Abstract
We studied modulation of undirected functional connectivity (uFC) in cortical-hippocampal sub-networks during associative learning. Nineteen healthy individuals were studied (fMRI acquired on a Siemens Verio 3T), and uFC was studied between nodes in a network of regions identified by standard activation models based on bivariate correlational analyses of time series data. The paradigm alternated between Memory Encoding, Rest and Retrieval. “Rest” intervals promoted covert consolidation. Over the task, performance was broadly separable into linear (Early) and asymptomatic (Late) regimes, with late performance reflecting successful memory consolidation. Significant modulation of uFC was observed during periods of covert consolidation. The sub-networks which were modulated constituted connections between frontal regions such as the dorsal prefrontal cortex (dPFC) and dorsal anterior cingulate cortex (dACC), the medial temporal lobe (hippocampus, HPC), the superior parietal cortex (SPC) and the fusiform gyrus (FG). uFC patterns were dynamic in that sub-networks modulated during Early learning (dACC ↔ SPC, dACC ↔ FG, dPFC ↔ HPC) were not identical to those modulated during Late learning (dACC ↔ HPC, dPFC ↔ FG, FG ↔ SPC). Covert consolidation exerts systematic effects, and these results add to emerging evidence for the constructive role of the brain’s “resting state” in potentiating action.
Keywords: Associative learning, Functional connectivity, Resting state, Cortical-hippocampal networks, fMRI
1. Introduction
Frontal-hippocampal interactions are central to the creation of episodic memories (Miller & D’Esposito, 2012; Sommer, Rose, Glascher, Wolbers, & Buchel, 2005; Woodcock, White, & Diwadkar, 2015) wherein the standard model is one of early hippocampal engagement followed by rapid reconsolidation in the neo-cortex (Bero et al., 2014). In human fMRI studies, associative memory tasks frequently employ paradigms wherein the experiment oscillates between periods (blocks) of Encoding (the presentation of memoranda to be associated) and Retrieval (the presentation of a recall cue) (Buchel, Coull, & Friston, 1999; Stanley et al., 2017; Wadehra, Pruitt, Murphy, & Diwadkar, 2013). Between these, the experimental structure includes Rest epochs involving instruction-free fixation (and which are typically used as baseline resting conditions in activation-based analyses). The accumulative nature of the task (requiring learning over time) promotes the covert rehearsal of to-be-remembered memoranda during these intervals, a process that is also driven by the general processing demands of memory consolidation. Moreover, reactivation of memory traces facilitates consolidation. Whereas some studies have shown relatively little if any effects of interference during the consolidation stage on memory consolidation (Varma et al., 2017), several others have shown that interference during memory rehearsal is highly disruptive to such consolidation (McFarlane & Humphreys, 2012; Scully, Napper, & Hupbach, 2017). Clearly, resting epochs are not passive, but active, and as such must present with a detectable neuronal signature. Previous fMRI investigations have extensively modeled brain network interactions during Encoding and Retrieval, demonstrating that both effective, and functional connectivity between cortical-hippocampal sub-networks are essential correlates of memory formation and retrieval (Banyai, Diwadkar, & Erdi, 2011; Buchel et al., 1999; Woodcock, Wadehra, & Diwadkar, 2016).
Here we adopt a focus that complements previous studies: We specifically interrogated brain network interactions during the “active” Rest epochs that envelope the task-specific epochs of memory Encoding and memory Retrieval. Our goal was to discover patterns of network interactions during these epochs that provide suggestive evidence of synchrony of network constituents despite subjects being in an instruction-free state, yet were likely to be engaged in covert memory consolidation.
The term “rest” is itself not a unitary one, and in the context of assessing brain networks, might be conceptualized in at least two distinct ways: (1) Periods of passive rest provide access to the brain’s “default modes” (Raichle et al., 2001), whereas (2) periods of rest between task-driven activity (Diwadkar, Asemi, Burgess, Chowdury, & Bressler, 2017), and as defined in the current study provide access to ongoing constructive processes in brain networks. Several studies have characterized spontaneous brain-activity during periods of rest (Biswal, Yetkin, Haughton, & Hyde, 1995; Cordes et al., 2000; Greicius, Krasnow, Reiss, & Menon, 2003), and data acquisition in these studies is unconstrained by task context, with participants instructed not to think of anything specific. These studies allow for estimation of what is deemed “intrinsic” connectivity (or “connectomics”) (Smith et al., 2013) between brain regions. These patterns of connectivity can predict behavioral performance/proficiency on tasks such as working memory (Zou et al., 2013) where performance data was acquired independently of fMRI data. By comparison, rest periods are almost universally employed in fMRI block designs to provide baseline conditions against which task-related activity during sensori-motor stimulation is contrasted (Amaro & Barker, 2006). Evidence suggests that at least depending on the nature of the primary target task, these resting periods are constructive in the sense that interactions between brain regions is systematic, and supports cognitive architectures associated with the task-related networks (Deco, Jirsa, & McIntosh, 2013; Diwadkar et al., 2017). Our work can be placed in this latter framework.
Our explorations were conducted based on analyzing the undirected functional connectivity (uFC) between regional time series relying on bivariate correlational models (Friston, 2011; Silverstein, Bressler, & Diwadkar, 2016; Whitfield-Gabrieli & Nieto-Castanon, 2012). Furthermore, behavioral proficiency on the task typically increases non-linearly (i.e., we observe a linear regime of learning followed by a transition to asymptotic performance), suggesting that performance transitions from early learning (with lower levels of proficiency and consolidation), to late learning (with higher levels of the same). These characteristics motivated a further interest on whether patterns of network interactions transition from more effortful (and lower consolidated) states of learning and memory (linear regime) to more consolidated states (asymptotic regime).
Our results provide evidence of significant supra-threshold correlations that are evident in cortical-hippocampal networks at rest. Moreover, we also demonstrate that these patterns of network connectivity are not static in the sense that the patterns of uFC observed during the linear (and presumably less consolidated) learning regime are different than those observed in the asymptotic (and presumably more consolidated) learning regime. In interpreting the results, we argue that they are consistent with an emerging literature emphasizing the active role of resting state networks in sub-serving potentiation for action in the dynamic brain (Deco et al., 2013; Diwadkar et al., 2017). In effect, these studies (and we) suggest that the brain between active states is under systematic potentiation for action. The fMRI signal is maximally sensitive to task-induced modulation (Logothetis, 2008). Therefore, appropriately structured experimental paradigms and tasks may help in discovering how network synchrony in the resting state sub serves function in task-relevant brain networks. Moreover, these results might also suggest a strategic reappraisal of using “resting” baselines, certainly within tasks such as ours, to identify activation profiles, given that these specific states appear to be covertly functionally active.
Given the goal-oriented nature of the learning task presented in this study and the need for memory consolidation for task mastery, we expected to observe some form of frontal-hippocampal synchrony during the Rest periods, especially in the Early phase when participants underwent a linear regime of learning.
2. Methods
2.1. Subjects
MRI/fMRI were acquired from 19 individuals (11 M, 8 F, mean age=25.0 years old, SD=1.8) from the greater Detroit area, recruited through advertisements. All individuals gave informed consent to take part in the fMRI dataset as healthy controls, and received remuneration for participating in all assessments. All procedures were approved by the Human Subjects Investigative Committee (HIC) at Wayne State University.
2.2. MR Protocol
Because fMRI responses are sensitive to circadian rhythmicity (Muto et al., 2016), all MRI/fMRI data were acquired in the morning within a narrow temporal window (9–11 am). Data were acquired on a 3 T Siemens Verio scanner with a 32-channel volume head coil. For the functional data, multiband gradient EPI fMRI was used (TR=3 s, TE=24.6 s, multiband factor=3, FOV=192 × 192mm2, matrix= 96 × 96, 64 axial slices, pixel resolution=2 × 2 × 2mm3). In addition, T1-weighted MRI images were collected for normalization and co-registration with the EPI scan (3D Magnetization Prepared Rapid Gradient Echo (MPRAGE) Sequence, TR=2150 ms, TE=3.5 ms, TI=1100 ms, flip angle=8 degrees, FOV=256 × 256 × 160mm3, 160 axial slices, pixel resolution=1 × 1 × 1mm3).
Associative Learning and memory Task
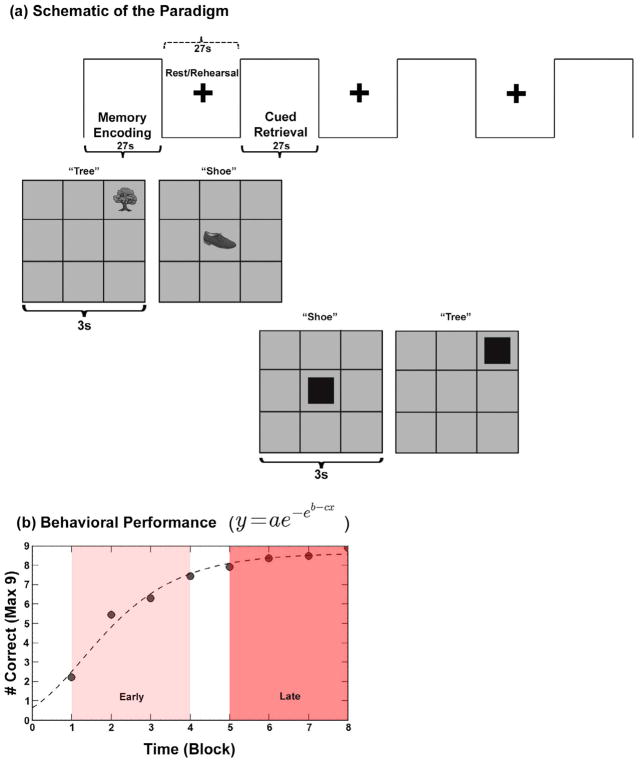
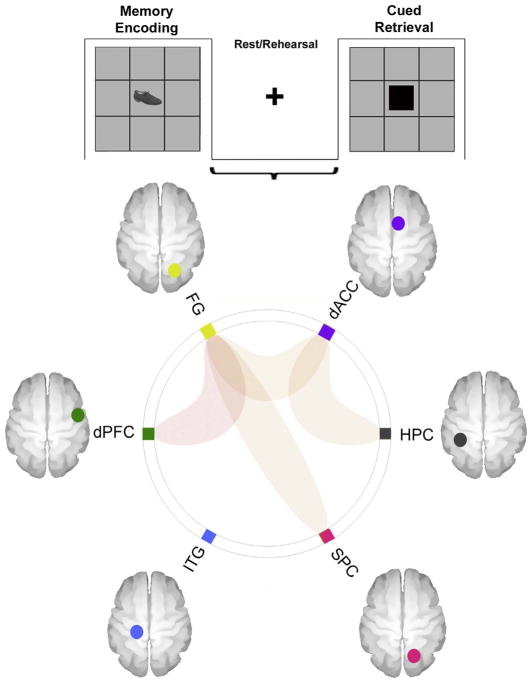
An established object-location associative learning and memory paradigm was employed (Diwadkar et al., 2016; Woodcock et al., 2016). During the paradigm, participants were required to learn associations between nine object-location pairs (nine objects each associated with a unique location in a 3×3 spatial grid). The task alternated between Encoding, Rest and Retrieval epochs (27 s each). During Encoding, each of the nine equi-familiar objects was presented (3 s/object), in its associated location for naming. An instruction-free Rest interval (27 s), followed during which a fixation marker was presented at the center of the screen. This “Rest” interval is of interest as it is assumed to constitute the primary window for rehearsing associations, given that it is positioned between Encoding and subsequent Retrieval epochs. Following the rest interval, a cued Retrieval epoch (27 s) was employed to test episodic memory. Locations were cued in random order and participants were required to name the object associated with it. A total of eight cycles were used to increase chances of participants reaching asymptotic performance. Fig. 1a provides a depiction of the paradigm (a description of Fig. 1b is provided in the results section).
Fig. 1.
(a) The figure provides a schematic depiction of the employed experimental paradigm used. Within a block design (each epoch 27 s), the task alternated between Memory Encoding, Rest/Rehearsal and Cued Retrieval. During Memory Encoding, nine objects (3s/object, 9 objects total) were presented in random sequence in their associated grid location for naming. An instruction free fixation interval followed. During cued Retrieval, grid locations were cued and participants were asked to recall (by naming) the object associated with the location. A total of eight epochs of each type were employed in sequence to maximize the chances of participants reaching asymptotic performance. (b) Averaged behavioral performance across the eight epochs is depicted (# correct) with a relational Gompertz function fitted to the mean performance curve of all 19 subjects (dashed line). As the shaded windows indicate, performance could be differentiated into early epochs (linearly increasing performance regime) and late epochs (asymptotic performance regime).
2.3. fMRI preprocessing
MR images were preprocessed and analyzed using SPM 8 (Statistical Parametric Mapping, Wellcome Department of Imaging and Neuroscience, London, UK) using established methods for temporal (slice timing correction) followed by spatial preprocessing. For spatial pre-processing, the EPI images were manually oriented to the AC-PC line with the reorientation vector applied across the EPI image set, realigned to a reference image to correct for head movement, and co-registered to the anatomical high resolution T1 image. This high-resolution T1 image was normalized to the MNI template, with the resultant deformations subsequently applied to the co-registered EPI images for normalization. Low frequency components were removed using a low-pass filter (128 s) and images were spatially smoothed using a Gaussian filter (8mm full-width half maximum; FWHM). An autoregressive AR(1) model was used to account for serial correlation.
2.4. fMRI modeling: Identifying nodes of interest based on activation profiles
First-level (within-subject) analyses included General Linear Model (GLM) fixed effects approach to model task periods, with a linear parametric modulator to model incremental effects in performance over time (Buchel et al., 1999). Regressors representing these processes of interest were modeled as vectors convolved with a canonical hemodynamic reference waveform, with the six motion parameters (3 for translation and 3 for rotation) from the co-registration used as covariates of no interest.
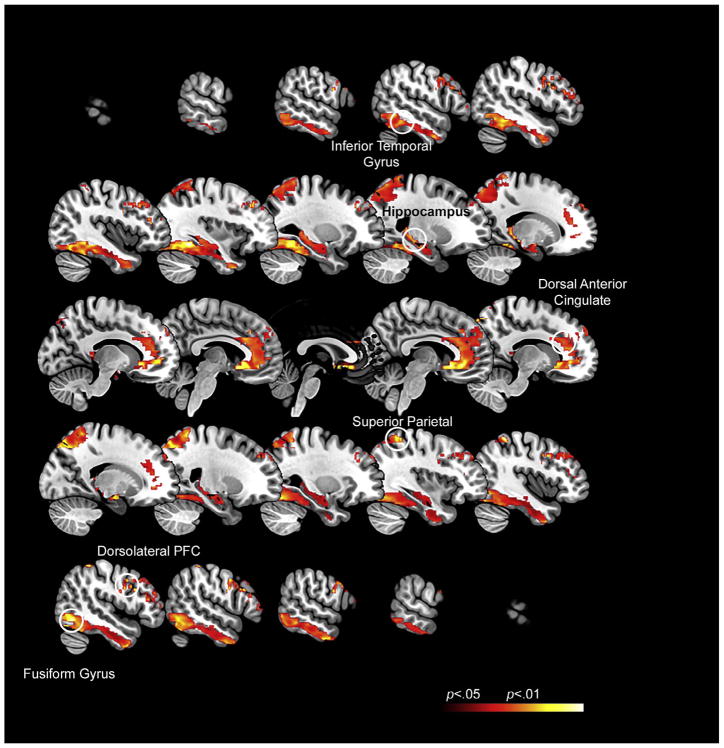
The first-level regressors representing the task-active conditions (Encoding and Retrieval) were forwarded to second level random effects repeated measures analyses with condition as the single factor of interest. The goal of the activation analysis was to identify task-relevant nodes co-activated across Encoding and Retrieval compared to Rest: This task active network would be submitted for interrogation of uFC during Rest. Therefore, co-activated nodes were identified based on a conjunction analyses using the minimum inference statistic (Nichols, Brett, Andersson, Wager, & Poline, 2005). Co-activated clusters were identified based on cluster-level thresholding (p < .05, cluster-level corrected for multiple comparisons) (Ward, 2000) and centroids (radius= 5 mm) for further analyses were established at the significance peaks, which are depicted in Fig. 2, Results. Significance peaks were found to be in the left hemisphere for HPC and ITG, and the right hemisphere for dACC, dPFC, SPC, & FG. Therefore, hemispheric laterality for each of the regions was driven by the activation analyses results. This learning network was forwarded for subsequent uFC assessment associated with Rest.
Fig. 2.
The map represents the activation profiles associated with the paradigm. As seen (and expected), the task induced robust engagement of multiple regions of interest including the dorsal anterior cingulate cortex (dACC), the dorsolateral prefrontal cortex (dPFC), the hippocampus (HPC), the fusiform gyrus (FG), the inferior temporal gyrus (ITG) and the superior parietal cortex (SPC). The insets denote the activation peaks (see Table 1) time series from which were represented in the subsequent uFC analyses.
2.5. uFC analyses
uFC of nodes in the network of interest was assessed using previously published methods (Whitfield-Gabrieli & Nieto-Castanon, 2012). In initial pre-processing, component-based noise correction methods (CompCorr) were used to correct for physiological noise, movement artifacts, and temporal covariates in the time series from the nodes of interest (Fig. 2). These time series were submitted to uFC analyses.
Two analyses consistent with our a priori motivations were conducted. First, we identified sub-networks the uFC of which was significantly modulated during the three conditions of interest: Encoding, Retrieval and Rest. A second motivation was to assess changes in coordinated sub-networks over time. As noted, based on average learning performance, the task can roughly be cleaved apart into early stages (when performance is increasing linearly) and late stages (when performance is asymptotic). As seen in Fig. 1 this division was effected by separating the first 4 epochs (Early) from the last four epochs (Late), resulting in equal numbers of volumes in each phase. Thus, we assessed the modulation of each pathway in the network of interest for each of the conditions separately during early and late periods of learning. All statistical inference was based on family-wise error correction using the False Discovery Rate (qFDR < 0.05).
3. Results
We organize the presentation of results as follows: (1) First, we discuss the behavioral effects observed across the experiment; (2) Next, we depict activation profiles across the experimental paradigm highlighting the network of interest derived from activation peaks; (3) Then, we depict connectomic profiles across the network. These profiles highlight network pairs, the uFC of which was positively modulated during each of Encoding, Rest and Retrieval. The data are presented in that order to be consistent with the temporal order in which those conditions appeared in each learning phase; (4) Finally we present stage-wise analyses of the modulation of uFC during the Early and the Late stages of learning to discover how uFC was modulated during the different phases of the task.
(1) Behavioral results. As anticipated from previous studies, behavioral performance across the experiment was characterized by negatively accelerated learning. Performance data in Fig. 1b was well predicted by a sigmoidal relational Gompertz function (Stanley et al., 2017), with three free parameters modeling estimated asymptotic performance (a), inflection point (c) and learning rate (b) from Fig. 1b. The dashed line reflects the results of the Gompertz fit across the averaged performance data (r2=0.978; parameter estimates: a=8.65, b=0.946, c=0.743).
(2) Activation profiles: Fig. 2 depicts regional activations (see Table 1 for statistical and location information) in a network of interest that includes (a) memoranda-specific regions such as the inferior temporal and fusiform gyri (associated with object identity), the superior parietal cortex (associated with spatial localization and processing), (b) regions involved in memory formation such as the hippocampus, and (c) regions such as the dorsal anterior cingulate and the dorsolateral prefrontal cortex involved in both memory formation, and cognitive and memory control.
Table 1.
This table shows the MNI coordinates, the t statistics at the cluster peak, and the cluster extent thresholds used in cluster formation for the activation profiles (see Fig. 2 for the activation map).
| Region of interest | MNI Coordinates X, Y, Z | t-statistic | Cluster Extent Threshold (k) | ||
|---|---|---|---|---|---|
| Hippocampus | −22 | −31 | 02 | 3.14 | 56 |
| Dorsal ACC | 10 | 12 | 40 | 3.07 | 29 |
| Fusiform Gyrus | 24 | −72 | −17 | 2.83 | 80 |
| Dorsal PFC | 54 | 0 | 24 | 3.30 | 21 |
| Inferior Temporal Gyrus | −40 | −43 | −11 | 2.80 | 54 |
| Superior Parietal | 20 | −73 | 58 | 6.02 | 241 |
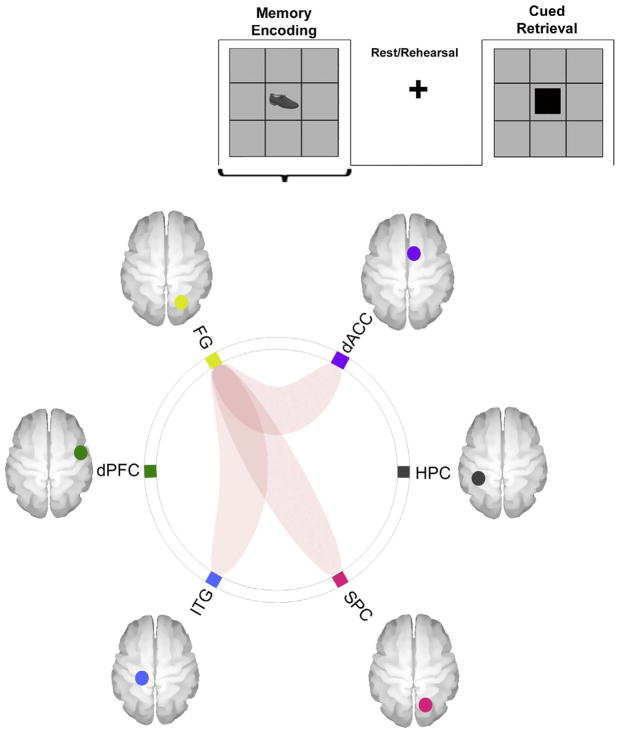
(3a) uFC Analyses – Memory Encoding: To understand the sub-network patterns across the different conditions of the task, bivariate correlations were performed for the six regions of interest across the three conditions (with bilateral peaks from each region forwarded for uFC analyses). Fig. 3 depicts sub-networks the uFC of which was significantly modulated (that is uFC greater than 0) during Memory Encoding (qFDR < 0.05). As seen, effects were observed between the dACC ↔ FG (t18=3.30, β = 0.16), the FG ↔ ITG (t18=3.03, β=0.14), and the FG ↔ SPC (t18=3.29, β =0.14).
Fig. 3.
The connectomic ring depicts sub-networks the uFC of which was significantly modulated during Encoding (across the entire experimental run). As is evident, effects were significant between the dACC ↔ FG, the FG ↔ SPC and the FG ↔ ITG.
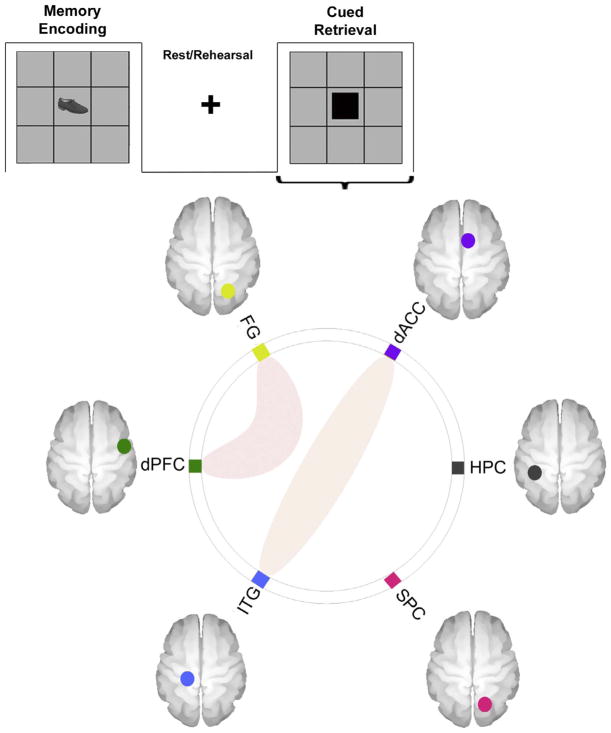
3b) uFC Analyses – Cued Retrieval: As seen in Fig. 4, during Retrieval, the uFC was significantly modulated between dACC ↔ ITG (t18=2.90, β = 0.11) and the dPFC ↔ FG (t18=3.76, β =0.19).
Fig. 4.
The connectomic ring depicts sub-networks the uFC of which was significantly modulated during the Rest/Rehearsal epochs (across the entire experimental run). As is evident, effects were significant between the dACC ↔ Hippocampus, the dACC ↔ FG, the dPFC ↔ FG, and the FG ↔ SPC.
(3c) uFC Analyses – Rest/Rehearsal: Notably, the uFC of multiple sub-networks was significantly modulated during the Rest/Rehearsal epochs (Fig. 5). As seen, significant effects were observed between the dACC ↔ HPC (t18=2.92, β =0.10), the dACC ↔ FG (t18=3.76, β = 0.19), the dPFC ↔ FG (t18=4.12, β= 0.19) and the SPC ↔ FG (t18=2.90, β = 0.11).
Fig. 5.
The connectomic ring depicts sub-networks the uFC of which was significantly modulated during the Retrieval epochs (across the entire experimental run). As is evident, effects were significant between the dACC ↔ ITG, and the dPFC ↔ FG.
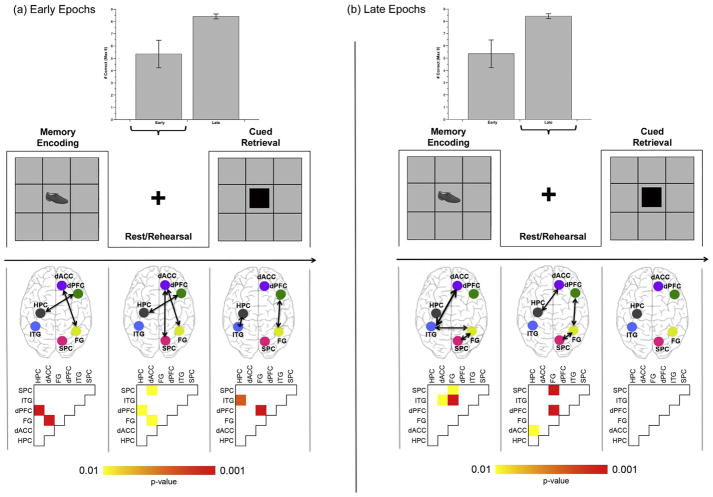
uFC Analyses during Early and Late Stages of Learning: Based on the expectation that distinct stages of behavior result in different patterns of the modulation of uFC during the conditions, we separately assessed uFC during Early stages of learning (when learning is largely linear) and Late stages (when learning is largely asymptotic) (Stanley et al., 2017). Fig. 6 provides an overview of the behavioral data with bar graphs depicting average performance during Early (first four) and Late (last four) epochs of the experiment. Below the depiction of the paradigm and aligned in columns with the phases of the task, we present the uFC profiles (in schematic with arrows depicting sub-networks the uFC of which was significantly modulated under the condition), and the probability matrix from which the uFC profiles were inferred. These are presented for each of the Early (a) and Late (b) stages of the task
Fig. 6.
Performance and uFC profiles are depicted during each of the (a) Early and (b) Late periods of the task. The bar graphs in each panel depict averaged performance across Early and Late epochs (see Fig. 1b for performance curve; error bars are ± sem). Following the depiction of the conditions of the paradigm, schematic brain images depict pathways, the uFC of which was significantly modulated under condition. The probability matrices below correspond to the brain images. As seen, the characteristics of uFC modulation during Rest/Rehearsal appear to be dynamic (as a function of task period). During early learning periods uFC of dACC ↔ SPC, dACC ↔ FG and dPFC ↔ Hippocampus is significantly modulated during Rest/Rehearsal. Later, these patterns of modulation shift to the dACC ↔ Hippocampus, dPFC ↔ FG and SPC ↔ FG pathways.
Several notable effects are evident. First, patterns of uFC modulation are dynamic, particularly during Rest/Rehearsal epochs: During the Early period, significant modulation was observed for dPFC ↔ HPC pathway (t18=2.25, β = 0.13), the dACC ↔ FG pathway (t18=2.64, β = 0.09) and the dACC ↔ SPC pathway (t18=2.34, β = 0.14), whereas during the Late period, these effects were shifted to dACC ↔ HPC (t18=2.58, β= 0.14), dPFC ↔ FG (t18=5.01, β =0.24) and the FG ↔ SPC (t18=4.36, β = 0.19).
The modulation of uFC during Encoding epochs also changed over the course of learning. Early encoding was associated with increased uFC in dPFC ↔ HPC (t18=4.19, β = 0.18) and dACC ↔ FG (t18=3.85, β = 0.21) sub-networks, but Late encoding was associated with increased uFC in dACC ↔ ITG (t18=2.12, β = 0.16), FG ↔ ITG (t18=3.95, β = 0.27) and FG ↔ SPC (t18=2.30, β= 0.16). Finally, during early Retrieval, increased uFC was observed in HPC ↔ ITG (t18=3.08, β=0.16) and dPFC ↔ FG (t18=5.19, β= 0.25) pathways, whereas no pathways were significant during Late Retrieval.
We also explored differences in uFC between Encoding and Rest for the connections that showed significant positive uFC, specifically dPFC ↔ FG, FG ↔ HPC, dACC ↔ FG, dACC ↔ HPC, FG ↔ ITG, and FG ↔ SPC. After correction for multiple comparisons, none of these inter-condition differences were significant. Similarly, planned comparisons explored between Retrieval and Rest, for the following connections: dPFC ↔ FG and dACC ↔ ITG were not significant.
4. Discussion
A principal aim of this study was to explore the modulation of undirected functional connectivity between sub-networks during (presumably) “active” rest states that are embedded within the paradigm itself. To maximize the facility of this exploration we specifically leveraged a previously employed associative learning paradigm (Diwadkar et al., 2008; Stanley et al., 2017; Woodcock, White, & Diwadkar, 2015; Woodcock et al., 2016). This task was characterized by increased proficiency over the course of the study as subjects acquire episodic memory for associations between different memoranda classes arbitrarily brought into association for the purposes of the study. The paradigm was explicitly structured to alternate between periods of Memory Encoding and Cued Retrieval, where each of these task-active conditions were characterized by sensorimotor (visual inputs and verbal outputs) and memorial processing. These conditions enveloped a “Rest/Rehearsal” period involving only the presentation of a fixation marker. The ostensible functional role of this Rest period is to permit “internally driven” rehearsal of the associated memoranda, thus making this a potentially “active” rest state.
Our exploration of the connectivity profiles of the learning network (Fig. 2), revealed: (1) Memory Encoding resulted in the modulation of uFC between the dACC and FG, the FG and the ITG, and the FG and the SPC (Fig. 3); (2) Cued Retrieval resulted in the modulation of the uFC between the dACC and ITG, and the dPFC and the FG (Fig. 4); (3) Of the greatest specific relevance to our aims, the uFC of several sub-networks was significantly modulated during Rest/Rehearsal (Fig. 5). These included the dPFC and the FG, the dACC and the FG, the dACC and the HPC, the FG and the SPC; (4) Finally, the patterns of uFC modulation appeared dynamic across the phases of learning (Fig. 6), with different patterns of modulation observed during Early Resting epochs.
These explorations (and results) do not conclusively establish a precise role for active resting states in sub serving “cognitive architectures” (Deco et al., 2013; Schlichting & Preston, 2016). Nevertheless, they suggest that functional transactions during particular rest states remain ongoing (Diwadkar et al., 2017), and that dynamic patterns of changes in uFC are broadly associated with behavioral changes during the task. Below, we attempt to unravel plausible mechanistic bases for these effects and place our results within a broader conceptualization of the notion of the brain’s “resting state.”
5. An active role for the rest state in cognitive architectures
Brain’s in principle never rest, yet characterizing the role of the brain’s resting state is a non-trivial challenge (Logothetis et al., 2009). Resting periods associated with both sleep (Stickgold, 2013) and wakefulness (Craig, Dewar, Della Sala, & Wolbers, 2015) play active functional roles, particularly in memory, suggesting that the neurobiological signatures of these mechanisms are ripe for discovery. Only recently have in vivo imaging studies turned attention to analyses of task-relevant resting state signals, as opposed to connectomic analyses (Smith et al., 2013). The recent efforts address the functional role that resting state network interactions might play, and this emergent evidence suggests that the resting state often assumes highly specific “active” roles and/or resting networks are associated with task-active networks in specific domains (Dosenbach et al., 2007; Hoffstaedter et al., 2014). In this process of discovery, the analyses of resting signals that are embedded within ongoing tasks can be particularly useful, as these periods are likely to reflect psychological refractory and/or constructive intervals in the ongoing and unfolding cognitive process.
These questions were addressed in a recent study evaluating directed functional connectivity (dFC; based on multivariate autoregressive models to time series data (Bressler & Seth, 2011; Diwadkar et al., 2017)) during both task- and resting epochs. Two classes of task epochs were used: basic motor control (Friedman et al., 2017) and working memory (Diwadkar et al., 2015). Of particular interest were interactions between dACC ↔ SMA during resting epochs embedded throughout the experiment, particularly given prior evidence that dFC from the dACC to the SMA is crucial in sub serving coordinated motor control, but not necessarily working memory (Asemi, Ramaseshan, Burgess, Diwadkar, & Bressler, 2015). Analyses of resting epochs revealed compelling differences in dFC from the SMA to the dACC; specifically, dFC during resting epochs was increased from the SMA to the dACC only for motor, but not memory tasks (Diwadkar et al., 2017). This result suggests that the directionality of FC in the resting state (SMA → dACC) complements that observed during task-active motor control (dACC → SMA), evidence for a relatively specific and constructive role for network interactions at rest.
Translating previous results into the domain of memory is challenging, even though attempts at understanding how the brain consolidates memories is one of the most active areas of research in the field of memory-related neuroscience (Eichenbaum, 2001). fMRI and other studies have suggested that initial consolidation is driven by short or long term synaptic potentiation of hippocampal cells (Bittner, Milstein, Grienberger, Romani, & Magee, 2017; Wirth et al., 2003) leading to modulation of the strength of synaptic circuits. Traces initially consolidated in the medial temporal lobe are thought to be distributed to the neo-cortex over time (Haist, Bowden Gore, & Mao, 2001). Though fMRI provides aggregate “neural” data over several orders of spatial and temporal scale, evidence suggests that fMRI responses to stimuli during encoding are predictive of subsequent recall, and hence consolidation (Dickerson et al., 2007). Our current results are only obliquely related to these seminal studies, yet can be more directly compared to (and are broadly consistent with) other studies in associative learning. When subjects are trained to associate faces with other objects, supra-threshold activation in the fusiform face area (FFA) is observed several seconds after the encoding pair, suggestive of “reactivation” of this region following paired-associate memory (Schlichting & Preston, 2014). Moreover, in the same study, resting state uFC between the FFA and the hippocampus predicted learning of generalized associations of one pair of the memoranda, to a novel item. These results imply that resting state FC may facilitate the integration of ongoing information in memory in both fear and non-fear related contexts (de Voogd, Fernandez, & Hermans, 2016; Schlichting & Preston, 2016; van Kesteren, Fernandez, Norris, & Hermans, 2010). Other recent work also demonstrates a role for the resting medial temporal lobe in memory formation. The correlational structure of hippocampal voxel time series immediately following associative encoding is more similar to the encoding period than to that observed in pre-encoding periods (Tambini & Davachi, 2013), suggesting that post-encoding activation sub-serves consolidation of recently acquired memories.
6. Cortical-hippocampal sub-networks during rest
In our results, aspects of the frontal lobe including the prefrontal cortex and the dorsal anterior cingulate, both of which are implicated in associative memory formation and recall (Woodcock et al., 2015; Woodcock et al., 2016) evinced significant uFC with other regions during rest/rehearsal (Fig. 5). These included “unimodal” regions such as the fusiform gyrus (dACC ↔ FG; dPFC ↔ FG), and the hippocampus itself (dACC ↔ Hippocampus). Additionally, we also observed increased uFC between the FG ↔ Superior Parietal cortex. These effects which are oriented around the dACC and the dPFC are consistent with the roles these structures play in memory and executive control (Anderson, Bunce, & Barbas, 2016; Botvinick, Braver, Barch, Carter, & Cohen, 2001). The dACC implicated in motor and cognitive control (Anderson, Fincham, Qin, & Stocco, 2008; Asemi et al., 2015; Carter, Botvinick, & Cohen, 1999), is seen as the interface between these domains (Paus, 2001). In the context of associative learning, the region appears to underpin many of the executive processing commitments that are in play during memory encoding including maintaining memoranda in transient memory, updating representations and executive attention. Indeed, dFC analyses using psychophysiological interaction (PPI) (Friston et al., 1997; Woodcock et al., 2015), have confirmed that the dACC exerts significant modulatory effects on multiple regions including the hippocampus and fusiform gyrus during memory encoding.
The dPFC has been associated with inhibitory and facilitatory control over memories (Anderson & Green, 2001), and is strongly engaged in tasks of memory suppression and retrieval. During associative memory and learning, frontal-hippocampal regions are crucial for consolidation of memories (Eichenbaum, 2014), though the functional contributions vary over time such that early memory formation is thought to be hippocampal-driven, whereas long-term consolidation is thought to happen over the cortex. Because the dPFC is integral to the long-term consolidation of memories, this structure (and the frontal lobe in general) is presumed to generate retrieval cues during episodic/associative memory retrieval (Simons & Spiers, 2003). Our observed uFC effects involving the dPFC are also consistent with independent analyses of connectivity during task-active epochs (Banyai et al., 2011). The dPFC’s network signature’s during Memory Encoding and Cued Retrieval have been established using both effective connectivity techniques, such as Dynamic Causal Modeling (Friston, 2011), and PPI, and the structure assumes a primal role in establishing network control during associative learning. It appears that many of these signatures may be at play in the resting state as well. Finally, increased uFC of FG ↔ SP is notable suggesting that the rehearsal of pairs during rest drives increased synchrony between regions associated with each memoranda class. In summary, increased uFC during rest was observed between nodes with multi-faceted aspects of relative functional specialization, ranging from executive processing and control (dPFC, dACC) to modality specific processing (FG, SPC), suggesting that these plausible network profiles of consolidation in the post memory encoding period are diverse. Moreover, componential analyses of temporal windows of the task revealed interesting “dynamics” of uFC: During the Early period, increased uFC was observed in dACC ↔ SPC, dACC ↔ FG and dPFC ↔ Hippocampus. During Late resting periods, these effects were observed in dACC ↔ Hippocampus, dPFC ↔ FG and FG ↔ SPC. It is notable that the temporal dynamics of these effects are broadly consistent with independent studies of the dynamics of modulated glutamate during learning (Stanley et al., 2017). Using functional 1H Magnetic Resonance Spectroscopy, a non-hemodynamics based method of estimating brain function, we have previously shown that an early increase in glutamate modulation is a principle advantage that rapid learners possess over slow learners.
7. Conclusions and limitations
Our results provide evidence of significant supra-threshold correlations that are evident in cortical-hippocampal networks during periods when participants are presumably covertly consolidating memories. Moreover, we also demonstrate that these patterns of network connectivity are not static in the sense that the patterns of uFC observed during the linear learning regime are different than those observed in the asymptotic learning regime (when memories are more strongly consolidated). We argue that our results are consistent with an emerging literature emphasizing the active role of networks in sub-serving potentiation for action in the dynamic brain, even in the absence of overt sensorimotor stimulation (Deco et al., 2013; Diwadkar et al., 2017). In effect, these studies (and we) suggest that between active states, brain networks are often revisiting overt processes, or are in preparatory stages for upcoming action. Because the fMRI signal is maximally sensitive to task-induced modulation (Logothetis, 2008), appropriately structured experimental paradigms and tasks may help in discovering how network synchrony in the resting state sub serves function in task-relevant brain networks. Moreover, these results might also suggest a re-evaluation of using “resting” baselines, certainly within tasks such as ours, to identify activation profiles, given that these specific states appear to be covertly functionally “active.”
Using fMRI to infer “mental” processes is an endeavor fraught with significant conceptual challenges (Price & Friston, 2005). Brain-Behavior (or Behavior-Brain) relationships are characterized by significant “degeneracy”, wherein brain regions and cognitive architectures do not sit in straightforward one-to-one relationships; diverse tasks can dynamically evoke responses in identical brain networks/regions (Park & Friston, 2013), and inferring “causality” in the classical sense is nearly impossible (Diwadkar, 2015; Mannino & Bressler, 2015). These conceptual challenges are compounded by the spatio-temporal and interpretational limitations of the fMRI signal. These limitations preclude inferences regarding the precise neuronal bases of the signal (Logothetis, 2008; Singh, 2012), and its relationship to the excitation and inhibition balance in the cortex. Moreover, to a large degree the fMRI signal is modulated simply by sensori-motor stimulation, and is affected by a mixture of feedforward and feedback processes (Logothetis, 2008). Resting state network profiles themselves are a cause of further controversy because in “pure” resting state acquisitions, it is unclear if correlated signals across regions reflect true intrinsic connectivity, or synchronization to common source inputs (Logothetis et al., 2009). Moreover, these correlations are themselves highly sensitive to basic sensory modulators such as whether eyes are open or closed during acquisition (Song et al., 2015; Zou et al., 2015).
In effect, functional connectivity methods, while attempting to capture interactions between system components, are higher order descriptions of data (Silverstein et al., 2016; Smith, 2012), with little consideration for estimating neurophysiological parameters or drivers of the hemodynamic signal. This is a significant limitation of this weak class of methods. Whereas effective connectivity models provide strong bases for assessing directional network interactions between regions (Friston, 2011; Silverstein et al., 2016), our motivations here were driven by the need for discovery motivated by general (as opposed to specific) hypotheses. Toward that end, a “weak” connectivity technique such as uFC that relies on exploring statistical dependencies between fMRI signals, serves our goals well. Of course, generative causal models of network interactions provide a better framework for specific hypotheses assessment (Stephan et al., 2010) and indeed in our past work we have relied on such approaches to assess frontal-striatal (Diwadkar et al., 2014), frontal-limbic (Diwadkar et al., 2012), frontal-thalamic (Jagtap & Diwadkar, 2016) and frontal-hippocampal network interactions (Banyai et al., 2011). We intend to use the current discoveries to motivate more detailed analyses of network interactions using effective connectivity techniques.
It is challenging if not impossible to ascribe precise psychological interpretations to the resting state correlations that we observed. It has been noted that fMRI is not a “mind reader” (Logothetis, 2008), yet modulations in fMRI signals are strongly effected by task-related processing. Thus, we suggest that our observed modulation of sub-network uFC during rest is likely to reflect an active and constructive process. The correlations are not general (i.e., network-wide), and in cases are exclusive to effects observed in Memory Encoding or Retrieval (see Fig. 5 vs. 3 and 4). This implies that our results do not merely reflect task-related reverberations from preceding Memory Encoding epochs, but profiles uniquely modulated at rest. uFC also appears more sensitive during the Rest epochs rather than the task-active states of Memory Encoding and Cued Retrieval. If in fact, task-active states induce higher frequency fluctuations, these may obscure the sensitivity of correlational analyses that are highly sensitive to lower frequency fluctuations that dominate resting state signals, and the relationships of which to task-active modulation is highly task-specific (Yuan et al., 2013). From this perspective, our current results, and other previous studies of this nature, can help elucidate what if any, active role is assumed when the brain is at “rest” (assuming it ever truly is) (Logothetis et al., 2009).
Acknowledgments
We thank Karthik Ramaseshan, Caroline Zajac-Benitez, Patricia Thomas and Usha Rajan for their time and effort in recruiting and assessing the participants, and helping with data acquisition and analyses. This work was supported by the National Institutes of Mental Health (MH111177 to J.A.S and V.A.D.), the Mark Cohen Neuroscience Endowment, the Children’s Hospital of Michigan Foundation, and the Lycaki-Young Funds from the State of Michigan.
Footnotes
Conflicts of interest
The authors have no conflicts of interest to report.
References
- Amaro E, Jr, Barker GJ. Study design in fMRI: Basic principles. Brain and Cognition. 2006;60(3):220–232. doi: 10.1016/j.bandc.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Anderson MC, Bunce JG, Barbas H. Prefrontal-hippocampal pathways underlying inhibitory control over memory. Neurobiology of Learning and Memory. 2016;134(Pt A):145–161. doi: 10.1016/j.nlm.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JR, Fincham JM, Qin Y, Stocco A. A central circuit of the mind. Trends in Cognitive Sciences. 2008;12(4):136–143. doi: 10.1016/j.tics.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MC, Green C. Suppressing unwanted memories by executive control. Nature. 2001;410(6826):366–369. doi: 10.1038/35066572. [DOI] [PubMed] [Google Scholar]
- Asemi A, Ramaseshan K, Burgess A, Diwadkar VA, Bressler SL. Dorsal anterior cingulate cortex modulates supplementary motor area in coordinated unimanual motor behavior. Frontiers in Human Neuroscience. 2015;9:309. doi: 10.3389/fnhum.2015.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banyai M, Diwadkar VA, Erdi P. Model-based dynamical analysis of functional disconnection in schizophrenia. Neuroimage. 2011;58(3):870–877. doi: 10.1016/j.neuroimage.2011.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bero AW, Meng J, Cho S, Shen AH, Canter RG, Ericsson M, et al. Early remodeling of the neocortex upon episodic memory encoding. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(32):11852–11857. doi: 10.1073/pnas.1408378111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bittner KC, Milstein AD, Grienberger C, Romani S, Magee JC. Behavioral time scale synaptic plasticity underlies CA1 place fields. Science. 2017;357(6355):1033–1036. doi: 10.1126/science.aan3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108(3):624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Bressler SL, Seth AK. Wiener-Granger causality: A well established methodology. Neuroimage. 2011;58(2):323–329. doi: 10.1016/j.neuroimage.2010.02.059. [DOI] [PubMed] [Google Scholar]
- Buchel C, Coull JT, Friston KJ. The predictive value of changes in effective connectivity for human learning. Science. 1999;283:1538–1541. doi: 10.1126/science.283.5407.1538. [DOI] [PubMed] [Google Scholar]
- Carter CS, Botvinick MM, Cohen JD. The contribution of the anterior cingulate cortex to executive processes in cognition. Reviews in the Neurosciences. 1999;10(1):49–57. doi: 10.1515/revneuro.1999.10.1.49. [DOI] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Wendt GJ, Turski PA, Moritz CH, et al. Mapping functionally related regions of brain with functional connectivity MR imaging. AJNR American Journal of Neuroradiology. 2000;21(9):1636–1644. [PMC free article] [PubMed] [Google Scholar]
- Craig M, Dewar M, Della Sala S, Wolbers T. Rest boosts the long-term retention of spatial associative and temporal order information. Hippocampus. 2015;25(9):1017–1027. doi: 10.1002/hipo.22424. [DOI] [PubMed] [Google Scholar]
- de Voogd LD, Fernandez G, Hermans EJ. Awake reactivation of emotional memory traces through hippocampal-neocortical interactions. Neuroimage. 2016;134:563–572. doi: 10.1016/j.neuroimage.2016.04.026. [DOI] [PubMed] [Google Scholar]
- Deco G, Jirsa VK, McIntosh AR. Resting brains never rest: computational insights into potential cognitive architectures. Trends in Neurosciences. 2013;36(5):268–274. doi: 10.1016/j.tins.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Miller SL, Greve DN, Dale AM, Albert MS, Schacter DL, et al. Prefrontal-hippocampal-fusiform activity during encoding predicts intraindividual differences in free recall ability: An event-related functional-anatomic MRI study. Hippocampus. 2007;17(11):1060–1070. doi: 10.1002/hipo.20338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwadkar VA. Critical perspectives on causality and inference in brain networks: Allusions, illusions, solutions?: Comment on: “Foundational perspectives on causality in large-scale brain networks” by M. Mannino and S. L. Bressler. Physics of Life Reviews. 2015;15:141–144. doi: 10.1016/j.plrev.2015.10.018. [DOI] [PubMed] [Google Scholar]
- Diwadkar VA, Asemi A, Burgess A, Chowdury A, Bressler SL. Potentiation of motor sub-networks for motor control but not working memory: Interaction of dACC and SMA revealed by resting-state directed functional connectivity. PLoS One. 2017;12(3):e0172531. doi: 10.1371/journal.pone.0172531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwadkar VA, Bakshi N, Gupta G, Pruitt P, White R, Eickhoff SB. Dysfunction and dysconnection in cortical-striatal networks during sustained attention: genetic risk for schizophrenia or bipolar disorder and its impact on brain network function. Frontiers in Psychiatry. 2014;5:50. doi: 10.3389/fpsyt.2014.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwadkar VA, Bellani M, Ahmed R, Dusi N, Rambaldelli G, Perlini C, et al. Chronological age and its impact on associative learning proficiency and brain structure in middle adulthood. Behavioural Brain Research. 2016;297:329–337. doi: 10.1016/j.bbr.2015.10.016. [DOI] [PubMed] [Google Scholar]
- Diwadkar VA, Burgess A, Hong E, Rix C, Arnold PD, Hanna GL, et al. Dysfunctional activation and brain network profiles in youth with obsessive-compulsive disorder: A focus on the dorsal anterior cingulate during working memory. Frontiers Human Neuroscience. 2015;9:149. doi: 10.3389/fnhum.2015.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwadkar VA, Flaugher B, Jones T, Zalanyi L, Ujfalussy B, Keshavan MS, et al. Impaired associative learning in schizophrenia: Behavioral and computational studies. Cognitive Neurodynamics. 2008;2(3):207–219. doi: 10.1007/s11571-008-9054-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwadkar VA, Wadehra S, Pruitt P, Keshavan MS, Rajan U, Zajac-Benitez C, et al. Disordered cortico-limbic interactions during affective processing in children and adolescents at risk for schizophrenia revealed by fMRI and Dynamic Causal Modeling. Archives of General Psychiatry. 2012;69(3):231–242. doi: 10.1001/archgenpsychiatry.2011.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, et al. Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(26):11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. The long and winding road to memory consolidation. Nature Neuroscience. 2001;4(11):1057–1058. doi: 10.1038/nn1101-1057. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. Time cells in the hippocampus: A new dimension for mapping memories. Nature Reviews Neuroscience. 2014;15(11):732–744. doi: 10.1038/nrn3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A, Burgess A, Ramaseshan K, Easter P, Khatib D, Chowdury A, et al. Brain network dysfunction in obsessive-compulsive disorder induced by simple uni-manual behavior: The role of the dorsal anterior cingulate cortex. Psychiatry Research: Neuroimaging. 2017;260:6–15. doi: 10.1016/j.pscychresns.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ. Functional and effective connectivity: A review. Brain Connectivity. 2011;1(1):13–36. doi: 10.1089/brain.2011.0008. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6(3):218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haist F, Bowden Gore J, Mao H. Consolidation of human memory over decades revealed by functional magnetic resonance imaging. Nature Neuroscience. 2001;4(11):1139–1145. doi: 10.1038/nn739. [DOI] [PubMed] [Google Scholar]
- Hoffstaedter F, Grefkes C, Caspers S, Roski C, Palomero-Gallagher N, Laird AR, et al. The role of anterior midcingulate cortex in cognitive motor control: Evidence from functional connectivity analyses. Human Brain Mapping. 2014;35(6):2741–2753. doi: 10.1002/hbm.22363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagtap P, Diwadkar VA. Effective connectivity of ascending and descending frontalthalamic pathways during sustained attention: Complex brain network interactions in adolescence. Human Brain Mapping. 2016;37(7):2557–2570. doi: 10.1002/hbm.23196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453(7197):869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Murayama Y, Augath M, Steffen T, Werner J, Oeltermann A. How not to study spontaneous activity. Neuroimage. 2009;45(4):1080–1089. doi: 10.1016/j.neuroimage.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Mannino M, Bressler SL. Foundational perspectives on causality in large-scale brain networks. Physics of Life Reviews. 2015;15:107–123. doi: 10.1016/j.plrev.2015.09.002. [DOI] [PubMed] [Google Scholar]
- McFarlane KA, Humphreys MS. Maintenance rehearsal: the key to the role attention plays in storage and forgetting. Journal of Experimental Psychology Learning, Memory, and Cognition. 2012;38(4):1001–1018. doi: 10.1037/a0026783. [DOI] [PubMed] [Google Scholar]
- Miller BT, D’Esposito M. Spatial and temporal dynamics of cortical networks engaged in memory encoding and retrieval. Frontiers in Human Neuroscience. 2012;6:109. doi: 10.3389/fnhum.2012.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto V, Jaspar M, Meyer C, Kusse C, Chellappa SL, Degueldre C, et al. Local modulation of human brain responses by circadian rhythmicity and sleep debt. Science. 2016;353(6300):687–690. doi: 10.1126/science.aad2993. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25(3):653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Park HJ, Friston K. Structural and functional brain networks: from connections to cognition. Science. 2013;342(6158):1238411. doi: 10.1126/science.1238411. [DOI] [PubMed] [Google Scholar]
- Paus T. Primate anterior cingulate cortex: Where motor control, drive and cognition interface. Nature Reviews Neuroscience. 2001;2(6):417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- Price CJ, Friston KJ. Functional ontologies for cognition: The systematic definition of structure and function. Cognitive Neuropsychology. 2005;22(3):262–275. doi: 10.1080/02643290442000095. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichting ML, Preston AR. Memory reactivation during rest supports upcoming learning of related content. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(44):15845–15850. doi: 10.1073/pnas.1404396111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichting ML, Preston AR. Hippocampal-medial prefrontal circuit supports memory updating during learning and post-encoding rest. Neurobiology of Learning and Memory. 2016;134(Pt A):91–106. doi: 10.1016/j.nlm.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scully ID, Napper LE, Hupbach A. Does reactivation trigger episodic memory change? A meta-analysis. Neurobiology of Learning and Memory. 2017;142(Pt A):99–107. doi: 10.1016/j.nlm.2016.12.012. [DOI] [PubMed] [Google Scholar]
- Silverstein B, Bressler S, Diwadkar VA. Inferring the dysconnection syndrome in schizophrenia: Interpretational considerations on methods for the network analyses of fMRI data. Frontiers in Psychiatry. 2016;7:132. doi: 10.3389/fpsyt.2016.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JS, Spiers HJ. Prefrontal and medial temporal lobe interactions in long-term memory. Nature Reviews Neuroscience. 2003;4(8):637–648. doi: 10.1038/nrn1178. [DOI] [PubMed] [Google Scholar]
- Singh KD. Which “neural activity” do you mean? fMRI, MEG, oscillations and neurotransmitters. Neuroimage. 2012;62(2):1121–1130. doi: 10.1016/j.neuroimage.2012.01.028. [DOI] [PubMed] [Google Scholar]
- Smith SM. The future of FMRI connectivity. Neuroimage. 2012;62(2):1257–1266. doi: 10.1016/j.neuroimage.2012.01.022. [DOI] [PubMed] [Google Scholar]
- Smith SM, Vidaurre D, Beckmann CF, Glasser MF, Jenkinson M, Miller KL, et al. Functional connectomics from resting-state fMRI. Trends in Cognitive Sciences. 2013;17(12):666–682. doi: 10.1016/j.tics.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer T, Rose M, Glascher J, Wolbers T, Buchel C. Dissociable contributions within the medial temporal lobe to encoding of object-location associations. Learning & Memory. 2005;12(3):343–351. doi: 10.1101/lm.90405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Zhou S, Zhang Y, Liu Y, Zhu H, Gao JH. Frequency-dependent modulation of regional synchrony in the human brain by eyes open and eyes closed resting-states. PLoS One. 2015;10(11):e0141507. doi: 10.1371/journal.pone.0141507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley JA, Burgess A, Khatib D, Ramaseshan K, Arshad M, Wu H, et al. Functional dynamics of hippocampal glutamate during associative learning assessed with in vivo 1H functional magnetic resonance spectroscopy. Neuroimage. 2017;153:189–197. doi: 10.1016/j.neuroimage.2017.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Penny WD, Moran RJ, den Ouden HE, Daunizeau J, Friston KJ. Ten simple rules for dynamic causal modeling. Neuroimage. 2010;49(4):3099–3109. doi: 10.1016/j.neuroimage.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickgold R. Parsing the role of sleep in memory processing. Current Opinion in Neurobiology. 2013;23(5):847–853. doi: 10.1016/j.conb.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambini A, Davachi L. Persistence of hippocampal multivoxel patterns into postencoding rest is related to memory. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(48):19591–19596. doi: 10.1073/pnas.1308499110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kesteren MT, Fernandez G, Norris DG, Hermans EJ. Persistent schema-dependent hippocampal-neocortical connectivity during memory encoding and postencoding rest in humans. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(16):7550–7555. doi: 10.1073/pnas.0914892107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma S, Takashima A, Krewinkel S, van Kooten M, Fu L, Medendorp WP, et al. Non-interfering effects of active post-encoding tasks on episodic memory consolidation in humans. Frontiers in Behavioral Neuroscience. 2017;11:54. doi: 10.3389/fnbeh.2017.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadehra S, Pruitt P, Murphy ER, Diwadkar VA. Network dysfunction during associative learning in schizophrenia: Increased activation, but decreased connectivity: An fMRI study. Schizophrenia Research. 2013;148(1–3):38–49. doi: 10.1016/j.schres.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Ward BD. Simultaneous inference for fMRI data. Milwaukee, WI: Medical College of Wisconsin; 2000. [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A. Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity. 2012;2(3):125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Wirth S, Yanike M, Frank LM, Smith AC, Brown EN, Suzuki WA. Single neurons in the monkey hippocampus and learning of new associations. Science. 2003;300(5625):1578–1581. doi: 10.1126/science.1084324. [DOI] [PubMed] [Google Scholar]
- Woodcock EA, Wadehra S, Diwadkar VA. Network profiles of the dorsal anterior cingulate and dorsal prefrontal cortex in schizophrenia during hippocampal-based associative memory. Frontiers in Systems Neuroscience. 2016;10:32. doi: 10.3389/fnsys.2016.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock EA, White R, Diwadkar VA. The dorsal prefrontal and dorsal anterior cingulate cortices exert complementary network signatures during encoding and retrieval in associative memory. Behavioural Brain Research. 2015;290:152–160. doi: 10.1016/j.bbr.2015.04.050. [DOI] [PubMed] [Google Scholar]
- Yuan R, Di X, Kim EH, Barik S, Rypma B, Biswal BB. Regional homogeneity of resting-state fMRI contributes to both neurovascular and task activation variations. Magnetic Resonance Imaging. 2013;31(9):1492–1500. doi: 10.1016/j.mri.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Q, Miao X, Liu D, Wang DJ, Zhuo Y, Gao JH. Reliability comparison of spontaneous brain activities between BOLD and CBF contrasts in eyes-open and eyes-closed resting states. Neuroimage. 2015;121:91–105. doi: 10.1016/j.neuroimage.2015.07.044. [DOI] [PubMed] [Google Scholar]
- Zou Q, Ross TJ, Gu H, Geng X, Zuo XN, Hong LE, et al. Intrinsic resting-state activity predicts working memory brain activation and behavioral performance. Human Brain Mapping. 2013;34(12):3204–3215. doi: 10.1002/hbm.22136. [DOI] [PMC free article] [PubMed] [Google Scholar]