Abstract
Despite the causal role of cigarette smoking in atherosclerotic cardiovascular disease (ASCVD), the underlying mechanisms are not fully understood. We evaluated the joint relationship between smoking and inflammatory markers with ASCVD risk. We tested cross-sectional associations of self-reported smoking status (never, former, current) and intensity (packs/day) with lipoprotein-associated phospholipase A2 (Lp-PLA2) activity and high-sensitivity C-reactive protein (hsCRP) in 10,506 ARIC participants at Visit 4 (1996–1998). Using Cox hazard models adjusted for demographic and traditional ASCVD risk factors, we examined the associations of smoking status and intensity with incident adjudicated ASCVD events (n=1,745 cases), over an average of 17 years, stratified by Lp-PLA2 and hsCRP categories. Greater packs/day smoked was linearly associated with higher levels of both Lp-PLA2 and hsCRP among current smokers. Compared to never smokers, the hazard ratio for incident ASCVD in current smokers was 2.04 (95%CI 1.76–2.35). Among current smokers, the risk for ASCVD per 1 pack/day greater was 1.39 (1.10–1.76). Both Lp-PLA2 activity ≥253 nmol/min/mL and hsCRP >3 mg/L identified current smokers at the highest risk for incident ASCVD, with similar hazard ratios. HsCRP better risk-stratified current smokers based on intensity. Among current smokers, hsCRP improved ASCVD prediction beyond traditional risk factors better than Lp-PLA2 (C-statistic 0.675 for hsCRP vs 0.668 for Lp-PLA2, p=0.001). In this large cohort with long follow-up, we found a dose-response relationship between smoking intensity with Lp-PLA2 activity, hsCRP and ASCVD events. While both Lp-PLA2 activity and hsCRP categories identified high-risk among current smokers, hsCRP may better stratify risk of future ASCVD.
Keywords: inflammation, smoking, atherosclerotic cardiovascular disease, risk prediction
INTRODUCTION
Cigarette smoking is one of the major preventable causes of death and atherosclerotic cardiovascular disease (ASCVD) globally.1,2 Some data have reported a dose-response relation between smoking intensity and burden with ASCVD.3,4 Other data suggested that the relation has a low “ceiling”, making smoking status more important than smoking burden or intensity.5–7 However, a recent study using modern statistical techniques found that smoking intensity (pack/day) may be a better way to model ASCVD outcomes compared to smoking status and pack-years.8 Although the causal role of smoking and ASCVD is well established, only about 33% of smokers develop a smoking-related cardiovascular illness.1 While all smokers should be offered guidance regarding smoking cessation, identification of higher-risk smokers might help triage allocation of more expensive resources (i.e. case management programs). Inflammation is on the causal pathway linking smoking to ASCVD.9 The objective of this study was to evaluate the usefulness of 2 inflammatory biomarkers approved for routine clinical use in ASCVD risk prediction – high sensitivity C-reactive protein (hsCRP)10 and lipoprotein associated phospholipase A2 (Lp-PLA2) activity11 – in identifying smokers at high-risk for ASCVD.
METHODS
The Atherosclerosis Risk in Communities (ARIC) study design, objectives, sampling strategies and examination techniques have been well described elsewhere.12 Briefly, ARIC is a large, ongoing, prospective, cohort of 15,792 participants, who were predominantly white or black race, aged 45–64 years, and recruited from four US communities; Forsyth County, North Carolina; Jackson, Mississippi; suburban Minneapolis, Minnesota; and Washington County, Maryland. The baseline examination (Visit 1) was conducted between 1987–1989, with four more examinations occurring between 1990–1992 (Visit 2), 1993–1995 (Visit 3), 1996–1998 (Visit 4) and 2011–2013 (Visit 5). The present analysis was done on participants who attended Visit 4 where Lp-PLA2 activity and hsCRP were measured in all ARIC participants.
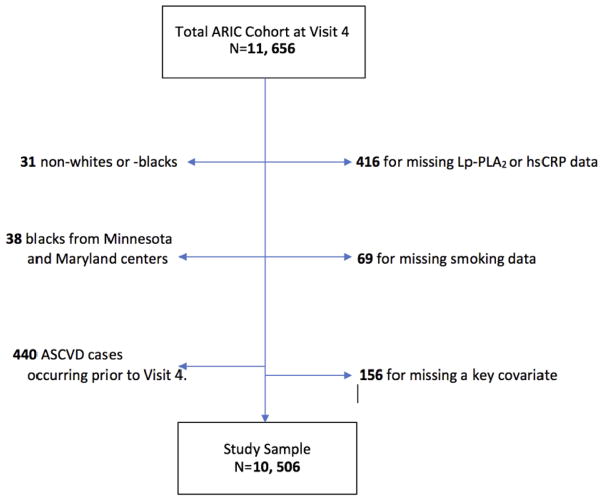
Of the 11,656 participants attending Visit 4, we excluded 41 for missing Lp-PLA2 activity data; 1 for missing hsCRP data; 69 for missing smoking data; 156 for missing data on any covariate adjusted in models; 31 participants who were neither black nor white; 38 blacks from Minnesota and Maryland field centers (because small numbers limited estimates for their race/center combinations); and 440 who had coronary heart disease (CHD) or stroke prior to Visit 4. Thus, the analytical sample included 10,506 participants for both cross sectional and longitudinal analyses (Figure 1).
Figure 1.
Flow diagram illustrating inclusion/exclusion criteria of participants
Smoking data were collected using interviewer-administered questionnaires at Visit 4. Participants were considered as smokers if they ever smoked at least 400 cigarettes in their lifetime and then further categorized as “current smokers” if they answered yes to currently smoking cigarettes, “former smokers” if last cigarette smoked was ≥ 3 months. “Never smokers” denied ever smoking cigarettes. Among current and former smokers, information on number of cigarettes smoked per day was also collected. We divided the number of cigarettes smoked per day by 20 to obtain number of packs per day.
Participants were asked to fast for 12 hours before their Visit 4 clinic appointment and plasma samples were stored for approximately 10 years at −70°C before being assayed. Lp-PLA2 activity was measured using an automated Colorimetric Activity Method assay (diaDexus Inc., South San Francisco, CA) using a Beckman Coulter (Olympus) AU400e autoanalyzer. The Lp-PLA2 activity assay had an inter-assay variation coefficient of 4.4% and a reliability coefficient of 0.92, based on 419-blinded replicate samples. HsCRP was measured by immunonephelometric assay (Dade Behring, Newark, Delaware). The reliability coefficient was 0.95.13 Both Lp-PLA2 activity and hsCRP are highly stable in plasma samples for more than 10 years from collection when stored at −70 °C.14,15 Thus, this approach has been standard practice in epidemiologic studies.
Demographics, medical history, anthropometrics, and blood pressure measurements were obtained at Visit 4 using a standardized protocol. Hypertension, diabetes and current alcohol use were defined as previously published.16 Total cholesterol and high-density lipoprotein cholesterol (HDL-C) were determined by enzymatic methods,17 and estimated glomerular filtration rate by the CKD-Epi formula.18
Participants were followed up for incident ASCVD events occurring between Visit 4 (1996–1998) and December 31, 2014. ARIC participants (or their proxy) were contacted annually by telephone. Reported hospitalizations and deaths related to possible CHD events or strokes in the previous year were identified, and hospital medical records and/or death certificates were obtained for review. Definite or probable CHD events were defined by rigorous computer algorithms and physician review using combinations of chest pain, ECG changes, and cardiac enzyme levels, as previously described.19 Potential CHD events were reviewed by 2 members of the ARIC Morbidity and Mortality Classification Committee, and any difference between reviewers were adjudicated by the committee chairperson.
Similarly, incident stroke occurring within the same follow-up period were identified. Transient ischemic attacks were not included. Abstractors recorded stroke signs and symptoms and photocopied neuroimaging (CT or MRI) if hospital discharge diagnoses included a cerebrovascular disease code, if a cerebrovascular condition or procedure was mentioned in the discharge summary, or if a cerebrovascular finding was noted on a CT or MRI report. Each eligible case was classified by computer algorithm and by a physician reviewer,20 according to criteria adapted from the National Survey of Stroke.21 Disagreements were adjudicated by another reviewer.
For our primary endpoint, we combined CHD and stroke to obtain overall ASCVD. Data were summarized using counts (proportions) for categorical variables, and means (standard deviations) or medians (interquartile intervals) for continuous variables. Chi-squared test, ANOVA, and Kruskal-Wallis testing were used for comparison across smoking categories where appropriate. HsCRP was skewed and thus natural log-transformed when considered as a continuous variable. Elevated Lp-PLA2 activity was defined as levels ≥ the clinical cutpoint of 225 nmol/min/mL,22 and elevated hsCRP was defined as levels >3 mg/L (corresponding to the AHA/CDC high-risk category).23
For cross-sectional analyses, a modified Poisson regression approach,24 given the high prevalence of elevated Lp-PLA2 activity and hsCRP (more than 10%) in our study population, was used to estimate their adjusted prevalence risk ratios (adjPRR) by smoking status. Models were adjusted for as follows: Model 1 adjusted for age, sex, and race-center. Model 2 adjusted for Model 1 in addition to current alcohol use, hypertension, body mass index, diabetes mellitus, total cholesterol, HDL-C, estimated glomerular filtration rate, and statin use. Furthermore, an adjusted restricted cubic spline model using Model 2 variables was used to illustrate the linear dose-response relationship of pack/day of cigarettes smoked among current smokers with Lp-PLA2 activity and hsCRP levels.
For longitudinal analyses, multivariable Cox proportional hazard models were used to estimate the hazard ratios (HR) for incident CHD, stroke and total ASCVD by smoking status; tertiles of packs/day of smoking (corresponding to ≤0.5 pack/day; >0.5–1 pack/day; >1 pack/day) among current smokers. Also, restricted cubic splines for packs/day were used to assess for a linear dose-response relationship of smoking intensity with CHD and stroke. Similarly, Cox models were used to estimate the association of smoking status and intensity with ASCVD, stratified by categories of Lp-PLA2 activity (tertiles) and hsCRP (AHA/CDC risk categories). Cox models in stratified analyses adjusted for variables in Model 2.
To test the incremental predictive value of adding Lp-PLA2 activity or hsCRP to a model including traditional ASCVD risk factors among smokers, we estimated inverse hazard rate variables for the 3 models in a random half of the data (training set) using Cox proportional hazard models. Harrell’s c indices were then determined in the test set for all 3 models and differences between Harrell’s c indices were calculated.
In sensitivity analyses, we tested for statistical interaction between measures of smoking behavior with age, sex, and race in their associations with Lp-PLA2 activity and hsCRP in cross-sectional analyses, and with events in longitudinal analyses. We visually assessed for the proportional hazards assumption for each Cox model. All statistical analyses were performed using Stata 13 (StataCorp LP, College Station, TX) and statistical significance was generally considered at a two-sided P value<0.05.
RESULTS
The baseline characteristics of the study population stratified by categories of smoking status are presented in Table 1. Briefly, current smokers were younger, less likely to be hypertensive, had lower mean body mass index, and smoked a median of 1 pack/day of cigarettes. On average, crude Lp-PLA2 activity and hsCRP levels were higher among current smokers compared to never smokers. Baseline characteristics by categories of smoking intensity (packs/day) among current smokers are summarized in Supplemental Table 1.
Table 1.
Characteristics* of the Atherosclerosis Risk in Communities (ARIC) Study According to Visit 4 (1996–1998) Smoking Status.
| Characteristic‡ | Never | Former | Current |
|---|---|---|---|
|
| |||
| (n=4459) | (n=4544) | (n=1503) | |
| Age (years), mean (SD) | 62.8 (5.6) | 63.1 (5.7) | 61.6 (5.5) |
| Women | 3189 (71.5%) | 1996 (43.9%) | 812 (54.0%) |
| Race-center | |||
| Minneapolis, Minnesota whites | 1132 (25.4%) | 1496 (32.9%) | 375 (25.0%) |
| Washington Co, Maryland whites | 1302 (29.2%) | 1237 (27.2%) | 353 (23.5%) |
| Forsyth Co, North Carolina whites | 955 (21.4%) | 1024 (22.5%) | 405 (26.9%) |
| Forsyth Co, North Carolina blacks | 96 (2.2%) | 91 (2.0%) | 48 (3.2%) |
| Jackson, Mississippi blacks | 974 (21.8%) | 696 (15.3%) | 322 (21.4%) |
| Current alcohol drinker | 1725 (38.7%) | 2673 (58.8%) | 838 (55.8%) |
| Hypertension | 2151 (48.2%) | 2094 (46.1%) | 629 (41.8%) |
| Body mass index (kg/m2), mean (SD) | 29.2 (5.8) | 29.0 (5.4) | 26.8 (5.2) |
| Diabetes mellitus | 699 (15.7%) | 768 (16.9%) | 200 (13.3%) |
| Total cholesterol (mg/dL), mean (SD) | 200 (38) | 196 (34) | 192 (38) |
| HDL cholesterol (mg/dL), mean (SD) | 53 (17) | 49 (17) | 49 (17) |
| eGFR category (mL/min/1.73m2) | |||
| ≥90 | 2029 (45.5%) | 2036 (44.8%) | 898 (59.7%) |
| 60–<90 | 2153 (48.3%) | 2219 (48.8%) | 538 (35.8%) |
| <60 | 277 (6.2%) | 289 (6.4%) | 67 (4.5%) |
| Used statin within 2 weeks prior to visit | 424 (9.5%) | 531 (11.7%) | 125 (8.3%) |
| Lp-PLA2 activity (nmol/min/mL), mean (SD) | 220.2 (63.0) | 234.4 (61.2) | 236.9 (60.8) |
| Lp-PLA2 activity ≥ 225 nmol/min/mL | 1964 (44.0%) | 2488 (54.8%) | 849 (56.5%) |
| C-Reactive Protein (mg/L), median (IQI) | 2.4 (1.0, 5.4) | 2.2 (1.0, 5.1) | 3.1 (1.4, 6.3) |
| C-Reactive Protein > 3 mg/L | 1930 (43.3%) | 1852 (40.8%) | 756 (50.3%) |
| Pack/day smoked, median (IQI) | … | … | 1.0 (0.5, 1.0) |
Results are presented as mean (SD), N (%), or median (Interquartile Interval),
Abbreviations: SD, standard deviation; CI, confidence interval; IQI, interquartile interval; eGFR, estimated glomerular filtration rate; HDL, high density lipoprotein; Lp-PLA2, lipoprotein-associated phospholipase A2
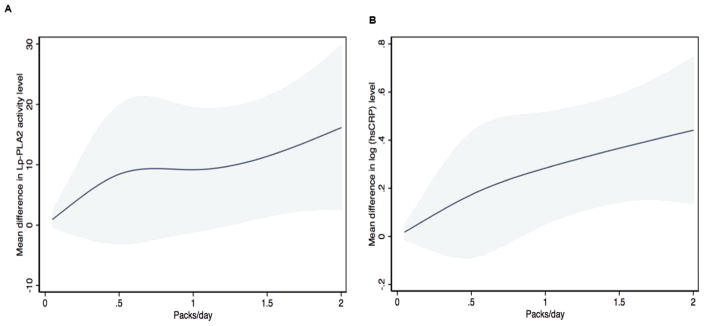
In a multivariable model adjusted for demographics and traditional ASCVD risk factors (Model 2), current smokers had a higher prevalence of elevated Lp-PLA2 activity ≥253 nmol/min/mL and elevated hsCRP >3 mg/L, compared to never smokers (Table 2). Also, higher number of packs of cigarettes smoked per day was linearly associated with higher levels of both Lp-PLA2 activity and hsCRP (Figure 2).
Table 2.
Cross-sectional Associations* of Smoking Status with the Prevalence of Elevated Lp-PLA2 Activity and HsCRP at Visit 4.
| Model 1† | Model 2‡ | |||
|---|---|---|---|---|
|
| ||||
| adjPRR (95% CI) | P-value | adjPRR (95% CI) | P-value | |
| Elevated Lp-PLA2§ | ||||
| Never Smokers | 1 (Ref) | 1 (Ref) | ||
| Former Smokers | 0.96 (.93, 1.00) | 0.05 | 0.98 (0.94, 1.02) | 0.26 |
| Current Smokers | 1.14 (1.08, 1.19) | <0.001 | 1.08 (1.03, 1.13) | 0.002 |
|
| ||||
| Elevated hsCRP|| | ||||
| Never Smokers | 1 (Ref) | 1 (Ref) | ||
| Former Smokers | 1.10 (1.05, 1.15) | <0.001 | 1.09 (1.04, 1.15) | <0.001 |
| Current Smokers | 1.27 (1.20, 1.35) | <0.001 | 1.43 (1.34, 1.51) | <0.001 |
Abbreviations: Lp-PLA2, lipoprotein-associated phospholipase A2; hsCRP, high-sensitivity C-Reactive Protein; adjPRR, adjusted prevalence risk ratio; CI, confidence interval
Model 1: Adjusted for age, sex, and race-center.
Model 2: Model 1 + current drinker, hypertension, body mass index, diabetes mellitus status, total cholesterol, HDL cholesterol, estimated glomerular filtration rate, and statin use.
Elevated Lp-PLA2 activity was defined as value ≥ 225 nmol/min/mL (clinical cut-point)
Elevated hsCRP was defined as value > 3 mg/L (AHA/CDC cut-point)
Figure 2.
Adjusted* restricted cubic splines for the cross-sectional associations of smoking intensity with Lp-PLA2 activity (Panel A) and log(hsCRP) (Panel B) as a function of pack/day of smoking among current smokers. *Model adjusted for age, sex, race-center, current drinker, hypertension, body mass index, diabetes mellitus status, total cholesterol, HDL-C, estimated glomerular filtration rate, and statin use. The x-axis for (packs/day) was truncated at 2 because of sparse data beyond this limit.
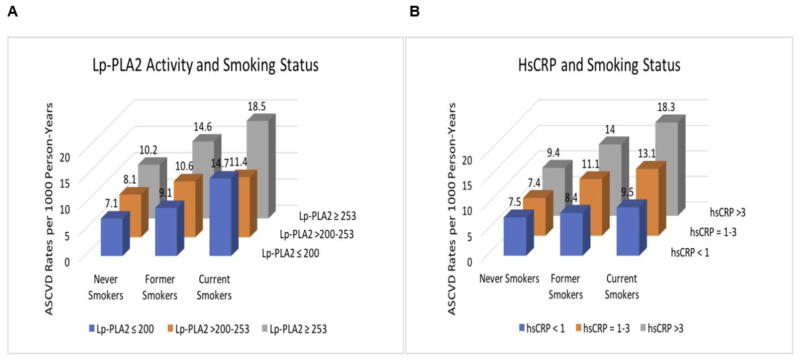
Over a median (interquartile interval) follow-up of 17 (13–22) years, 1,196 CHD and 685 stroke cases occurred, totaling 1,745 ASCVD cases. Crude ASCVD incidence rates (per 1000 person-years) were 8.3 for never smokers, 10.8 for former smokers and 15.0 for current smokers. When stratified by tertiles of Lp-PLA2 activity, the lowest ASCVD crude incidence rate occurred among never smokers with Lp-PLA2 activity level ≤200 nmol/min/mL, and the highest ASCVD incidence rate occurred among current smokers with Lp-PLA2 activity levels ≥253 nmol/min/mL (Figure 3A). Of note, current smokers had the highest crude ASCVD incidence rates compared to former and never smokers within the same stratum of Lp-PLA2 activity. Similar crude ASCVD estimates and trends were noted for smoking status when stratified by hsCRP (Figure 3B).
Figure 3.
Crude ASCVD incidence rates (per 1000 person-years) by smoking status. A, ASCVD incidence rates stratified by Lp-PLA2 activity (tertiles). B, ASCVD incidence rates stratified by hsCRP (AHA/CDC risk categories).
After accounting for demographics and adjusting for other potential confounders, both former and current smokers had an increased risk for incident ASCVD (Table 3). However, stronger associations with incident CHD, stroke, ASCVD were observed for current smokers than former smokers (Table 3). For smoking intensity among current smokers, the adjusted HRs in the highest tertile of smoking intensity (more than 1 pack/day) were significantly increased compared to the lowest tertile (less than half a pack/day) for stroke and total ASCVD (Table 3). The association of smoking intensity with CHD, stroke, and ASCVD was generally linear as shown in fully-adjusted restricted cubic spline models in Supplemental Figure 1. Each unit higher in number of packs/day smoked was associated with a 29% increase in the hazard for CHD, 57% for stroke and 39% when CHD and stroke were combined (ASCVD) (Table 3).
Table 3.
Hazard Ratios (HR) for CHD, Stroke and ASCVD by Smoking Status and Intensity over 17-year mean follow-up*
| Model | Smoking parameter | CHD | Stroke | ASCVD |
|---|---|---|---|---|
| Smoking status | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| 1† | ||||
| Never smokers | 1 (Ref) | 1 (Ref) | 1 (Ref) | |
| Former smokers | 1.26 (1.10, 1.44) | 1.10 (0.92, 1.29) | 1.18 (1.06, 1.31) | |
| Current smokers | 1.95 (1.65, 2.31)** | 1.89 (1.51, 2.36)** | 1.93 (1.68, 2.21)** | |
| P for linear trend | <0.001 | <0.001 | <0.001 | |
|
| ||||
| 2‡ | ||||
| Never smokers | 1 (Ref) | 1 (Ref) | 1 (Ref) | |
| Former smokers | 1.19 (1.04, 1.36) | 1.12 (0.94, 1.33) | 1.14 (1.02, 1.28) | |
| Current smokers | 2.11 (1.78, 2.51)** | 1.93 (1.53, 2.43)** | 2.04 (1.76, 2.35)** | |
| P for linear trend | <0.001 | <0.001 | <0.001 | |
|
| ||||
| Smoking intensity | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| 1† | ||||
| ≤ 0.5 pack/day | 1 (Ref) | 1 (Ref) | 1 (Ref) | |
| >0.5–1 pack/day | 1.05 (0.78, 1.41) | 1.37 (0.89, 2.12) | 1.09 (0.85, 1.40) | |
| >1pack/day | 1.38 (0.93, 2.07) | 2.01 (1.09, 3.70) | 1.57 (1.11, 2.23)** | |
| P for linear trend | 0.11 | 0.025 | 0.010 | |
| Packs/day continuous | 1.33 (1.02, 1.74) | 1.44 (0.98, 2.11) | 1.39 (1.11, 1.75)** | |
|
| ||||
| 2‡ | ||||
| ≤ 0.5 pack/day | 1 (Ref) | 1 (Ref) | 1 (Ref) | |
| >0.5–1 pack/day | 1.04 (0.76, 1.41) | 1.43 (0.92, 2.23) | 1.11 (0.86, 1.43) | |
| >1 pack/day | 1.33 (0.88, 2.01) | 2.29 (1.22, 4.30)** | 1.60 (1.13, 2.28)** | |
| P for linear trend | 0.17 | 0.010 | 0.009 | |
| Packs/day continuous | 1.29 (0.97, 1.71) | 1.57 (1.05, 2.33) | 1.39 (1.10, 1.76)** | |
Bolded items are statistically significant. Abbreviations: ASCVD, atherosclerotic cardiovascular disease; CHD, coronary heart disease; CI, confidence interval; Lp-PLA2, lipoprotein-associated phospholipase A2; hsCRP, high-sensitivity C-Reactive Protein
P-value < 0.001
Model 1: Adjusted for age, sex, and race-center.
Model 2: Model 1 + current drinker, hypertension, body mass index, diabetes mellitus status, total cholesterol, HDL cholesterol, estimated glomerular filtration rate, and statin use.
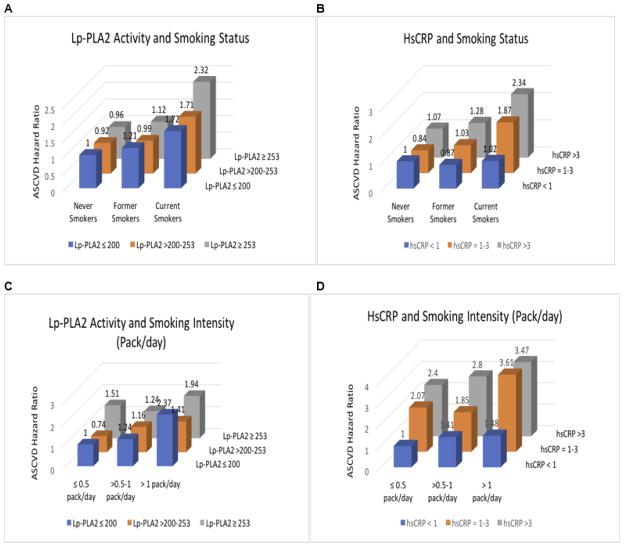
Figure 4 and Supplemental Table 2 illustrate the adjusted HRs for ASCVD by smoking status and intensity, stratified by levels of Lp-PLA2 activity and hsCRP. The risk for future ASCVD among former smokers was comparable to never smokers when stratification was done by either Lp-PLA2 activity or hsCRP (Supplemental Table 2; Figure 4A, B). Among current smokers, both Lp-PLA2 activity ≥253 nmol/min/mL and hsCRP >3 mg/L were associated with over a 2-fold increase in risk for future ASCVD. However, among smoking intensity categories (packs/day), event rates were inconsistent when stratified by Lp-PLA2 activity (Figure 4C), which contrasts with the generally monotonic increases in risk for future ASCVD by hsCRP strata among each smoking intensity category (Figure 4D). Overall, current smokers who smoked >1 pack/day and had elevated hsCRP (1–3 mg/L or >3 mg/L) had about a 3.5-fold increase risk for future ASCVD (compared to ≤0.5 pack/day and hsCRP <1 mg/L) (Figure 4D). Similar trends were observed for CHD, stroke and ASCVD when the analyses were restricted to each of the pack/day categories, after stratification by Lp-PLA2 activity and hsCRP (Supplemental Table 3).
Figure 4.
Adjusted* ASCVD Hazard Ratios by smoking status and intensity: stratified by Lp-PLA2 activity (tertiles) and hsCRP (AHA/CDC risk categories). A, ASCVD Hazard Ratios by smoking status, stratified by Lp-PLA2. B, ASCVD Hazard Ratios by smoking status, stratified by hsCRP. C, ASCVD Hazard Ratios by smoking intensity among current smokers, stratified by Lp-PLA2 activity. D, ASCVD Hazard Ratios by smoking intensity among current smokers, stratified by hsCRP. For Figures A and B, never smokers in the lowest inflammatory category served as reference group. For Figure C and D, participants with ≤0.5 packs/day in the lowest inflammatory category served as reference group. *Models adjusted for age, sex, race-center, current drinker, hypertension, body mass index, diabetes mellitus status, total cholesterol, HDL-C, estimated glomerular filtration rate, and statin use.
Among current smokers, the addition of Lp-PLA2 activity to a model including traditional risk factors (Model B) did not significantly improve the predictive power compared to a model with traditional risk factors alone (Model A) (difference between Harrell’s c indices= −0.002, p=0.19; Table 4). In contrast, the addition of hsCRP to a model including traditional risk factors (Model C) significantly improved the predictive power, albeit absolute improvement was marginal (difference between Harrell’s c indices= −0.009, p<0.001; Table 4).
Table 4.
Comparing the Predictive Power of Survival Models Including Traditional Risk Factors with and without Lp-PLA2 Activity or HsCRP among Current Smokers.
| Model | Harrell’s c indices | 95% Conf. Interval | P-value |
|---|---|---|---|
| A* | 0.666 | 0.643, 0.689 | <0.001 |
| B† | 0.668 | 0.645, 0.690 | <0.001 |
| C‡ | 0.675 | 0.652, 0.698 | <0.001 |
|
| |||
| Differences between Harrell’s c indices | |||
|
| |||
| A–B | −0.002 | −0.004, 0.001 | 0.19 |
| A–C | −0.009 | −0.126, −0.005 | <0.001 |
| B–C | −0.007 | −0.012, −0.003 | 0.001 |
Model A was adjusted for age, sex, race-center, current drinker, hypertension, body mass index, diabetes mellitus status, total cholesterol, HDL cholesterol, estimated glomerular filtration rate, and statin use.
Model B: traditional risk factors plus Lp-PLA2 activity
Model C: traditional risk factors plus hsCRP
There was no evidence for effect modification by age, sex, or race for any of the associations tested.
DISCUSSION
In this large community-based cohort free of clinical ASCVD at baseline, current smokers had higher prevalence of elevated Lp-PLA2 activity and hsCRP levels, two reliable inflammatory biomarkers of ASCVD risk. Greater intensity of smoking (packs/day) among current smokers was linearly associated with higher Lp-PLA2 activity and hsCRP levels. In addition to confirming an increased risk for future ASCVD among current smokers compared to never smokers, we also observed that greater smoking intensity (packs/day) was associated with increased risk for future cardiovascular events. Finally, while both Lp-PLA2 activity ≥253 nmol/min/mL and hsCRP >3mg/L identified high-risk current smokers who may benefit from targeted and more-intensive prevention strategies, hsCRP may better stratify risk of future ASCVD among current smokers. Moreover, hsCRP improved the ability to predict the onset of future cardiovascular events beyond traditional ASCVD risk factors.
Our data are consistent with prior evidence suggesting that inflammation is on the causal pathway linking cigarette smoking to clinical CVD.3,4,9 In the Multi-Ethnic Study of Atherosclerosis (MESA) study, McEvoy et al. reported that two powerful markers of ASCVD risk; hsCRP and coronary artery calcium (CAC) scores, could be useful in identifying high-risk smokers needing intensive smoking cessation efforts.4 Our study provides more evidence to support a dose-response relation of smoking intensity with inflammatory biomarkers of cardiovascular risk and clinical ASCVD events.
Most importantly, although prior studies have evaluated the effects of cigarette smoking on hsCRP and other biomarkers of ASCVD risk,3,4,25 this study is the first to our knowledge to explore the relation of smoking with Lp-PLA2 activity level, a biomarker recently approved by the FDA for routine clinical use in CHD risk stratification.11 Lp-PLA2 is a serine-dependent lipase secreted by macrophages and found in atherosclerotic plaques.22 It is considered to be a marker of plaque vulnerability and its activity has been found to independently predict the risk for incident CHD, stroke and all-cause mortality.22 However, despite this, a recent clinical trial found that darapladib, a selective oral inhibitor of Lp-PLA2, did not reduce ASCVD events compared to placebo in a population of stable CHD patients.26
We found that across categories of both Lp-PLA2 and hsCRP, the risk for future ASCVD among former smokers was similar to never smokers. This finding is consistent with previous studies which have shown that the risk of cardiovascular events for former smokers is comparable to never smokers after 5 to 10 years of cessation.27,28 Among current smokers, our data demonstrate that both Lp-PLA2 activity and hsCRP categories can identify high-risk groups who may benefit from aggressive smoking cessation efforts. However, hsCRP was consistently associated with greater risk for future ASCVD within each tertile of cigarette smoking intensity (packs/day) and also improved prediction for future ASCVD incremental to traditional risk factors. From our data, current smokers with hsCRP categories of 1 to 3 as well as >3 have a 3.5-fold increase in the risk for future ASCVD (compared to ≤0.5 pack/day and hsCRP <1 mg/L).
Results of this study may have important implications for tobacco regulatory science. The FDA’s authority for tobacco regulation has now been extended to include electronic nicotine delivery systems, cigars, and hookahs.29 HsCRP has increasingly been identified as a sensitive biomarker for the study and subsequent regulation of novel tobacco products, prior to the availability of long-term cardiovascular events data. The findings in this well-characterized cohort confirms the potential role of hsCRP and Lp-PLA2 activity as sensitive biomarkers which could be used to study the potential cardiovascular toxicity of novel tobacco products. Given the linear dose-response association of packs/day with both Lp-PLA2 activity and hsCRP found in this study, measuring these biomarkers among users of electronic cigarettes and comparing these to current combustible cigarette users and non-tobacco using controls might be considered for use to determine the potential cardiovascular risk profile of such products prior to availability of long-term cardiovascular outcomes.
This study was conducted in a large cohort with an average follow-up of 17 years, thus enabling a better estimation of the longer-term cardiovascular risks of smoking compared to prior studies.4 Also, events were well adjudicated and ASCVD risk factors were well characterized. Nonetheless, several limitations to this study must be acknowledged. First, smoking exposure was self-reported and did not include information on urine cotinine levels to corroborate self-reported smoking behaviors and avoid misclassification. However, measurement of cotinine would potentially have identified participants who reported being never or former smokers that were actively using tobacco which would serve to correct misclassification resulting in a difference between groups larger than what was observed. Second, despite adjusting for a wide variety of potential confounders, residual confounding cannot be excluded in this observational study. For instance, some biologically plausible confounders of the relation between inflammation and ASCVD such as socioeconomic status, intravenous drug use, physical activity level and others were not adjusted for in our models. Finally, Lp-PLA2 activity and hsCRP were measured at only one time point (Visit 4). Although ASCVD events were updated annually based on telephone calls and medical record adjudications, there was a ~14 year lag between in-person visits 4 and 5, which did not allow covariates (notably smoking status) to be updated. Thus, ASCVD risk assessment was based on a single time point, similar to a real world office-based risk assessment.
In conclusion, we found a dose-response relation of smoking intensity with biomarkers of ASCVD risk and cardiovascular events. While both Lp-PLA2 activity and hsCRP categories identify high-risk current smokers who may benefit from targeted and more-intensive prevention strategies, hsCRP may better stratify risk for future ASCVD.
Supplementary Material
Adjusted* restricted cubic spline analysis of smoking intensity with the hard ratio for incident coronary heart disease (CHD) (A), stroke (B) and combined CHD and stroke (C). The reference value for packs/day was set at the 10th percentile (0.2 packs/day or 4 cigarettes/day). The x-axis for (packs/day) was truncated at 2 because of sparse data beyond this limit. *Model adjusted for age, sex, race-center, current drinker, hypertension, body mass index, diabetes mellitus status, total cholesterol, HDL cholesterol, estimated glomerular filtration rate, and statin use. The dark line represents hazard ratio; dotted line, 95% confidence and histogram, the distribution of packs/day. ASCVD; atherosclerotic cardiovascular disease.
Acknowledgments
Sources of Funding:
This analysis was supported by funding from the American Heart Association Tobacco Regulation and Addiction Center (A-TRAC, NIH 1 P50 HL120163-01), a member of the Food and Drug Administration (FDA) Tobacco Centers of Regulatory Science for Research Relevant to the Family Smoking Prevention and Tobacco Control Act (P50). The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. (HHSN268201700001I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I, HHSN2682017000021). Dr. Michos and Dr. Zhao were supported by the Blumenthal Scholars Fund for Preventive Cardiology Research. Dr. Hoogeveen received a research grant from DiaDexus, which provided reagents to conduct the LpPLA2 activity assays. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors thank the staff and participants of the ARIC Study for their important contributions.
Footnotes
Disclosures:
Dr. Michos and Dr. Blaha report receiving an honorarium from Siemens Healthcare Diagnostics (unrelated to this topic). Dr. Blaha’s disclosures include Advisory Boards for Novartis, Amgen, Sanofi/Regeneron, MedImmune, Akcea and grant funding from Amgen and the Aetna Foundation. Dr. Hoogeveen has previously received a research grant from diaDexus. However, diaDexus played no role in the design, data analysis or data interpretation of this study. Dr. Hoogeveen reports receiving a research grant from Denka Seiken (unrelated to this topic). Dr. Ballantyne has received consultant fees/honoraria from Abbott Diagnostic, Amarin, Amgen, Astra Zeneca, Boehringer Ingelheim, Eli Lilly, Esperion, Ionis, Matinas Bio Pharma Inc, Merck, Novartis, Pfizer, Regeneron, Roche Diagnostic, Sanofi-Synthelabo. Dr. Ballantyne has received research grants from Abbott Diagnostic, Amarin, Amgen, Eli Lilly, Esperion, Ionis, Novartis, Pfizer, Regeneron, Roche Diagnostic, Sanofi-Synthelabo. Other authors did not report any disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Courtney R. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General, 2014. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. Drug and Alcohol Review. 2015;34:694–695. http://www.surgeongeneral.gov/library/reports/50-years-of-progress. [Google Scholar]
- 2.GBD 2015 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1659–1724. doi: 10.1016/S0140-6736(16)31679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al Rifai M, DeFilippis AP, McEvoy JW, Hall ME, Acien AN, Jones MR, Keith R, Magid HS, Rodriguez CJ, Barr GR, Benjamin EJ, Robertson RM, Bhatnagar A, Blaha MJ. The relationship between smoking intensity and subclinical cardiovascular injury: The Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2017;258:119–130. doi: 10.1016/j.atherosclerosis.2017.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McEvoy JW, Nasir K, DeFilippis AP, Lima JA, Bluemke DA, Hundley WG, Barr RG, Budoff MJ, Szklo M, Navas-Acien A, Polak JF, Blumenthal RS, Post WS, Blaha MJ. Relationship of cigarette smoking with inflammation and subclinical vascular disease: the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35:1002–1010. doi: 10.1161/ATVBAHA.114.304960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shiels MS, Katki HA, Freedman ND, Purdue MP, Wentzensen N, Trabert B, Kitahara CM, Furr M, Li Y, Kemp TJ, Goedert JJ, Chang CM, Engels EA, Caporaso NE, Pinto LA, Hildesheim A, Chaturvedi AK. Cigarette smoking and variations in systemic immune and inflammation markers. J Natl Cancer Inst. 2014;106:dju294. doi: 10.1093/jnci/dju294. https://doi.org/10.1093/jnci/dju294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inoue-Choi MLL, Reyes-Guzman C, Hartge P, Caporaso N, Freedman N. Association of long-term low-intensity smoking with all-cause and cause-specific mortality in the NIH-AARP Diet and Health Study. JAMA Intern Med. 2017;177:87–95. doi: 10.1001/jamainternmed.2016.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tibuakuu M, Kamimura D, Kianoush S, DeFilippis AP, Al Rifai M, Reynolds LM, White WB, Butler KR, Mosley TH, Turner ST, Kullo IJ, Hall ME, Blaha MJ. The association between cigarette smoking and inflammation: The Genetic Epidemiology Network of Arteriopathy (GENOA) study. PLoS One. 2017;12:e0184914. doi: 10.1371/journal.pone.0184914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nance R, Delaney J, McEvoy JW, Blaha MJ, Burke GL, Navas-Acien A, Kaufman JD, Oelsner EC, McClelland RL. Smoking intensity (pack/day) is a better measure than pack-years or smoking status for modeling cardiovascular disease outcomes. J Clin Epidemiol. 2017;81:111–119. doi: 10.1016/j.jclinepi.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.King CC, Piper ME, Gepner AD, Fiore MC, Baker TB, Stein JH. Longitudinal Impact of Smoking and Smoking Cessation on Inflammatory Markers of Cardiovascular Disease Risk. Arterioscler Thromb Vasc Biol. 2017;37:374–379. doi: 10.1161/ATVBAHA.116.308728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ridker PM, Glynn RJ, Hennekens CH. C-reactive protein adds to the predictive value of total and HDL cholesterol in determining risk of first myocardial infarction. Circulation. 1998;97:2007–2011. doi: 10.1161/01.cir.97.20.2007. [DOI] [PubMed] [Google Scholar]
- 11.Young K. FDA Clears Test to Help Predict Coronary Heart Disease Risk. [Accessed January 7, 2018];NEJM Journal Watch. 2014 Webpage: https://www.jwatch.org/fw109648/2014/12/17/fda-clears-test-help-predict-coronary-heart-disease-risk.
- 12.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 13.Ballantyne CM, Hoogeveen RC, Bang H, Coresh J, Folsom AR, Heiss G, Sharrett AR. Lipoprotein-associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident coronary heart disease in middle-aged men and women in the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2004;109:837–842. doi: 10.1161/01.CIR.0000116763.91992.F1. [DOI] [PubMed] [Google Scholar]
- 14.Persson M, Nilsson JA, Nelson JJ, Hedblad B, Berglund G. The epidemiology of Lp-PLA(2): distribution and correlation with cardiovascular risk factors in a population-based cohort. Atherosclerosis. 2007;190:388–396. doi: 10.1016/j.atherosclerosis.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 15.Doumatey AP, Zhou J, Adeyemo A, Rotimi C. High sensitivity C-reactive protein (Hs-CRP) remains highly stable in long-term archived human serum. Clin Biochem. 2014;47:315–318. doi: 10.1016/j.clinbiochem.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Folsom AR, Yamagishi K, Hozawa A, Chambless LE. Absolute and attributable risks of heart failure incidence in relation to optimal risk factors. Circ Heart Fail. 2009;2:11–17. doi: 10.1161/CIRCHEARTFAILURE.108.794933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown SA, Hutchinson R, Morrisett J, Boerwinkle E, Davis CE, Gotto AM, Jr, Patsch W. Plasma lipid, lipoprotein cholesterol, and apoprotein distributions in selected US communities. The Atherosclerosis Risk in Communities (ARIC) Study. Arterioscler Thromb. 1993;13:1139–1158. doi: 10.1161/01.atv.13.8.1139. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. 2010;55:622–627. doi: 10.1053/j.ajkd.2010.02.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, Higgins M, Williams OD, Tyroler HA. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years’ experience. J Clin Epidemiol. 1996;49:223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 20.Rosamond WD, Folsom AR, Chambless LE, Wang CH, McGovern PG, Howard G, Copper LS, Shahar E. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999;30:736–743. doi: 10.1161/01.str.30.4.736. [DOI] [PubMed] [Google Scholar]
- 21.The National Survey of Stroke. National Institute of Neurological and Communicative Disorders and Stroke. Stroke. 1981;12:I1–91. [PubMed] [Google Scholar]
- 22.Thompson A, Gao P, Orfei L, Watson S, Di Angelantonio E, Kaptoge S, Ballantyne C, Cannon CP, Criqui M, Cushman M, Hofman A, Packard C, Thompson SG, Collins R, Danesh J. Lipoprotein-associated phospholipase A(2) and risk of coronary disease, stroke, and mortality: collaborative analysis of 32 prospective studies. Lancet. 2010;375:1536–1544. doi: 10.1016/S0140-6736(10)60319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr, Taubert K, Tracy RP, Vinicor F. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 24.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 25.Nadruz W, Jr, Goncalves A, Claggett B, Querejeta Roca G, Shah AM, Cheng S, Heiss G, Ballantyne CM, Solomon SD. Influence of cigarette smoking on cardiac biomarkers: the Atherosclerosis Risk in Communities (ARIC) Study. Eur J Heart Fail. 2016;18:629–637. doi: 10.1002/ejhf.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Investigators S. White HD, Held C, Stewart R, Tarka E, Brown R, Davies RY, Budaj A, Harrington RA, Steg PG, Ardissino D, Armstrong PW, Avezum A, Aylward PE, Bryce A, Chen H, Chen MF, Corbalan R, Dalby AJ, Danchin N, De Winter RJ, Denchev S, Diaz R, Elisaf M, Flather MD, Goudev AR, Granger CB, Grinfeld L, Hochman JS, Husted S, Kim HS, Koenig W, Linhart A, Lonn E, Lopez-Sendon J, Manolis AJ, Mohler ER, 3rd, Nicolau JC, Pais P, Parkhomenko A, Pedersen TR, Pella D, Ramos-Corrales MA, Ruda M, Sereg M, Siddique S, Sinnaeve P, Smith P, Sritara P, Swart HP, Sy RG, Teramoto T, Tse HF, Watson D, Weaver WD, Weiss R, Viigimaa M, Vinereanu D, Zhu J, Cannon CP, Wallentin L. Darapladib for preventing ischemic events in stable coronary heart disease. N Engl J Med. 2014;370:1702–1711. doi: 10.1056/NEJMoa1315878. [DOI] [PubMed] [Google Scholar]
- 27.Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jha P, Ramasundarahettige C, Landsman V, Rostron B, Thun M, Anderson RN, McAfee T, Peto R. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368:341–350. doi: 10.1056/NEJMsa1211128. [DOI] [PubMed] [Google Scholar]
- 29.Federal Register. Deeming Tobacco Products To Be Subject to the Federal Food, Drug, and Cosmetic Act, as Amended by the Family Smoking Prevention and Tobacco Control Act; Restrictions on the Sale and Distribution of Tobacco Products and Required Warning Statements for Tobacco Products: a rule by the Food and Drug Administration on 05/10/2016. [Accessed January 7, 2018]; Webpage: https://www.federalregister.gov/d/2016-10685. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Adjusted* restricted cubic spline analysis of smoking intensity with the hard ratio for incident coronary heart disease (CHD) (A), stroke (B) and combined CHD and stroke (C). The reference value for packs/day was set at the 10th percentile (0.2 packs/day or 4 cigarettes/day). The x-axis for (packs/day) was truncated at 2 because of sparse data beyond this limit. *Model adjusted for age, sex, race-center, current drinker, hypertension, body mass index, diabetes mellitus status, total cholesterol, HDL cholesterol, estimated glomerular filtration rate, and statin use. The dark line represents hazard ratio; dotted line, 95% confidence and histogram, the distribution of packs/day. ASCVD; atherosclerotic cardiovascular disease.