Abstract
We aimed to understand drivers of HIV-infection in pregnant women in Malawi. The study was conducted in antenatal and labor and delivery wards. HIV-infected women and their partners (cases) were frequency matched in a 1:2 ratio based on age and screening location to HIV-uninfected women and their partners (controls) in a prevalent case-control study. Characteristics associated with female HIV infection were assessed using logistic regression modeling. At screening, HIV-infected women were more likely to have partners outside Lilongwe than HIV-uninfected women (24% versus 0%, p<0.0001). Case females were more likely to have HIV-infected study partners than control females (75% versus 4%, p<0.0001). The odds of female HIV-infection were higher if either couple member reported ≥2 lifetime marriages (OR=9.0, CI=2.6–30.9) or ≥3 lifetime partners (OR=18.0, CI=3.1–103.6) and lower if either reported past couple HIV testing and counseling (OR=0.1, CI=0.04–0.3). Targeting women with migrating partners, promoting couple HIV testing and counseling, and limiting partners could slow HIV transmission.
Keywords: HIV, counseling, testing, prevention, risk, couple
INTRODUCTION
In sub-Saharan Africa, the region hardest hit by the HIV epidemic, tremendous progress has been made in the prevention of mother to child transmission (PMTCT) with a four-fold reduction in pediatric infections from 2000 to 2015 (1, 2). In Malawi, following the introduction of Option B+, a PMTCT program that initiates all HIV-infected pregnant and breastfeeding women on free lifelong antiretroviral therapy, there has been more than a 70% reduction in MTCT (2). However, Malawi’s PMTCT success is largely due to its successful identification and treatment of HIV-infected pregnant women, rather than the prevention of HIV acquisition (1, 2). Between 2012 and 2016, the number of newly diagnosed HIV-infected pregnant women remained essentially unchanged, suggesting stable HIV incidence (3).
Prevention of HIV acquisition in women of reproductive age is the first prong of PMTCT approaches, but has historically been overlooked due to the absence of female-controlled prevention technologies and difficulties identifying women at highest risk. Additionally, HIV acquisition during pregnancy has been estimated in excess of 3 infections per 100 person years (4, 5), the recommended threshold for offering oral pre-prophylaxis (PrEP) (6). As female-controlled prevention technologies, such as PrEP, become available, understanding which pregnant women, are at highest risk of HIV acquisition is key.
A range of individual, partner, and couple factors have been associated with incident and prevalent HIV infection in pregnant and postpartum women. Individual factors (female attributes) include having multiple sexual partnerships or marriages (7–13), alcohol use (7, 12), and STI symptoms or diagnoses (7, 9, 12, 13). Partnership factors (male attributes reported by women) include having other partners (7, 8, 13), traveling (13), and using alcohol frequently (13). Couple factors (dyadic attributes reported by women) include shorter relationship durations (9, 11) and larger male-female age differences (7, 9, 13). However, these findings are based on female reports of male and partnership characteristics. This secondary reporting is subject to measurement error. Because primary marital and cohabiting male partners are often the source of HIV infection in women (14), reports by both partners on more characteristics can provide new insights.
We conducted a case-control study to understand the individual, partner, and couple characteristics associated with HIV infection among pregnant women in Malawi, as reported by both partnership members. Within each category, demographic, socioeconomic, behavioral, and HIV care-seeking characteristics were assessed.
METHODS
Study Setting
The study was conducted from December 2015-December 2016 at Bwaila District Hospital, a high-volume urban maternity hospital in Lilongwe, Malawi. Main findings were published previously (15). The study was based at the antenatal care (ANC) and labor and delivery (L&D) wards. During this period, approximately 1000 women attended each setting monthly. As standard of care at ANC, all women without a documented HIV-positive status were offered HIV testing. During the study period, ANC HIV prevalence was 11% with approximately half of these HIV-infected women already on ART and the other half testing HIV-positive at that visit. At L&D, all women, including those with a documented HIV-positive status, were tested for HIV as standard of care. HIV testing was conducted while women were awaiting delivery or just afterwards. At L&D, approximately 10% were confirmed HIV-positive, and 0.6% were new HIV diagnoses.
Study Design, Participants and Procedures
We conducted a prevalent case-control study comparing HIV-infected women and their partners with HIV-uninfected women and their partners.
In both ANC and L&D, HIV-infected women who tested HIV-positive on that day were approached by study staff and screened for eligibility. Eligibility criteria included being HIV-infected, pregnant, ≥18 years, having a current male sexual partner ≥18 years, not having received couple HIV testing and counseling (CHTC) at that visit, willingness to undergo CHTC with a partner in the next month, believing both partners would both be in Lilongwe for ≥2 months, and being interested in study participation. Eligible women interested in participation provided informed consent after eligibility was confirmed. Each consented woman was given one invitation for a male partner to present to the clinic for important family health information. Women provided their partners’ phone numbers, when available, and could elect to have the clinic call their partners right away or wait one week. Because this was a couple study, only women who presented with a partner after their screening visit enrolled. Enrolled couples with an HIV-infected pregnant woman were referred to as “case couples.”
We also enrolled “control couples,” those with an HIV-uninfected pregnant woman. These women were enrolled in a 1:2 ratio (one HIV-uninfected woman for every two HIV-infected women). HIV-uninfected women were selected using frequency matching based on HIV-infected women’s screening locations (ANC or L&D) and age categories (18–19, 20–24, 25–29, 30–34, and ≥35 years). Each potential HIV-uninfected woman was screened using the same eligibility criteria as HIV-infected women. If they consented, they were provided with the same invitation, and their partners were traced and enrolled using the same procedures.
All enrolled couples had two visits: a first visit approximately one week after they were screened and a second visit one month later. In this analysis, we only used information from the first visit. At the first visit, the two partners initially met separately with same-sex interviewers. The male partner provided informed consent (the female partner had done so at screening) and each partner participated in separate interviewer-administered behavioral surveys, which included questions about demographics; socioeconomic status; and alcohol, sexual, and HIV care-seeking behaviors. Afterwards, the couple was offered opt-out CHTC, based on Malawi’s guidelines. Data were collected on Android tablets using Open Data Kit software and uploaded to an encrypted password-protected web-based server. Data were downloaded bi-weekly and stored on a secure server.
Measures
The primary outcome of interest was female HIV status, assessed at the initial screening visit using the eligibility assessment. Individual and partner factors were obtained from the female and male behavioral surveys as independent variables of interest. Couple factors were developed as a composite from both behavioral surveys.
Eligibility factors included whether the woman had a male sex partner, whether she and her partner were ≥18 years, whether she and her partner were intending to be in Lilongwe for two months, whether they had already received CHTC on that day, and whether she was interested in study participation. Women who were screened out of the study were not asked further questions, and therefore no additional information was available.
Individual and partner demographic variables included age, religion, and duration living in their current home. Women were also asked about the number of prior pregnancies and living children. Couple variables included male minus female age difference, mean relationship length, and whether the couple had children together.
Individual and partner socioeconomic variables included education, earning status, floor material, and hunger in the last month. The only couple-level socioeconomic variable included was relative educational achievement.
Individual and partner behavioral variables included age at first intercourse and number of lifetime sexual partners and marriages. In addition, alcohol consumption, number of sexual partners in the last year, presence of sexual concurrency while with the study partner, and presence of transactional sex in the last month were included for male partners. Couple-level variables included whether either couple member had ≥2 marriages or ≥3 lifetime sexual partners, including their study partner.
Individual and partner HIV care-seeking behaviors included whether the person had a past HIV test, the timing and location of that test, and whether the person ever had received CHTC with any partner prior to enrolling in the study. Couple-level variables included whether either had undergone these behaviors. Male HIV status was ascertained through CHTC after behavioral survey administration. Men who knew they were HIV-infected prior to the study, were asked if they were taking antiretroviral therapy.
Statistical Methods
The first set of analyses was conducted among all screened women. The proportions of HIV-infected and HIV-uninfected women ineligible for each reason were compared using Fisher’s exact tests.
The remaining analyses were conducted among enrolled couples on a dataset with one record per couple. This record included female HIV status (the primary outcome); all individual, partner, and couple characteristics; and design variables (screening location and female age category). First, bivariable analyses were conducted using Fisher’s exact tests. Next, we used logistic regression models to estimate unadjusted and adjusted odds ratios (ORs) and 95% confidence intervals (CIs). In separate models, we explored individual and partner factors associated with female HIV status using a backward elimination approach. Separate models were used due to the small sample size and large number of covariates. In each, we started with a full model with all variables that had bivariable p-values ≤0.15. Variables were retained if multivariable p-values were ≤0.15. This relaxed threshold was used due to the small sample size and large number of variables. Unadjusted and adjusted models controlled for design variables.
We then implemented a multivariable logistic regression model estimating couple-level factors associated with female HIV status, also using backward elimination. The initial model included dyadic specifications of variables from the final individual or partner multivariable models. The modeling process was the same as that described above.
Because there were only two HIV-infected men from control couples, including male HIV status produced unstable estimates. However, due to the importance of this variable, we added this variable after the modeling process was complete as a sensitivity analysis. As a second sensitivity analysis, we implemented the final models among ANC couples only.
All analyses were performed using SAS version 9.4 (Cary, North Carolina, USA).
Ethics
The study received approval from our University’s Institutional Review Board and the National Health Science Research Committee in Malawi.
RESULTS
Study Population
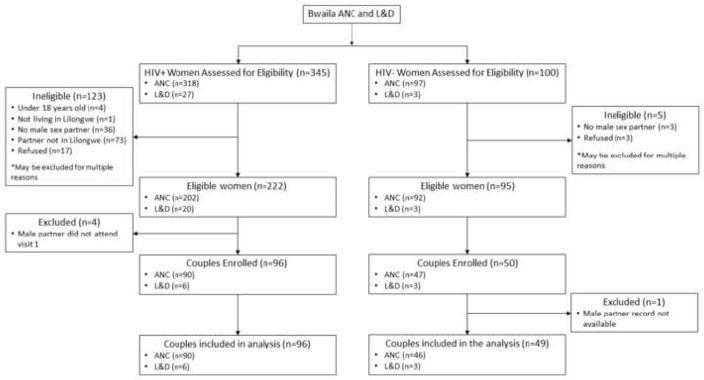
Overall, 222/345 (64%) of the HIV-infected women and 95/100 (95%) of the HIV-uninfected women were eligible (p<0.01) (Table I). HIV-infected women were more likely to not have a male sexual partner (10% versus 3%, p=0.03) or have a sexual partner outside of Lilongwe (24% versus 0%, p<0.0001). HIV-infected and HIV-uninfected women had comparable rates of participation disinterest (5% versus 3%, p=0.6) and enrollment (43% versus 53%, p=0.1).
Table I.
Eligibility characteristics of women screened for the study
| Female HIV+ (N=345) | Female HIV− (N=100) | Fisher’s Exact Test | |||
|---|---|---|---|---|---|
| N | % | N | % | p-value | |
| Age | |||||
| < 18 years old | 4 | (1.2%) | 0 | (0.0%) | |
| ≥ 18 years old | 341 | (98.8%) | 100 | (100.0%) | 0.6 |
| Recipient of CHTC at the screening visit | |||||
| Yes | 0 | (0.0%) | 0 | (0.0%) | |
| No | 345 | (100.0%) | 100 | (100.0%) | 1 |
| Female partner staying in Lilongwe for the next two months | |||||
| No | 1 | (0.3%) | 0 | (0.0%) | |
| Yes | 344 | (99.7%) | 100 | (100.0%) | > 0.9 |
| Has a male sex partner | |||||
| No | 36 | (10.4%) | 3 | (3.0%) | |
| Yes | 309 | (89.6%) | 97 | (97.0%) | 0.03 |
| Age of male sex partner | |||||
| < 18 years old | 0 | (0.0%) | 0 | (0.0%) | |
| ≥ 18 years old | 309 | (100.0%) | 97 | (100.0%) | 1 |
| Male sex partner in Lilongwe | |||||
| No | 73 | (23.6%) | 0 | (0.0%) | |
| Yes | 236 | (76.4%) | 97 | (100.0%) | < 0.0001 |
| Interest in study participation | |||||
| No | 17 | (4.9%) | 3 | (3.0%) | |
| Yes | 328 | (95.1%) | 97 | (97.0%) | 0.6 |
By design 100 case women and 50 control women were enrolled (Figure 1). Four case partners and one control partner lacked male behavioral survey data and were excluded, resulting in 96 case couples and 49 control couples. Among enrolled women, median age was 25 years and, by design, age distribution and recruitment site were nearly identical between cases and controls (p>0.9 for both) (Table II). Ninety-four percent of couples were recruited from ANC. Demographic characteristics of the full sample have been published previously (15).
Figure 1.
Table II.
Individual characteristics associated with female HIV infection
| Case Women (N=96) | Control Women (N=49) | Fisher’s Exact Test | Unadjusteda | Adjustedb | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | p-value | OR | (95% CI) | p-value | OR | (95% CI) | p-value | |
| Study Design | |||||||||||
| Variables | |||||||||||
| HIV Status | |||||||||||
| Positive | 96 | (100%) | 0 | (0.0%) | |||||||
| Negative | 0 | (0.0%) | 49 | (100.0%) | < 0.0001 | ||||||
| Location of HIV Diagnosis | |||||||||||
| Antenatal Care | 90 | (93.8%) | 46 | (93.9%) | 1. | 1. | |||||
| Postnatal | 6 | (6.3%) | 3 | (6.1%) | > 0.9 | 1.0 | (0.2, 4.3) | > 0.9 | 5.1 | (0.7, 34.6) | 0.1 |
| Age Category | |||||||||||
| 18–19 | 5 | (5.2%) | 2 | (4.1%) | 1.3 | (0.2, 7.4) | 0.8 | 1.3 | (0.2, 8.3) | 0.8 | |
| 20–24 | 38 | (39.6%) | 20 | (40.8%) | 1. | 1. | |||||
| 25–29 | 27 | (28.1%) | 14 | (28.6%) | 1.0 | (0.4, 2.4) | > 0.9 | 0.5 | (0.2, 1.4) | 0.2 | |
| 30–34 | 20 | (20.8%) | 10 | (20.4%) | 1.1 | (0.4, 2.7) | 0.9 | 0.3 | (0.08, 1.2) | 0.08 | |
| ≥35 | 6 | (6.3%) | 3 | (6.1%) | > 0.9 | 1.1 | (0.2, 4.7) | 0.9 | 0.2 | (0.02, 1.4) | 0.1 |
| Demographics | |||||||||||
| Religion | |||||||||||
| Protestant | 57 | (59.4%) | 24 | (49.0%) | 1. | ||||||
| Catholic | 18 | (18.8%) | 7 | (14.3%) | 1.0 | (0.4, 2.9) | 0.9 | ||||
| Muslim | 8 | (8.3%) | 5 | (10.2%) | 0.6 | (0.2, 2.3) | 0.5 | ||||
| Other | 13 | (13.5%) | 13 | (26.5%) | 0.2 | 0.4 | (0.2, 1.0) | 0.06 | |||
| Length of residence in current home | |||||||||||
| < 1 year | 23 | (24.0%) | 7 | (14.3%) | 1. | ||||||
| 1–2 years | 21 | (21.9%) | 8 | (16.3%) | 0.8 | (0.2, 2.6) | 0.7 | ||||
| 2–5 years | 27 | (28.1%) | 17 | (34.7%) | 0.5 | (0.2, 1.4) | 0.2 | ||||
| 5–10 years | 7 | (7.3%) | 8 | (16.3%) | 0.3 | (0.07, 1.0) | 0.05 | ||||
| ≥ 10 Years | 18 | (18.8%) | 9 | (18.4%) | 0.3 | 0.6 | (0.2, 2.0) | 0.4 | |||
| Number of prior pregnancies | |||||||||||
| 0 | 17 | (17.7%) | 9 | (18.4%) | 1. | ||||||
| 1–2 | 51 | (53.1%) | 24 | (49.0%) | 1.2 | (0.4, 3.5) | 0.8 | ||||
| 3–4 | 24 | (25.0%) | 14 | (28.6%) | 0.9 | (0.2, 3.5) | 0.8 | ||||
| ≥ 5 | 4 | (4.2%) | 2 | (4.1%) | > 0.9 | 0.9 | (0.09, 10.2) | > 0.9 | |||
| Number of living children | |||||||||||
| 0 | 6 | (7.6%) | 3 | (7.5%) | 1. | ||||||
| 1–2 | 54 | (68.4%) | 24 | (60.0%) | 1.1 | (0.2, 4.7) | 0.9 | ||||
| ≥ 3 | 19 | (24.1%) | 13 | (32.5%) | 0.6 | 0.6 | (0.1, 3.2) | 0.5 | |||
| Socioeconomic Status | |||||||||||
| Education | |||||||||||
| Less than Secondary | 81 | (84.4%) | 39 | (79.6%) | 1.4 | (0.6, 3.5) | 0.5 | ||||
| Secondary Complete | 15 | (15.6%) | 10 | (20.4%) | 0.5 | 1. | |||||
| Earning Status | |||||||||||
| Non-Wage Earner | 82 | (85.4%) | 34 | (69.4%) | 1.9 | (0.6, 5.9) | 0.2 | ||||
| Partial Wage Earner | 4 | (4.2%) | 8 | (16.3%) | 0.4 | (0.08, 1.7) | 0.2 | ||||
| Full Wage Earner | 10 | (10.4%) | 7 | (14.3%) | 0.03 | 1. | |||||
| Floor Material | |||||||||||
| Cement or tile | 71 | (74.0%) | 41 | (83.7%) | 1. | ||||||
| Dirt or dung | 15 | (15.6%) | 6 | (12.2%) | 1.4 | (0.5, 4.0) | 0.5 | ||||
| Other | 10 | (10.4%) | 2 | (4.1%) | 0.4 | 3.0 | (0.6, 14.6) | 0.2 | |||
| Any Hunger in the Last Month | |||||||||||
| No | 82 | (85.4%) | 45 | (91.8%) | 1. | ||||||
| Yes | 14 | (14.6%) | 4 | (8.2%) | 0.3 | 1.9 | (0.6, 6.3) | 0.3 | |||
| Behavioral Factors | |||||||||||
| Age at first intercourse | |||||||||||
| < 15 years old | 9 | (9.4%) | 3 | (6.1%) | 1.6 | (0.4, 6.4) | 0.5 | ||||
| 15–17 years old | 36 | (37.5%) | 20 | (40.8%) | 0.9 | (0.4, 1.9) | 0.8 | ||||
| ≥ 18 years old | 51 | (53.1%) | 26 | (53.1%) | 0.9 | 1. | |||||
| Number of lifetime sexual partners | |||||||||||
| 1–2 | 47 | (49.0%) | 37 | (75.5%) | 1. | 1. | |||||
| ≥ 3 | 49 | (51.0%) | 12 | (12.5%) | 0.003 | 3.3 | (1.5, 7.2) | 0.003 | 2.0 | (0.8, 5.1) | 0.1 |
| Number of lifetime marriages | |||||||||||
| 1 | 51 | (53.7%) | 43 | (87.8%) | 1. | 1. | |||||
| ≥ 2 | 44 | (46.3%) | 6 | (12.2%) | < 0.0001 | 8.6 | (3.0, 24.2) | < 0.0001 | 9.1 | (2.4, 34.2) | 0.001 |
| Missing | 1 | 0 | |||||||||
| HIV care-seeking | |||||||||||
| Time since last HIV test | |||||||||||
| Never | 27 | (30.7%) | 13 | (27.1%) | 1. | 1. | |||||
| ≤1 Year ago | 11 | (12.5%) | 17 | (35.4%) | 0.3 | (0.1, 0.9) | 0.03 | 0.3 | (0.08, 1.2) | 0.1 | |
| > 1 Year ago | 50 | (56.8%) | 18 | (37.5%) | 0.007 | 1.4 | (0.6, 3.5) | 0.5 | 1.9 | (0.7, 5.8) | 0.2 |
| Missing | 8 | 1 | |||||||||
| Location of last HIV test | |||||||||||
| ANC | 38 | (55.1%) | 21 | (58.3%) | 1. | ||||||
| L&D | 6 | (8.7%) | 2 | (5.6%) | 1.7 | (0.3, 9.6) | 0.5 | ||||
| VCT facility | 9 | (13.0%) | 3 | (8.3%) | 1.8 | (0.4, 7.6) | 0.4 | ||||
| Other Healthcare Setting | 15 | (21.7%) | 10 | (27.8%) | 0.8 | 0.8 | (0.3, 2.2) | 0.7 | |||
| Missing | 1 | 0 | |||||||||
| Couple HIV testing and counseling | |||||||||||
| Never | 92 | (95.8%) | 37 | (75.5%) | 1. | 1. | . | ||||
| Ever | 4 | (4.2%) | 12 | (24.5%) | 0.0004 | 0.1 | (0.04, 0.4) | 0.0009 | 0.2 | (0.04, 0.9) | 0.03 |
Adjusted for female age category and recruitment site
Adjusted for female age category, recruitment site, and all covariates with p <= 0.1
Considered for final adjustment set but not included in final model (p > 0.15)
Female Individual Characteristics
Nearly all women were married and most had a living child (Table II). Few completed secondary school or earned a wage. Thirty-five percent reported ≥2 lifetime marriages, 42% reported ≥3 lifetime partners, and 21% reported both (Pearson’s correlation coefficient=0.3). Four women reported a concurrent partner while with the study partner. Most had tested for HIV previously, but few had tested ≤1 year ago and few had ever received CHTC.
There were no individual demographic factors associated with HIV infection. Female earning status was the only socio-economic factor associated with HIV infection: HIV-infected women were less likely to be wage earners. Two behavioral variables were associated with HIV infection: ≥3 lifetime sexual partners and ≥2 lifetime marriages. Two HIV care-seeking behaviors were associated with HIV infection: HIV-infected women were less likely to have tested in the last year and less likely to have ever received CHTC.
In the multivariable model, all variables were retained except wage earning status. Holding all else constant, the odds of female HIV infection were higher among women with ≥3 lifetime partners compared to those with <3 lifetime partners (OR: 2.0, CI: 0.8–5.1) and higher among those with ≥2 lifetime marriages compared to those with <2 (OR: 9.1, CI: 2.4–34.2). Women who had tested in the last year had lower odds of HIV infection compared to those who never tested (OR: 0.3, CI 0.08–1.2), as did women reporting CHTC compared to those not reporting CHTC (OR: 0.2, CI: 0.04–0.9).
Male Partner Characteristics
Male median age was 32 years. Nearly all men were married. The majority had completed secondary education, were full-time wage earners, and reported no hunger in the last month (Table III). Forty-six percent reported ≥2 lifetime marriages, 84% reported ≥3 lifetime partners, and 43% reported both (Pearson’s correlation coefficient=0.3). Few men reported a concurrent partner while with the study partner and few reported recent paying of money or goods for sex. Most men had tested for HIV previously, but few had tested within the last year, and few had ever received CHTC.
Table III.
Partner-level characteristics associated with female HIV infection
| Case Men (N=96) | Control Men (N=49) | Fisher’s Exact Test | Unadjusteda | Adjustedb | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | p-value | OR | (95% CI) | p-value | OR | (95% CI) | p-value | |
| Demographics | |||||||||||
| Male Age | |||||||||||
| 18–24 | 15 | (15.6%) | 8 | (16.3%) | 1. | ||||||
| 25–29 | 24 | (25.0%) | 15 | (30.6%) | 1.0 | (0.3, 3.3) | > 0.9 | ||||
| 30–34 | 25 | (26.0%) | 10 | (20.4%) | 1.7 | (0.6, 4.9) | 0.3 | ||||
| 35–39 | 17 | (17.7%) | 13 | (26.5%) | 1.0 | (0.3, 3.0) | > 0.9 | ||||
| ≥ 40 | 15 | (15.6%) | 3 | (6.1%) | 0.4 | 5.0 | (0.9, 28.4) | 0.07 | |||
| Religion | |||||||||||
| Protestant | 34 | (35.8%) | 16 | (33.3%) | 1. | ||||||
| Catholic | 18 | (19.0%) | 12 | (25.0%) | 0.7 | (0.3, 1.8) | 0.4 | ||||
| Muslim | 7 | (7.4%) | 5 | (10.4%) | 0.6 | (0.2, 2.4) | 0.5 | ||||
| Other | 36 | (37.9%) | 15 | (31.3%) | 0.7 | 1.1 | (0.5, 2.7) | 0.8 | |||
| Missing | 1 | 1 | |||||||||
| Length of residence in current home | |||||||||||
| < 1 year | 13 | (13.5%) | 4 | (8.2%) | 1. | ||||||
| 1–2 years | 20 | (20.8%) | 7 | (14.3%) | 0.9 | (0.2, 3.7) | 0.9 | ||||
| 2–5 years | 23 | (24.0%) | 12 | (24.5%) | 0.6 | (0.2, 2.2) | 0.4 | ||||
| 5–10 years | 15 | (15.6%) | 8 | (16.3%) | 0.5 | (0.1, 2.3) | 0.4 | ||||
| ≥ 10 Years | 25 | (26.0%) | 18 | (36.7%) | 0.6 | 0.4 | (0.1, 1.5) | 0.2 | |||
| Socioeconomic Status | |||||||||||
| Education | |||||||||||
| Less than Secondary | 28 | (29.2%) | 29 | (40.8%) | 3.6 | (1.7, 7.5) | 0.0006 | 2.2 | (1.0, 5.1) | 0.06 | |
| Secondary Complete | 68 | (70.8%) | 20 | (59.2%) | 0.0006 | 1. | 1. | ||||
| Earning Status c | |||||||||||
| Non-Wage Earner | 3 | (3.1%) | 1 | (2.0%) | 2.1 | (0.2, 22.1) | 0.5 | ||||
| Part-time Wage Earner | 42 | (43.8%) | 12 | (24.5%) | 2.5 | (1.2, 5.4) | 0.02 | ||||
| Full-time Wage Earner | 51 | (53.1%) | 36 | (73.5%) | 0.05 | 1 | |||||
| Floor Material | |||||||||||
| Cement or tile | 73 | (76.0%) | 41 | (83.7%) | 1 | ||||||
| Dirt or dung | 22 | (22.9%) | 8 | (16.3%) | 0.4 | 1.5 | (0.6, 3.8) | 0.4 | |||
| Missing | 1 | 0 | |||||||||
| Any Hunger in the Last Month c | |||||||||||
| No | 63 | (65.6%) | 41 | (83.7%) | 1. | ||||||
| Yes | 33 | (34.4%) | 8 | (16.3%) | 0.03 | 2.8 | (1.2, 6.9) | 0.02 | |||
| Behavioral Factors | |||||||||||
| Alcohol Consumption | |||||||||||
| No Drinking | 55 | (57.3%) | 31 | (63.3%) | 1. | ||||||
| No Binge Drinking | 20 | (20.8%) | 10 | (20.4%) | 1.1 | (0.5, 2.7) | 0.8 | ||||
| Binge Drinking | 21 | (21.9%) | 8 | (16.3%) | 0.7 | 1.5 | (0.6, 3.8) | 0.4 | |||
| Age at first intercourse | |||||||||||
| < 15 years old | 5 | (5.2%) | 5 | (10.2%) | 0.5 | (0.1, 2.0) | 0.3 | ||||
| 15–17 years old | 33 | (34.4%) | 13 | (26.5%) | 1.4 | (0.6, 3.1) | 0.4 | ||||
| ≥ 18 years old | 58 | (60.4%) | 31 | (63.3%) | 0.4 | 1. | |||||
| Number of sexual partners in the last year | |||||||||||
| 1 | 72 | (75.0%) | 41 | (83.7%) | 1. | ||||||
| ≥ 2 | 24 | (25.0%) | 8 | (16.3%) | 0.3 | 1.8 | (0.7, 4.5) | 0.2 | |||
| Number of lifetime sexual partners | |||||||||||
| 1–2 | 7 | (7.3%) | 15 | (30.6%) | 1. | 1. | |||||
| ≥ 3 | 89 | (92.7%) | 34 | (69.4%) | 0.0004 | 6.0 | (2.2, 16.3) | 0.0005 | 4.6 | (1.5, 14.0) | 0.007 |
| Number of lifetime marriages | |||||||||||
| 1 | 41 | (42.7%) | 37 | (75.5%) | 1. | 1. | |||||
| ≥ 2 | 55 | (57.3%) | 12 | (24.5%) | 0.0002 | 4.7 | (2.1, 10.4) | 0.0002 | 4.0 | (1.6, 10.3) | 0.004 |
| Concurrency while with the study partner | |||||||||||
| No | 76 | (79.2%) | 41 | (83.7%) | 1. | ||||||
| Yes | 20 | (20.8%) | 8 | (16.3%) | 0.7 | 1.3 | (0.5, 3.6) | 0.5 | |||
| Exchange of money or gifts for sex with any partner | |||||||||||
| No | 81 | (84.4%) | 44 | (89.8%) | 1. | ||||||
| Yes | 15 | (15.6%) | 5 | (10.2%) | 0.5 | 1.7 | (0.6, 5.1) | 0.4 | |||
| HIV care-seeking | |||||||||||
| Time since last HIV test | |||||||||||
| Never | 31 | (34.8%) | 13 | (26.5%) | 1. | ||||||
| < 1 Year | 22 | (24.7%) | 13 | (26.5%) | 0.7 | (0.3, 1.9) | 0.5 | ||||
| ≥ 1 Year | 36 | (40.5%) | 23 | (46.9%) | 0.6 | 0.6 | (0.3, 1.6) | 0.4 | |||
| Missing | 7 | 0 | |||||||||
| Location of last HIV test | |||||||||||
| VCT facility | 7 | (10.9%) | 6 | (16.7%) | 0.7 | (0.2, 2.7) | 0.6 | ||||
| Healthcare Setting | 35 | (54.7%) | 16 | (44.4%) | 1.4 | (0.6, 3.7) | 0.5 | ||||
| Other | 22 | (34.4%) | 14 | (38.9%) | 0.5 | 1. | |||||
| Couples-HIV Testing and Counseling | |||||||||||
| Never | 77 | (81.0%) | 31 | (63.3%) | 1. | 1. | |||||
| Ever | 18 | (19.0%) | 18 | (36.7%) | 0.03 | 0.4 | (0.2, 0.9) | 0.02 | 0.3 | (0.1, 0.7) | 0.007 |
| Missing | 1 | 0 | |||||||||
| HIV Status | |||||||||||
| Negative | 23 | (24.7%) | 46 | (95.8%) | 1. | ||||||
| Positive not on ART | 70 | (75.3%) | 2 | (4.2%) | < 0.0001 | 90.3 | (18.1, 451.4) | < 0.0001 | |||
| Unknown | 3 | 1 | |||||||||
Adjusted for female age category, and recruitment site
Adjusted for female age category, recruitment site, and all covariates with p <= 0.1, except Male HIV Status
Considered for final adjustment set but not included in final model (p > 0.15)
The strongest independent predictor of female HIV infection was male HIV status. After CHTC, 75% of HIV-infected women had a partner diagnosed as HIV-infected compared to four percent of HIV-uninfected women (p<0.0001). Of these men, only 7% of men in case couples and 50% of men in control couples were on antiretroviral therapy.
In bivariable analyses, no partner demographic factors and three partner socioeconomic factors were associated with female HIV infection (Table III). Men without secondary education, who were not full-time wage earners, and who experienced hunger in the last month were more likely to have an HIV-infected partner. Two male partner behavioral factors were associated with female HIV infection: having ≥3 lifetime sexual partners and ≥2 lifetime marriages. One HIV care-seeking variable was protective: history of ever receiving CHTC.
In multivariate analysis, hunger and earning status were dropped. Holding all else constant, the odds of female HIV infection were higher among those with male partners who did not complete secondary education compared to those with male partners who did complete secondary education (OR: 2.2, CI: 1.0–5.1). The odds were also higher among men with ≥3 lifetime partners compared to those with <3 lifetime partners (OR: 4.6, CI: 1.5–14.0) and higher among men with ≥2 lifetime marriages compared to those with <2 lifetime marriages (OR: 4.0, CI: 1.6, 10.3). Past male CHTC was protective against female HIV infection (OR: 0.3, CI: 0.01–0.7). When male HIV status was added to the model, other coefficients remained similar and confidence intervals widened, suggesting that male HIV status was not confounding these other relationships. The odds of female HIV infection were considerably higher if her male partner was also HIV infected (OR=105, CI: 16.6, 664.0).
Couple Characteristics
The male partner was ≥10 years older in 15% of couples (Table IV). Most couple members were married to each other and living together. Approximately half reported having a child together. Median relationship duration was 3.5 year. In most couples, male partners had equal or higher educational achievement than female partners. In most couples, one or both had ≥3 lifetime partners and in approximately half, one or both partner had ≥2 lifetime marriages.
Table IV.
Couple-level factors associated with female HIV infection
| Case Couples (N=96) | Control Couples (N=49) | P-value | Unadjusted a | Adjusted b | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | Fisher’s exact test | OR | (95% CI) | p-value | OR | (95% CI) | p-value | |
| Demographic factors | |||||||||||
| Age Difference (male minus female) | |||||||||||
| < 10 years | 71 | (74.0%) | 45 | (91.8%) | 1. | 1. | |||||
| ≥ 10 years | 25 | (26.0%) | 4 | (8.2%) | 0.01 | 4.3 | (1.4, 13.3) | 0.01 | 9.4 | (1.8, 49.9) | 0.008 |
| Relationship duration~ | |||||||||||
| ≤ 2 years | 36 | (37.5%) | 8 | (16.3%) | 1. | ||||||
| 3–5 years | 34 | (35.4%) | 15 | (30.6%) | 0.4 | (0.1, 1.2) | 0.09 | ||||
| ≥ 5 years | 26 | (27.1%) | 26 | (53.1%) | 0.004 | 0.1 | (0.04, 0.4) | 0.0003 | |||
| Socioeconomic factors | |||||||||||
| Difference in educational achievement | |||||||||||
| Male Higher achievement | 44 | (45.8%) | 30 | (61.2%) | 1. | 1. | |||||
| Male Equal achievement | 28 | (29.2%) | 16 | (32.7%) | 1.2 | (0.5, 2.6) | 0.7 | 1.3 | (0.4, 3.9) | 0.7 | |
| Male Lower achievement | 24 | (25.0%) | 3 | (6.1%) | 0.01 | 5.8 | (1.6, 21.8) | 0.009 | 6.9 | (1.3, 36.0) | 0.02 |
| Behavioral Factors | |||||||||||
| Lifetime sexual partnerships | |||||||||||
| Neither has ≥ 3 lifetime partners | 3 | (3.1%) | 15 | (30.6%) | 1. | 1. | |||||
| At least one has ≥ 3 lifetime partners | 93 | (96.9%) | 34 | (69.4%) | < 0.0001 | 16.3 | (4.2, 62.6) | < 0.0001 | 18.0 | (3.1, 103.6) | 0.001 |
| Lifetime marriages | |||||||||||
| Neither has ≥ 2 lifetime marriages | 31 | (32.3%) | 35 | (71.4%) | 1. | 1. | |||||
| At least one has ≥ 2 lifetime marriages | 65 | (67.7%) | 14 | (28.6%) | < 0.0001 | 6.7 | (2.9, 15.3) | < 0.0001 | 9.0 | (2.6, 30.9) | 0.0005 |
| HIV Care-seeking | |||||||||||
| HIV testing c | |||||||||||
| Neither tested in the past year | 57 | (65.5%) | 24 | (50.0%) | 1. | ||||||
| One tested in the past year | 27 | (31.0%) | 18 | (37.5%) | 0.6 | (0.3, 1.4) | 0.2 | ||||
| Both tested in the past year | 3 | (3.5%) | 6 | (12.5%) | 0.07 | 0.2 | (0.04, 0.8) | 0.03 | |||
| Missing | 9 | 1 | |||||||||
| Couple HIV testing and counseling | |||||||||||
| Neither reports CHTC | 75 | (79.0%) | 28 | (57.1%) | 1. | 1. | |||||
| At least one reports CHTC | 20 | (21.0%) | 21 | (42.9%) | 0.01 | 0.3 | (0.2, 0.7) | 0.1 | (0.04, 0.3) | < 0.001 | |
| Male HIV Status | |||||||||||
| Negative | 23 | (24.7%) | 46 | (95.8%) | 1. | ||||||
| Positive | 70 | (75.3%) | 2 | (4.2%) | < 0.0001 | 90.3 | (18.1, 451.4) | < 0.0001 | |||
| Unknown | 3 | 1 | |||||||||
Adjusted for female age category and recruitment site
Adjusted for female age category, recruitment site, and all covariates with p <= 0.1, except Male HIV Status
Considered for final adjustment set but not included in final model (p > 0.15)
In bivariable analysis, two couple demographic variables were associated with female HIV infection: ≥10 year age difference and shorter relationship duration (Table IV). Female HIV infection was also associated with male partners having lower educational achievement than female partners. Two couple behavioral factors were associated with female HIV status: one or both having ≥3 lifetime sexual partners and one or both having ≥2 lifetime marriages. Female HIV infection was lower if at least one partner reported ever receiving CHTC prior to study participation.
In multivariate analysis, relationship duration was dropped. Holding all else constant, the odds of female HIV infection were higher if men were ≥10 years older than their female partner (OR 9.4, CI: 1.8–49.9) or if the male partner had lower educational achievement than the female partner (OR: 6.9, CI: 1.3, 36.0). The odds of female HIV infection were also higher if at least one partner reported ≥3 lifetime partners (OR: 18.0, CI: 3.1, 103.6) or ≥2 lifetime marriages (OR: 9.0 95% CI: 2.6, 30.9). The odds of female HIV infection were lower if one or both partners reported CHTC (OR 0.1: CI: 0.04, 0.3). When analyses were restricted to observations from ANC, the same variables remained in the model, odds ratios remained similar, and confidence intervals widened, suggesting similar drivers of HIV infection among couples in both settings.
DISCUSSION
In a sample of Malawian pregnant women and their male partners, individual, partner, and couple factors were associated with the female partner being HIV-infected. HIV-infected women were more likely to have a partner outside of the catchment area. Multiple marriages and lifetime partnerships were each strongly associated with female HIV infection. Either partner receiving CTHC was highly protective against female HIV infection. And larger male-female age gaps (with older males) and male-female socioeconomic gaps (with higher earning women) were associated with female HIV infection. All of these factors remained important, even after adjusting for male HIV status, the factor most strongly associated with female HIV infection.
Male partner’s HIV-positive status was the strongest predictor of female HIV infection. Among the 70 HIV-infected men in case couples, only 7% reported being on antiretroviral therapy, and were therefore were unlikely to be virologically suppressed; they are a likely source of transmission to the female partner (16). Although approximately half of HIV-infected Malawian men are suppressed (17), identifying the remaining half, engaging them in care, and supporting adherence are critical to ensure viral suppression. HIV-infected pregnant women could be an excellent liaison for identifying and accessing this hard-to-reach population, an observation consistent with other findings in ANC settings in the region (18–20)
Having a male partner outside of Lilongwe was strongly associated with female HIV infection. We hypothesize that many of these partners were engaging in sexual activity with casual partners while they were away from their primary partners, a phenomenon observed throughout the region (21–23). However, because these couples were excluded from the study, this hypothesis cannot be explored, and our results generalize to women with partners in Lilongwe. Future work is needed to understand reasons, locations, and durations of partner separation, as well as the risk behaviors in both partners during these periods.
For both men and women, having at least two marriages and three lifetime partners were strongly associated with a female HIV infection, an observation made in other antenatal and postpartum populations (7–13). Multiple marriages and multiple partnerships were weakly correlated in our data, and may contribute to HIV risk in different ways. Multiple partnerships are an indicator of more possible exposures to an HIV-infected person. At a national level, there is a dose response relationship with more partners being associated with higher HIV prevalence (24). Multiple marriages may indicate greater likelihood of past exposure to a person at very high risk of HIV infection, which has been associated with divorce and widowhood in prospective analysis (22, 25) and nationally representative cross-sectional samples (24).
Either partner receiving CHTC before study participation was strongly associated with lower rates of female HIV infection. One possible explanation is that less risky couples were more inclined to test together. This explanation would indicate that CHTC is the result of a safer relationship, rather than the cause of it. However, CHTC appears protective against HIV acquisition (26), primarily due to substantial increases in consistent condom use (27–29), CHTC could play an important role in the treatment and prevention cascades, observations leading the WHO to issue guidance encouraging CHTC (30).
Few demographic and socioeconomic indicators were associated with female HIV infection. Greater male-female age differences were associated with higher female HIV infection. This phenomenon has been observed previously in other antenatal settings in sub-Saharan Africa (7, 9, 13) and in Malawi’s general population. This may be due to older male partners having more time to become infected, as well as greater male-female power differentials that make sexual negotiation difficult.
Collecting data from male partners is a unique strength of our study. Although multiple analyses have assessed factors associated with HIV infection during pregnancy or postpartum, data have been collected solely from women. Collecting data from male partners offers two important advantages. First, self-report is likely more accurate than a partner’s report of the same characteristics. Second, data from both partners allows for dyadic analyses, and certain variables appeared to operate at a dyadic level. For example, the relative age and education levels between men and women was more strongly associated with female HIV status than absolute levels of either.
Our findings must be interpreted in light of several limitations. Our results generalize to those couples who were able to present together; those who did not may have been busier, more resistant to testing, or more violent (20). However, refusal and participation were similar among eligible cases and controls, so this may not have biased estimates. Additionally, although it is not known whether results generalize beyond our setting, the risk factors we observed are comparable to those observed in other antenatal settings in the region (7–13) and in national population surveys in Malawi (24). Next, because our sample size was small, many estimates were imprecise and we lacked statistical power to detect small differences. The small sample also limited our ability to conduct hierarchical modeling with several levels of analysis in a single model. Finally, we captured prevalent HIV cases, rather than incident HIV cases, and as a result, it is impossible to determine whether or not these “risk factors” caused or even preceded HIV infection.
CONCLUSIONS
Taken together, these findings offer important insights into individual, partner, and dyadic factors that may put HIV-uninfected women of childbearing age at risk for HIV acquisition. A clear understanding of these factors is an essential first step for determining which HIV-uninfected women are at highest risk of HIV acquisition, and ultimately in greatest need of biomedical and combination HIV prevention.
Acknowledgments
This study was funded by X the National Institute of Mental Health (K99MH104154).
The study and NER were supported by the National Institute of Mental Health (K99MH104154). LAG and MCH were supported by the National Institute of Child Health and Human Development (4T32HD052468-09, R01HD080485 respectively). AW was supported by the Doris Duke International Clinical Research Fellowship. We would like to thank Lighthouse Trust and Lilongwe District Health Office for their support. We would like to thank Nivedita Bhushan for designing the database and Mary Kacheyo for providing HIV testing and counseling.
Footnotes
COMPLIANCE WITH ETHICAL STANDARDS
Authors NER, LAG, AW, NM, CEG, SM, MT, LC, IFH, MCH, and WCM declare that they have no conflict of interest.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent was obtained from all individual participants included in the study.
References
- 1.UNAIDS. The Gap Report. 2014. [Google Scholar]
- 2.UNAIDS. On the Fast Track to an AIDS Free Generation. 2016. [Google Scholar]
- 3.Government of Malawi Ministry of Health. Integrated HIV Program Report. Jan-Mar. 2016. [Google Scholar]
- 4.Keating MA, Hamela G, Miller WC, Moses A, Hoffman IF, Hosseinipour MC. High HIV incidence and sexual behavior change among pregnant women in Lilongwe, Malawi: implications for the risk of HIV acquisition. PloS one. 2012;7(6):e39109. doi: 10.1371/journal.pone.0039109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drake AL, Wagner A, Richardson B, John-Stewart G. Incident HIV during pregnancy and postpartum and risk of mother-to-child HIV transmission: a systematic review and meta-analysis. PLoS medicine. 2014 Feb;11(2):e1001608. doi: 10.1371/journal.pmed.1001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. Geneva, Swizerland: 2015. [PubMed] [Google Scholar]
- 7.Businge CB, Longo-Mbenza B, Mathews V. Risk factors for incident HIV infection among antenatal mothers in rural Eastern Cape, South Africa. Global health action. 2016 Jan;9(1):29060. doi: 10.3402/gha.v9.29060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Schacht C, Hoffman HJ, Mabunda N, et al. High rates of HIV seroconversion in pregnant women and low reported levels of HIV testing among male partners in Southern Mozambique: results from a mixed methods study. PloS one. 2014;9(12):e115014. doi: 10.1371/journal.pone.0115014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kinuthia J, Drake AL, Matemo D, et al. HIV acquisition during pregnancy and postpartum is associated with genital infections and partnership characteristics. AIDS. 2015 Sep 24;29(15):2025–33. doi: 10.1097/QAD.0000000000000793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kinuthia J, Kiarie JN, Farquhar C, et al. Cofactors for HIV-1 incidence during pregnancy and postpartum period. Current HIV research. 2010 Oct;8(7):510–4. doi: 10.2174/157016210793499213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumogola Y, Slaymaker E, Zaba B, Mngara J, Isingo R, Changalucha J, et al. Trends in HIV & syphilis prevalence and correlates of HIV infection: results from cross-sectional surveys among women attending ante-natal clinics in Northern Tanzania. BMC public health. 2010 Sep 13;10:553. doi: 10.1186/1471-2458-10-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawi JD, Mirambo MM, Magoma M, et al. Sero-conversion rate of Syphilis and HIV among pregnant women attending antenatal clinic in Tanzania: a need for re-screening at delivery. BMC pregnancy and childbirth. 2015 Jan 22;15:3. doi: 10.1186/s12884-015-0434-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Msuya SE, Mbizvo E, Hussain A, Uriyo J, Sam NE, Stray-Pedersen B. HIV among pregnant women in Moshi Tanzania: the role of sexual behavior, male partner characteristics and sexually transmitted infections. AIDS research and therapy. 2006 Oct 17;3:27. doi: 10.1186/1742-6405-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellan SE, Fiorella KJ, Melesse DY, Getz WM, Williams BG, Dushoff J. Extra-couple HIV transmission in sub-Saharan Africa: a mathematical modelling study of survey data. Lancet. 2013 May 4;381(9877):1561–9. doi: 10.1016/S0140-6736(12)61960-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenberg NE, Graybill LA, Wesevich A, et al. The Impact of Couple HIV Testing and Counseling on Consistent Condom Use among Pregnant Women and their Male Partners: An Observational Study. J Acquir Immune Defic Syndr. Aug 1;75(4):417–425. doi: 10.1097/QAI.0000000000001398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. The New England journal of medicine. 2011 Aug 11;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.President’s Emergency Plan for AIDS Relief, ICAP, CDC, Center for Social Responsibility, Naitonal Statistics Office, COM-JHP. Malawi Population-Based HIV Impact Assessment, MPHIA 2015–2016. Malawi Population-Based HIV Impact Assessment: A Drop that Counts. 2016 [Google Scholar]
- 18.Henley C, Forgwei G, Welty T, et al. Scale-up and case-finding effectiveness of an HIV partner services program in Cameroon: an innovative HIV prevention intervention for developing countries. Sexually transmitted diseases. 2013 Dec;40(12):909–14. doi: 10.1097/OLQ.0000000000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osoti AO, John-Stewart G, Kiarie J, et al. Home visits during pregnancy enhance male partner HIV counselling and testing in Kenya: a randomized clinical trial. Aids. 2014 Jan 2;28(1):95–103. doi: 10.1097/QAD.0000000000000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenberg NE, Mtande TK, Saidi F, et al. Recruiting male partners for couple HIV testing and counselling in Malawi’s option B+ programme: an unblinded randomised controlled trial. The Lancet HIV. 2015 Nov;2(11):e483–91. doi: 10.1016/S2352-3018(15)00182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grabowski MK, Lessler J, Redd AD, et al. The role of viral introductions in sustaining community-based HIV epidemics in rural Uganda: evidence from spatial clustering, phylogenetics, and egocentric transmission models. PLoS medicine. 2014 Mar;11(3):e1001610. doi: 10.1371/journal.pmed.1001610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anglewicz P. Migration, marital change, and HIV infection in Malawi. Demography. 2012 Feb;49(1):239–65. doi: 10.1007/s13524-011-0072-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Helleringer S, Kohler HP, Chimbiri A. Characteristics of external/bridge relationships by partner type and location where sexual relationship took place. AIDS. 2007 Nov 30;21(18):2560–1. doi: 10.1097/QAD.0b013e3282f112bd. [DOI] [PubMed] [Google Scholar]
- 24.Macro and National Statistics Office. Malawi Demographic and Health Survey 2010. Zomba, Malawi and Calverton, Maryland, USA: 2011. [Google Scholar]
- 25.Anglewicz P, Reniers G. HIV status, gender, and marriage dynamics among adults in Rural Malawi. Studies in family planning. 2014 Dec;45(4):415–28. doi: 10.1111/j.1728-4465.2014.00005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenberg NE, Hauser BM, Ryan J, Miller WC. The effect of HIV counselling and testing on HIV acquisition in sub-Saharan Africa: a systematic review. Sexually transmitted infections. 2016 doi: 10.1136/sextrans-2016-052651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenberg NE, Pettifor AE, Bruyn GD, Westreich D, Delany-Moretlwe S, Behets F, et al. HIV Testing and Counseling Leads to Immediate Consistent Condom Use Among South African Stable HIV-Discordant Couples. Journal of acquired immune deficiency syndromes. 2013 Feb 1;62(2):226–33. doi: 10.1097/QAI.0b013e31827971ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kennedy CE, Medley AM, Sweat MD, O’Reilly KR. Behavioural interventions for HIV positive prevention in developing countries: a systematic review and meta-analysis. Bulletin of the World Health Organization. 2010 Aug 1;88(8):615–23. doi: 10.2471/BLT.09.068213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Denison JA, O’Reilly KR, Schmid GP, Kennedy CE, Sweat MD. HIV voluntary counseling and testing and behavioral risk reduction in developing countries: a meta-analysis, 1990–2005. AIDS and behavior. 2008 May;12(3):363–73. doi: 10.1007/s10461-007-9349-x. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization. Couples HIV Testing and Counseliling Including Antiretroviral Therapy for Treatment and Prevention in Serodiscordant Couples: Recommendations for a Public Health Approach. 2012 [PubMed] [Google Scholar]