Abstract
In this study, relative germination percentage (RGP) and delayed mean germination time (DMGT) were measured in various rice accessions at the germination stage and carried out association analysis to identify candidate genes related to low temperature germination (LTG) using a natural population comprising 137 rice cultivars and inbred lines selected from the Korean rice core set. Genome-wide association study using ~ 1.44 million high-quality SNPs, which were identified by re-sequencing all rice collections, revealed 48 candidate genes on chromosome 10 and 55 candidate genes on chromosome 11 in the high peak SNP sites of associated loci for RGP and DMGT, respectively. By detecting highly associated variations located inside genic regions and performing functional annotation of the genes, we detected 23 candidate genes for RGP and 18 genes for DMGT for LTG. In addition, the haplotype and sequence analysis of the candidate gene (Os10g0371100) with RGP trait and the candidate gene (Os11t0104240-00) with DMGT revealed correlation between sequences of functional variations and phenotypes. Several novel LTG-related candidate genes previously were known for the function during rice germination and uncovered their substantial natural variations. These candidate genes represent valuable resources for molecular breeding and genetic improvement of cold tolerance during rice germination.
Electronic supplementary material
The online version of this article (10.1007/s13205-018-1252-9) contains supplementary material, which is available to authorized users.
Keywords: Oryza sativa L., Low temperature germination, Re-sequencing, GWAS, Haplotype
Introduction
Rice (Oryza sativa L.) is a monocotyledonous model plant that serves as a source of food for more than half of the world’s population. Rice is a major crop and staple food in Asia, where it is cultivated in temperate regions and at high altitudes in tropical regions. In rice-growing regions, the temperature of irrigation water during the seeding period is frequently below 15 °C, while the optimum temperature for rice during germination and early seedling growth ranges from 25 to 35 °C (Chen et al. 2006).
Seed germination is usually a very important stage in establishing stable seedling stands, and it determines the success of crop production (Almansouri et al. 2001). Direct-seeding cultivation has become increasingly important and popular in many rice-growing countries in Asia as an alternative to conventional transplanting due to its reduced labor requirements and lower production costs (Fujino 2004; Jiang et al. 2006). However, since water and soil temperatures at sowing time in Korea, Japan, northern China, and the tropical and subtropical regions at high altitude or high latitude locations are frequently below 15 °C, direct sowing often leads to poor establishment due to low germination rates at low temperatures (Ji et al. 2009). The effect of low temperatures on seed germination is extremely complex, involving various physical and biochemical cues. In general, low temperature stress during rice cultivation leads to reduced yields and an increase in weed competition (Bosetti et al. 2012). Similarly, low temperatures during germination and seedling growth can lead to a series of problems: less vigorous germination, increased seedling mortality, seedling stunting, leaf yellowing or wilting, and slow growth, which subsequently reduce yields (Fujino 2004; Andaya and Tai 2006; Lou et al. 2007). Cold temperatures during germination have become an increasingly important problem for rice cultivation. Therefore, improved cold tolerance at the germination and seedling stages has become an important agronomic character in rice breeding programs. Accordingly, vigorous germination under cold stress is an important trait in rice under direct seedling cultivation in low temperature areas, where rice is sown directly into fields under relatively low temperature conditions (Chen et al. 2006).
Many efforts have been made to improve seed germination and seedling vigor, including QTL (quantitative trait locus) analysis for low temperature germination (LTG) in rice, which has revealed many LTG-related QTLs in various populations (Sasaki et al. 1974; Miura et al. 2001; Cui et al. 2002; Fujino 2004; Zhang et al. 2004; Jiang et al. 2006, 2017; Ji et al. 2008; Wang et al. 2011; Li et al. 2013; Yang et al. 2013; Satoh et al. 2016). Miura et al. (2001) detected five LTG-related QTLs, and Zhang et al. (2004) identified 34 QTLs associated with seedling vigor traits using three temperature regimes (15, 20, and 25 °C). Jiang et al. (2006) identified 11 putative QTLs, and Ji et al. (2008) detected 11 QTLs as well. Iwata and Fujino (2010) identified two major QTLs on chromosomes 3 and 11, qLTG3-1 and qLTG11. Wang et al. (2011) identified two minor QTLs responsible for cold tolerance at germination, and Li et al. (2013) found three QTLs associated with LTG. Borjas et al. (2016) identified 49 QTLs distributed over ten chromosomes for 11 traits. Satoh et al. (2016) detected four QTLs responsible for LTG, and Jiang et al. (2017) detected six QTLs in the Nipponbare genome. Among these, only one QTL (qLTG3-1) has been cloned, and the genetic mechanism of the genes harboring these QTLs is still unknown (Fujino et al. 2008). Indeed, LTG is a complex quantitative trait that is affected by many genes and environmental conditions (Nguyen et al. 2012). Moreover, all of these LTG-related QTLs were identified using traditional markers, making it difficult to obtain complete, precise positional information about these LTG-related QTLs (Mao et al. 2015). Therefore, it is important to survey landraces and existing varieties of rice derived from different subspecies and diverse areas to identify additional LTG-related genes and introduce them into high-quality cultivars, and to develop new types of markers to facilitate fine mapping and cloning of these QTLs (Jiang et al. 2017).
Genome-wide association study (GWAS) is an efficient method for detecting valuable natural variations in trait-associated loci, which makes it possible to explore the correlations between genetic markers and phenotypic variation for fine-scale localization of QTLs (Huang et al. 2010). Instead of traditional simple sequence repeat markers, SNPs (single-nucleotide polymorphisms) have become more popular for use in GWAS due to the rapid development of next-generation sequencing and the identification of high-density SNPs by re-sequencing (Huang et al. 2012). Some studies have been successfully performed in rice to dissect complex trait variations through GWAS (Huang et al. 2012; Wang et al. 2016; Shakiba et al. 2017).
Here, we applied GWAS mapping using ~ 1.44 million high-quality SNPs covering all 12 rice chromosomes in a diverse rice collection to reveal natural variations and to uncover candidate genes that may contribute to LTG in rice during germination, with the aim of guiding the breeding of cold-tolerant rice varieties and facilitating direct-seeding cultivation.
Materials and methods
Plant materials
137 accessions (19 Tropical japonica, 62 Temperate japonica, 43 Indica, 8 Aus, 3 Aromatic, 2 Admixture; Supplementary Table S1) of the Korean rice core set (KRICE_CORE) selected from 25,604 rice accessions in the Genebank Information Center of the Rural Development Administration (RDA-Genebank, Republic of Korea) were re-sequenced in the current study (Kim et al. 2007, 2016; Zhao et al. 2010). A field experiment was conducted during the rice-growing season at an experimental farm. Young leaves from a single plant per sample were collected and immediately frozen at − 80 °C prior to genomic DNA extraction using a DNeasy Plant Mini Kit (Qiagen). After quality checking, the DNA was sent for whole genome re-sequencing.
Whole genome re-sequencing and variation detection
The genomes of all 137 accessions were sequenced on the Illumina HiSeq 2500 Sequencing Systems Platform (Illumina Inc. USA), with an average coverage of approximately 7.8×. Raw reads were processed to obtain an average quality score (QS) per read ≤ 20 by trimming the 3′-ends of reads using SICKLE (https://github.com/najoshi/sickle). High-quality reads were aligned against the rice reference genome (IRGSP 1.0) (Kawahara et al. 2013) for genotype calling using the Burrows–Wheeler Aligner (BWA) (version 0.7.5a). To generate the genotype dataset for GWAS, only SNPs without missing values and a minor allele frequency (MAF) > 0.05 and containing genotype calls for all 137 accessions were used. Finally, ~ 1.44 million high-quality SNPs were obtained and subjected to further GWAS (Kim et al. 2016).
Phenotypic evaluation of LTG
To evaluate LTG, seeds from the 137 rice accessions were germinated under two conditions: 13 °C for 15 days (treatment) and 28 °C for 7 days (control) (Cruz and Milach 2004; Hyun et al. 2017). Prior to planting, all seeds were rinsed with water, sterilized in 1% sodium hypochlorite solution for 10 min, and rinsed three times with deionized distilled water. The seeds (100 seeds per accessions, with three replications) were placed on filter paper in a 9-cm Petri dish (SPL Life Sciences, 10090), followed by the addition of 20 ml distilled water. The Petri dishes were placed in a random order in an incubator, with three replicates per accession under control and treatment conditions. Seed germination was measured daily, and the filter paper was replaced as needed. Plumule emergence (when the plumule reached ~ 1 mm) was used as an index of germination. Based on these experiments, several germination stage-related phenotypes (listed below) were calculated and subjected to GWAS. The mean value of three biological replicates was calculated and used in subsequent analysis. The standards for evaluation of germination characteristics were calculated using the following formulas:
where n is the number of seeds germinated, and N is the total number of seeds placed (Mousavizadeh et al. 2013).
where n is the number of seeds germinated on D day, and D is the number of days counted (Ellis and Roberts 1981).
The standards for evaluation of LTG were calculated using the following formulas:
where a comparison was made between the average germination percentage at 13 °C (treatment) and the average germination percentage at 28 °C (control).
where the difference between mean germination time at 13 °C (treatment) and mean germination time at 28 °C (control) was calculated.
Data analysis was performed using SAS software (version 9.4; SAS Institute, Inc., Cary, NC, USA). Analysis of variance (ANOVA) was performed using the general linear model (GLM). Pearson’s correlation analysis and Duncan’s new multiple-range test (DRMT) were used to determine significant differences.
Principal component analysis and GWAS
Principal component analysis (PCA) of the genotypes based on ~ 1.44 million SNPs and LTG phenotypes was conducted using GAPIT and Trait Analysis by Association, Evolution and Linkage (TASSEL) 5.0 (Bradbury et al. 2007). The optimized number of principal components (PCs) in genotypic and phenotypic PCA was also calculated using GAPIT and TASSEL 5 (Bradbury et al. 2007).
GWAS was performed using the GAPIT package (Genome Association and Prediction Integrated Tool) (Lipka et al. 2012). The compressed mixed linear model (CMLM), which uses a kinship (K) matrix as the variance–covariance matrix between the individuals, was selected, complemented with population structure (PCA). Only SNPs with adjusted P values < 0.05 were considered significant. Gene loci containing the SNPs in highly associated peaks were considered to be candidate genes related to LTG.
Linkage disequilibrium (LD) block and haplotype analysis
LD analysis was performed using TASSEL 5 based on the high-quality (no missing data or heterozygotes and minor allele frequency > 0.05) variations in a 400 kb range determined using the most closely associated SNPs. An LD block was recognized when the top 95% confidence bounds of the D′ value exceeded 0.98 and the lower bounds exceeded 0.70 (Gabriel et al. 2002). Loci with significant variations harbored by LD blocks were then defined as the candidate genes.
Haplotyping of the identified candidate genes was performed with an approximately 7.3 × depth of genome coverage. Nucleotide variations in the entire region of each target gene were captured based on the rice reference genome (IRGSP 1.0).
Results
Phenotypic evaluation of LTG
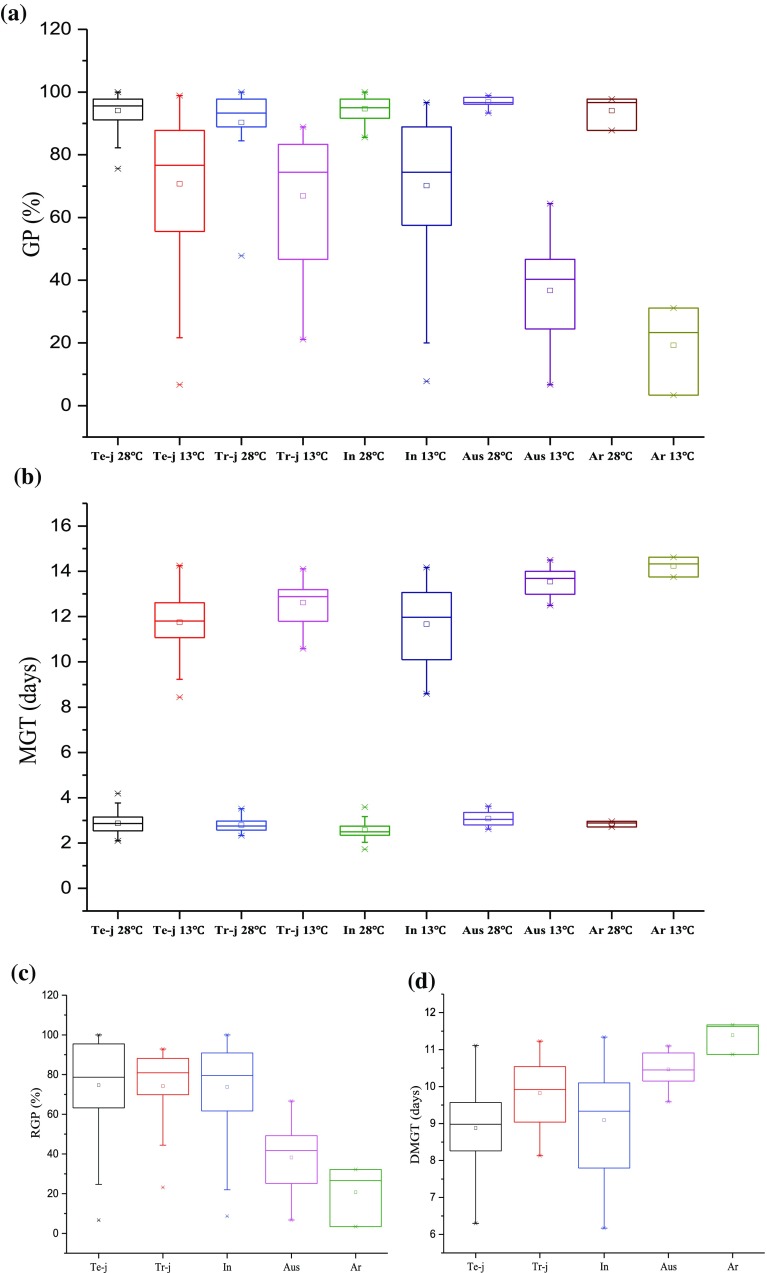
We investigated the germination characteristics of 137 rice accessions in seeds incubated at 13 °C for 15 days (treatment) and 28 °C for 7 days (control). All investigated characters showed marked differences under cold vs. optimal temperature conditions (Fig. 1). The treatment groups (13 °C) exhibited lower values for GP and higher values for MGT than the control groups (28 °C), suggesting that different rice accessions were influenced differently by cold stress (Fig. 1a, b). The results indicated that low temperature is a major environment for rice germination. We evaluated LTG based on the following germination characteristics: RGP and DMGT. The tropical japonica subspecies and the temperate japonica subspecies had slightly higher cold tolerance than the indica accessions and markedly higher than the Aus and aromatic accessions, but there was variability within subspecies (Fig. 1c); this result is consistent with previous reports (Cruz and Milach 2004). The DMGT for tropical japonica subspecies was similar to the indica. However, the indica lines had shorter DMGT than the tropical japonica subspecies (Fig. 1d). These findings reveal phenotypic differences between control and cold stress conditions, suggesting that rice growth at the seed germination stage is markedly inhibited by cold stress. This inhibition may suppress seed germination, especially in some direct sowing areas, and may markedly reduce plant density and yields. Therefore, developing rice varieties with cold-tolerant seeds would prevent cold-mediated plant and yield losses at the early stage of growth.
Fig. 1.
Determination of germination characteristics in different groups under 28 and 13 °C. a Germination percentage (GP); b mean germination time (MGT); c relative germination percentage (RGP); d delayed mean germination time (DMGT), Te-j: Temperate japonica; Tr-j: Tropical japonica; In: Indica; Ar: Aromatic
The correlation analysis was conducted among the tested phenotypes under control and cold conditions (Table 1). The phenotypic correlations among all traits were significant (P < 0.01), except for RGP, DMGT, MGT-28 °C and MGT-13 °C in the control trait GP-28 °C, which were not significantly correlated. MGT-28 °C and MGT-13 °C were positive correlated with DMGT and negatively correlated with RGP, while there were significant positive correlations between MGT-28 °C and MGT-13 °C, suggesting that the phenotypes evaluated in this study could be used for GWAS separately or in combination. The phenotypic correlations among all traits were highly significant (P < 0.001) under cold conditions, suggesting that the same genes or factors control these LTG-related traits (Hu et al. 2016).
Table 1.
Pearson correlation coefficients among traits under control (28 °C) and cold stress (13 °C) conditions
| Trait | GP28 | GP13 | MGT28 | MGT13 | RGP | DMGT |
|---|---|---|---|---|---|---|
| GP28 | 1 | |||||
| GP13 | 0.254** | 1 | ||||
| MGT28 | ns | − 0.510*** | 1 | |||
| MGT13 | ns | − 0.586*** | 0.536*** | 1 | ||
| RGP | ns | 0.980*** | − 0.524*** | − 0.613*** | 1 | |
| DMGT | ns | − 0.493*** | 0.266** | 0.956*** | − 0.519*** | 1 |
GP germination percentage, MGT mean germination time, RGP relative germination percentage, DMGT delayed mean germination time
**, ***ns: significant at the 0.01, 0.001 probability level and not significant, respectively
Genetic structures of various rice accessions
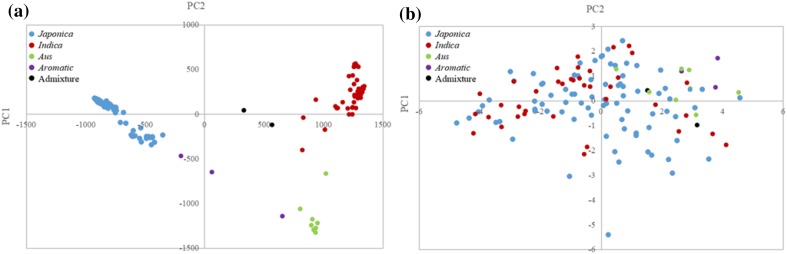
We performed PCA using the 1.44 million high-quality SNPs to mine the population structures in the 137 rice accessions. Two components were suggested by examining the scree plot obtained through GAPIT (Supplementary Fig. S1a). Clear subpopulation structures were observed based on the first two PCs (PC1 and PC2), which generated two subpopulation groups, namely, indica and japonica; Aus and admixture are located in the indica group, and the aromatic accessions are located between the two groups (Fig. 2a).
Fig. 2.
Principal component analysis (PCA) using genotype and phenotype. a For genotype of 137 accessions, they were divided into japonica and indica based on PC1 and PC2 along with Aus, aromatic and admixture groups; b for phenotype of 133 accessions (excluding 4 missing data), no clear grouping was observed
For PCA based on phenotype, we examined correlations in LTG between subspecies using two major phenotypes that drove the differences among accessions. We used TASSEL to perform PCA of RGP and DMGT in the rice collection. Most of the phenotypic variation (> 80%) in the accessions was explained by the first two PCs (Supplementary Fig. S1b). Thus, we generated a PCA plot using the first two PCs (PC1 and PC2). However, unlike the results of genotypic PCA, phenotypic PCA showed no clear grouping of accessions (Fig. 2b), indicating that cold tolerance levels in rice at the germination stage are not strongly correlated with the indica or japonica subgroups.
GWAS and candidate gene identification
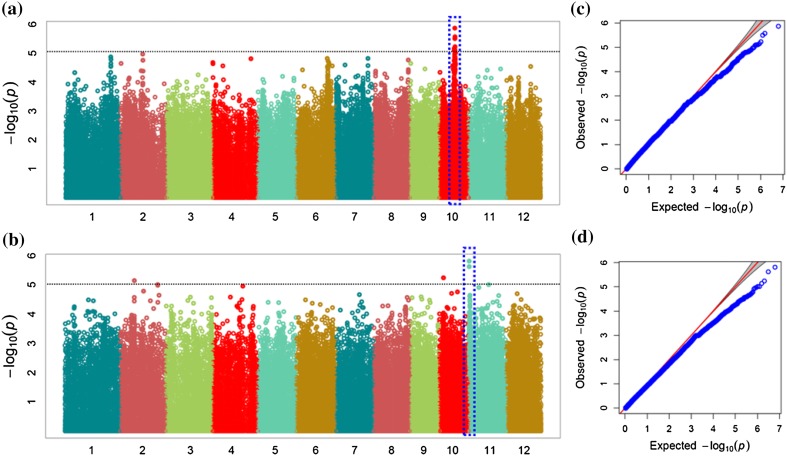
We identified more than ~ 1.4 million SNPs across the 137 accessions, which were used to generate the genotypic dataset for GWAS, with the CMLM controlling population structure (Lipka et al. 2012). We also examined the associations held by the peaks with − log10 (P) value > 5 and adjusted P value (false discovery rate, FDR) < 0.05, given that the cutoff of the − log10 (P) value was 5 when the FDR ≤ 0.05. The associated loci for RGP and DMGT detected in this GWAS were mainly interspersed on the chromosome 10 and 11, respectively (Fig. 3). In total, seven and two SNPs were significant for RGP and DMGT traits on chromosome 10 and 11, respectively. For RGP, we detected one GWAS peak containing seven SNPs (chr10_12401691, chr10_12401749, chr10_12493993, chr10_12493994, chr10_12400200, chr10_12489259, and chr10_11890928) on chromosome 10 (Table 2), while for DMGT, we identified one GWAS peak containing two SNPs (chr11_141191 and chr11_140283) on chromosome 11 (Table 3).
Fig. 3.
Genome-wide association study analysis of low-temperature germinability in 137 rice accessions. a Manhattan plots of SNPs association for relative germination percentage (RGP) using the CMLM. b Manhattan plots of SNPs association for delayed mean germination time (DMGT) under cold stress condition using CMLM. The x-axis represents the relative density of SNPs physically mapped on 12 chromosomes. The y-axis denotes the − log10 (P) value for significant association of SNPs with LTG trait. The SNPs revealing significant association with LTG trait at cut-off − log10 (P) value > 5 are demarcated with dotted lines. c, d Quantile–quantile plots of expected and observed − log10 (P) values at a FDR cut-off ≤ 0.05. The gray areas are the 95% confidence intervals under the null hypothesis of no association between the SNP and the trait. The red line is the expected line under the null distribution. The blue points are the observed distribution
Table 2.
Genome-wide significant associations for RGP under low temperature using CMLM
| SNPa | Chromosome | Positionb | P value | − Log10(P) | MAF | R 2 |
|---|---|---|---|---|---|---|
| chr10_12401691 | 10 | 12,401,691 | 1.35E−06 | 5.87 | 0.2197 | 0.1499 |
| chr10_12401749 | 10 | 12,401,749 | 2.69E−06 | 5.57 | 0.2159 | 0.1407 |
| chr10_12493993 | 10 | 12,493,993 | 2.69E−06 | 5.57 | 0.2159 | 0.1407 |
| chr10_12493994 | 10 | 12,493,994 | 2.69E−06 | 5.57 | 0.2159 | 0.1407 |
| chr10_12400200 | 10 | 12,400,200 | 3.14E−06 | 5.50 | 0.2197 | 0.1386 |
| chr10_12489259 | 10 | 12,489,259 | 5.92E−06 | 5.23 | 0.1780 | 0.1302 |
| chr10_11890928 | 10 | 11,890,928 | 6.89E−06 | 5.16 | 0.1288 | 0.1282 |
MAF minor allele frequency
aThe SNP positions were based on the annotation data on Os-Nipponbare-Reference-IRGSP-1.0 (RAP-DB, http://rapdb.dna.affrc.go.jp/)
bPosition of the SNP showing the most significant association for RGP
Table 3.
Genome-wide significant associations for DMGT under low temperature using CMLM
| SNPa | Chromosome | Positionb | P value | − Log10(P) | MAFc | R 2 |
|---|---|---|---|---|---|---|
| chr11_141191 | 11 | 141,191 | 1.53E−06 | 5.81 | 0.0871 | 0.1492 |
| chr11_140283 | 11 | 140,283 | 2.37E−06 | 5.62 | 0.0909 | 0.1433 |
MAF minor allele frequency
aThe SNP positions were based on the annotation data on Os-Nipponbare-Reference-IRGSP-1.0 (RAP-DB, http://rapdb.dna.affrc.go.jp/)
bPosition of the SNP showing the most significant association for DMGT
We used LD (linkage disequilibrium) scores to analyze the candidate peak regions and to identify LD blocks harboring significant SNPs (highest − log10 (P) > 5 with P < 0.05) the last step in the characterization of candidate regions. A LD block was detected in a 400 kb range centered on the highest − log10 (P) value. Using the CMLM approach, we successfully identified associations as well as new candidate loci in the rice genome. We identified 48 candidate genes on chromosome 10 and 55 candidate genes on chromosome 11 in the peak SNP sites (or adjacent to those sites) of associated loci for RGP and DMGT, respectively, which we annotated using RAP-DB (IRGSP 1.0). Among the 48 candidate genes associated with RGP, 24 were annotated as having specific functions. Other remaining genes are non-protein-coding transcripts or hypothetical (genes) or predicted proteins that are currently predicted as nonfunctional gene. Among the genes associated with DMGT, 33 were annotated as having specific functions, the other are hypothetical (genes) or non-protein-coding transcripts.
Haplotype and sequence analysis of the natural variations in candidate genes
Based on these results, we identified many candidate genes that might be associated with LTG in rice during the germination stage. To mine the functional and novel candidate genes by detecting the highly associated variations located inside genic regions, we investigated the 23 final candidate genes for RGP (Table 4) and the 18 final candidate genes for DMGT (Table 5), which contained highly associated SNPs within their coding region.
Table 4.
Candidate genes with highly associated with RGP in the coding region
| Chr_Postion | Trait | P value | Gene ID | Descriptions |
|---|---|---|---|---|
| Os10g0380100 | Transferase family protein. THT2; tryptamine hydroxycinnamoyl transferase 2 | |||
| Os10g0380800 | Tryptophan decarboxylase. Variants = Os10t0380800-00 | |||
| Os10g0381700 | OsFbox548%2C,F-boxprotein548 | |||
| Os10g0382100 | Ribosome-inactivating protein domain-containing protein. Variants = Os10t0382100-01 | |||
| Os10g0382300 | Signal peptide-containing large protein with proline stretches | |||
| Os10g0384600 | Cyclin-like F-box domain-containing protein | |||
| Os10g0370700 | Nitrate transporter (fragment) | |||
| Os10g0370800 | Exo-1%2C3-beta-glucanase precursor (EC 3.2.1.58) (fragment) | |||
| Os10g0371000 | Pollen proteins Ole e I family protein%2C expressed | |||
| Os10g0371100 | AP2 domain-containing protein%2C expressed. ERF51 | |||
| Os10g0374200 | Paired amphipathic helix domain-containing protein | |||
| Chr 10_11890928 | RGP | 6.89E-06 | Os10g0374450 | Barren inflorescence2-like serine/threonine protein kinase (fragment) |
| Os10g0375000 | YK19 | |||
| Os10g0375400 | Leucine-rich repeat domain-containing protein | |||
| Os10g0375600 | ER66 protein (fragment) | |||
| Os10g0375700 | Alpha/beta hydrolase fold-1 domain-containing protein | |||
| Os10g0376400 | Phosphate-induced protein 1 conserved region-containing protein. Os10t0376400-00 | |||
| Os10g0376900 | Helix-loop-helix DNA-binding domain-containing protein | |||
| Os10g0377150 | ARABINOGALACTAN PROTEIN 9; OsAGP9 | |||
| Os10g0377300 | Homeobox-leucine zipper protein HOX8 | |||
| Os10g0377400 | GTP-binding protein OsRab 11E1 | |||
| Os10g0377500 | Nucleic acid-binding%2C OB-fold domain-containing protein | |||
| Os10g0377800 | Pyridoxamine 5-phosphate oxidase |
Table 5.
Candidate genes with highly associated with DMGT in the coding region
| Chr_Postion | Trait | P value | Gene ID | Descriptions |
|---|---|---|---|---|
| Os11g0l00l00 | Autophagy-related protein 8D | |||
| Os11g0102200 | NPH1-1 | |||
| Os11g0102700 | Peptidase C48%2C SUMO/sentrin/Ubll family protein | |||
| Os11g0103000 | Pentatricopeptide repeat domain-containing protein | |||
| Os11g0103400 | IBR domain-containing protein | |||
| Os11g0103700 | EF-Hand type domain-containing protein | |||
| Os11g0104240 | CsAtPR5. (Os11t0104240-00) | |||
| Os11g0104300 | Substrate of the SCFD3 ubiquitination complex, repressor of strigolactone (SL) signaling (Os11t0104300-01) | |||
| Os11g0104400 | W-3 fatty acid desaturase | |||
| Chrl l_141191 | DMGT | 1.53E−06 | Os11g0104800 | OsTBL10%2C TBL10;trichome birefringence-like 10 |
| Os11g0104866 | Clathrin heavy chain. Clathrin heavy chain binding | |||
| Os11g0105750 | Quinoprotein alcohol dehydrogenase-like domain containing-protein. 1t0105750-01); transcript variants; Os11t0105750-01 | |||
| Os11g0105800 | Phosphatidate cytidylyltransferase family protein | |||
| Os11g0105901 | EF-hand-like domain-containing protein | |||
| Os11g0106200 | Outer membrane protein%2C OMP85 family protein | |||
| Os11g0106400 | Ubiquitin-activating enzyme El 2 | |||
| Os11g0107000 | Xylan acetyltransferase, resistance to bacterial leaf blight disease (Os11t0107000-01) | |||
| Os11g0107266 | Sodium/calcium exchanger protein. Ca(2+)/H(+) EXCHANGER 7 |
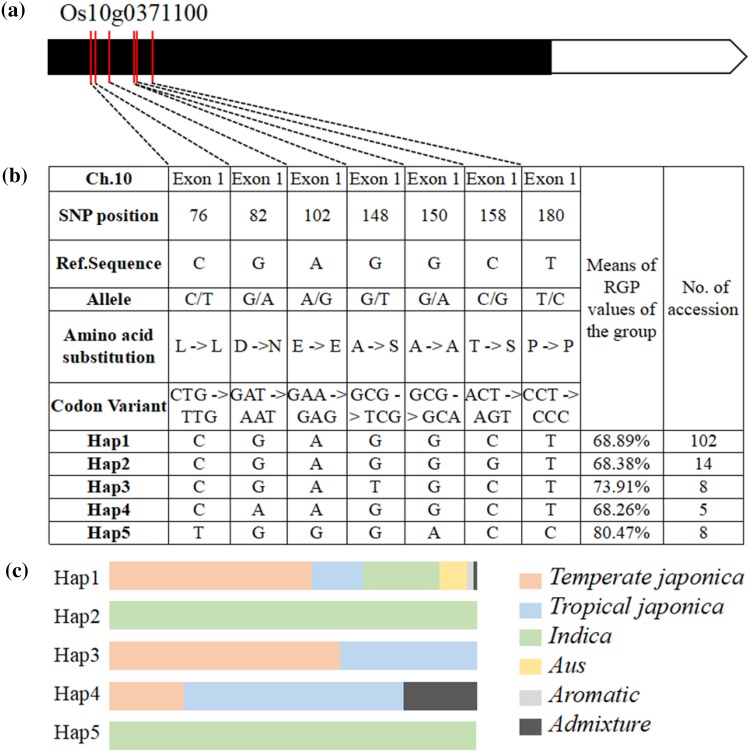
Among these, we identified a transcription factor gene (Os10g0371100) associated with RGP encoding an AP2 domain-containing protein that plays a crucial role in the transcriptional regulation of diverse biological processes. These processes are related to plant growth, development, and responses to environmental stimuli. This gene is rapidly upregulated in response to low temperatures, but not in response to ABA, NaCl, or dehydration treatment (Chen et al. 2003); the haplotype of Os10g0371100 was investigated to detect five haplotypes where five different types of variation were in its exon region (Fig. 4). Most of accessions were belonged to type 1 sequence (reference sequence), followed by type 2, type 3, type 5 and type 4 (Fig. 4b). Phenotypic differences among accessions with the five different haplotypes of sequences were compared. The mean RGP values of the groups were 68.89, 68.38, 73.91, 68.26, and 80.47%, respectively (Fig. 4b), and type 3 and type 5 showed significantly different RGP values compared with reference allele.
Fig. 4.
Haplotyping and sequence analysis of Os10g0371100, which was correlated with a phenotypic difference. a Gene structure of Os10g0371100. White boxes represent untranslated region, black boxes represent coding region, and red lines represent the location of amino acids. b Average RGP by each group and results of haplotype analysis. c The distribution of 137 accessions in these five haplotypes
Os11t0104240-00 detected as to be associated with DMGT encodes a member of a group of defense-related proteins related to many stresses, such as P stress, Cd stress, and others (Singh and Jwa 2013). The haplotype analysis (Fig. 5) revealed six different haplotypes. The most of accessions were belonged to type 1 (reference sequence), followed by type 5 and type 2, respectively. For type 3, type, 4, type, only 3 accessions were belonged to each type. The phenotypic differences of each haplotypes were investigated to reveal that the average DMGT values of the groups were 9.19, 9.01, 11.25, 9.80, 9.07 and 10.57 days, respectively (Fig. 5b). Sequence analysis of the natural variations in the candidate gene revealed the following: type 1 (reference sequence) and type 2 (variation) sequences contain one natural SNP substitution (G/A) at − 956 bp, which causes a C/Y amino acid change; type 3 contains one natural SNP substitution (G/A) at − 157 bp, which causes an A/T amino acid change; type 4 contains one natural SNP substitution (T/C) at − 136 bp, which causes a synonymous amino acid substitution; type 5 contains two natural SNP substitutions, including (A/G) at − 1093 bp, which causes a K/E amino acid change, and (T/C) at − 136 bp, which causes a synonymous amino acid substitution; type 6 contains four natural SNP substitutions, including (T/C) at − 136 bp and (G/A) at − 165 bp, which cause synonymous amino acid substitutions, and (C/T) at − 172 bp and (G/T) at − 180 bp, which cause L/F and D/Y amino acid changes, respectively.
Fig. 5.
Haplotyping and sequence analysis of Os11t0104240-00, which was correlated with a phenotypic difference. a Gene structure of Os11t0104240-00. Black boxes represent coding regions, solid lines represent introns and red lines represent the location of amino acids. b Average DMGT by each group and results of haplotype analysis. c The distribution of 137 accessions in these six haplotypes
We identified other functional SNPs in the 41 candidate genes, which were mined and screened for functional variations after checking the positions of the variations in the genes and the corresponding amino acid changes (data not shown). These candidate genes might be related to the seed germination rate in rice under low temperature conditions based on previous reports and the natural variation detected in the current study.
Discussion
SNPs associated with LTG in rice
LTG is an important character for breeding rice varieties that could be widely used for direct-seeding cultivation. Low temperature leads to a delay in seed germination (during which the coleoptile emerges from the embryo) or an increase in the time needed for this process to occur. Temperate japonica rice varieties are usually grown in East Asian countries such as Korea, where the water temperature in the field during the sowing season is often below 15 °C. Therefore, tolerance to low temperatures during germination is important for rapid, uniform crop establishment (Hyun et al. 2017). Previous studies of low temperature germinability have revealed the complex inheritance of the trait (Miura et al. 2001). To improve rice productivity in such areas, novel genes and alleles associated with complex quantitative cold tolerance traits must be identified in diverse rice accessions, and low temperature-tolerant varieties should be bred. An alternate, complementary approach is GWAS, which takes advantage of historical recombination events that have accumulated over thousands of generations in historical populations, thus enabling high-resolution mapping to identify target genomic regions for complex quantitative traits in rice (Huang et al. 2012).
In the current study, we identified nine SNPs that are significant for RGP and DMGT traits on chromosome 10 and 11. To date, many QTLs for rice LTG traits have been reported, but it is difficult to directly compare the chromosomal locations of the marker–trait associations detected in the current study with previously reported QTLs due to the use of different genetic materials, descriptive traits, marker types, and molecular mapping techniques (Borjas et al. 2016). Many QTLs have been reported on chromosome 10 and 11 that control LTG, cold tolerance at the seedling stage, and seedling vigor (Miura et al. 2001; Wang et al. 2016; Borjas et al. 2016; Shakiba et al. 2017; Jiang et al. 2017). These QTLs are all located on small genomic regions, but we found some SNPs that were located in similar or proximal regions, such as the significant SNPs (chr10_12401691, chr10_12401749, chr10_12493993, chr10_12493994, chr10_12400200, chr10_12489259) near qRtL-GH-10 identified by Borjas et al. (2016) on chromosome 10, and SNPs (chr11_141191 and chr11_140283) near qCTGERM11 identified by Borjas et al. (2016) on chromosome 11 (Shakiba et al. 2017). Wang et al. (2016) and Borjas et al. (2016) found additional QTLs on chromosome 11, suggesting that this location on chromosome 11 might be a hot spot for genes responsible for cold tolerance at germination and the seedling stage; in the current study, we found two SNPs in this region. Our study provides new insights into the genetic structure of LTG in rice. In addition, we identified SNP markers highly associated with cold tolerance that could be used for rice improvement.
Candidate genes linked to marker loci for LTG
Plants have developed several strategies at the molecular and cellular levels to survive in cool and cold temperatures, such as undergoing biochemical and physiological changes. Exposure to low temperatures causes variations in the expression of genes and alters the functions of their products to enhance tolerance to cold stress (Zhang et al. 2014). Apart from the SNPs associated with previously identified QTLs for cold tolerance, a few SNPs that affect specific genes with known roles in the cold stress response are related to changes in metabolism. For example, the candidate gene, Os11g0104400, encodes a protein belonging to the w-3 fatty acid desaturase (FAD) family. Rice mutants deficient in FAD8 fail to acclimate to cold temperatures (Tovuu et al. 2016). The rice WUSCHEL-related homeobox gene Os11g0102100 is involved in reproductive organ development, hormone signaling, and abiotic stress responses (Cheng et al. 2014). Os11g0106100 belongs to the MYB transcription factor family. Several MYB transcription factors increase cold stress tolerance in rice (Su et al. 2010). The homeodomain leucine zipper (HD-Zip) genes (such as Os10g0377300) encode transcription factors that play diverse roles in plant development and have often been implicated in stress adaptation (Agalou et al. 2008). The candidate genes Os11g0103700, Os11g0105000, and Os11g0105901 belong to the EF-hand-containing protein family, which is involved in Ca2+ signaling to regulate many cellular and developmental processes (Boonburapong and Buaboocha 2007) and responses to early chilling stress (Lindlöf et al. 2015). The candidate gene Os10g0371100, encoding an AP2/EREBP-type transcription factor, is specifically induced by cold stress (Chen et al. 2003). We also identified a candidate gene (Os11t0104240-00) encoding a defense-related protein (Pathogenesis-related protein, PR5) (Singh and Jwa 2013), as well as candidate genes related to photosynthesis (Os11g0102200: NPH1-1), vitamin B biosynthesis (Os10g0377800: pyridoxamine 5-phosphate oxidase), transport (Os11g0107266: sodium/calcium exchanger protein), hormone responses (Os10g0374450: serine/threonine protein kinase), and energy (Os10g0377400: GTP-binding protein and Os10g0377150: OsAGP9) (Singh and Jwa 2013). These results suggest that the candidate genes play essential roles in various cold tolerance mechanisms.
Novel natural variations in candidate genes
Natural variations in some key genes in rice can result in breakthroughs in genetic, functional, or domestication processes. For example, a G/T substitution in sh4 leads to reduced grain shattering in cultivated rice (Li et al. 2006). Using the results of GWAS and LD analysis, the haplotypes of candidate genes can be targeted and the functional alleles involved in various responses can be identified. Other new alleles in rice have been reported (Konishi et al. 2006; Huang et al. 2009), providing insight for researchers and breeders about the underlying mechanisms and facilitating the breeding of improved varieties. Os10g0371100 is a cold-inducible and a AP2/EREBP transcriptional factor gene OsDREBL and is specifically regulated by low temperature (Chen et al. 2003). According to the previously reports for the expression patterns of Os10g0371100, the transcripts of Os10g0371100 in shoots and roots in seedling stage accumulated rapidly within 30 min under low temperature (4 °C) treatment and reached a maximum at 5 h, then the gene transcript levels remained at a very low level within 32 h after initiation of the cold treatments however, the transcript level of Os10g0371100 was reported to be increased slightly in 30 day old plant after 10 h and remained at this low level within 32 h after initiation of the cold treatments (Chen et al. 2003). It is not clear that there are correlation among the transcription level, cold treatment, and the developmental stage of rice. However, the reports of the increase of transcript of Os10g0371100 and the results of association between the haplotypes and cold resistant detected in this study suggests that Os10g0371100 was associated with low temperature tolerance in rice at the seed germination stage and Os10g0371100 might encode a transcription factor that functions in the cold stress response during rice germination stage. In addition, the type 5 of haplotype of Os10g0371100 consisted only indica accessions, showing the higher level of RGP compared with that of japonica accessions (Fig. 4). According to the reports by Chen et al. (2003), the transcription level under low temperature (4 °C) treatment was higher in indica than japonica subspecies in both shoots and roots. This concord report suggests that the differential expression of this gene in these two subspecies may reflect their different levels of cold tolerance because of these natural variations occurred in these haplotypes. Therefore, the mechanism of OsDREBL gene and other candidate genes identified here should be investigated further. These natural variations that occurred during rice domestication can be utilized to help breeders select rice varieties with the best traits for food production. Investigating new natural variations in important traits can lead to the identification of functional alleles in a tolerant variety that can be transferred to other, less-tolerant varieties. Breeding methods can then be used to transfer these attributes to elite lines to produce tolerant varieties.
Conclusions
We used a heuristic core set of rice accessions (137 accessions) to perform GWAS of LTG-related phenotypes in rice during the germination stage. We identified 23 and 18 final candidate genes for low temperature tolerance at the germination stage with high association peaks for RGP and DMGT phenotypes, respectively. In addition, we performed haplotype and sequence analysis of Os10g0371100 and Os11t0104240-00 to explore their possible roles in germination at low temperatures. The results of this study provide an important resource for molecular breeding and functional analysis of LTG tolerance in rice.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by National Research Foundation of Korea (NRF) grant funded by the Korean government (NRF-2015R1C1A1A01054699).
Compliance with ethical standards
Conflict of interest
All the authors declare that they have on conflict of interest in the publication.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s13205-018-1252-9) contains supplementary material, which is available to authorized users.
References
- Agalou A, Purwantomo S, Övernäs E, Johannesson H, Zhu X, Estiati A, et al. A genome-wide survey of HD-Zip genes in rice and analysis of drought-responsive family members. Plant Mol Biol. 2008;66(1–2):87–103. doi: 10.1007/s11103-007-9255-7. [DOI] [PubMed] [Google Scholar]
- Almansouri M, Kinet JM, Lutts S. Effect of salt and osmotic stresses on germination in durum wheat (Triticum durum Desf.) Plant soil. 2001;231(2):243–254. doi: 10.1023/A:1010378409663. [DOI] [Google Scholar]
- Andaya VC, Tai TH. Fine mapping of the qCTS12 locus, a major QTL for seedling cold tolerance in rice. Theor Appl Genet. 2006;113(3):467–475. doi: 10.1007/s00122-006-0311-5. [DOI] [PubMed] [Google Scholar]
- Boonburapong B, Buaboocha T. Genome-wide identification and analyses of the rice calmodulin and related potential calcium sensor proteins. BMC Plant Biol. 2007;7(1):4. doi: 10.1186/1471-2229-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borjas AH, De Leon TB, Subudhi PK. Genetic analysis of germinating ability and seedling vigor under cold stress in US weedy rice. Euphytica. 2016;208(2):251–264. doi: 10.1007/s10681-015-1584-z. [DOI] [Google Scholar]
- Bosetti F, Montebelli C, Novembre ADL, Chamma HP, Pinheiro JB. Genetic variation of germination cold tolerance in Japanese rice germplasm. Breed Sci. 2012;62(3):209–215. doi: 10.1270/jsbbs.62.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury PJ, Zhang Z, Kroon DE, Casstevens TM, Ramdoss Y, Buckler ES. TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics. 2007;23(19):2633–2635. doi: 10.1093/bioinformatics/btm308. [DOI] [PubMed] [Google Scholar]
- Chen JQ, Dong Y, Wang YJ, Liu Q, Zhang JS, Chen SY. An AP2/EREBP-type transcription-factor gene from rice is cold-inducible and encodes a nuclear-localized protein. Theor Appl Genet. 2003;107(6):972–979. doi: 10.1007/s00122-003-1346-5. [DOI] [PubMed] [Google Scholar]
- Chen L, Lou QJ, Sun ZX, Xing YZ, Yu XQ, Luo LJ. QTL mapping of low temperature on germination rate of rice. Rice Sci. 2006;13(2):93–98. [Google Scholar]
- Cheng S, Huang Y, Zhu N, Zhao Y. The rice WUSCHEL-related homeobox genes are involved in reproductive organ development, hormone signaling and abiotic stress response. Gene. 2014;549(2):266–274. doi: 10.1016/j.gene.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Cruz RPD, Milach SCK. Cold tolerance at the germination stage of rice: methods of evaluation and characterization of genotypes. Sci Agricola. 2004;61(1):1–8. doi: 10.1590/S0103-90162004000100001. [DOI] [Google Scholar]
- Cui K, Peng S, Xing Y, Xu C, Yu S, Zhang Q. Molecular dissection of seedling-vigor and associated physiological traits in rice. TAG Theor Appl Genet. 2002;105(5):745–753. doi: 10.1007/s00122-002-0908-2. [DOI] [PubMed] [Google Scholar]
- Ellis RH, Roberts EH (1981) The quantification of ageing and survival in orthodox seeds. Seed Sci Technol (Neth)
- Fujino K. A major gene for low temperature germinability in rice (Oryza sativa L.) Euphytica. 2004;136(1):63–68. doi: 10.1023/B:EUPH.0000019519.43951.67. [DOI] [Google Scholar]
- Fujino K, Sekiguchi H, Matsuda Y, Sugimoto K, Ono K, Yano M. Molecular identification of a major quantitative trait locus, qLTG3–1, controlling low-temperature germinability in rice. Proc Natl Acad Sci. 2008;105(34):12623–12628. doi: 10.1073/pnas.0805303105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, et al. The structure of haplotype blocks in the human genome. Science. 2002;296(5576):2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- Hu S, Lübberstedt T, Zhao G, Lee M. QTL mapping of low-temperature germination ability in the maize IBM Syn4 RIL population. PloS One. 2016;11(3):e0152795. doi: 10.1371/journal.pone.0152795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Qian Q, Liu Z, Sun H, He S, Luo D, Fu X. Natural variation at the DEP1 locus enhances grain yield in rice. Nat Genet. 2009;41(4):494. doi: 10.1038/ng.352. [DOI] [PubMed] [Google Scholar]
- Huang X, Sang T, Zhao Q, Feng Q, Zhao Y, Li C, et al. Genome-wide association studies of 14 agronomic traits in rice landraces. Nat Genet. 2010;42(11):961–967. doi: 10.1038/ng.695. [DOI] [PubMed] [Google Scholar]
- Huang X, Zhao Y, Li C, Wang A, Zhao Q, Li W, et al. Genome-wide association study of flowering time and grain yield traits in a worldwide collection of rice germplasm. Nat Genet. 2012;44(1):32–39. doi: 10.1038/ng.1018. [DOI] [PubMed] [Google Scholar]
- Hyun DY, Oh M, Choi YM, Lee S, Lee MC, Oh S. Morphological and molecular evaluation for germinability in rice varieties under low-temperature and anaerobic conditions. J Crop Sci Biotechnol. 2017;20(1):21–27. doi: 10.1007/s12892-016-0083-1. [DOI] [Google Scholar]
- Iwata N, Fujino K. Genetic effects of major QTLs controlling low-temperature germinability in different genetic backgrounds in rice (Oryza sativa L.) Genome. 2010;53(10):763–768. doi: 10.1139/G10-060. [DOI] [PubMed] [Google Scholar]
- Ji SL, Jiang L, Wang YH, Liu SJ, Liu X, Zhai HQ, Yoshimura A, Wan JM. QTL and epistasis for low temperature germinability in rice. Acta Agron Sin. 2008;34(4):551–556. [Google Scholar]
- Ji SL, Jiang L, Wang YH, Zhang WW, Liu X, Liu SJ, et al. Quantitative trait loci mapping and stability for low temperature germination ability of rice. Plant Breed. 2009;128(4):387–392. doi: 10.1111/j.1439-0523.2008.01533.x. [DOI] [Google Scholar]
- Jiang L, Liu S, Hou M, Tang J, Chen L, Zhai H, et al. Analysis of QTLs for seed low temperature germinability and anoxia germinability in rice (Oryza sativa L.) Field Crops Res. 2006;98(1):68–75. doi: 10.1016/j.fcr.2005.12.015. [DOI] [Google Scholar]
- Jiang N, Shi S, Shi H, Khanzada H, Wassan GM, Zhu C, et al. Mapping QTL for seed germinability under low temperature using a new high-density genetic map of rice. Front Plant Sci. 2017 doi: 10.3389/fpls.2017.01223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara Y, de la Bastide M, Hamilton JP, Kanamori H, McCombie WR, Ouyang S, et al. Improvement of the Oryza sativa Nipponbare reference genome using next generation sequence and optical map data. Rice. 2013;6(1):4. doi: 10.1186/1939-8433-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KW, Chung HK, Cho GT, Ma KH, Chandrabalan D, Gwag JG, et al. PowerCore: a program applying the advanced M strategy with a heuristic search for establishing core sets. Bioinformatics. 2007;23(16):2155–2162. doi: 10.1093/bioinformatics/btm313. [DOI] [PubMed] [Google Scholar]
- Kim TS, He Q, Kim KW, Yoon MY, Ra WH, Li FP, et al. Genome-wide resequencing of KRICE_CORE reveals their potential for future breeding, as well as functional and evolutionary studies in the post-genomic era. BMC Genom. 2016;17(1):408. doi: 10.1186/s12864-016-2734-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi S, Izawa T, Lin SY, Ebana K, Fukuta Y, Sasaki T, Yano M. An SNP caused loss of seed shattering during rice domestication. Science. 2006;312(5778):1392–1396. doi: 10.1126/science.1126410. [DOI] [PubMed] [Google Scholar]
- Li C, Zhou A, Sang T. Rice domestication by reducing shattering. Science. 2006;311(5769):1936–1939. doi: 10.1126/science.1123604. [DOI] [PubMed] [Google Scholar]
- Li L, Liu X, Xie K, Wang Y, Liu F, Lin Q, et al. qLTG-9, a stable quantitative trait locus for low-temperature germination in rice (Oryza sativa L.) Theor Appl Genet. 2013;126(9):2313–2322. doi: 10.1007/s00122-013-2137-2. [DOI] [PubMed] [Google Scholar]
- Lindlöf A, Chawade A, Sikora P, Olsson O. Comparative transcriptomics of Sijung and Jumli Marshi rice during early chilling stress imply multiple protective mechanisms. PloS One. 2015;10(5):e0125385. doi: 10.1371/journal.pone.0125385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipka AE, Tian F, Wang Q, Peiffer J, Li M, Bradbury PJ, et al. GAPIT: genome association and prediction integrated tool. Bioinformatics. 2012;28(18):2397–2399. doi: 10.1093/bioinformatics/bts444. [DOI] [PubMed] [Google Scholar]
- Lou Q, Chen L, Sun Z, Xing Y, Li J, Xu X, et al. A major QTL associated with cold tolerance at seedling stage in rice (Oryza sativa L.) Euphytica. 2007;158(1–2):87–94. doi: 10.1007/s10681-007-9431-5. [DOI] [Google Scholar]
- Mao D, Yu L, Chen D, Li L, Zhu Y, Xiao Y, et al. Multiple cold resistance loci confer the high cold tolerance adaptation of Dongxiang wild rice (Oryza rufipogon) to its high-latitude habitat. Theor Appl Genet. 2015;128(7):1359–1371. doi: 10.1007/s00122-015-2511-3. [DOI] [PubMed] [Google Scholar]
- Miura K, Lin SY, Yano M, Nagamine T. Mapping quantitative trait loci controlling low temperature germinability in rice (Oryza sativa L.) Breed Sci. 2001;51(4):293–299. doi: 10.1270/jsbbs.51.293. [DOI] [Google Scholar]
- Mousavizadeh SJ, Sedaghathoor S, Rahimi A, Mohammadi H. Germination parameters and peroxidase activity of lettuce seed under stationary magnetic field. Int J Biosci. 2013;3(4):199–207. doi: 10.12692/ijb/3.4.199-207. [DOI] [Google Scholar]
- Nguyen HN, Park IK, Yeo SM, Yun YT, Ahn SN. Mapping quantitative trait loci controlling low-temperature germinability in rice. Korean J Agric Sci. 2012;39(4):477–482. doi: 10.7744/cnujas.2012.39.4.477. [DOI] [Google Scholar]
- Sasaki T, Kinoshita T, Takahashi ME. Estimation of the number of genes in the germination ability at low temperature in rice: genetical studies in rice plant, LVII. J Fac Agric Hokkaido Univ. 1974;57(3):301–312. [Google Scholar]
- Satoh T, Tezuka K, Kawamoto T, Matsumoto S, Satoh-Nagasawa N, Ueda K, et al. Identification of QTLs controlling low-temperature germination of the East European rice (Oryza sativa L.) variety Maratteli. Euphytica. 2016;207(2):245–254. doi: 10.1007/s10681-015-1531-z. [DOI] [Google Scholar]
- Shakiba E, Edwards JD, Jodari F, Duke SE, Baldo AM, Korniliev P, et al. Genetic architecture of cold tolerance in rice (Oryza sativa) determined through high resolution genome-wide analysis. PloS One. 2017;12(3):e0172133. doi: 10.1371/journal.pone.0172133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Jwa NS. Understanding the responses of rice to environmental stress using proteomics. J Proteome Res. 2013;12(11):4652–4669. doi: 10.1021/pr400689j. [DOI] [PubMed] [Google Scholar]
- Su CF, Wang YC, Hsieh TH, Lu CA, Tseng TH, Yu SM. A novel MYBS3-dependent pathway confers cold tolerance in rice. Plant Physiol. 2010;153(1):145–158. doi: 10.1104/pp.110.153015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovuu A, Zulfugarov IS, Wu G, Kang IS, Kim C, Moon BY, et al. Rice mutants deficient in u-3 fatty acid desaturase (FAD8) fail to acclimate to cold temperatures. Plant Physiol Biochem. 2016;109:525–535. doi: 10.1016/j.plaphy.2016.11.001. [DOI] [PubMed] [Google Scholar]
- Wang L, Wang A, Huang X, Zhao Q, Dong G, Qian Q, et al. Mapping 49 quantitative trait loci at high resolution through sequencing-based genotyping of rice recombinant inbred lines. Theor Appl Genet. 2011;122(2):327–340. doi: 10.1007/s00122-010-1449-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Liu J, Li C, Kang H, Wang Y, Tan X, et al. Genome-wide association mapping of cold tolerance genes at the seedling stage in rice. Rice. 2016;9(1):61. doi: 10.1186/s12284-016-0133-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Huang D, Tang W, Zheng Y, Liang K, Cutler AJ, et al. Mapping of quantitative trait loci underlying cold tolerance in rice seedlings via high-throughput sequencing of pooled extremes. Plos One. 2013;8(7):e68433. doi: 10.1371/journal.pone.0068433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZH, Qu XS, Wan S, Chen LH, Zhu YG. Comparison of QTL controlling seedling vigour under different temperature conditions using recombinant inbred lines in rice (Oryza sativa) Ann Bot. 2004;95(3):423–429. doi: 10.1093/aob/mci039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Chen Q, Wang S, Hong Y, Wang Z. Rice and cold stress: methods for its evaluation and summary of cold tolerance-related quantitative trait loci. Rice. 2014;7(1):24. doi: 10.1186/s12284-014-0024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Cho GT, Ma KH, Chung JW, Gwag JG, Park YJ. Development of an allele-mining set in rice using a heuristic algorithm and SSR genotype data with least redundancy for the post-genomic era. Mol Breed. 2010;26(4):639–651. doi: 10.1007/s11032-010-9400-x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.