Abstract
Recent evidence suggests that adolescent and young adult females may be particularly responsive to nicotine use interventions that include exercise or environmental enrichment. This possibility was addressed in the current study by comparing the efficacy of exercise versus non-exercise environmental enrichment (saccharin) during abstinence at reducing subsequent nicotine-seeking/relapse vulnerability in an adolescent-onset rat model. The efficacy of each intervention was examined as a function of estrous cycle phase given findings indicating that hormonal status influences relapse vulnerability and treatment outcome in females. Once adolescent female rats acquired nicotine self-administration, they were given 23-hr/day access to nicotine (0.01 mg/kg/infusion) for 10 days. Following the last self-administration session, rats began a 10-day forced abstinence period with 2-hr/day access to an unlocked wheel (exercise, n=15), a bottle containing a saccharin-sweetened solution (0.25%; saccharin, n=19), or without access to a wheel or saccharin (control, n=20). Nicotine-seeking, as assessed under an extinction/cued-induced reinstatement procedure, was examined on day 11 of abstinence. Levels of nicotine-seeking were highest in females tested during estrus as compared to females tested during non-estrus phases. Exercise or saccharin during abstinence reduced nicotine-seeking in females tested during estrus, but neither affected the low levels of nicotine-seeking observed in females tested during non-estrus phases, presumably due to a floor effect. These results demonstrate that exercise or saccharin during abstinence decrease nicotine-seeking, and suggest that either would be effective as an early intervention for nicotine use and addiction in females.
Keywords: Adolescent-Onset, Estrous cycle, Female, Intervention, Nicotine Addiction
Introduction
Tobacco use is the leading cause of preventable death in the United States, with 1 of every 5 deaths attributable to smoking (US Department of Health and Human Services 2014). Most smokers initiate use during adolescence, and although 80% of smokers report wanting to quit, very few are successful at doing so (US Department of Health and Human Services 2014). The tobacco industry spends billions of dollars each year on advertisements, promotions, and product development to encourage tobacco use, focusing particularly on adolescents (Federal Trade Commission 2015). These efforts have been effective: greater than 2% of middle school students and 9% of high school students report current cigarette use (Centers for Disease Control and Prevention 2016; Johnston et al. 2016). Rates of e-cigarette use are even higher, having surpassed rates of cigarette use in 2014, with greater than 5% of middle school students and 16% of high school students reporting current e-cigarette use (Centers for Disease Control and Prevention 2016; Johnston et al. 2016). These trends are expected to continue with this cohort into adulthood and throughout their lifetime (US Department of Health and Human Services 2012). Adolescent tobacco and nicotine use has also been shown to be predictive of illicit drug use in adulthood (Kandel and Kandel 2014; Kandel et al. 1992; Lewinsohn et al. 1999). Therefore, early intervention may be critical for not only preventing the progression of nicotine addiction into adulthood, but also for reducing the risk of illicit drug use and abuse.
Early nicotine use intervention may be particularly beneficial for females. Although women have historically maintained lower rates of tobacco use as compared to men, sex differences have narrowed over time (Allen 2014; McIntosh 1997; Pampel 2006), and among adolescent populations, rates of current smoking are now equivalent between females and males (ages 12 to 17; 8.4% versus 8.9%, respectively; Garrett et al. 2013). These findings are significant considering that females are at greater risk for tobacco-related diseases as compared to males (Allen et al. 2014; US Department of Health and Human Services 2001). Female smokers are also less likely than male smokers to try to quit smoking, and are more likely to relapse when they do attempt to quit (Allen 2014; Allen et al. 2014; Perkins et al. 1999; Perkins 2001). Preclinical results corroborate an enhanced vulnerability in females with results showing that adolescent and adult female rats acquire nicotine self-administration faster, are more motivated to obtain nicotine, and self-administer higher levels of nicotine under extended access conditions as compared to their male counterparts (Donny et al. 2000; Li et al. 2014; Lynch 2009; Sanchez et al. 2014). These parallel findings suggest that the enhanced vulnerability in females is biologically based, and indicate the utility of preclinical studies using adolescent females for identifying interventions for nicotine addiction in this vulnerable population.
Interventions aimed at reducing nicotine use and addiction typically focus on replacing or mimicking the effects of nicotine, the primary addictive component in tobacco products. However, such pharmacological approaches are not very effective for females (Perkins and Scott 2008), and are not feasible for adolescents due to concerns with ongoing brain development (Kaplan and Ivanov 2011). Exercise has been suggested as a non-pharmacological intervention for nicotine addiction that may be particularly suited as an intervention for females (Lynch et al. 2017; also see Bock et al. 2012; Patten et al. 2017; Prapavessis et al. 2016). For example, the weight control benefits of exercise may be particularly attractive to girls/young women who report weight gain concerns as a significant contributor to maintenance smoking and relapse to smoking (French and Jeffery 1995; Hussaini et al. 2011; Torchalla et al. 2012). Females also experience greater negative affect during nicotine withdrawal, and are more likely to attribute relapse to withdrawal relief or to reduce stress or negative affect as compared to men (Allen et al. 2014; McVay and Copeland 2011). Exercise is known to decrease anxiety and nicotine withdrawal severity (Bock et al. 2012; Bernard et al. 2013; Prapavessis et al. 2014; Roberts et al. 2012); thus, individuals may be able to mimic the anxiolytic effects of smoking with exercise.
Although few studies have specifically examined exercise as an early intervention for nicotine addiction in girls/young adult females, recent findings in women and adult populations that include women support its potential efficacy (for review see Lynch et al. 2017). For example, numerous studies have shown that exercise during abstinence reduces cigarette craving and the likelihood of relapse (Haasova et al. 2014; Roberts et al. 2012; Williams et al. 2011), and improves treatment outcome when used as an adjunct to other treatments (Bock et al. 2012; Patten et al. 2017; Tritter et al. 2015; Williams et al. 2010). Results from preclinical studies also support the potential utility of exercise as an intervention for drug addiction including nicotine addiction (Ogbonmwan et al. 2015; Peterson et al. 2014; Sanchez et al. 2013; 2014; 2015; Sobieraj et al. 2016; Zlebnik and Carroll 2015). For example, we showed in an adolescent-onset model of nicotine addiction that wheel-running exercise (2-hr/day) during forced abstinence decreased subsequent nicotine-seeking in both males and females (Sanchez et al. 2013; 2014). Interestingly, in females, but not males, levels of nicotine-seeking were also decreased under a locked wheel control condition as compared to a no-wheel condition indicating that the presence of the wheel in the environment (i.e., as an enrichment) was sufficient to reduce nicotine-seeking in females (Sanchez et al. 2014). These findings parallel results reported in a community intervention study showing that while boys showed a reduction in smoking behavior when the enrichment program had an exercise component, girls benefitted from programs with and without an exercise component (Horn et al. 2011; Werch et al. 2005). These findings suggest that exercise or non-exercise environmental enrichment may be effective early interventions for reducing nicotine use and addiction in females.
The purpose of this study was to determine in an adolescent-onset female rat model of nicotine addiction whether the efficacy of exercise as an intervention differs from non-exercise environmental enrichment. A saccharin-sweetened solution (0.25%) was selected for non-exercise enrichment because it is a highly palatable, non-caloric reward that, unlike a running wheel (locked or unlocked), does not induce exercise or play behavior (Lenoir et al. 2007; Sanchez et al. 2014). As with the exercise intervention, rats assigned to the saccharin intervention were given 2-hr/day access to the saccharin solution throughout the 10-day abstinence period. Additional control rats were housed in polycarbonate cages without access to running wheels or saccharin. As in our previous studies, we used extended access (23-hr/day) self-administration conditions that result in levels of nicotine intake that are comparable to those observed in humans (Valentine et al. 1997), and that induce significant extinction and cue-induced reinstatement responding following abstinence (Abdolahi et al. 2010; Sanchez et al. 2013; 2014).
The efficacy of exercise versus saccharin during abstinence was examined as a function of estrous cycle phase given findings indicating that hormonal status influences both relapse vulnerability and treatment outcome in females (e.g., Saladin et al. 2015; for review see Wetherill et al. 2016). For example, numerous preclinical studies have shown that levels of motivation for nicotine (Lynch 2009; but see Donny et al. 2000), and other stimulants such as cocaine vary across the estrous cycle (Lacy et al. 2016; Lynch et al. 2008; Roberts et al. 1989), with highest levels reported during estrus, a period associated with a higher ratio of estradiol to progesterone (Feltenstein and See 2007). Although few studies have included a large enough sample size to examine estrous cycle phase differences in levels of nicotine-seeking (~3–7/group; Feltenstein et al. 2012), previous research with other stimulants has shown that levels of drug-seeking are also highest in females tested during estrus as compared to non-estrus phases (Feltenstein and See 2007; Kerstetter et al. 2008; Kippin et al. 2005; Peterson et al. 2014). Importantly, levels of nicotine craving are cyclically regulated in women (Allen et al. 2009; Franklin et al. 2015), and like findings in rats, in women, they are highest when the ratio of estradiol to progesterone is high (i.e., during the follicular phase; Wetherill et al. 2016).
Based on previous findings showing that environmental enrichment, including access to sweet rewards, reduces relapse vulnerability for other drugs of abuse (Thiel et al. 2009), as well as findings showing that girls benefitted from both exercise and non-exercise based environmental interventions (Horn et al. 2011), we predicted that exercise or saccharin during abstinence would decrease subsequent nicotine-seeking. Furthermore, based on our previous findings in females showing that access to a locked wheel was equally effective at reducing drug-seeking as running in an unlocked wheel (Sanchez et al. 2014; Peterson et al. 2014), we predicted that saccharin would produce a similar decrease in nicotine-seeking as compared to exercise. Finally, based on findings indicating that hormonal status influences relapse vulnerability and treatment outcome in women (Wetherill et al. 2016; Allen et al. 2014; Lynch and Sofuoglu 2010), we hypothesized that levels of nicotine-seeking would be highest during estrus as compared to non-estrus phases, and that the efficacy of exercise versus saccharin during abstinence would vary between females tested during estrous versus non-estrous phases.
Material and Methods
Animals
Adolescent female Sprague-Dawley rats (N = 63) arrived on postnatal day (PND) 22 from Charles River Laboratories (Portage, ME, USA). Females were selected for two reasons. First, females are under-represented in preclinical studies, and there have been recent requests to increase their use in research relevant to human health (Clayton and Collins 2014). This is particularly true for preclinical studies on nicotine addiction where very few studies have included females, and even fewer have examined estrous cycle effects (e.g., Feltenstein et al. 2012). Second, as discussed in the introduction, results from both clinical and preclinical studies demonstrate a specific need to examine exercise, and other potential interventions for nicotine addiction, in females as a function of hormonal status (e.g., Allen et al. 2009; Feltenstein and See 2007; Franklin et al. 2015; Sanchez et al. 2014).
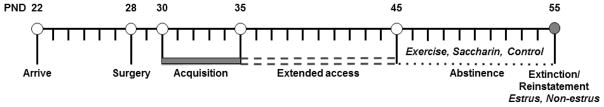
Upon arrival, animals were housed in individual self-administration chambers (ENV-018M, Med Associates, St Albans, VT, USA) within sound-attenuating boxes (ENV-008CT). Rats were maintained on a 12-hr light/dark schedule (house lights and room lights on at 7:00 am) with ad libitum access to food and water. They were trained to lever-press for sucrose pellets (45-mg) prior to surgery in order to facilitate the acquisition of nicotine self-administration. Pre-training methods were performed as previously described (i.e., fixed ratio 1, FR1, 23-hr/day sessions; Lynch 2008) with sessions continuing daily until acquisition occurred (defined as 2 days with 50 or more pellets; completed in approximately 4 sessions). Health was monitored daily and animals were weighed three times per week. All procedures were approved by the University of Virginia Animal Care and Use Committee. The experimental timeline is summarized in Figure 1.
Figure 1.
Time-line of experimental events as a function of postnatal day (PND). Rats arrived on PND 22, and after recovery from the catheter implantation surgery (PND 28), began a 5-day acquisition period (PND 30) during which 20 infusions of nicotine were available/day under a fixed ratio 1 schedule. Rats then began a 10-day extended access self-administration period (23-hr/day; PND 35) during which there was no limit on the number of infusions available/day. During the 10-day abstinence period rats had 2-hour/day access to an unlocked running wheel (exercise), a saccharin solution (saccharin), or without access to a wheel or saccharin (control; PND 45–54). After the last exercise/saccharin/control session rats were returned to their self-administration boxes. Nicotine-seeking was assessed the next day (PND 55) in females during estrus versus non-estrus phases (assessed 30-min prior to the session) using a within-session extinction/cue-induced reinstatement procedure.
Apparatus
Each self-administration chamber (31 × 24 × 21 cm) contained a water bottle holder, house light (4.76 W), retractable lever and lever light (4.74 W), a stationary lever (inactive) and lever light (4.74 W), and a counterbalanced metal arm. Each chamber was housed within a ventilated sound-attenuating box that contained a 10-ml drug syringe driven by an automatic pump (PHM-100, Med Associates, St. Albans, VT, USA). Tygon tubing was used to connect the syringe to a swivel (Instech Laboratories Inc., Plymouth Meeting, PA, USA) which was attached to the metal arm at the top of the chamber. A metal spring (C313CS; PlasticsOne, Roanoke, VA, USA) encased the tubing from the swivel to a 22-gauge guide (C313G; PlasticsOne, Roanoke, VA, USA) that was embedded in a harness (CIH95AB; Instech Laboratories Inc., Plymouth Meeting, PA, USA) that each animal wore during the self-administration component of this study. A subset of animals were given access to running wheels (35.6 cm diameter; ENV-046; Med Associates, St. Albans, VT, USA) during abstinence that were equipped with a counter for revolutions and an attachment for a polycarbonate cage.
Drugs
Nicotine bitartrate was obtained from Sigma-Aldrich (St. Louis, MO, USA), dissolved in 0.9% sterile saline (pH 7.4), and stored in the dark at room temperature during self-administration (stock stored at 4°C). Each intravenous infusion delivered 0.01 mg/kg (freebase weight) at a rate of 0.1 ml/sec with infusion durations adjusted three times per week according to the animals’ weight. This dose was previously shown to induce rapid rates of acquisition and to elicit robust levels of nicotine-seeking after abstinence (Abdolahi et al. 2010; Lynch 2009; Sanchez et al. 2013; 2015). Saccharin was obtained from Sigma-Aldrich (St. Louis, MO, USA), dissolved in tap water, and presented at room temperature. A 0.25% concentration was selected based on work showing that this concentration induces high levels of consumption and preferences in adult and adolescent females (Lenoir et al. 2007; Tordoff et al. 2008; Vendruscolo et al. 2010).
Surgery
Catheterization of the right jugular vein was performed on PND 28 using methods previously described (Lynch 2008). Rats were given an analgesic (2.0 mg/kg ketoprofen, subcutaneous) and an antibiotic (5.5 mg/kg gentamicin, intravenous) on the day of surgery and the next two days. Catheter patency was verified the day after surgery and every Monday, Wednesday, and Friday thereafter by flushing the catheter with a heparinized saline and the n pulling back until blood appeared in the line. If catheter patency was lost (i.e., no blood appeared in the line), the rat was replaced until there were a minimum of 15 rats/group (or 6 rats/group tested for extinction/reinstatement responding during estrus).
Nicotine Self-Administration
On PND 30, rats began training to self-administer nicotine (0.01 mg/kg/infusion) on a FR1 schedule as described previously (Sanchez et al. 2013). Sessions began with the extension of the active lever into the chamber. Responses on the active lever produced an immediate delivery of nicotine that was paired with a light cue above the lever and the sound of the pump activation. A response of the other lever was recorded, but had no programmed consequence. A maximum of 20 infusions were available during the first five training sessions. Sessions began at 12:00 pm and lasted until all 20 infusions were obtained or until 10-min before the next daily session (11:50 am). The active lever retracted from the self-administration chamber at the end of each session. Following the fifth training session, rats were given unrestricted access to infusions of nicotine during 23-hr sessions using the same conditions as described above. These extended access sessions began on PND 35 and were continued until PND 44 (10 days). Nine of the 63 rats tested either did not acquire nicotine self-administration within the 5-day training period (defined as two consecutive days where all 20 infusions were obtained) or lost catheter patency during the self-administration period, and their data were excluded from all analyses.
Interventions During Abstinence
On PND 45, rats were housed individually in polycarbonate cages for a 10-day forced abstinence period. We have previously shown that 10-days of abstinence is sufficient for inducing extinction and cue-induced reinstatement responding in our adolescent-onset model (Sanchez et al. 2013; 2014). Animals were randomly assigned to wheel (N = 15), saccharin (N = 19), or no-wheel/no-saccharin control (N = 20) conditions.
Exercise
Rats assigned to the exercise group were given 2-hr/day access to an unlocked running wheel beginning at 10:00 am. We have previously shown that these exercise conditions result in moderate levels of running during abstinence (~1–2 km/session) and reduce subsequent nicotine-seeking in females (Sanchez et al. 2014). A gate that separated the polycarbonate cage from the wheel was manually removed and replaced each day by the experimenter in order to allow and discontinue access to the wheel. Rats were free to move between the wheel and their polycarbonate cage during the exercise sessions. The number of revolutions completed during each session was recorded daily. This group was compared to a no-wheel condition, rather than a locked-wheel condition, given our previous findings in females showing that the presence of a wheel, locked or unlocked, decreased subsequent drug-seeking (Sanchez et al. 2014; Peterson et al. 2014). Indeed, these findings set the premise for the current study to address the efficacy of exercise versus non-exercise enrichment as potential interventions for nicotine-seeking in females. Numerous recent studies have also used a no-wheel control, rather than a locked-wheel control, to examine addiction-related behaviors (Aarde et al. 2015; Balter and Dykstra 2012; Darlington et al. 2016; Engelmann et al. 2014; Gallego et al. 2015; Lacy et al. 2014; Miladi-Gorji et al. 2012; Mokhtari-Zaer et al. 2014; Ogbonmwan et al. 2015; Smith et al. 2012; Smith and Pitts 2012; Smith and Witte 2012; Sobieraj et al. 2016) since a locked wheel may serve as enrichment and/or a modified exercise condition, particularly in females (Sanchez et al. 2014).
Saccharin
Rats assigned to the saccharin group were given 2-hr/day access to a bottle containing a saccharin-sweetened solution (0.25%). This was given in addition to their water bottle, and in concurrence with the exercise group schedule (i.e., sessions began at 10:00 am). The position of saccharin relative to water was alternated daily, and intake of both was monitored at the start and end of each session.
Control
Rats assigned to the control condition were housed in polycarbonate cages without access to a wheel or saccharin solution. These animals received similar handling as compared to the exercise and saccharin groups.
Extinction and Cue-Induced Reinstatement of Nicotine-Seeking
Rats were placed back in their original self-administration chambers on the tenth day of abstinence following the last exercise, saccharin, or control session. Levels of extinction and cue-induced reinstatement of nicotine-seeking were assessed the following day on PND 55 using a within-session procedure as previously described (Abdolahi et al. 2010; Sanchez et al. 2014). Briefly, extinction responding was assessed in a minimum of five, 1-hr sessions beginning at 10:00 a.m. Each extinction session was separated by a 5-min timeout period, with sessions continuing until responding extinguished (defined as fewer than 15 responses/1-hr session). Reinstatement of nicotine-seeking was assessed in a 1-hr session that began 5-min after the last extinction session. Each response during this session produced a delivery of the cues formerly associated with nicotine (i.e., light above lever and sound of pump) under a FR1 schedule. Each rat was tested in just one extinction/reinstatement session.
Estrous cycle phase determination
To determine the association between nicotine-seeking and estrous cycle phase, rats were vaginally swabbed 30-min prior to the extinction/reinstatement session. However, in order to habituate rats to this procedure, and to track the estrous cycle leading up to the test session, rats were vaginally swabbed on three additional consecutive days prior to the extinction/reinstatement session. Swabs were conducted as described previously (Lynch 2008), and were microscopically examined to determine estrous cycle phase based on previously published criteria (Feder 1981). Females tested during proestrus, diestrus, and metestrus were combined into one non-estrus group and compared to females tested during estrus. This strategy of comparing estrus to non-estrus females has been used by our group (Peterson et al. 2014) and others (Kerstetter et al. 2008), and is based on results from multiple studies with nicotine and other stimulants showing that females tested during metestrus, diestrus, and proestrus do not differ on levels of motivation for drug or drug-seeking (Donny et al. 2000; Feltenstein et al. 2012; Kippin et al. 2005; Lacy et al. 2016; Lynch 2008; 2009; Peterson et al. 2014; Roberts et al. 1989). Females tested during these phases also did not differ on levels of nicotine-seeking (or other measures) in the present study. Six of the 15 rats in the exercise group, seven of the 19 rats in the saccharin group, and six of the 20 rats in the polycarbonate groups were categorized as in estrus at the start of the extinction/reinstatement session. Data from one of the non-estrus control females and one of the non-estrus saccharin females were not available for the reinstatement session due to a technical issue.
Data analysis
All data presented are mean ± standard error of mean (SEM). Group differences were analyzed with repeated measures analysis of variance (ANOVA). Between group factors included intervention during abstinence (exercise, saccharin, control) and estrous cycle phase (estrus, non-estrus), and dependent measures included responding and intake during the 10 extended access self-administration sessions, daily distance run during abstinence, daily saccharin intake and preference (defined as saccharin consumption/total consumption × 100) during abstinence, responses during the first five extinction sessions, responses during the last extinction session versus during the reinstatement session, and body weights from the beginning of the study to the end (recorded every Monday, Wednesday, and Friday). In order to examine changes in levels of nicotine intake, wheel running, and saccharin intake/preference over time, levels observed on day 1 were compared to the average of those observed during the last 3 sessions using paired samples t-tests. Univariate ANOVA was used to compare total levels of extinction responding during each of the 5–9 sessions completed between intervention groups and estrous cycle phases. We also analyzed differences from control levels of extinction responding (total during the first 5 sessions) and reinstatement responding for the exercise and saccharin groups using univariate ANOVA with posthoc comparison made using a one-sample t-test. Post-hoc comparisons to control were made using Dunnett’s t-test, and one-tailed t-tests were used for a priori predicted hypotheses correcting for family-wise error. Data were analyzed in SPSS with alpha set at 0.05.
Results
Extended Access Nicotine Self-Administration
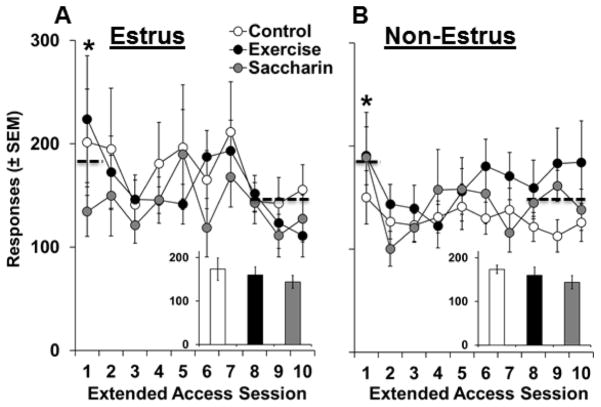
Before abstinence and the subsequent exercise, saccharin, or control interventions, each of the groups showed a similar pattern of responding over the 10-day period of extended access self-administration (Figure 2A). Females that were subsequently tested on nicotine-seeking during estrus versus non-estrus phases within each of these groups also didn’t differ on levels of responding for nicotine (Figure 2B) or levels of nicotine intake (average mg/kg/day ± SEM; exercise, estrus: 1.3 ± 0.1, non-estrous: 1.2 ± 0.1; saccharin, estrus: 1.2 ± 0.1, non-estrus: 1.1 ± 0.1; control, estrus: 1.4 ± 0.2, non-estrous: 1.1 ± 0.1; p’s>0.05). Results from a repeated measures ANOVA comparing responses over the 10-day extended access period revealed non-significant overall and interactive effects of intervention group and estrous cycle phase (p’s>0.05), but a significant effect of day (F9, 432 = 2.4, p < 0.05). Subsequent analysis of responses collapsed across groups revealed that responding was higher on the first day of access (~180 responses) as compared to during the last 3 days of access (~140 responses; t53 = 2.3, p < 0.05). Thus, patterns of responding and levels of nicotine intake were similar between groups prior to abstinence and subsequent interventions.
Figure 2.
Prior to abstinence and subsequent exercise, saccharin or control interventions, daily patterns and levels of responding for nicotine did not vary between the intervention groups or between females subsequently tested during estrus (exercise, n=6; saccharin, n=7; and control, n=6) versus non-estrus phases (exercise, n=9; saccharin, n=12; control, n=14). Mean (±SEM) number of responses for nicotine as a function of extended-access day for females subsequently tested on extinction/reinstatement responding during estrus (A) versus non-estrus phases (B) in each of the three intervention groups. Insets show mean (±SEM) daily nicotine intake (mg/kg) across the 10-day extended-access period for females tested during estrus versus non-estrus phases in each of the groups. A dashed line and an asterisk indicates a significant decrease in responding from the 1st session to the last 3 sessions (p<0.05).
Levels of Wheel Running and Saccharin Intake/Preference During Abstinence
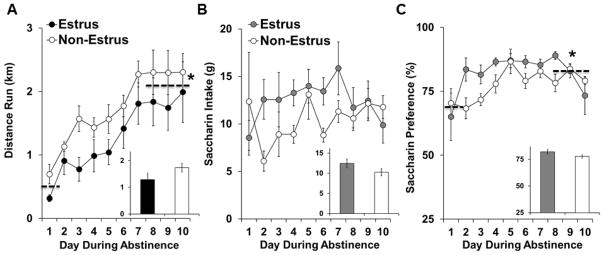
While there was considerable variability in levels of wheel running within each group (average distance ranged from 0.4 to 2.5 km/day), the average distance run was similar between females subsequently tested on extinction/reinstatement responding during as estrus versus non-estrus phases, and both groups showed a similar increase in levels of running over time (Figure 3A). Results from a repeated measures ANOVA comparing distance run over the 10-day abstinence period revealed non-significant overall and interactive effects of estrous cycle phase (p’s>0.05), and a significant effect of day (F9, 117 = 17.9, p < 0.001). Subsequent analysis of wheel running collapsed across estrous cycle phase revealed that distance run was significantly lower during the first exercise session (~0.5 km) as compared to the average distance run during the last 3 sessions (~2.1 km; t14 = 6.7, p < 0.001).
Figure 3.
Daily patterns and levels of wheel running and saccharin intake and preference during abstinence did not differ between females subsequently tested on extinction/reinstatement responding during estrus versus non-estrus phases (exercise: n=6 and 9; saccharin: n=7 and 12, respectively). Mean (±SEM) distance run for each of the exercise sessions (A) and mean (±SEM) saccharin intake (g) and preference (%) for each of the saccharin sessions (B and C, respectively) for females subsequently tested during estrus versus non-estrus phases. Insets show mean values (±SEM) across sessions for females tested during estrus versus non-estrus phases in the exercise (A) and saccharin group (B,C). A dashed line and an asterisk indicates a significant increase in distance run (A) and saccharin preference (C) over time (1st session versus average of last 3 sessions; p<0.05).
Like levels of running, there was considerable variability in levels of saccharin intake (average intake ranged from 6.7 to 20.0 g/day) and preference for saccharin over water (average preference ranged from 70.5 to 89.6%); however, both intake and preference were similar between females subsequently tested on extinction/reinstatement responding during estrus or non-estrus phases, and both groups showed a similar increase in preference for saccharin over time (Figure 3B and C). Repeated measures ANOVA of saccharin intake over the 10-day abstinence period revealed non-significant overall and interactive effects of estrous cycle phase and day (p’s>0.05). Analysis of saccharin preference also revealed non-significant overall and interactive effects of estrous cycle phase (p’s>0.05), but a significant effect of day (F9, 153 = 4.4, p < 0.001). Subsequent analysis of saccharin preference collapsed across estrous cycle phase revealed that preference was significantly lower during first the session (~69%) as compared to the average observed during the last 3 sessions (~81%; t18 = 2.8, p < 0.05).
Each of the groups also weighed a similar amount and showed similar increases in body weight over the course of the experiment (at surgery: 83.3 ± 2.4 g exercise; 84.3 ± 2.1 g saccharin; 87.9 ± 1.5 g control; at the end of the study: 193.7 ± 6.3 g exercise, 192.8 ± 3.2 g saccharin, 191.0 ± 4.1 g control). Repeated measures ANOVA comparing body weights from the beginning of the study to the end revealed a significant effect of day (F11, 528 = 418.2, p < 0.001), but non-significant overall and interactive effects of group and estrous cycle phase (p’s>0.05).
Thus, females that were tested on nicotine-seeking during estrus versus non-estrus phases did not differ prior to testing with regard to body weight, levels of running (exercise group), or saccharin intake/preference (saccharin group).
Effect of Exercise versus Saccharin During Abstinence on Nicotine-Seeking
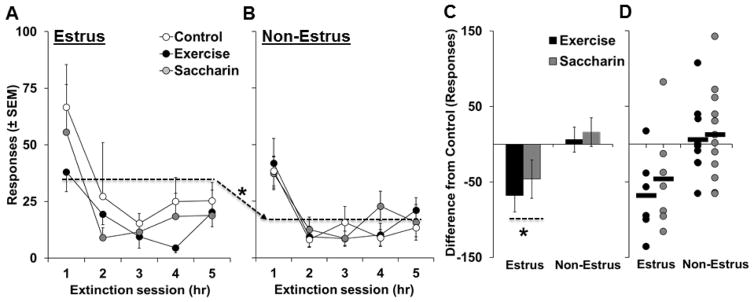
Although levels and patterns of extinction responding were statistically similar between each of the groups during first 5 extinction sessions (p’s>0.05; Figure 4A and B), total levels of extinction responding during each of the sessions completed (five to nine sessions) were 46% higher on average in females testing during the estrus phase (143 ± 22) versus females tested during non-estrus phases (98 ± 10) across all groups. Results from a univariate ANOVA comparing total levels of extinction responding between each of the groups revealed a significant effect of estrous cycle phase (F1, 48 = 4.2, p < 0.05), but non-significant overall and interactive effects of intervention (p’s>0.05). Planned comparison within the control group also revealed significantly higher levels of extinction responding in estrus versus non-estrus females for both total responses during all sessions (approximately 75% higher, 163 ± 50 versus 93 ± 13, respectively; t18 = 4.4, p < 0.05) and responses during the first 5 sessions (approximately 92% higher, 161 ± 50 versus 84 ± 9, respectively; t18 = 3.6, p < 0.05). Non-significant effects of estrous cycle phase were observed within the exercise and saccharin groups for both measures of extinction responding (p’s>0.05) indicating that each intervention blocked the estrus-induced increase in extinction responding. Similarly, an analysis of difference from control levels of extinction responding revealed a significant effect of estrous cycle phase (F1, 30 = 5.3, p < 0.05) with subsequent analysis revealing a significant decrease from control in estrus (t12 = 3.4, p < 0.01), but not non-estrus females (p > 0.05; Figure 4C). The analysis of difference from control levels of responding also revealed a non-significant overall effect of intervention group (p>0.05) indicating that exercise and saccharin similarly decreased extinction responding in females tested during estrus. Thus, levels of extinction responding were higher in females tested during estrus versus non-estrus phases, and exercise and saccharin during abstinence similarly blocked the estrus-induced increase in extinction responding.
Figure 4.
Wheel running exercise or saccharin consumption during abstinence decreased subsequent estrus-induced increases in extinction responding (estrus: exercise, n=6; saccharin, n=7; control, n=6; non-estrus, exercise, n=9; saccharin, n=12; control, n=14). Mean number of responses during each of the 5, 1-hr extinction sessions for females tested during estrus (A) versus non-estrus phases (B) in each of the intervention groups. Difference from control responses (±SEM) during the first five extinction sessions averaged across groups (C) and for each of the females tested (D) during estrus versus non-phases in the exercise and saccharin groups. Dashed lines with an arrow and an asterisk (A and B) indicate a significant difference between levels of extinction responding in control females tested during estrus versus non-estrus phases (p<0.05). A dashed line with an asterisk (C) indicates a significant decrease from control (p<0.05).
Similar effects of estrous cycle phase and intervention group were observed for reinstatement responding (Figure 5A and B). Results from a repeated measures ANOVA comparing responses during the last extinction session to responses during the reinstatement session revealed significant overall effects of session (F1, 46 = 45.8, p < 0.001), intervention group (F2, 46 = 3.8, p < 0.05), and estrous cycle phase (F1, 46 = 4.8, p < 0.05), as well as a significant interaction of session by group by estrous cycle phase (F2, 46 = 6.7, p < 0.01). Subsequent analysis within the control, exercise, and saccharin groups revealed significant overall effects of session for each group (F1, 17 = 21.7, p < 0.001; F1, 13 = 7.9, p < 0.05; F1, 16 = 22.9, p < 0.001; respectively) indicating that responding was reinstated by nicotine-associated cues for each of the groups.
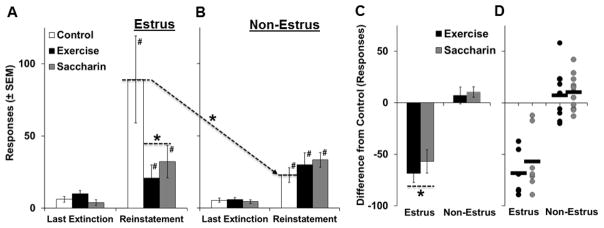
Figure 5.
Wheel running exercise or saccharin consumption during abstinence decreased subsequent estrus-induced increases in cue-induced reinstatement responding (estrus: exercise, n=6; saccharin, n=7; control, n=6; non-estrus, exercise, n=9; saccharin, n=11; control, n=13). Mean number of responses (±SEM) during the last extinction session as compared to the reinstatement session for females testing during estrus (A) versus non-estrus phases (B) in each of the three intervention groups. Difference from control responses (±SEM) during the reinstatement sessions averaged across groups (C) and for each of the females tested (D) during estrus versus non-estrus phases in the exercise and saccharin groups. Number signs (#) indicate a significant increase from the last extinction session (A and B). Dashed lines with an arrow and an asterisk (A and B) indicate a significant difference between levels of reinstatement responding in control females tested during estrus versus non-estrus phases (p<0.05). A dashed line with an asterisk (A and C) indicates a significant decrease from control (p<0.05).
Significant effects of estrous cycle phase and its interaction with session were also observed in the control group (F1, 17 = 10.0, p < 0.01; F1, 17 = 9.3, p < 0.01, respectively), but not in the exercise or saccharin group (p’s>0.05) indicating that both interventions blocked the estrus-induced increase in reinstatement responding. Additionally, while no significant differences were observed within the last extinction session (p’s>0.05), total levels of cue-induced reinstatement responding were 65% higher on average in estrus females (47 ± 12) as compared to non-estrus females (28 ± 3); Within the control condition this difference increased to 290% on average. Univariate analysis within the reinstatement session revealed a significant overall effect of intervention group (F2, 46 = 4.0, p < 0.05), and estrous cycle phase (F1, 46 = 4.1, p < 0.05), as well as a significant interaction of estrous cycle phase by intervention group (F 2, 46 = 6.8, p < 0.01). Subsequent analysis within estrus females revealed a significant effect of group (F2, 16 = 3.7, p < 0.01), with posthoc comparison to control revealing significant decreases in responses for both the exercise (t10 = 4.4, p < 0.05) and the saccharin (t11 = 3.8, p < 0.05) groups interventions. There were no significant differences within non-estrus females (p’s>0.05). Similarly, an analysis of difference from control levels of reinstatement responding revealed a significant effect of estrous cycle phase (F1, 29 = 74.5, p < 0.001), but a non-significant overall effect of intervention group (p>0.05) indicating that exercise and saccharin similarly decreased reinstatement responding. Subsequent analysis collapsed across intervention group revealed a significant difference within females tested during estrus (t12 = 8.5, p < 0.001), but not non-estrus phases (p>0.05; Figure 5C). Thus, levels of reinstatement responding were markedly higher in females tested during estrus versus non-estrus phases, and exercise and saccharin during abstinence similarly blocked this estrus-induced increase.
Discussion
The goals of this study were to determine whether the efficacy of exercise at reducing nicotine-seeking differed from non-exercise based environmental enrichment (saccharin), and to determine whether the efficacy of these interventions varied between females tested during estrus versus non-estrus phases. Consistent with our hypotheses, we found that levels of nicotine-seeking were highest during estrus, and that exercise or saccharin during abstinence decreased subsequent nicotine-seeking. Surprisingly, the ability of exercise and saccharin to decrease nicotine-seeking was restricted to the heightened levels observed in females tested during estrus; neither intervention affected nicotine-seeking in females tested during non-estrus phases. The lack of an effect in females tested during non-estrus phases, however, is likely due to a floor effect since levels of nicotine-seeking under both extinction and reinstatement testing were low in females tested during non-estrus phases in each of the groups, including control females housed without wheels or saccharin. These results demonstrate that exercise or saccharin during abstinence decrease nicotine-seeking, and suggest that that either would be effective as an early intervention for nicotine use and addiction in females.
Levels of nicotine-seeking under extinction testing conditions and in response to nicotine-associated cues were highest in females tested during estrus versus non-estrus phases. These findings were predicted based on results in female smokers and female animals self-administering other stimulant drugs which indicate that a high ratio of estradiol to progesterone, characteristic of the follicular/estrus phase, contributes to increased drug-seeking/craving (DeVito et al. 2014; Feltenstein and See 2007; Lynch and Sofuoglu 2010; Wetherill et al. 2016). These findings are also consistent with our previous work showing that motivation for nicotine is highest during estrus, and positively associated with the ratio of estradiol to progesterone (Lynch 2008), and with previous work in women showing that nicotine-associated cues induce craving and neural activity in women tested during the follicular, but not the luteal phase, of the menstrual cycle (Franklin et al. 2015). They are, however, in contrast to results from a previous study in adult female rats showing that levels of nicotine-seeking did not vary with estrous cycle phase (Feltenstein et al. 2012). While this previous study was likely under-powered for detecting estrous cycle effects on nicotine-seeking (Feltenstein et al. 2012), it is also possible that the influence of estrous cycle phase on nicotine-seeking changes from adolescence to adulthood. Future studies are needed to examine this possibility.
Exercise versus saccharin during abstinence similarly decreased extinction and cue-induced reinstatement responding. These findings were predicted by results showing that girls benefitted from anti-smoking interventions with and without an exercise component (Horn et al. 2011), and our recent findings in female rats showing that access to a wheel during abstinence, whether it was locked or unlocked, effectively reduced subsequent nicotine- and cocaine-seeking (Sanchez et al. 2014; Peterson et al. 2014). These results add to a growing body of work supporting exercise as a promising intervention for drug addiction (Lynch et al. 2013), and further indicate the potential utility of exercise as an early intervention for nicotine use and addiction in females. These results also show for the first time that brief daily access to a saccharin solution during abstinence is effective at reducing subsequent nicotine-seeking. Saccharin solution was selected for the comparison since, unlike toys, a locked wheel, and other forms of enrichment that are typically used in environmental enrichment studies (Solinas et al. 2010), it would not engender play or exercise-like behaviors (i.e., climbing, hanging). This addresses a limitation of our previous work using locked versus unlocked wheel access (Sanchez et al. 2014; Peterson et al. 2014), as well as previous work from others examining the effects of environmental enrichment (Solinas et al. 2010), where it has not been possible to determine the contribution of exercise versus enrichment effects. Saccharin and other sweet rewards have been shown to reduce drug-taking when concurrently available with the drug (Carroll et al. 2016), with results demonstrating that rats show an almost exclusive preference for saccharin versus drug under choice procedures (Cantin et al. 2010; Huynh et al. 2017). In fact, numerous recent studies have used saccharin solution and other highly palatable solutions as an alternative choice to the drug, including nicotine, as a means to maintain voluntary abstinence (Cantin et al. 2010; Huynh et al. 2017). Our current findings indicate that saccharin during abstinence, even a modest level of access, will itself reduce subsequent drug-seeking and relapse vulnerability. This may be particularly apparent in studies with females, however, considering that males in our previous work did not respond to the locked wheel enriched condition. This idea is supported by findings showing that prior access to saccharin is more protective against subsequent cocaine self-administration in female versus male rats (Cason and Grigson 2013), as well as findings in males showing that sucrose reward during abstinence does not affect subsequent cocaine-seeking behavior (Nicolas et al. 2016).
Our results also show that the efficacy of the exercise versus saccharin intervention varied between females tested during estrus versus non-estrus phases. While each intervention decreased the high levels of nicotine-seeking observed in females tested during estrus, neither affected the low levels observed in females tested during non-estrus phases. We also recently observed estrous cycle phase-dependent effects of wheel access (unlocked or locked) during abstinence on subsequent cocaine-seeking (Peterson et al. 2014). However, in contrast to the results observed here, results from this previous study showed that 2-hr/day access to a locked or unlocked wheel decreased cocaine-seeking in females tested during non-estrus phases, but not in females tested during estrus. Levels of drug-seeking were also highest during estrus in that study, but the levels observed in non-estrus females were much higher in the previous study (~50 reinstatement responses) as compared to those observed here. It is notable that in the previous study, the estrus-induced increases in levels of drug-seeking could be surmounted by increasing the length of daily access to the wheel (6–24-hr/day). Thus, it is possible that modest interventions can effectively reduce drug-seeking and prevent relapse in individuals that exhibit modest levels of drug-seeking (e.g., adolescent/young adult females), but that more intense interventions may be needed to combat drug-seeking in individuals that exhibit high levels of drug-seeking (e.g., adult women, particularly during the follicular phase). This interpretation is also consistent with recent findings in women showing that the efficacy of nicotine replacement therapy, which only modestly improves smoking cessation (Walker et al. 2016), depended on menstrual cycle phase (Saladin et al. 2015); whereas, varenicline, which more robustly enhances smoking cessation (Walker et al. 2016), was effective during each phase of the menstrual cycle (Saladin et al. 2015).
It is possible that exercise/saccharin during abstinence would provide long-lasting protection against nicotine-seeking such that females would continue to show reduced nicotine-seeking, even when tested during estrus at later abstinence points (when seeking is expected to be higher; Funk et al. 2016). This possibility is based on findings in male rats showing that wheel running during abstinence provides persistent protection against drug-seeking (Beiter et al. 2016), with evidence to suggest that it does so by preventing neuroadaptations that develop over abstinence that underlie enhanced drug-seeking/relapse vulnerability (Peterson et al. 2014; Lynch et al. 2017). For example, our recent findings with nicotine indicate that the efficacy of wheel running to reduce nicotine-seeking is associated with its ability to normalize markers of glutamatergic plasticity in the nucleus accumbens that are associated with enhanced nicotine-seeking (unpublished results). Our findings with cocaine further show that wheel running during early abstinence (days 1–7) persistently decreases drug-seeking following protracted abstinence (assessed on day 15 of abstinence). Interestingly, exercise during early abstinence produced a similar decrease in drug-seeking as compared to exercise throughout abstinence (days 1–14) indicating that its effects on neuroadaptations during early abstinence may be particularly critical for long-term protection. Further research is needed to determine whether similar effects also occur in females and in response to sucrose consumption during abstinence.
The impact of hormonal status on nicotine addiction has been an expanding area of study over the last decade. A better understanding of this relationship is crucial for developing appropriately timed interventions for nicotine craving and relapse in females. Our findings demonstrate that levels of nicotine-seeking vary across the estrous cycle, with the highest levels observed during estrus, and that exercise or saccharin during abstinence block this estrus-induced increase in nicotine-seeking. These findings support the potential utility of exercise or environmental enrichment as an intervention for nicotine use and addiction in adolescent and young adult female smokers. Future research is needed to determine whether the efficacy of exercise versus enrichment can be increased when combined together or with other pharmacotherapeutic interventions.
Highlights.
In females, treatment outcome for nicotine addiction may depend on hormonal status.
We compared the efficacy of exercise vs saccharin during abstinence on nicotine-seeking.
Efficacy was examined as a function of estrous cycle phase.
Both exercise and saccharin blocked estrus-induced increases in nicotine-seeking.
Exercise or enrichment may effectively reduce nicotine craving in human females.
Acknowledgments
Funding: This study was supported by the Virginia Foundation for Healthy Youth (grant 8520893; DHB and WJL), and the National Institute on Drug Abuse (grants R01DA024716 and R01DA039093; WJL).
Footnotes
Conflict of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdolahi A, Acosta G, Breslin FJ, Hemby SE, Lynch WJ. Incubation of nicotine seeking is associated with enhanced protein kinase A-regulated signaling of dopamine- and cAMP-regulated phosphoprotein of 32 kDa in the insular cortex. Eur J Neurosci. 2010;31(4):733–41. doi: 10.1111/j.1460-9568.2010.07114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen SS. Cigarette smoking among women: how can we help? Minn Med. 2014;97(3):41–3. [PubMed] [Google Scholar]
- Allen SS, Allen AM, Lunos S, Hatsukami DK. Patterns of self-selected smoking cessation attempts and relapse by menstrual phase. Addict Behav. 2009;34(11):928–931. doi: 10.1016/j.addbeh.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen AM, Oncken C, Hatsukami D. Women and smoking: the effect of gender on the epidemiology, health effects, and cessation of smoking. Curr Addict Rep. 2014;1(1):53–60. doi: 10.1007/s40429-013-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard P, Ninot G, Moullec G, Guillaume S, Courtet P, Quantin X. Smoking cessation, depression, and exercise: empirical evidence, clinical needs, and mechanisms. Nicotine Tob Res. 2013;15(10):1635–50. doi: 10.1093/ntr/ntt042. [DOI] [PubMed] [Google Scholar]
- Bock BC, Fava JL, Gaskins R, Morrow KM, Williams DM, Jennings E, Becker BM, Tremont G, Marcus BH. Yoga as a complementary treatment for smoking cessation in women. J Womens Health. 2012;21(2):240–8. doi: 10.1089/jwh.2011.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantin L, Lenoir M, Augier E, Vanhille N, Dubreucq S, Serre F, Vouillac C, Ahmed SH. Cocaine is low on the value ladder of rats: possible evidence for resilience to addiction. PLoS One. 2010;5(7):e11592. doi: 10.1371/journal.pone.0011592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cason AM, Grigson PS. Prior access to a sweet is more protective against cocaine self-administration in female rats than in male rats. Physiol Behav. 2013;112–113:96–103. doi: 10.1016/j.physbeh.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Collins M, Kohl EA, Johnson S, Dougen B. Sex and menstrual cycle effects on chronic oral cocaine self-administration in rhesus monkeys: Effects of a nondrug alternative reward. Psychopharmacology. 2016;233(15–16):2973–84. doi: 10.1007/s00213-016-4343-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Tobacco Use Among Middle and High School Students—United States, 2011–2015. Morbidity and Mortality Weekly Report. 2016;65(14):361–7. doi: 10.15585/mmwr.mm6514a1. [DOI] [PubMed] [Google Scholar]
- Clayton JA, Collins FS. NIH to balance sex in cell and animal studies. Nature. 2014;509(7500):282–283. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito EE, Herman AI, Waters AJ, Valentine GW, Sofuoglu M. Subjective, physiological, and cognitive responses to intravenous nicotine: effects of sex and menstrual cycle phase. Neuropsychopharmacology. 2014;39(6):1431–40. doi: 10.1038/npp.2013.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Rowell PP, Gharib MA, Maldovan V, Booth S, Mielke MM, Hoffman A, McCallum S. Nicotine self-administration in rats: estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology. 2000;151(4):392–405. doi: 10.1007/s002130000497. [DOI] [PubMed] [Google Scholar]
- Feder HH. Estrous cyclicity in mammals. In: Alder NT, editor. Neuroendocrinology of reproduction: physiology and behavior. Springer; New York: 1981. pp. 280–290. [Google Scholar]
- Federal Trade Commission. Federal Trade Commission Cigarette Report for 2014. Washington: Federal Trade Commission; 2015. [Google Scholar]
- Feltenstein MW, See RE. Plasma progesterone levels and cocaine-seeking in freely cycling female rats across the estrous cycle. Drug Alcohol Depend. 2007;89(2–3):183–9. doi: 10.1016/j.drugalcdep.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, Ghee SM, See RE. Nicotine self-administration and reinstatement of nicotine-seeking in male and female rats. Drug Alcohol Depend. 2012;121(3):240–6. doi: 10.1016/j.drugalcdep.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Sofuoglu M, Morgan PT, Tuit KL, Sinha R. The effects of exogenous progesterone on drug craving and stress arousal in cocaine dependence: impact of gender and cue type. Psychoneuroendocrinology. 2013;38(9):1532–44. doi: 10.1016/j.psyneuen.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Jagannathan K, Wetherill RR, Johnson B, Kelly S, Langguth J, Mumma J, Childress AR. Influence of menstrual cycle phase on neural and craving responses to appetitive smoking cues in naturally cycling females. Nicotine Tob Res. 2015;17(4):390–7. doi: 10.1093/ntr/ntu183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French SA, Jeffery RW. Weight concerns and smoking: A literature review. Ann Behav Med. 1995;17(3):234–44. doi: 10.1007/BF02903918. [DOI] [PubMed] [Google Scholar]
- Funk D, Coen K, Tamadon S, Hope BT, Shaham Y, Lê AD. Role of central amygdala neuronal ensembles in incubation of nicotine craving. J Neurosci. 2016;36(33):8612–23. doi: 10.1523/JNEUROSCI.1505-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett BE, Dube SR, Winder C, Caraballo RS. Centers for Disease Control and Prevention Cigarette smoking - United States, 2006–2008 and 2009–2010. MMWR Suppl. 2013;62(3):81–4. [PubMed] [Google Scholar]
- Haasova M, Warren FC, Ussher M, Janse Van Rensburg K, Faulkner G, Cropley M, Byron-Daniel J, Everson-Hock ES, Oh H, Taylor AH. The acute effects of physical activity on cigarette cravings: exploration of potential moderators, mediators and physical activity attributes using individual participant data (IPD) meta-analyses. Psychopharmacology. 2014;231(7):1267–75. doi: 10.1007/s00213-014-3450-4. [DOI] [PubMed] [Google Scholar]
- Horn K, Dino G, Branstetter SA, Zhang J, Noerachmanto N, Jarrett T, Taylor M. Effects of physical activity on teen smoking cessation. Pediatrics. 2011;128(4):e801–11. doi: 10.1542/peds.2010-2599. [DOI] [PubMed] [Google Scholar]
- Hussaini AE, Nicholson LM, Shera D, Stettler N, Kinsman S. Adolescent obesity as a risk factor for high-level nicotine addiction in young women. J Adolesc Health. 2011;49(5):511–7. doi: 10.1016/j.jadohealth.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Huynh C, Fam J, Ahmed SH, Clemens KJ. Rats quit nicotine for a sweet reward following an extensive history of nicotine use. Addict Biol. 2017;22(1):142–151. doi: 10.1111/adb.12306. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use, 1975–2015: Overview, key findings on adolescent drug use. Ann Arbor: Institute for Social Research, The University of Michigan; 2016. [Google Scholar]
- Kandel DB, Kandel ER. A molecular basis for nicotine as a gateway drug. N Engl J Med. 2014;371(21):2038–9. doi: 10.1056/NEJMc1411785. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Yamaguchi K, Chen K. Stages of progression in drug involvement from adolescence to adulthood: further evidence for the gateway theory. J Stud Alcohol. 1992;53(5):447–57. doi: 10.15288/jsa.1992.53.447. [DOI] [PubMed] [Google Scholar]
- Kaplan G, Ivanov I. Pharmacotherapy for substance abuse disorders in adolescence. Pediatr Clin North Am. 2011;58:243–258. doi: 10.1016/j.pcl.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Kerstetter KA, Aguilar VR, Parrish AB, Kippin TE. Protracted time-dependent increases in cocaine-seeking behavior during cocaine withdrawal in female relative to male rats. Psychopharmacology. 2008;198(1):63–75. doi: 10.1007/s00213-008-1089-8. [DOI] [PubMed] [Google Scholar]
- Kippin TE, Fuchs RA, Mehta RH, Case JM, Parker MP, Bimonte-Nelson HA, See RE. Potentiation of cocaine-primed reinstatement of drug seeking in female rats during estrus. Psychopharmacology. 2005;182(2):245–52. doi: 10.1007/s00213-005-0071-y. [DOI] [PubMed] [Google Scholar]
- Lacy RT, Strickland JC, Feinstein MA, Robinson AM, Smith MA. The effects of sex, estrous cycle, and social contact on cocaine and heroin self-administration in rats. Psychopharmacology. 2016;233(17):3201–10. doi: 10.1007/s00213-016-4368-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir M, Serre F, Cantin L, Ahmed SH. Intense sweetness surpasses cocaine reward. PLoS One. 2007;2(8):e698. doi: 10.1371/journal.pone.0000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn PM, Rohde P, Brown RA. Level of current and past ado lescent cigarette smoking as predictors of future substance use disorders in young adulthood. Addiction. 1999;94(6):913–21. doi: 10.1046/j.1360-0443.1999.94691313.x. [DOI] [PubMed] [Google Scholar]
- Li S, Zou S, Coen K, Funk D, Shram MJ, Lê AD. Sex differences in yohimbine- induced increases in the reinforcing efficacy of nicotine in adolescent rats. Addict Biol. 2014;19(2):156–64. doi: 10.1111/j.1369-1600.2012.00473.x. [DOI] [PubMed] [Google Scholar]
- Lynch WJ. Acquisition and maintenance of cocaine self-administration in adolescent rats: effects of sex and gonadal hormones. Psychopharmacology. 2008;197(2):237–46. doi: 10.1007/s00213-007-1028-0. [DOI] [PubMed] [Google Scholar]
- Lynch WJ. Sex and ovarian hormones influence vulnerability and motivation for nicotine during adolescence in rats. Pharmacol Biochem Behav. 2009;94:43–50. doi: 10.1016/j.pbb.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Sofuoglu M. Role of progesterone in nicotine addiction: evidence from initiation to relapse. Exp Clin Psychopharmacol. 2010;18(6):451–61. doi: 10.1037/a0021265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Peterson AB, Sanchez V, Abel J, Smith MA. Exercise as a novel treatment for drug addiction: a neurobiological and stage-dependent hypothesis. Neurosci Biobehav Rev. 2013;37(8):1622–44. doi: 10.1016/j.neubiorev.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Abel J, Robinson AM, Smith MA. Invited Review: Sex differences in the efficacy of exercise as a treatment for drug addiction. Special Section, Current Addiction Reports: Women and Addictions. 2017 in press. [Google Scholar]
- McIntosh H. For women smokers, the gender gap is narrowing. J Natl Cancer Inst. 1997;89(2):120–1. doi: 10.1093/jnci/89.2.120. [DOI] [PubMed] [Google Scholar]
- McVay MA, Copeland AL. Smoking cessation in peri- and postmenopausal women: a review. Exp Clin Psychopharmacol. 2011;19(3):192–202. doi: 10.1037/a0023119. [DOI] [PubMed] [Google Scholar]
- Nicolas C, Lafay-Chebassier C, Solinas M. Exposure to sucrose during periods of withdrawal does not reduce cocaine-seeking behavior in rats. Sci Rep. 2016;6:23272. doi: 10.1038/srep23272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogbonmwan YE, Schroeder JP, Holmes PV, Weinshenker D. The effects of post-extinction exercise on cocaine-primed and stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2015;232(8):1395–403. doi: 10.1007/s00213-014-3778-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pampel FC. Global patterns and determinants of sex differences in smoking. Int J Comp Sociol. 2006;47(6):466–487. doi: 10.1177/0020715206070267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten CA, Bronars CA, Vickers Douglas KS, Ussher MH, Levine JA, Tye SJ, Hughes CA, Brockman TA, Decker PA, DeJesus RS, Williams MD, Olson TP, Clark MM, Dieterich AM. Supervised, vigorous intensity exercise intervention for depressed female smokers: a pilot study. Nicotine Tob Res. 2017;19(1):77–86. doi: 10.1093/ntr/ntw208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Scott J. Sex differences in long-term smoking cessation rates due to nicotine patch. Nicotine Tob Res. 2008;10(7):1245–50. doi: 10.1080/14622200802097506. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Smoking cessation in women. Special considerations. CNS Drugs. 2001;15(5):391–411. doi: 10.2165/00023210-200115050-00005. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Donny E, Caggiula AR. Sex differences in nicotine effects and self-administration: review of human and animal evidence. Nicotine Tob Res. 1999;1(4):301–15. doi: 10.1080/14622299050011431. [DOI] [PubMed] [Google Scholar]
- Peterson AB, Hivick DP, Lynch WJ. Dose-dependent effectiveness of wheel running to attenuate cocaine-seeking: impact of sex and estrous cycle in rats. Psychopharmacology. 2014;231(13):2661–70. doi: 10.1007/s00213-014-3437-1. [DOI] [PubMed] [Google Scholar]
- Prapavessis H, De Jesus S, Harper T, Cramp A, Fitzgeorge L, Mottola MF, Ussher M, Faulkner G, Selby P. The effects of acute exercise on tobacco cravings and withdrawal symptoms in temporary abstinent pregnant smokers. Addict Behav. 2014;39(3):703–8. doi: 10.1016/j.addbeh.2013.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prapavessis H, De Jesus S, Fitzgeorge L, Faulkner G, Maddison R, Batten S. Exercise to enhance smoking cessation: the getting physical on cigarette randomized control trial. Ann Behav Med. 2016;50(3):358–69. doi: 10.1007/s12160-015-9761-9. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Bennett SA, Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology. 1989;98(3):408–11. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- Roberts V, Maddison R, Simpson C, Bullen C, Prapavessis H. The acute effects of exercise on cigarette cravings, withdrawal symptoms, affect, and smoking behaviour: systematic review update and meta-analysis. Psychopharmacology. 2012;222(1):1–15. doi: 10.1007/s00213-012-2731-z. [DOI] [PubMed] [Google Scholar]
- Saladin ME, McClure EA, Baker NL, Carpenter MJ, Ramakrishnan V, Hartwell KJ, Gray KM. Increasing progesterone levels are associated with smoking abstinence among free-cycling women smokers who receive brief pharmacotherapy. Nicotine Tob Res. 2015;17(4):398–406. doi: 10.1093/ntr/ntu262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez V, Moore CF, Brunzell DH, Lynch WJ. Effect of wheel-running during abstinence on subsequent nicotine-seeking in rats. Psychopharmacology. 2013;227(3):403–11. doi: 10.1007/s00213-012-2964-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez V, Moore CF, Brunzell DH, Lynch WJ. Sex differences in the effect of wheel running on subsequent nicotine-seeking in a rat adolescent-onset self-administration model. Psychopharmacology. 2014;231(8):1753–62. doi: 10.1007/s00213-013-3359-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez V, Lycas MD, Lynch WJ, Brunzell DH. Wheel running exercise attenuates vulnerability to self-administer nicotine in rats. Drug Alcohol Depend. 2015;156:193–8. doi: 10.1016/j.drugalcdep.2015.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobieraj JC, Kim A, Fannon MJ, Mandyam CD. Chronic wheel running-induced reduction of extinction and reinstatement of methamphetamine seeking in metha mphetamine dependent rats is associated with reduced number of periaqueductal gray dopamine neurons. Brain Struct Funct. 2016;221(1):261–76. doi: 10.1007/s00429-014-0905-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Thiriet N, Chauvet C, Jaber M. Prevention and treatment of drug addiction by environmental enrichment. Prog Neurobiol. 2010;92(4):572–92. doi: 10.1016/j.pneurobio.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Thiel KJ, Sanabria F, Pentkowski NS, Neisewander JL. Anti-craving effects of environmental enrichment. Int J Neuropsychopharmacol. 2009;12(9):1151–6. doi: 10.1017/S1461145709990472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torchalla I, Okoli CT, Bottorff JL, Qu A, Poole N, Greaves L. Smoking cessation programs targeted to women: a systematic review. Women Health. 2012;52(1):32–54. doi: 10.1080/03630242.2011.637611. [DOI] [PubMed] [Google Scholar]
- Tordoff MG, Alarcon LK, Lawler MP. Preferences of 14 rat strains for 17 taste compounds. Physiol Behav. 2008;95(3):308–32. doi: 10.1016/j.physbeh.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritter A, Fitzgeorge L, Prapavessis H. The effect of acute exercise on cigarette cravings while using a nicotine lozenge. Psychopharmacology. 2015;232(14):2531–9. doi: 10.1007/s00213-015-3887-0. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services. Women and Smoking: A Report of the Surgeon General. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2001. [Google Scholar]
- US Department of Health and Human Services. Preventing Tobacco Use Among Youth and Young Adults: A Report of the Surgeon General. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2012. [Google Scholar]
- US Department of Health and Human Services. The Health Consequences of Smoking--50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- Valentine JD, Hokanson JS, Matta SG, Sharp BM. Self-administration in rats allowed unlimited access to nicotine. Psychopharmacology. 1997;133(3):300–4. doi: 10.1007/s002130050405. [DOI] [PubMed] [Google Scholar]
- Vendruscolo LF, Gueye AB, Darnaudéry M, Ahmed SH, Cador M. Sugar overconsumption during adolescence selectively alters motivation and reward function in adult rats. PLoS One. 2010;5(2):e9296. doi: 10.1371/journal.pone.0009296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werch CC, Moore MJ, DiClemente CC, Bledsoe R, Jobli E. A multihealth behavior intervention integrating physical activity and substance use prevention for adolescents. Prev Sci. 2005;6(3):213–26. doi: 10.1007/s11121-005-0012-3. [DOI] [PubMed] [Google Scholar]
- Wetherill RR, Franklin TR, Allen SS. Ovarian hormones, menstrual cycle phase, and smoking: a review with recommendations for future studies. Curr Addict Rep. 2016;3(1):1–8. doi: 10.1007/s40429-016-0093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DM, Whiteley JA, Dunsiger S, Jennings EG, Albrecht AE, Ussher MH, Ciccolo JT, Parisi AF, Marcus BH. Moderate intensity exercise as an adjunct to standard smoking cessation treatment for women: a pilot study. Psychol Addict Behav. 2010;24(2):349–54. doi: 10.1037/a0018332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DM, Dunsiger S, Whiteley JA, Ussher MH, Ciccolo JT, Jennings EG. Acute effects of moderate intensity aerobic exercise on affective withdrawal symptoms and cravings among women smokers. Addict Behav. 2011;36(8):894–7. doi: 10.1016/j.addbeh.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlebnik NE, Carroll ME. Prevention of the incubation of cocaine seeking by aerobic exercise in female rats. Psychopharmacology. 2015;232(19):3507–13. doi: 10.1007/s00213-015-3999-6. [DOI] [PMC free article] [PubMed] [Google Scholar]