Abstract
Clinical trials that led to ibrutinib’s approval for the treatment of chronic lymphocytic leukemia showed that its side effects differ from those of traditional chemotherapy. Reasons for discontinuation in clinical practice have not been adequately studied. We conducted a retrospective analysis of chronic lymphocytic leukemia patients treated with ibrutinib either commercially or on clinical trials. We aimed to compare the type and frequency of toxicities reported in either setting, assess discontinuation rates, and evaluate outcomes. This multicenter, retrospective analysis included ibrutinib-treated chronic lymphocytic leukemia patients at nine United States cancer centers or from the Connect® Chronic Lymphocytic Leukemia Registry. We examined demographics, dosing, discontinuation rates and reasons, toxicities, and outcomes. The primary endpoint was progression-free survival. Six hundred sixteen ibrutinib-treated patients were identified. A total of 546 (88%) patients were treated with the commercial drug. Clinical trial patients were younger (mean age 58 versus 61 years, P=0.01) and had a similar time from diagnosis to treatment with ibrutinib (mean 85 versus 87 months, P=0.8). With a median follow-up of 17 months, an estimated 41% of patients discontinued ibrutinib (median time to ibrutinib discontinuation was 7 months). Notably, ibrutinib toxicity was the most common reason for discontinuation in all settings. The median progression-free survival and overall survival for the entire cohort were 35 months and not reached (median follow-up 17 months), respectively. In the largest reported series on ibrutinib- treated chronic lymphocytic leukemia patients, we show that 41% of patients discontinued ibrutinib. Intolerance as opposed to chronic lymphocytic leukemia progression was the most common reason for discontinuation. Outcomes remain excellent and were not affected by line of therapy or whether patients were treated on clinical studies or commercially. These data strongly argue in favor of finding strategies to minimize ibrutinib intolerance so that efficacy can be further maximized. Future clinical trials should consider time-limited therapy approaches, particularly in patients achieving a complete response, in order to minimize ibrutinib exposure.
Introduction
Ibrutinib is an orally bioavailable, irreversible inhibitor of Bruton tyrosine kinase (BTK). It is a standard of care in the treatment of chronic lymphocytic leukemia (CLL) in the relapsed/refractory1–3 as well as front-line4 settings.
The toxicities of ibrutinib and reasons for its discontinuation were initially defined through several landmark studies comparing ibrutinib to chlorambucil (front-line, RESONATE 2),4 ofatumumab (RESONATE)2 and bendamustine and rituximab +/− ibrutinib versus placebo (HELIOS).5 Higher proportions of patients discontinuing therapy (ranging from 25% to 51%) have been found with long-term follow-up (median follow-ups ranging between 20 months and approximately 5 years) of these and other ibrutinib clinical trials. Although the percentage of discontinuations due to progression of disease (21% to 45%) remained similar, there were high proportions of discontinuations due to adverse events, ranging from 12% to 32%.6–10
Woyach et al. suggested that discontinuation due to CLL progression (distinguished from disease transformation) occurred later in the disease course (cumulative incidence of 7.3% at 2 years, 19.1% at 4 years) while other reasons for discontinuation, such as Richter transformation, peaked early and reached a plateau by 3 years (18.7% at 2 years, 23.9% at 3 years).8 In clinical practice, adverse events were found to be the most common cause of ibrutinib discontinuation among 143 patients, approaching 50%.11 Atrial fibrillation, infectious complications, and cytopenias were the most commonly described adverse events.11 High percentages of patients discontinuing therapy, ranging from 19–41%, were encountered in additional studies conducted in the USA and outside of the USA; however, these studies were limited by relatively small sample sizes and short follow-up periods.12–15
We, therefore, aimed to characterize patterns of care among ibrutinib-treated CLL patients in clinical practice in the USA focusing on rates and reasons for discontinuation, and how these affect outcomes. To our knowledge, this series is the largest report on ibrutinib-treated CLL patients.
Methods
We conducted a multicenter, retrospective cohort study of CLL patients at nine USA cancer centers and from the Connect® CLL Registry (199 USA centers, 80% community sites) who were treated with ibrutinib either as part of a clinical trial or with commercially available drug.16 The institutional review board of each participating institution approved this study. Investigators at each institution were asked to utilize chart review, institutional electronic medical records and clinical/pathological databases to obtain required information for all CLL patients treated with ibrutinib. Data collected included: patients’ demographics, genetic characteristics, number of prior therapies, dosing and dose adjustments, discontinuation rates and reasons, toxicities and outcomes. The period of enrollment was January 2014 to August 2016.
The primary study endpoint was progression-free survival, which was defined as time from ibrutinib treatment to progression or death from any cause as per the Kaplan Meier method.17 Patients were otherwise censored, regardless of progression status, at the time of last follow-up and at the time of next therapy. When interpreting medical records, investigators were advised to use the International Workshop on Chronic Lymphocytic Leukemia (IWCLL) criteria to define response and progression of disease.18,19 Patients were stratified by line of therapy (front-line versus relapsed/refractory), reason for discontinuation (intolerance versus progressive disease), clinical trial participation versus commercial use, and depth of response (complete response versus partial response and partial response with lymphocytosis). Secondary endpoints included overall survival and reasons for ibrutinib discontinuation. Overall survival was defined as the time in months from initiation of ibrutinib to death.
Reasons for ibrutinib discontinuation were categorized as follows: toxicity, progressive disease, Richter transformation to either diffuse large B-cell lymphoma or Hodgkin lymphoma, planned cellular therapy (allogeneic hematopoietic stem cell transplantation or chimeric antigen receptor genetically modified T-cell therapy), secondary malignancies, physician’s or patient’s preference, financial concerns, and other/unrelated death. Toxicities leading to discontinuation were categorized as: hematologic toxicity, infection, atrial fibrillation, congestive heart failure, drug- induced pneumonitis, drug-induced colitis, transaminitis, bleeding, arthralgia/myalgia, dermatological, neurotoxicity, other, or unknown.
Survival data were compared using the log-rank test.20 Univariate Cox regression analyses were used to estimate hazard ratios.21 All other comparison analyses were descriptive. All tests were two-sided at the 5% level. Statistical analyses were performed using STATA 10.1 (Stata Statistical Software: Release 10. 2007; StataCorp LP, College Station, TX, USA).
Results
Patients
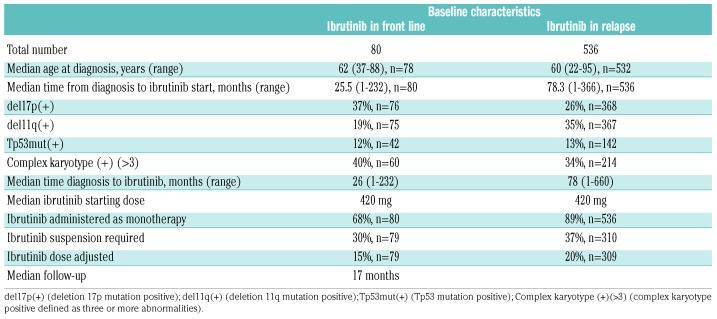
We identified 616 patients who received ibrutinib, including 536 relapsed-refractory and 80 previously untreated patients. Data from the nine contributing academic centers were collected retrospectively and included information for 399 patients treated with ibrutinib; data from the Connect CLL registry were collected prospectively and included information on 217 patients largely collected from community sites (80% community). The patients’ baseline characteristics stratified by line of therapy are available in Table 1. A total of 546 (88%) patients were treated with commercially available drug/off study. Clinical trial patients were younger (mean age 58 versus 61 years, P=0.01), had a similar time from diagnosis to treatment with ibrutinib (mean 85 versus 87 months, P=0.8) and were more consistently initiated at a dose of 420 mg daily (100% versus 89%).
Table 1.
Baseline characteristics.
Reasons for discontinuation, toxicities and timing of events
At a median follow-up of 17 months (range, 1–60 months), 41% of patients discontinued ibrutinib. The median time to ibrutinib discontinuation was 7 months (range, 0.1–41), with a median time of 6 months for patients who discontinued due to intolerance and 10 months for those who discontinued due to progression of disease (Online Supplementary Figure S1). Among patients on ibrutinib monotherapy, 41% discontinued therapy while the percentage of discontinuation among patients receiving ibrutinib-based combination therapy was 43.9%. Ibrutinib starting dose (420 mg daily versus <420 mg daily) did not correlate with the proportion of patients who discontinued ibrutinib due to toxicity (51% versus 50%) or disease progression (19.6% versus 21.4%).
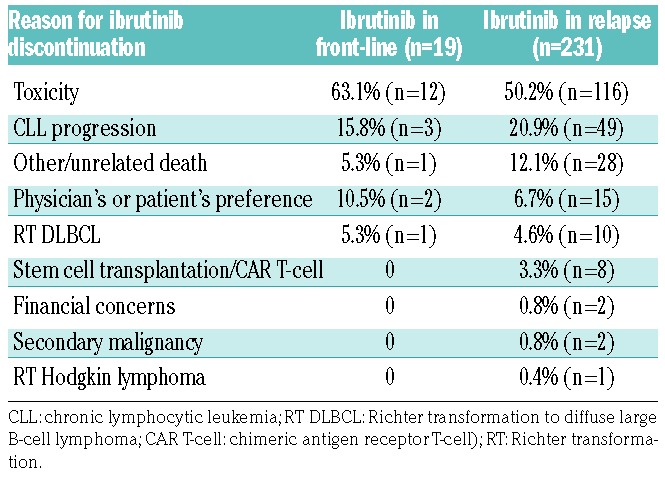
Reasons for ibrutinib discontinuation are listed in Table 2. Percentages listed indicate the proportion of discontinuations due to each category. Toxicity was the most common reason for discontinuation in all settings, accounting for 63.1% of discontinuations in front-line use (n=12/80 front-line patients) and 50.2% of discontinuations in relapsed/refractory use (n=116/536 relapsed/refractory patients). Toxicity was the most common reason for discontinuation in several settings including: commercial use and clinical trial use (50% of discontinuations in front-line commercial use, 77.7% of discontinuations in front-line clinical trial use, 52.5% of discontinuations in relapsed/refractory commercial use, and 39.7% of discontinuations in relapsed/refractory trial use). Notably, the proportion of discontinuations due to progressive disease was lower: 15.8% in the front-line setting and 20.9% in relapsed/refractory use. Richter transformation to diffuse large B-cell lymphoma or Hodgkin lymphoma accounted for 5.3% of the discontinuations in the front-line setting and 5.0% in the relapsed/refractory setting.
Table 2.
Reasons for Ibrutinib discontinuation.
Among the patients treated front-line with ibrutinib, the three most common toxicities leading to discontinuation were arthralgia (41.6%), atrial fibrillation (25%), and rash (16.7%). In the relapsed/refractory population, the most common toxicities leading to discontinuation were atrial fibrillation (12.3%), infection (10.7%), pneumonitis (9.9%), bleeding (9%) and diarrhea (6.6%).
The median time to ibrutinib discontinuation varied by toxicity: bleeding (8 months), diarrhea (7.5 months), atrial fibrillation (7 months), infection (6 months), arthralgia (5 months), pneumonitis (4.5 months) and rash (3.5 months).
Outcomes
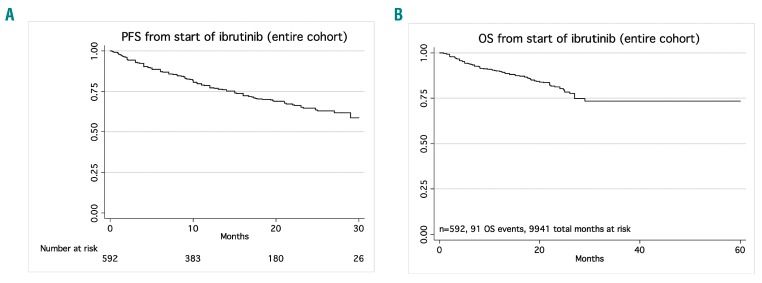
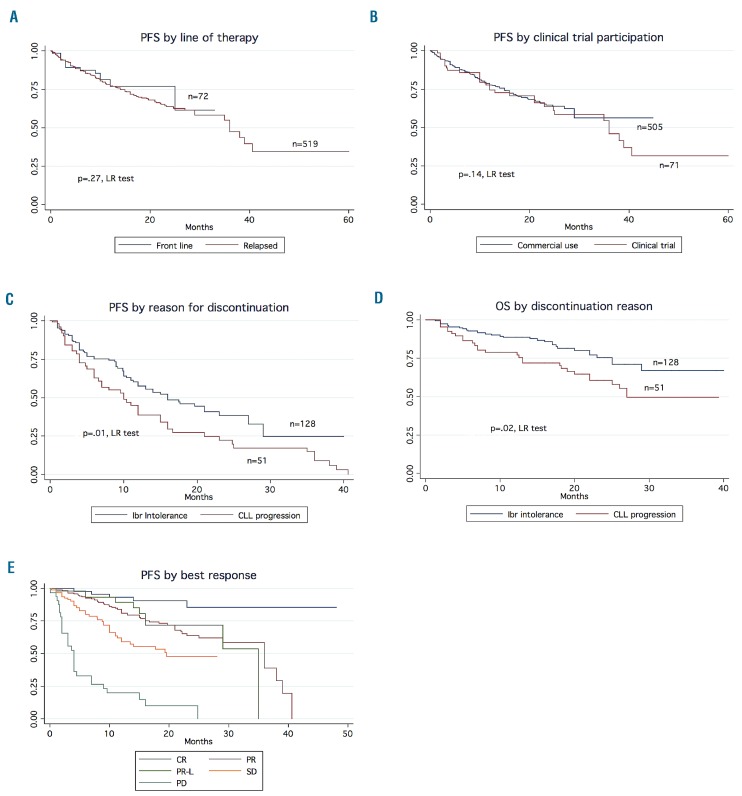
At a median follow-up of 17 months, the median progression-free and overall survival for the entire cohort were 35 months and not reached, respectively (Figure 1A,B). Overall survival from the start of ibrutinib therapy, stratified by whether the drug was being used in the front-line versus relapsed/refractory setting, is shown in Online Supplementary Figure S2A,B. Notably, there was no significant difference in progression-free survival by front-line versus relapsed/refractory use (P=0.27, log-rank test) (Figure 2A) or at first, second or third relapse (P=0.45) (Online Supplementary Figure S3). Progression-free survival was similar when stratified by ibrutinib use in the clinical practice setting as compared to the clinical trial setting (P=0.14, log-rank test) (Figure 2B). Patients who discontinued due to toxicity had significantly longer progression-free survival and overall survival than those who discontinued due to disease progression (P=0.01 and P=0.02, respectively, log-rank test) (Figure 2C,D). Investigator-assessed depth of response (complete response versus partial response versus partial response with lymphocytosis versus stable disease versus progressive disease) appeared to correlate with a longer progression-free survival (Figure 2E). We also stratified progression-free survival by deletion 17p status and complex karyotype status (≥3 abnormalities) in CLL patients treated in the relapsed/refractory setting. Progression-free survival was not significantly different in patients with deletion 17p (P=0.70), but was significantly shorter in patients with a complex karyotype (hazard ratio=1.8, 95% confidence interval: 1.1–3.0, P=0.01). The Kaplan Meier curves for these analyses are shown in Online Supplementary Figure S4A-C.
Figure 1.
Outcomes for the entire cohort. Kaplan Meier curves at a median follow-up of 17 months showing (A) progression-free survival (PFS) for the entire cohort and (B) overall survival (OS) for the entire cohort.
Figure 2.
Outcomes stratified by line of therapy, clinical trial participation, reason for discontinuation and depth of response. Kaplan Meier curves showing outcomes stratified by (A) line of therapy (progression-free survival), (B) clinical trial participation (progression-free survival), (C) reason for discontinuation (progression-free survival), (D) reason for discontinuation (overall survival), and (E) depth of response. CR: complete response; PR: partial response; PR-L: partial response with lymphocytosis; SD: stable disease; PD: progressive disease.
Discussion
In the largest reported series of ibrutinib-treated CLL patients so far, we found that the median progression-free survival was 35 months. Interestingly, this outcome was comparable between previously untreated and relapsed/refractory patients. Our observed progression- free survival of 35 months is shorter than that previously described in clinical trials in which the median progression-free survival was 52 months in relapsed/refractory disease.9 These observations suggest that outcomes vary when comparing clinical trial patients and those treated in clinical practice, underscoring the need to better understand outcomes and toxicities in a real-world setting.
Patient-specific factors, including molecular prognostic markers, performance status and prior therapies, may partially account for these discrepancies. Our front-line cohort of patients had a greater number of molecular abnormalities than typically seen in the treatment-naïve population; 37% of patients had del17p and 40% had a complex karyotype. Both features are associated with an inferior progression-free survival.22 For the relapsed cohort, patients may have received prior idelalisib which could have affected their subsequent response to ibrutinib; notably, this was prohibited in the earliest clinical trials of ibrutinib.11
At a median follow-up of 17 months, overall discontinuation in our study was high at 41%, suggesting that ibrutinib discontinuation is an emerging issue in clinical practice. This mirrors the high overall discontinuation patterns seen in longer follow-up studies of clinical trial patients,6–9 particularly the 51% estimated discontinuations seen at a median follow-up of 3.4 years by Woyach et al.8 In addition, 15% of front-line and 20% of relapsed ibrutinib- treated patients required a dose reduction, which is significantly higher than the 4% of patients requiring dose reduction due to adverse events in the RESONATE trial due to adverse events (4%).2
Early ibrutinib trials suggested that progressive disease was the cause of the majority of cases of discontinuation,2 a pattern that has persisted in many,6,8,9 but not all,5,7,10 studies including longer follow-up periods. Surprisingly, our results demonstrate that intolerance (50.2% in the relapsed/refractory setting, 63% in the front-line setting), rather than progressive disease (21% in the relapsed/refractory setting, 16% in the front-line setting), accounts for the majority of cases of discontinuation. Similar findings were previously noted in a smaller series reported by our group.11 With respect to adverse events leading to discontinuation, these values are greater than those reported in a pooled analysis from the phase III relapsed RESONATE and front-line RESONATE-2 studies in which the discontinuation rate was 10%.23 Because at least 50% of discontinuations are due to intolerance rather than progression of disease, it is unlikely that these patients harbor ibrutinib resistance mutations and, therefore, should likely be sensitive to alternate kinase inhibitors with different side effect profiles. Two clinical trials are being conducted at this time studying umbralisib (NCT02742090) and acalabrutinib (NCT02717611) in kinase inhibitor-intolerant patients.
For the first time we have established a timeline associated with time to discontinuation due to specific ibrutinib- related toxicities. Similar to the experience with idelalisib where specific toxicities appear to occur after different periods of time on treatment,24 these data may be of value in developing monitoring strategies for specific toxicities and educational material related to ibrutinib toxicities.
Reasons for discontinuation also varied by line of therapy. Our analysis revealed a strikingly higher number of discontinuations due to toxicity in the front-line setting; 63% compared to the previously reported 9% in RES- ONATE-2.4 Similar findings, although not as marked, were noted in the relapsed setting; 50% of patients in our analysis discontinued therapy due to intolerance compared to 12% discontinuing due to adverse events in the 4-year follow-up of RESONATE data.6 In line with prior reports, we found that atrial fibrillation and pulmonary complications were common reasons for discontinuation. Arthralgias and rash were frequently noted as well, particularly in treatment-naïve patients. The higher discontinuation rate for toxicity may reflect lack of physicians’ comfort in toxicity management, a higher incidence of toxicity in clinical practice, differences in patients’ comorbidities and age, or a lower threshold for discontinuation given an increasing number of available alternative treatment choices. This may be in contrast to the limited number of therapies available to ibrutinib-treated patients in early clinical trials. In a recent series by Lampson et al. of treatment-naïve patients treated with idelalisib plus ofatumumab adverse events (particularly liver toxicity) were the most common reasons for discontinuation.25 This study suggested that younger patients’ age and intact immunity may lead to autoimmune treatment-related toxicities in treatment-naïve patients.25
Progression of disease was the second most common indication for ibrutinib discontinuation. Sixteen percent of previously untreated patients experienced progression compared to 10% progressions/deaths at an 18-month follow-up in the front-line RESONATE-2 study.4 This difference may be related to the exclusion of patients with del17p from the RESONATE-2 study.4 Rates of discontinuation due to progression in the relapsed population in our cohort were more comparable with the 27% found in the 4-year follow-up of the RESONATE study.6 Outcomes of patients in our series who discontinued therapy due to toxicity were superior to those of patients who discontinued due to progressive disease.
We also considered limitations in the study design. Conducted by physicians and research coordinators from several institutions in a retrospective manner, data collection may have been affected by inconsistencies in chart interpretation, as well as clinical experience and practice style. There were a disproportionate number of relapsed/refractory patients compared to front-line patients.
It is vital to develop strategies to mitigate ibrutinib intolerance so that efficacy can be further maximized. Examples include the creation of guidelines for the evaluation and management of problematic side effects such as atrial fibrillation, rash, and arthralgias. An educational forum focused on oncologists, physician educators, and nurses should be implemented. In addition, the design of future clinical trials should allow for cessation of therapy in order to minimize ibrutinib exposure, particularly in the small subset of patients who achieve complete remission. This strategy has been successfully demonstrated in patients receiving venetoclax who were able to achieve minimal residual disease negativity.26 For example, the incorporation of BCL-2 inhibitors and/or anti-CD20 monoclonal antibody therapies in combination with ibrutinib may enable patients to experience minimal residual disease-negative responses that may translate into shorter durations of treatment.27,28
Supplementary Material
Acknowledgments
The authors thank Joseph and Cindy Riggs for their ongoing support of this work. They also thank the Center for CLL, University of Pensylvania.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/103/5/874
References
- 1.Byrd J, Furman R, Coutre S, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Byrd J, Brown J, O’Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Brien S, Jones J, Coutre S, et al. Ibrutinib for patients with relapsed or refractory chronic lymphocytic leukaemia with 17p deletion (RESONATE-17): a phase 2, openlabel, multicentre study. Lancet Oncol. 2016;17(10):1409–1418. [DOI] [PubMed] [Google Scholar]
- 4.Burger J, Tedeschi A, Barr P, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia N Engl J Med. 2015;373(25):2425–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chanan-Khan A, Cramer P, Demirkan F, et al. Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): a randomised, double-blind, phase 3 study. Lancet Oncol. 2016;17(2):200–211. [DOI] [PubMed] [Google Scholar]
- 6.Byrd J, Hillmen P, O’Brien S, et al. Long-term efficacy and safety with ibrutinib (ibr) in previously treated chronic lymphocytic leukemia (CLL): up to four years follow-up of the RESONATE study. J Clin Oncol. 2017;35(suppl; abstr 7510). [Google Scholar]
- 7.Maddocks K, Ruppert A, Lozanski G, et al. Etiology of ibrutinib therapy discontinuation and outcomes in patients with chronic lymphocytic leukemia. JAMA Oncol. 2015; 1(1):80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woyach J, Ruppert A, Guinn D, et al. BTKC481S-mediated resistance to ibrutinib in chronic lymphocytic leukemia. J Clin Oncol. 2017;35(13):1437–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Brien S, Furman R, Coutre S, et al. Five- year experience with single-agent ibrutinib in patients with previously untreated and relapsed/refractory chronic lymphocytic leukemia/small Lymphocytic Leukemia. Blood. 2016; 128:233. [Google Scholar]
- 10.Jain P, Thompson P, Keating M, et al. Long- term outcomes for patients with chronic lymphocytic leukemia who discontinue ibrutinib. Cancer. 2017;123(12): 2268–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mato A, Nabhan C, Barr P, et al. Outcomes of CLL patients treated with sequential kinase inhibitor therapy: a real world experience. Blood. 2016;128(18):2199–2205. [DOI] [PubMed] [Google Scholar]
- 12.Parikh S, Chaffee K, Call T, et al. Ibrutinib therapy for chronic lymphocytic leukemia (CLL): an analysis of a large cohort of patients treated in routine clinical practice. Blood. 2015;126(23):2935. [Google Scholar]
- 13.Sandoval-Sus J, Chavez J, Dalia S, et al. Outcomes of patients with relapsed/refractory chronic lymphocytic leukemia after ibrutinib discontinuation outside clinical trials: a single institution experience. Blood. 2015;126:2945. [Google Scholar]
- 14.Winqvist M, Asklid A, Andersson P, et al. Real-world results of ibrutinib in patients with relapsed or refractory chronic lymphocytic leukemia: data from 95 consecutive patients treated in a compassionate use program. Haematologica. 2016;101(12): 1573–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.UK CLL Forum. Ibrutinib for relapsed/refractory chronic lymphocytic leukemia: a UK and Ireland analysis of outcomes in 315 patients. Haematologica. 2016;101(12):1563–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mato A, Nabhan C, Kay N, et al. Real-world clinical experience in the Connect chronic lymphocytic leukaemia registry: a prospective cohort study of 1494 patients across 199 US centres. Br J Haematol. 2016;175(5):892–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bland J, Altman D. Survival probabilities (the Kaplan-Meier method). BMJ. 1998;317(7172):1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hallek M, Cheson B, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. 2008;111(12):5445–5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheson B, Byrd J, Rai K, et al. Novel targeted agents and the need to refine clinical end points in chronic lymphocytic leukemia. J Clin Oncol. 2012;30(23):2820–2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matthews D, Farewell V. 7 The log-rank or Mantel-Haenszel test for the comparison of survival curves in: Basel S, Karger A, editors. Using and Understanding Medical Statistics 2007:67–75. [Google Scholar]
- 21.Anderson P, Gill R. Cox’s regression model for counting processes: a large sample study. Ann Statist. 1982;10(4):1100–1120. [Google Scholar]
- 22.Thompson PA, O’Brien SM, Wierda WG, et al. Complex karyotype is a stronger predictor than del(17p) for an inferior outcome in relapsed or refractory chronic lymphocytic leukemia patients treated with ibrutinib- based regimens. Cancer. 2015;121(20): 3612–3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Brien SM, Byrd JC, Hillmen P, et al. Outcomes with ibrutinib by line of therapy in patients with CLL: analyses from phase III data. J Clin Oncol. 2016;34(15_suppl): 7520. [Google Scholar]
- 24.Coutré SE, Barrientos JC, Brown JR, et al. Management of adverse events associated with idelalisib treatment: expert panel opinion. Leuk Lymphoma. 2015;56(10): 2779–2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lampson B, Kasar S, Matos T, et al. Idelalisib given front-line for treatment of chronic lymphocytic leukemia causes frequent immune-mediated hepatotoxicity. Blood 2016;128(12):195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seymour JF, Ma S, Brander DM, et al. Venetoclax plus rituximab in relapsed or refractory chronic lymphocytic leukaemia: a phase 1b study. Lancet Oncol. 1017;18(2): 230–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharman J, Brander D, Mato A, et al. Ublituximab and ibrutinib for previously treated genetically high-risk chronic lymphocytic leukemia: results of the GENUINE phase 3 study. J Clin Oncol. 2017;35(Supplemental, ASCO abstract 7504). [Google Scholar]
- 28.Hillmen P, Rawstron A, Munir T, et al. The initial report of bloodwise tap clarity study combining ibrutinib and venetoclax in relapsed, refractory CLL shows acceptable safety and promising early indications EHA Oral Presentation June 22–25, Madrid Spain 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.