA13 (n=4), C. difficile-infected mice (n=11), C. difficile-infected mice treated with s.c. CSA13 (n=9), and C. difficile-infected mice treated with oral CSA13 (n=5). Mean±SEM. (B) Principal coordinates plot of weighted UniFrac for all mice (left panel) or only C. difficile colonized mice (right panel). The significance of differences in microbial composition (beta diversity) across groups was determined using a permutational method (PERMANOVA) and the p-values are shown in the plots. (C) Genus-level taxonomic summary of mean abundances for each group. Family is shown for microbes that could only be assigned at the family level. (D) Log2 fold change is shown for genera that were enriched or depleted in C. difficile colonized mice after SC CSA13 treatment vs. untreated mice at a false discovery rate threshold of 0.1 or better in negative binomial models implemented in DESeq2. Dots in red indicate genera that were also significantly changed after oral (PO) CSA13-Eudragit treatment (blue indicates significance only with SC treatment). Dot size represents relative abundance of the genus. Only genera with abundance greater than 0.001 are shown. (E) Co-occurrence network of genera with abundance greater than 0.001 in C. difficile infected mice. Edges represent statistically significant correlations between genera across samples after adjustment for CSA13 treatment. Genera that are affected by CSA13 are colored. (F) Left panel: C. difficile spores (105 cfu) were incubated with CSA13 in BHIS-taurocholate media (1mL) for 24 hours. The viability of C. difficile was determined by absorbance reading at 600nm. Right panel: The same culture (100μL) was inoculated to BHIS-taurocholate-agar and incubated for 24 hours. The presence of colonies is shown. Negative control showed no colony. Results represent three independent experiments.
In uninfected mice, subcutaneous CSA13 treatment for 3 days significantly changed the microbial composition and abundance of many bacterial genera (Figure 3B–C). In a separate experiment, oral CSA13-Eudragit administration for 10 days did not significantly change the richness, evenness, and microbial composition of the uninfected mice (Supplementary Figure 2A–C). Oral CSA13-Eudragit treatment increased the abundance of Akkermansia and Lactobacillus genera, and reduced the abundance of Lachnospiraceae family in the uninfected mice, compared to those on day 0.
CSA13 inhibited C. difficile spore germination and cell viability starting at 4μg/ml (Figure 3F, left panel). However, the minimum inhibitory concentration (MIC) of CSA13 against C. difficile was 60μg/ml (Figure 3F, right panel).
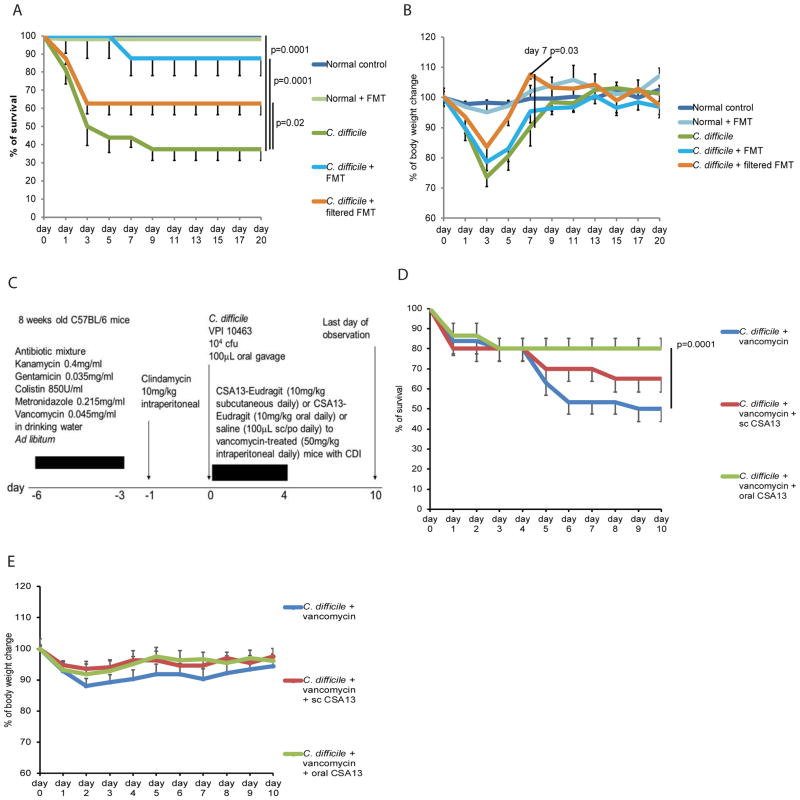
To determine whether the intestinal microbiota in the CSA13-treated mice are responsible for the protective effects of CSA13 in CDI, the freshly-collected fecal materials from C. difficile-infected donor mice with subcutaneous CSA13 treatment were transplanted to the C. difficile-infected recipient mice without CSA13 treatment. Fecal materials from subcutaneous CSA13-treated donor mice should have reduced carryover of intestinal CSA13 via the fecal microbiota transplantation (FMT). FMT reduced mortality with 90% survival, but did not prevent body weight loss in CDI (Figure 4A–B).
Figure 4. Sterile fecal filtrate treatment improved survival and reduced body weight loss in mice with CDI.
(A) Survival curve. Survival curves of normal groups overlap since all of them had 100% survival. (B) Body weight of mice. (C) Experimental plan of the vancomycin-dependent CDI relapse model. (D) Survival curve in a vancomycin-dependent relapse model. (E) Body weight of mice in a vancomycin-dependent relapse model. Each group consisted of 8 mice.
Interestingly, sterile fecal filtrates from the same donor also significantly reduced mortality with 60% survival and faster recovery of body weight (day 7) in the recipient mice, compared to untreated C. difficile-infected mice (Figure 4A–B). This finding led us to hypothesize that CSA13, via microbial interactions, generated protective chemicals against CDI.
CSA13 prevents vancomycin-dependent CDI relapse
Vancomycin is effective for treating primary CDI infection; however, relapse often occurs after drug withdrawal 11. To determine whether CSA13 can prevent vancomycin-dependent relapse, we treated the mice with CSA13 subcutaneously and orally during vancomycin treatment (Figure 4C). Twenty percent of mice died during vancomycin treatment, and an additional 30% of mice developed relapse and died after vancomycin withdrawal (Figure 4D). Oral CSA13-Eudragit, but not subcutaneous CSA13, significantly improved the survival rate after vancomycin withdrawal during day 5–10 (Figure 4D). Relapse occurred randomly after vancomycin withdrawal; body weight changes of individual relapsing mice did not significantly affect the average values of body weight of the groups (Figure 4E). No additional mortality was observed in all groups during day 11–20 (data not shown).
CSA13 has a mild protective effect against toxin A
CDI involves the interactions between C. difficile bacteria, C. difficile toxins, intestinal microbiota, and host cells. The toxin A-mediated enteritis model demonstrated a direct interaction between toxin A and CSA13 without bacterial involvement. Co-incubation of mouse ileal loops with CSA13 (3μM) moderately ameliorated toxin A-induced enteritis and abolished TNFα expression (Supplementary Figure 3A–B). CSA13 (3μM) significantly reduced toxin A-mediated inflammasome gene Pycard (ASC) and NF-κB p65 (RELA) mRNA expression (Supplementary Figure 3C). In mouse fibroblasts, toxin A-mediated intestinal crypt organoid rupture, cell rounding, and actin cytoskeletal disruption was prevented by CSA13 (Supplementary Figure 3D).
CSA13 significantly inhibited TNFα protein expression in mouse macrophages at 10μM (Supplementary Figure 4A). CSA13 (3μM) significantly reduced C. difficile-conditioned media-induced inflammasome activity (Supplementary Figure 4B). NF-κB p65 phosphorylation were also inhibited by CSA13 in macrophages and colonic tissues of C. difficile-infected mice (Supplementary Figure 4C–D). CSA13 slightly increased human colonic epithelial cell viability at low concentrations (Supplementary Figure 4E). In general, CSA13 may have a mild direct cytoprotective effect against toxin A.
Structurally similar CSA44 protects mice without cytoprotective and anti-inflammatory effects
To differentiate between antibacterial and anti-inflammatory effects mediating the initial protective effect of CSA13 against CDI, we compared the protective effects between CSA13 and its structurally similar analog CSA44 (Supplementary Figure 5A). Intracolonic administration of CSA13 and CSA44 protected the mice from CDI with high survival rate and minimal body weight loss (Supplementary Figure 5B–C). CSA44 inhibited C. difficile spore germination and cell viability with the same MIC value as CSA13 (i.e. 60μg/ml) (Supplementary Figure 5D–E).
However, CSA44 (3μM) did not prevent toxin A-mediated cell rounding in 3T3 fibroblasts and did not inhibit toxin A-mediated TNFα expression in mouse macrophages, suggesting the lack of cytoprotective and anti-inflammatory effects (Supplementary Figure 6A–B). High concentration of CSA44 (10μM) significantly inhibited cell viability of human colonic epithelial cells (Supplementary Figure 6C).
CSA13 ameliorates CDI colitis by inhibiting endocannabinoid production and enhancing production of protective metabolites in the intestine
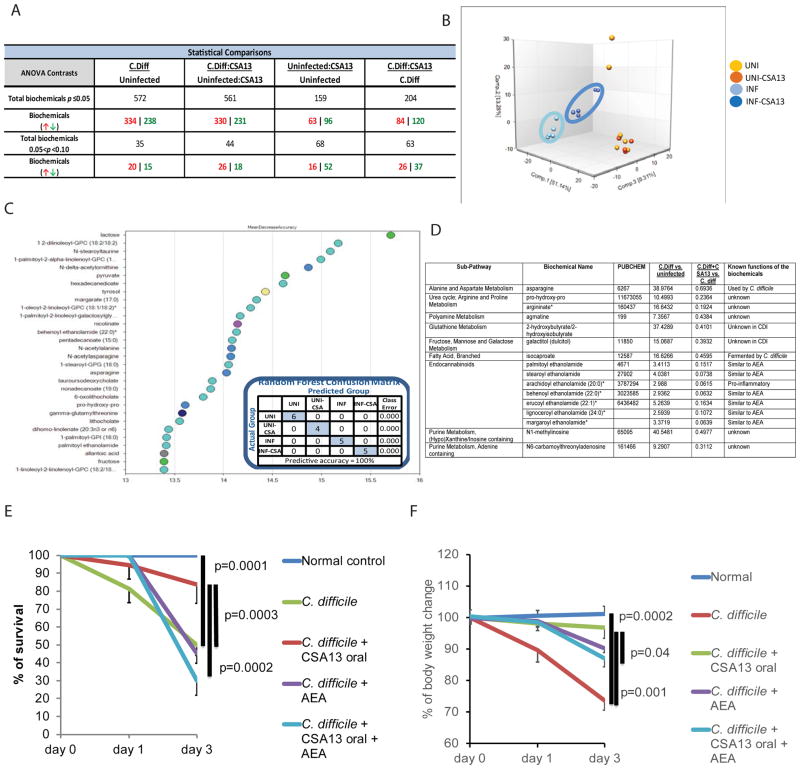
Fecal metabolomic analysis was performed on sterile fecal filtrates from CSA13-treated mice to identify chemicals potentially protective against CDI. The results showed that many metabolites were affected by CDI and oral CSA13-Eudragit treatment (Figure 5A). Oral CSA13-Eudragit significantly changed the metabolite composition of fecal materials (Figure 5B). Random Forest Analysis indicated the most relevant metabolites affected by CDI and CSA13 treatment (Figure 5C). Most notably, fecal levels of many endocannabinoids were increased by CDI, which were then significantly reduced by oral CSA13-Eudragit treatment (Figure 5D). A single intracolonic administration of a representative endocannabinoid (arachidoyl ethanolamide or AEA) significantly reversed the oral CSA13-Eudragit-mediated protection of CDI mortality and body weight loss (Figure 5E–F), suggesting that CSA13 at least partially ameliorated CDI colitis by reducing fecal endocannabinoid levels. Colonic TPRV1 mRNA expression was not affected by CDI or CSA13 treatment (Supplementary Figure 4F).
Figure 5. CSA13 reduced fecal pro-inflammatory endocannabinoids in mice with CDI.
(A) Summary of metabolomic analysis of fecal samples, collected on day 3. (B) Principle Component Analysis of all groups showing all groups are separate from one another. (C) Random Forest Analysis shows top 30 metabolites which most strongly contribute to the binning of individual samples into groups. (D) Fecal metabolites that were significantly increased by CDI and reduced by CSA13 treatment. (E) Mice with CDI were treated with a single intracolonic administration of AEA (10mg/kg) on day 0. Oral CSA13-Eudragit was given from day 0 to day 3. Survival curve of one-time AEA treatment. (F) Body weight change of one-time AEA treatment. Each group consisted of 8 mice.
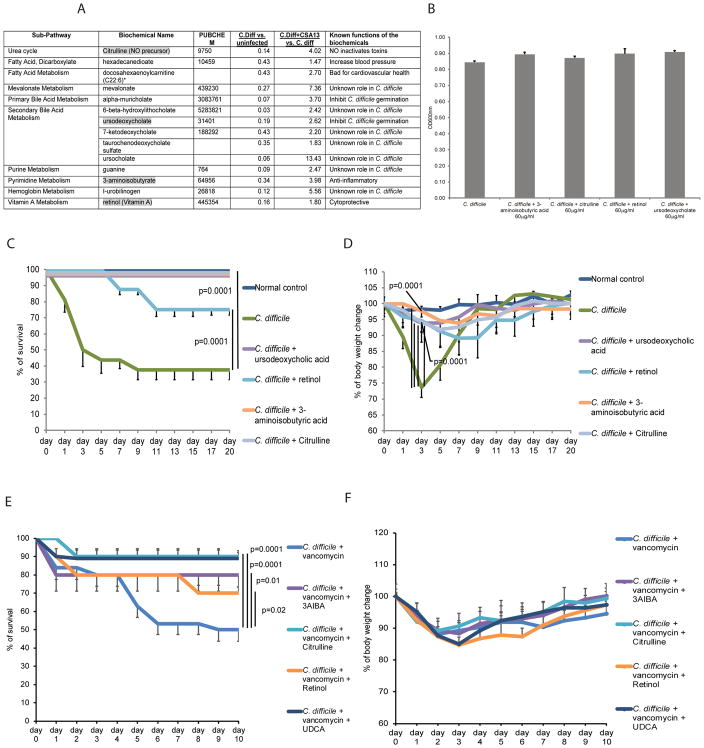
On the other hand, fecal levels of some metabolites were reduced in CDI and this reduction was counteracted by CSA13-Eudragit treatment (Figure 6A). Four metabolites (3AIBA, citrulline, retinol, and UDCA) were chosen for further investigation. C. difficile germination and viability were not affected by these four metabolites (Figure 6B), indicating no antibacterial effect against C. difficile.
Figure 6. CSA13-enhanced fecal metabolites are associated with amelioration of CDI.
(A) Fecal metabolites that were reduced by CDI and enhanced by CSA13 treatment. (B) C. difficile spores (105 cfu) were incubated with metabolites (60mg/L) in BHIS-taurocholate media (1mL) for 24 hours. The viability of C. difficile was determined by absorbance reading at 600nm. Metabolites did not affect C. difficile viability. (C) Survival curve of oral metabolite treatment in primary CDI model. (D) Body weight change of oral metabolite treatment in primary CDI model. (E) Survival curve of oral metabolite treatment in the vancomycin-dependent relapse model. (F) Body weight of mice in the vancomycin-dependent relapse model. Each group consisted of 8 mice.
Oral administration of metabolites from day 0 to day 10 significantly reduced CDI mortality and body weight loss (day 3) (Figure 6C–D). No relapse occurred after the withdrawal of oral metabolite administration through day 20 (Figure 6C–D). When the mice were treated with oral metabolites along with vancomycin administration from day 0 to day 4, all four oral metabolites significantly improved survival against vancomycin-dependent relapse (Figure 6E). As relapse occurred randomly during day 5–10, there were no significant difference in body weight between the control and metabolite-treated groups (Figure 6F). No additional mortality was observed in any groups between day 11–20 (data not shown). Experimental plans of oral metabolite administration in the primary CDI model and vancomycin-dependent relapse model are shown in Supplementary Figure 7A–B).
Although none of these four metabolites directly reversed toxin A-mediated cell rounding in cultured fibroblasts (Supplementary Figure 7C), the sterile fecal filtrates of metabolite-treated mice resulted in less cell rounding than those of untreated C. difficile-infected mice (Supplementary Figure 7D), suggesting reduced cytotoxicity. Citrulline, retinyl acetate (water-soluble form of retinol), and UDCA significantly inhibited toxin A-mediated TNFα protein secretion in mouse macrophages (Supplementary Figure 7E), suggesting anti-inflammatory effects. 3AIBA had no anti-inflammatory effect against toxin A (Supplementary Figure 7E), but it moderately promoted colonic epithelial cell migration (wound healing) with or without the presence of toxin A (Supplementary Figure 8).
Discussion
This study demonstrated the effectiveness of subcutaneous CSA13 and oral CSA13-Eudragit administration for inhibiting CDI. CSA13 may mediate its protective effects via three mechanisms: (1) inhibition of C. difficile; (2) reduction of pro-inflammatory metabolite levels; and (3) increase of protective metabolite levels in the intestine.
The sustained protection against CDI relapse after withdrawal might be mediated by the establishment of the protective intestinal microbiome. Our FMT experiments suggest the involvement of microbiota (Figure 4A–B). CSA13-enhanced Peptostreptococcaceae and Clostridiaceae families consist of many species, and their functions in CDI are unknown. Other studies demonstrated that Peptostreptococcaceae and Clostridiaceae families are associated with inflammatory bowel disease and colorectal cancer, but their functional roles in these diseases had not been validated 21–23. As CSA13 affected many bacterial genera, it is not clear how CSA13-modulated microbiome collectively inhibited C. difficile abundance.
As shown in toxin A-mediated enteritis model (Supplementary Figure 3A and B), direct cytoprotective and anti-inflammatory effects of CSA13 against toxin A is limited. Inflammasome (Pycard/ASC) and NF-κB inhibition is linked to prevention of cell death and tissue damages in response to C. difficile toxins 10, 24–26. The involvement of NF-κB and inflammasome in the CSA13-mediated cytoprotective and anti-inflammatory effect is unknown and beyond the scope of this investigation. Comparing the differences between CSA13 and CSA44 (Supplementary Figure 3–6), direct cytoprotective and anti-inflammatory effects of CSA13 and CSA44 are not essential in the protection against CDI. We believe that the CSA13-mediated reduction of intestinal TNFα expression should be secondary to histological improvement (Figure 2C and Supplementary Figure 3B).
The short period of high fecal CSA13 levels at 6 hours after subcutaneous and oral CSA13 administration (Figure 1B) was consistent with gastrointestinal transit time of mice 27. Oral CSA13-Eudragit in the intestine may exert temporary antibacterial effect against C. difficile. Colonic bioavailability of CSA13 in the subcutaneous CSA13 group may be too low to exert direct antibacterial effect against C. difficile (Figure 1B). As the therapeutic effect of subcutaneous and oral CSA13 administration depends on the establishment of modified intestinal microbiome, continuous maintenance of antibacterial CSA13 levels is not necessary.
We speculate that the insufficient protection against vancomycin-dependent relapse in the subcutaneous CSA13-treated group was caused by low colonic bioavailability of CSA13 (Figure 4D). A recent report demonstrated that failure of vancomycin tapering increases the failure rate of FMT in CDI patients, suggesting the link between the disturbed intestinal microbiome and CDI recurrence 28. As vancomycin treatment disturbed the intestinal microbiome 29, the low colonic level of CSA13 failed to establish a protective microbiome environment in the presence of strong interference from vancomycin.
CSA13-dependent modifications of intestinal microbiome are different with and without CDI (Figure 3A–E and Supplementary Figure 2). CSA13-mediated intestinal microbiome and metabolomic profiles in other colitis models are also distinct from this CDI model 30. Therefore, the therapeutic effects of CSA13 in other gastrointestinal diseases may depend on the other microbial species and metabolites.
Compared to the MICs of vancomycin and metronidazole (MIC <2μg/ml) 31, 32, the direct antibacterial effect of CSA13 against C. difficile is weak (Figure 3F). When the normal gut microflora is disrupted, inhibition of C. difficile alone may help ameliorate primary infection but cannot prevent relapse. Although FMT can treat CDI 5, the pathogenicity (bacteria, fungi, and virus) of the FMT materials have not been fully characterized 33. It is also difficult to remove pathogens in the FMT materials. There is no consensus about how to optimize the microbial composition of FMT materials as the functions of FMT microbiota and their interactions in CDI patients are not fully understood 34. CSA13, as a modulator of intestinal microbiota, may circumvent the disadvantages of FMT.
Metabolomic analysis also provided insight into the protective mechanisms of CSA13. Toxin A was shown to stimulate endocannabinoid expression 35. Endocannabinoids bind to the capsaicin receptor TRPV1 and trigger the release of pro-inflammatory Substance P, leading to colitis 35. The CSA13-mediated inhibition of endocannabinoid expression may be associated with the amelioration of colonic inflammation.
The core functions of the four CSA13-dependent protective metabolites have been revealed in various animal experiments or clinical trials. 3AIBA reduces hepatic inflammation in obese mice and hepatic apoptosis in diabetic mice 36, 37. Retinol is cytoprotective in epithelial cells exposed to toxin A 38. Citrulline is converted to nitric oxide, which neutralizes C. difficile toxins 39, 40. UDCA prevents C. difficile germination 41. The anti-inflammatory effects of three metabolites in mouse macrophages and the pro-healing effect of 3AIBA in colonic epithelial cells have not been previously reported (Supplementary Figure 7–8). However, the interactions between intestinal metabolites in the CSA13-treated mice remain complex. Many fecal metabolites have multiple functions in CDI. Identification of the specific metabolites responsible for modulating the intestinal microbiota is a goal of future work. A high-throughput screening platform may be necessary to discover new functions of these metabolites.
In conclusion, CSA13 is a new drug against CDI with pleiotropic mechanisms. The therapeutic effect of CSA13 is mediated by both direct antibacterial effect against C. difficile and modification of intestinal microbiome and its metabolomic environment. CSA13 has direct cytoprotective and anti-inflammatory effects against toxin A, but they are not essential in vivo. Oral CSA13-Eudragit is more convenient to use than subcutaneous and intracolonic CSA13. Oral CSA13-Eudragit is superior to vancomycin and metronidazole for the prevention of relapse. Some of the CSA13-dependent fecal metabolites modulate the development of CDI.
Supplementary Material
Acknowledgments
Grant Support:
This work was supported by NIH K01 grant (DK084256), R03 grant (DK103964), and National Center for Advancing Translational Sciences UCLA CTSI Grant (UL1TR001881) to HWK.
Funding sponsors were not involved in the study design and collection, analysis, and interpretation of data.
We thank Prof. Charalabos Pothoulakis, MD for technical and financial assistance to this project. We thank Prof. Paul Savage (Brigham Young University, Utah) for providing pure CSA13, pure CSA44, and BODIPY-labeled CSA13 and Dr. Xingguo Cheng (Southwest Research Institute, Texas) for providing the CSA13-Eudragit. We thank N8 Medical, Inc. for collaborating with us in the gastrointestinal research.
Footnotes
Disclosure: All authors have nothing to disclose. No conflict of interest exists.
Author contributions:
J.W., S.G., C.X., C.C.M., C.O., E.C.L., D.H.T. contributed the animal study and cell culture data.
J.P.J. and V.L. performed microbiome analysis.
K.F.F. and T.M. performed LC/MS analysis.
M.R. performed immunophenotyping.
X.C. produced toxins A and B.
H.W.K. supervised the study, drafted the manuscript, and gave approval of the submitted and the final version.
All authors reviewed and approved the final version of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.El Feghaly RE, Bangar H, Haslam DB. The molecular basis of Clostridium difficile disease and host response. Curr Opin Gastroenterol. 2015;31:24–9. doi: 10.1097/MOG.0000000000000131. [DOI] [PubMed] [Google Scholar]
- 2.Kaiser AM, Hogen R, Bordeianou L, et al. Clostridium Difficile Infection from a Surgical Perspective. J Gastrointest Surg. 2015;19:1363–77. doi: 10.1007/s11605-015-2785-4. [DOI] [PubMed] [Google Scholar]
- 3.Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. The American journal of gastroenterology. 2013;108:478–98. doi: 10.1038/ajg.2013.4. quiz 499. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein EJ, Babakhani F, Citron DM. Antimicrobial activities of fidaxomicin. Clin Infect Dis. 2012;55(Suppl 2):S143–8. doi: 10.1093/cid/cis339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borgia G, Maraolo AE, Foggia M, et al. Fecal microbiota transplantation for Clostridium difficile infection: back to the future. Expert Opin Biol Ther. 2015;15:1001–14. doi: 10.1517/14712598.2015.1045872. [DOI] [PubMed] [Google Scholar]
- 6.Epand RM, Epand RF, Savage PB. Ceragenins (cationic steroid compounds), a novel class of antimicrobial agents. Drug News Perspect. 2008;21:307–11. doi: 10.1358/dnp.2008.21.6.1246829. [DOI] [PubMed] [Google Scholar]
- 7.Carmona-Ribeiro AM, de Melo Carrasco LD. Cationic antimicrobial polymers and their assemblies. Int J Mol Sci. 2013;14:9906–46. doi: 10.3390/ijms14059906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bucki R, Niemirowicz K, Wnorowska U, et al. Bactericidal Activity of Ceragenin CSA-13 in Cell Culture and in an Animal Model of Peritoneal Infection. Antimicrob Agents Chemother. 2015;59:6274–82. doi: 10.1128/AAC.00653-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibson PR, Fixa B, Pekarkova B, et al. Comparison of the efficacy and safety of Eudragit-L-coated mesalazine tablets with ethylcellulose-coated mesalazine tablets in patients with mild to moderately active ulcerative colitis. Aliment Pharmacol Ther. 2006;23:1017–26. doi: 10.1111/j.1365-2036.2006.02861.x. [DOI] [PubMed] [Google Scholar]
- 10.Hing TC, Ho S, Shih DQ, et al. The antimicrobial peptide cathelicidin modulates Clostridium difficile-associated colitis and toxin A-mediated enteritis in mice. Gut. 2013;62:1295–305. doi: 10.1136/gutjnl-2012-302180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warren CA, van Opstal EJ, Riggins MS, et al. Vancomycin treatment’s association with delayed intestinal tissue injury, clostridial overgrowth, and recurrence of Clostridium difficile infection in mice. Antimicrob Agents Chemother. 2013;57:689–96. doi: 10.1128/AAC.00877-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koon HW, Ho S, Hing TC, et al. Fidaxomicin inhibits Clostridium difficile toxin A-mediated enteritis in the mouse ileum. Antimicrob Agents Chemother. 2014;58:4642–50. doi: 10.1128/AAC.02783-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pothoulakis C, Castagliuolo I, LaMont JT, et al. CP-96,345, a substance P antagonist, inhibits rat intestinal responses to Clostridium difficile toxin A but not cholera toxin. Proc Natl Acad Sci U S A. 1994;91:947–51. doi: 10.1073/pnas.91.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tong M, Jacobs JP, McHardy IH, et al. Sampling of intestinal microbiota and targeted amplification of bacterial 16S rRNA genes for microbial ecologic analysis. Curr Protoc Immunol. 2014;107:7 41 1–11. doi: 10.1002/0471142735.im0741s107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–6. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson MJ. A new method for non-parametric multivariate analysis of variance. Austral Ecology. 2001;26:32–46. [Google Scholar]
- 17.Yoo JH, Ho S, Tran DH, et al. Anti-fibrogenic effects of the anti-microbial peptide cathelicidin in murine colitis-associated fibrosis. Cell Mol Gastroenterol Hepatol. 2015;1:55–74. e1. doi: 10.1016/j.jcmgh.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koon HW, Su B, Xu C, et al. Probiotic Saccharomyces boulardii CNCM I-745 prevents outbreak-associated Clostridium difficile-associated cecal inflammation in hamsters. Am J Physiol Gastrointest Liver Physiol. 2016 doi: 10.1152/ajpgi.00150.2016. ajpgi 00150 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koon HW, Zhao D, Na X, et al. Metalloproteinases and transforming growth factor-alpha mediate substance P-induced mitogen-activated protein kinase activation and proliferation in human colonocytes. J Biol Chem. 2004;279:45519–27. doi: 10.1074/jbc.M408523200. [DOI] [PubMed] [Google Scholar]
- 20.Koon HW, Shih DQ, Hing TC, et al. Human monoclonal antibodies against Clostridium difficile toxins A and B inhibit inflammatory and histologic responses to the toxins in human colon and peripheral blood monocytes. Antimicrob Agents Chemother. 2013;57:3214–23. doi: 10.1128/AAC.02633-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen W, Liu F, Ling Z, et al. Human intestinal lumen and mucosa-associated microbiota in patients with colorectal cancer. PLoS One. 2012;7:e39743. doi: 10.1371/journal.pone.0039743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tannock GW, Lawley B, Munro K, et al. Comprehensive analysis of the bacterial content of stool from patients with chronic pouchitis, normal pouches, or familial adenomatous polyposis pouches. Inflamm Bowel Dis. 2012;18:925–34. doi: 10.1002/ibd.21936. [DOI] [PubMed] [Google Scholar]
- 23.Peters BA, Dominianni C, Shapiro JA, et al. The gut microbiota in conventional and serrated precursors of colorectal cancer. Microbiome. 2016;4:69. doi: 10.1186/s40168-016-0218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cowardin CA, Kuehne SA, Buonomo EL, et al. Inflammasome activation contributes to interleukin-23 production in response to Clostridium difficile. MBio. 2015:6. doi: 10.1128/mBio.02386-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ng J, Hirota SA, Gross O, et al. Clostridium difficile toxin-induced inflammation and intestinal injury are mediated by the inflammasome. Gastroenterology. 2010;139:542–52. 552e1–3. doi: 10.1053/j.gastro.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 26.Koon HW, Wang J, Mussatto CC, et al. Fidaxomicin and OP-1118 inhibit C. difficile toxin A- and B-mediated inflammatory responses via inhibition of NF-kappaB activity. Antimicrob Agents Chemother. 2017 doi: 10.1128/AAC.01513-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Padmanabhan P, Grosse J, Asad AB, et al. Gastrointestinal transit measurements in mice with 99mTc-DTPA-labeled activated charcoal using NanoSPECT-CT. EJNMMI Res. 2013;3:60. doi: 10.1186/2191-219X-3-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patron RL, Hartmann CA, Allen S, et al. Vancomycin Taper and Risk of Failure of Fecal Microbiota Transplant in Patients With Recurrent Clostridium difficile Infection. Clin Infect Dis. 2017 doi: 10.1093/cid/cix511. [DOI] [PubMed] [Google Scholar]
- 29.Lewis BB, Buffie CG, Carter RA, et al. Loss of Microbiota-Mediated Colonization Resistance to Clostridium difficile Infection With Oral Vancomycin Compared With Metronidazole. J Infect Dis. 2015;212:1656–65. doi: 10.1093/infdis/jiv256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu C, Ghali S, Wang J, et al. CSA13 inhibits colitis-associated intestinal fibrosis via a formyl peptide receptor like-1 mediated HMG-CoA reductase pathway. Sci Rep. 2017;7:16351. doi: 10.1038/s41598-017-16753-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aspevall O, Lundberg A, Burman LG, et al. Antimicrobial susceptibility pattern of Clostridium difficile and its relation to PCR ribotypes in a Swedish university hospital. Antimicrob Agents Chemother. 2006;50:1890–2. doi: 10.1128/AAC.50.5.1890-1892.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buchler AC, Rampini SK, Stelling S, et al. Antibiotic susceptibility of Clostridium difficile is similar worldwide over two decades despite widespread use of broad-spectrum antibiotics: an analysis done at the University Hospital of Zurich. BMC Infect Dis. 2014;14:607. doi: 10.1186/s12879-014-0607-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manges AR, Steiner TS, Wright AJ. Fecal microbiota transplantation for the intestinal decolonization of extensively antimicrobial-resistant opportunistic pathogens: a review. Infect Dis (Lond) 2016;48:587–92. doi: 10.1080/23744235.2016.1177199. [DOI] [PubMed] [Google Scholar]
- 34.Khoruts A, Sadowsky MJ, Hamilton MJ. Development of fecal microbiota transplantation suitable for mainstream medicine. Clin Gastroenterol Hepatol. 2015;13:246–50. doi: 10.1016/j.cgh.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 35.McVey DC, Schmid PC, Schmid HH, et al. Endocannabinoids induce ileitis in rats via the capsaicin receptor (VR1) J Pharmacol Exp Ther. 2003;304:713–22. doi: 10.1124/jpet.102.043893. [DOI] [PubMed] [Google Scholar]
- 36.Shi CX, Zhao MX, Shu XD, et al. beta-aminoisobutyric acid attenuates hepatic endoplasmic reticulum stress and glucose/lipid metabolic disturbance in mice with type 2 diabetes. Sci Rep. 2016;6:21924. doi: 10.1038/srep21924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Begriche K, Massart J, Abbey-Toby A, et al. Beta-aminoisobutyric acid prevents diet-induced obesity in mice with partial leptin deficiency. Obesity (Silver Spring) 2008;16:2053–67. doi: 10.1038/oby.2008.337. [DOI] [PubMed] [Google Scholar]
- 38.Maciel AA, Oria RB, Braga-Neto MB, et al. Role of retinol in protecting epithelial cell damage induced by Clostridium difficile toxin A. Toxicon. 2007;50:1027–40. doi: 10.1016/j.toxicon.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee JY, Hirota SA, Glover LE, et al. Effects of nitric oxide and reactive oxygen species on HIF-1alpha stabilization following clostridium difficile toxin exposure of the Caco-2 epithelial cell line. Cell Physiol Biochem. 2013;32:417–30. doi: 10.1159/000354448. [DOI] [PubMed] [Google Scholar]
- 40.Savidge TC, Urvil P, Oezguen N, et al. Host S-nitrosylation inhibits clostridial small molecule-activated glucosylating toxins. Nat Med. 2011;17:1136–41. doi: 10.1038/nm.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weingarden AR, Chen C, Zhang N, et al. Ursodeoxycholic Acid Inhibits Clostridium difficile Spore Germination and Vegetative Growth, and Prevents the Recurrence of Ileal Pouchitis Associated With the Infection. J Clin Gastroenterol. 2016;50:624–30. doi: 10.1097/MCG.0000000000000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.