Abstract
The present work summarizes different sources of biomass used as raw material for the production of biogas, focusing mainly on the use of plants that do not compete with the food supply. Biogas obtained from edible plants entails a developed technology and good yield of methane production; however, its use may not be sustainable. Biomass from agricultural waste is a cheap option, but in general, with lower methane yields than those obtained from edible plants. On the other hand, the use of algae or aquatic plants promises to be an efficient and sustainable option with high yields of methane produced, but it necessary to overcome the existing technological barriers. Moreover, these last raw materials have the additional advantage that they can be obtained from wastewater treatment and, therefore, they could be applied to the concept of biorefinery. An estimation of methane yield per hectare per year of the some types of biomass and operational conditions employed is presented as well. In addition, different strategies to improve the yield of biogas, such as physical, chemical, and biological pretreatments, are presented. Other alternatives for enhanced the biogas production such as bioaugmentation and biohythane are showed and finally perspectives are mentioned.
Keywords: Biogas, Lignocellulosic, Aquatic plants, Algae, Pretreatments
Introduction
Climate change has been directly related to fossil fuel combustion, which generates CO2 and it is the energy production predominating worldwide since the last century (DeMarco 2017). Due to this, research done on the subject to obtain diverse clean energies has been intensified, with a particular focus on renewable energy sources in the few last decades. The use of biomass from edible plants to biofuels production has been a topic of debate, because it could increase the compete for availability of agricultural lands and water bodies and displace food crops. Because of this, works have been carried out to explore the use of different biomass sources for biogas production that will not compete with the food supply and, at the same time, could contribute to the development of sustainable processes.
As a result of the concerned mentioned above of food crops competing with biogas crops, the interest to obtain biogas from lignocellulosic biomass through anaerobic digestion has increased owing to the fact that anaerobic digestion is one of the most abundant sources of renewable biomass in the world, and methane is one of the main products. Through anaerobic digestion, complex polymers can be transformed in simple molecules and finally in biogas, which is mainly formed by CH4 (60–70%) and CO2 (30–40%) (Brennan and Owende 2010). The enzymatic capacity of anaerobic microorganisms is utilized for breaking down organic matter through complex interactions that occur among microorganisms. The manner in which those microorganisms interact can define the performance and effectiveness of the process. Biogas obtained in this way is, therefore, a renewable form of energy that may contribute to mitigate environmental pollution (Jiang et al. 2011), and simultaneously can be utilized to produce electricity, heat, or fuel for vehicles.
However, the obtaining biogas from lignocellulosic biomass is difficult, because lignocellulose is recalcitrant to microbial or enzymatic biodegradation, due to its structure and composition (Hendriks and Zeeman 2009). Lignocellulosic biomass is composed mainly by cellulose, hemicellulose, and lignin. Cellulose is a biopolymer formed of crystalline and amorphous parts, while hemicelluloses are amorphous and have heterogeneous complex structures formed by different polymers such as pentoses, hexoses, among others. Hemicellulose serves as a connection between cellulose and lignin; and, therefore, it provides more rigidity (Laureano-Perez et al. 2005). Lignin is an amorphous aromatic heteropolymer whose function is to provide support and impermeability to the plant as well as resistance to microbial attack and oxidative stress (Hendriks and Zeeman 2009). Due to this structural complexity, lignin is difficult to break down.
The aim of this work is, first, to present an update review on biogas production from different types of biomass such as edible plants, agricultural waste, and non-food plant species, focusing mainly on the use of aquatic plants and algae. Second, it is also presented different strategies which have been used to improve the process, for instance chemical, physical, physicochemical, and biological pretreatments. The applications of alternative techniques such as bioaugmentation are presented too.
Production of biogas from different biomass sources
The production of biogas is an attractive alternative for energy production in terms of energy yield. The total supply of biomass in 2014 was estimated at 59.2 EJ representing the 10.3% of all energy supply globally (WBA 2017). Forestry, agriculture, and municipal solid-waste sectors contribute 87, 10, and 3%, respectively, to the supply of biomass (WBA 2017). Biofuels can be classified according to the type of biomass from which they come from: first-, second-, and third-generation biofuels. First-generation biofuels are those that come from edible plants. Second-generation biofuels come from agricultural waste and non-edible plants. Finally, third-generation biofuels are those that come from algal biomass. Biofuels of second and third generations are also called advanced biofuels, because their production does not compete with food supplies and, in many cases, are produced from biomass resulting from remediation processes.
Edible plants
The first generation of biofuels is obtained from terrestrial crops such as grains (rice, wheat, and corn), potatoes, and sugarcane, among others (Brennan and Owende 2010). Biogas obtained from this type of biomass presents advantages, since it uses the highest technological development and it also has competitive cost and scalable process. Biogas production from energy crops has been suggested to give more net energy yield per hectare per year (up to two times), in comparison with obtaining bioethanol from wheat (Börjesson and Mattiasson 2008). In Table 1, several examples of feedstock for the first-generation biogas production are shown, including operational conditions and methane yield. The studies presented in this table were conducted under mesophilic conditions at temperature ranging 30–38 °C, at a interval of pH between 7 and 8 with hydraulic residence time (HRT) of 30–60 days, the operating conditions used were close to the optimal values reported to favor the methane production (Chandra et al. 2012). In Table 1, it is shown that the highest methane yield per hectare is reached when maize is used. This means that utilizing of maize as a raw material to obtain biogas will require a smaller surface area compared to crops such as barley. Maize is one of the most used crops from which a high yield of methane formation can be obtained. A modern study in Germany showed that maize is the most common co-substrate used with manure in biogas plants (Weiland 2006). Sugar beet and sorghum are other types of crops which can have the higher values of yield methane per hectare per year (Table 1). On the other hand, extensive agricultural areas are required to obtain a sufficient quantity of biogas to be able to gain ground in the use of fossil fuels and, consequently, this could cause a competition with food crops in terms of the land and water that is required (Correa et al. 2017). Consequently, the use of edible plants as raw material for the generation of biogas is not a sustainable process.
Table 1.
Different feedstock for the production of first-generation biogas and its performance
| Biomass | Inoculum | Operation conditions | Type of reactor | Pretreatment | Methane yield | Methane yielda, m3 ha−1 year−1 | Crop yield t DS ha−1 year−1 | References |
|---|---|---|---|---|---|---|---|---|
| Maize and amaranth | Mixture of microorganisms | 37.5 °C | Batch assays (100 mL syringes) | Ensiling techniques | 349.5 mL CH4 g−1 ODM | – | – | Haag et al. (2015) |
| Maize | Sludge | 36 °C, 30 days | Batch assays | – | 379.0 mL g−1 VS | 3411–7505 | 10–22b,c | Pakarinen et al. (2011) |
| Maize silage | Methanogenic | 37 °C, pH 7.2, 21 days | Batch (1 L) | Microbial consortium with high cellulolytic activity | 393.3 mL g−1 | 3933–8652 | 10–22b,c | Poszytek et al. (2016) |
| Zea mays (maize) | Anaerobic sludge | 39 °C, HRT = 60 days | Continuously stirred tank reactors (CSTRs) | Ensiling | 330.0 mL CH4 g−1 VS | 2970–6536 | 10–22b,c | Klimiuk et al. (2010) |
| Sorghum | Digestates | 35 °C, 30 days | Batch (2 L Glass vessel) | Silage | 341.0–378.0 mL g−1 ODM | 6479.0–7182 | 19c | Herrmann et al. (2011) |
| Barley | Inoculum from anaerobic reactor | 37 °C | Batch | Milled | 314.8 mL g−1 VS | 1416 | 5d | Himanshu et al. (2017) |
| Sugar beet | Digestate | 35 °C, pH 8.1, 30 days | Batch (2 L) | Silage | 350.4–399.4 mL g−1 ODM | 4905–5591 | 14e | Herrmann et al. (2016) |
| Sunflowers | Digestate | 35 °C, pH 8.1, 30 days | Batch (2 L) | Silage | 210–286.1 mL g−1 ODM | 2100–3147 | 10–11d,e | Herrmann et al. (2016) |
| Winter wheat | Digestate | 35 °C, pH 8.1, 30 days | Batch (2 L) | Silage | 269.2–327.6 mL g−1 ODM | 1346–3277 | 5–10d,e | Herrmann et al. (2016) |
Non-edible plants or wastes
The second-generation biogas is produced from lignocellulosic biomass derived from agricultural waste, forest waste, municipal and industrial waste, and non-edible plants such as grass and aquatic plants. Moreover, all these resources share the characteristic that they are formed from non-food resources. This type of biomass presents advantage of low cost and abundance; nonetheless, it is not yet cost-effective attractive by a number of barrier techniques that require to be surpassed (Naik et al. 2010). Among the different barriers which limit their commercialization are: government policies, added value from non-fuel co-products, high production costs, and competition with fossil fuels (Chen and Smith 2017).
Agricultural wastes
Biogas production from different agricultural wastes through anaerobic digestion has increased worldwide due to not compete with food supply, and is environmentally friendly. Furthermore, this method present other advantages like contributing to reduce pollution produced by organic waste, waste ceasing to be garbage, and becoming value-added products that are converted into clean energy. Agricultural waste includes the non-edible portions of plant, such as the leaves, corn stover, straw, etc. Large quantities of crop residues are generated from agricultural activities annually worldwide. In 2006, it was estimated that 75.73 million tons of dry matter was produced from 20 crops in Mexico (Valdez-Vazquez et al. 2010). Furthermore, this process contributes to the management of agricultural waste, which, not having been treated properly, is decomposed and methane is released to the environment of non-controlled manner.
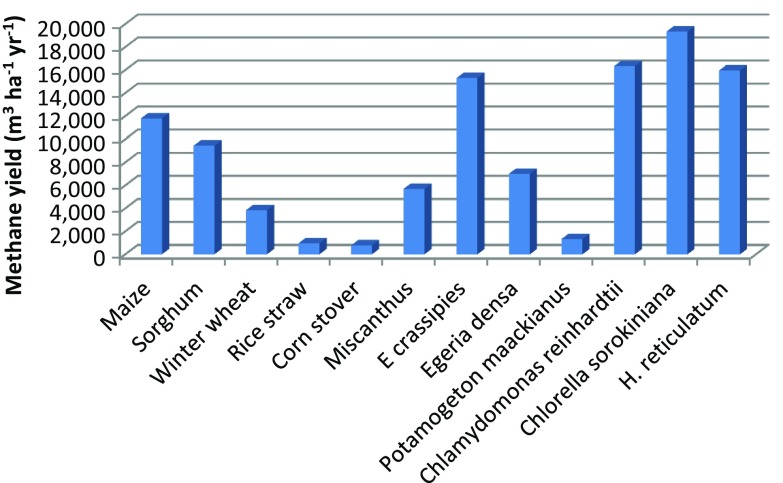
In Table 2, several feedstocks from agricultural waste are shown It can be observed that mesophilic or thermophilic conditions were employed, at pH range of 6.5–7 with TRH from 30 to 63 days. The methane yield obtained per unit g VS from corn stover is 1.8 higher than the one obtained from rice straw, but the yield per unit hectare of the rice straw is greater than corn stover. In other words, corn stover will take up more agricultural lands in comparison with rice straw. This means that to have a complete scenery of the performance of the process, it is necessary not only to calculate the yield per unit weight of biomass used, but also to know the yield per surface unit. In addition, the methane yield obtained per hectare per year from Miscanthus sacchariflorus reaches value that is even higher than the value obtained from wheat winter (Fig. 1). Miscanthus is a plant species that has been identified as an ideal fuel crop because of the ease of its growth and harvest and it produces high dry matter content (McKendry 2002). In general, the methane yield per hectare obtained from agricultural waste is lower than the one obtained from crop grains such as maize (Fig. 1). However, its use does not put the supply of food at risk, so it is necessary to develop a technology that allows the increase of methane yields from this type of raw material. In this sense, it is recommended to utilize a pretreatment to enhance the accessibility of holocelluloses (Monlau et al. 2013a).
Table 2.
Different feedstock of agricultural waste used for production of second-generation biogas and its performance
| Biomass | Inoculum | Operation conditions | Type of reactor | Pretreatment | Methane yield | Methane yielda, m3 ha−1 year−1 | Crop yield t DS ha−1 year−1 | References |
|---|---|---|---|---|---|---|---|---|
| Rice straw | Anaerobic sludge | 35 °C at 100 rpm | Batch flasks | Citric acid to (100–140 °C) | 322.1 mL biogas g−1 rice straw | 128.8–966.3 | 0.4–3b | Amnuaycheewa et al. (2016) |
| Rice straw | Sludge from manure compost (seed) | 55 °C, pH 6.8 | Semi-batch bioreactor of 250 mL containing carbon fiber textile | Premilled nanofiltration | 260 mLCH4 g−1 VS | 104–780 | 0.4–3b | Sasaki et al. (2016) |
| Corn stover | Mixture from biogas plant | 37.5 °C, 49 days | Batch fermenters | Steam explosion (160 °C for 2 min) | 585 mL g−1 VS | 783.9 | 1.34c | Lizasoain et al. (2017) |
| Grass silage | Manure and crops | 55 °C, 63 days | Anaerobic inocula | 405 mLCH4 g−1 VS | 4374 | 12d | Voelklein et al. (2016) | |
| Agave tequilana bagasse | Anaerobic granular sludge | 35 °C pH 7, 4 g COD L−1 day and HRT 4–5 days and 30 g VSS L−1 | UASB | Acid or enzymatic hydrolysis | 240 mL CH4 g−1 COD | Arreola-Vargas et al. (2016) | ||
| Wheat straw Sugarcane bagasse |
Sludge wastewater | 35.1 °C, pH 6.5–7.0 30 days |
Batch (2 L) | Thermal Acid Alkaline (30%) Alkaline-peroxide |
200–240 mL CH4 g−1 VS | 612–2304 | 3.4–9.6e | Bolado-Rodríguez et al. (2016) |
| Sunflower stalks | Granular sludge | 35 °C, pH 7 | Batch anaerobic flasks | Acid and thermal (170 °C) | 302 mLCH4 g−1 VS | Monlau et al. (2013a) | ||
| Miscanthus sacchariflorus | Anaerobic sludge | 39 °C, HRT = 60 days | Continuously stirred tank reactors (CSTRs) | Ensilage | 190 mLCH4 g−1 VS | 2223–5700 | 13–30f | Klimiuk et al. (2010) |
COD chemical oxygen demand, HRT hydraulic retention time
aCalculated value assuming 90% volatile solid content
b http://www2.gec.jp/gec/en/Activities/FY2009/ietc/wab/wab_day3-5.pdf
c http://www.dairy.missouri.edu/drought/StoverFAQ.pdf
dSmyth et al. (2009)
eDai et al. (2016)
fMcKendry (2002)
Fig. 1.
Methane yield per hectare per year of different types of biomass
Floating, submerged, or emergent aquatic plants
Floating aquatic plants are considered to be invasive plants owing to their rapid and uncontrollable growth. However, aquatic plants can be used in the phytofiltration to remove pollutants; for example, one phytofiltration lagoon at scale pilot has been used for the removal of COD, reaching values in the range of 47.8–88.0%, depending on the season (Olguín et al. 2017). Aquatic plants perform the removal of contaminants through physical, chemical, and microbiological process which take place in the roots (Akinbile and Yusoff 2012). Moreover, aquatic plants can be used as a source of biomass for biogas production; nonetheless, it is required to be careful, so the growth of these plants is controlled at all times. In addition, it has been suggested that aquatic plants are a good option as feedstock for biogas production, due to the possibility to obtain from them a high content of volatile fatty acids (Hernández-García et al. 2015). In this way, biomass produced through the phytofiltration process or the biomass of aquatic plants harvested in sites like lakes or freshwater bodies can be used as feedstock for biogas production. However, it is desirable for their controlled production and the way in which the concept of biorefinery can be applied. The controlled use of aquatic plants does not put the food supply at risk, because it does not compete with water or crop land. The utilization of aquatic plants presents two disadvantages: their low mechanical strength and high moisture content that reached values of 95% (Koyama et al. 2017a). In Table 3 methane production from several aquatic plants is shown; the studies were carried out in a temperature range of 30–38 °C and interval of pH between 7 and 8. In addition, highest yield of methane was obtaining from Elodea nuttallii.
Table 3.
Different aquatic plants used as feedstock for the production of biogas
| Biomass | Inoculum | Operation conditions | Type of reactor | CH4 conversion efficiency (%) | Yield | Methane yielda, m3 ha−1 year−1 | Crop yield t DS ha year−1 | References |
|---|---|---|---|---|---|---|---|---|
| Ipomoea aquatica and Eichhornia crassipes | Cow dung slurry | Agitation manual twice daily, 25.5–35.5 °C, 119 days | Batch assays working volume of 15 dm3 | – | 290 mL biogas kg−1 VS days−1 | – | – | Adanikin et al. (2017) |
| Typha latifolia | Anaerobic sludge | 37 °C, 60 days | Batch assays | – | 151 mL CH4 g−1 VS | 2147 | 15.8b | Nkemka et al. (2015) |
| Eichhornia crassipes | Sludge of wastewater | 38 °C, pH 7–8 | Pilot scale, batch | – | 140 mL CH4 g−1 VS | 7560–12,600 | 60–100c | O’Sullivan et al. (2010) |
| Eichhornia crassipes | Sludge | 35 °C | Batch | – | 170 mL CH4 g−1 VS | 9180–15,300 | 60–100c | Gao et al. (2013) |
| Cabomba | Sludge of wastewater | 38 °C, pH 7–8 | Pilot scale, batch | – | 109 mL CH4 g−1 VS | – | – | O’Sullivan et al. (2010) |
| Elodea nuttallii | Anaerobic sludge | 37 °C and 100 rpm, 14 days | Batch assays | 61.4 | 299 mL CH4 g−1 TS | – | – | Koyama et al. (2014) |
| Egeria densa | Anaerobic sludge | 37 °C and 100 rpm, 14 days | Batch assays | 60.6 | 234 mL CH4 g−1 TS | 7020 | 30d | Koyama et al. (2014) |
| Potamogeton malaianu | Anaerobic sludge | 37 °C and 100 rpm, 14 days | Batch assays | 72.2 | 156 mL CH4 g−1 TS | 528.8–1332.4 | 3.39–8.54e | Koyama et al. (2014) |
| Duckweed (aquatic plant):cattle dung in a 1:1 ratio | Cattle dung | 38 °C, pH 7.2, 55 days | Batch | – | 580 mL days−1 | – | – | Yadav et al. (2017) |
| Egeria densa | Anaerobic sludge | 35 °C, 300 rpm, HRT = 45 days | Semi-continuous reactor | – | 231 mL CH4 g−1 VS | 6930 | 30d | Kobayashi et al. (2015) |
| Potamogeton maackianus | Anaerobic sludge | – | Semi-continuous operation | 53.6 | 255.9 mL CH4 g−1 VS | 857.5–2185 | 3.39–8.54e | Koyama et al. (2017b) |
The other additional advantage that comes with using aquatic plants is that they contain a greater amount of biodegradable protein than other plants (Kobayashi et al. 2015). However, a few studies have been carried out with floating or submerged aquatic plants. Of these works, the most studied plant is Eichhornia crassipes, while other species of aquatic plants have been studied to a lesser extent. Jiang et al. (2014) evaluated seven species of wetland aquatic plants, and their results showed a negative correlation between biogas production and hemicelluloses or lignin content, whereas the correlation between biogas production and starch carbohydrate content was positive. The authors reported that the greatest biogas production was obtained from Colocasia tonoimo Nakai, and it reached up to 629.4 mL g−1 volatile solid (VS). Koyama et al. (2017a) evaluated the effect of dissolved lignin (0–5 g L−1) extracted with an alkaline pretreatment of an aquatic macrophyte (Potamogeton maackianus) on methanogenesis, acidogenesis, and hydrolysis. They found that regarding acidogenesis and methanogenesis, the efficiency decreased up to 15%, while the hydrolysis decreased up to 35% at the higher concentration tested (5 g L−1) with respect to the control. In other words, it seems that the production of biogas was inversely proportional to the lignin content.
In addition, co-digestion of cow manure with sewage sludge and Eichhornia crassipes has been evaluated, the finding that accelerated the reaction and improved methane content (Tasnim et al. 2017). Moreover, Yadav et al. (2017) evaluated biogas production from the co-digestion of duckweed with cattle dung, where different proportions were studied for maintaining an optimal C/N ratio of between 25 and 30; the best mixing for biogas production was a relation of 1:1. On the other hand, Ali et al. (2014) suggested the combination of different plants (cob heart and Eichhornia crassipes) combined with a pretreatment that consisted of delignification with Volveriella diplasia and Phanerochaete chrysosporium, which could be a good source of energy and fertilizer. However, it was observed that the seeds of Eichhornia crassipes can be recovered after the process of anaerobic digestion with germination values of 1.00%, which is why its use may not be completely safe (Albano et al. 2015). Therefore, more studies are needed to evaluate the potential risks, as well as to evaluate other aquatic plants that have been studied to a lesser extent, because they could be good alternatives for biogas production.
The development and strengthening of this technology could represent some great advantages: first, the treatment of contaminated water bodies, and second, obtaining of biomass which could potentially assist in the generation of cheap and sustainable energy. If we compare the yield per hectare obtained from E. crassipes, we can see that it can even be higher than the one obtained from maize; consequently, we can say that aquatic plants can have great potential to produce energy (Fig. 1). However, efficient technologies for cultivation and harvesting in contaminated water bodies must be sought; being careful that growth is controlled to avoid environmental problems. If they are cultivated in artificial lagoons, then it is necessary to create an efficient and sustainable infrastructure that does not involves high construction and operational costs. It is desirable for the design of lagoons that occupy a small area and allow a high yield biomass high. Furthermore, the development of strategies to control the cultivation of aquatic plants is necessary; likewise, pilot-scale studies are required to determine whether the application of this technology is feasible of carrying it out.
Algae biomass
In the last few years, the interest for obtaining biofuel from algal biomass has considerably increased, as they are considered the third-generation biofuels. Algae are organisms that can be classified in macroalgae and microalgae. These photosynthetic organisms transform inorganic carbon (CO2) or simple compounds directly into higher organic compounds, and have simple nutritional requirements: light, sugars, CO2, N, P, and K (Brennan and Owende 2010; Ghimire et al. 2017). The macroalgae are multicellular organisms, while microalgae are unicellular organisms. The use of microalgae biomass presents several advantages: they can grow up to 10 times faster that terrestrial plants (Kröger and Müller-Langer 2012); they can be cultured without competing with food production; they can grow in wastewater using the nutrients present in them; therefore, the biogas production can be coupled wastewater remediation (Mahdy et al. 2016). In addition, the residual biomass algae generated from processes for liquid biofuel production can be used as a feedstock in anaerobic digestion for biogas production (Ghimire et al. 2017). Moreover, the study of microalgae has recently been increased due to microalgae biomass being considered a lignocellulosic-type feedstock that has a lignin-deficient cell wall that allows more permeability in comparison with other types of lignocellulosic biomass (Chen et al. 2013).
Microalgae cultures fix the CO2, contributing to mitigate environmental pollution, which can be economically attractive (Brennan and Owende 2010). Different studies have shown the use of different types of microalgae for biogas production, obtaining yields per hectare per year higher to those found when edible plants are used (Table 4). However, some factors can affect the formation of biogas, such as the C/N ratio; for example, it has been suggested that it is possible to balance C/N in the optimum interval between 20 and 25 for the co-digestion of algal sludge and agricultural waste (Yen and Brune 2007). The temperature affects methane production and can generally be enhanced when this is increased (González-Fernández et al. 2012).
Table 4.
Production of methane from different microalgae
| Biomass | Source | Pretreatment | Operation conditions | System | Methane yield | Methane yielda, m3 ha−1 year−1 | COD removal efficiency | References |
|---|---|---|---|---|---|---|---|---|
| 35% Scenedesmus, 30% Dictyosphaerium, 15% Keratococcus, 10% Oscillatoria, 8% Monoraphidium and 2% Nitzchia | Phoreactors, illumination of 54 W daylight neon lamp at intensity of 5000 lumens. 28 °C, pH 8 for 8 days | Ozonation 382 mg O3 g−1 VS | 35 °C and 150 rpm | Batch assays | 432.7 mL CH4g−1 VS | 15,572 | – | Cardeña et al. (2017) |
| Chlorella vulgaris | Race-ways at 25 °C | Enzymatic | Semi-continuous | Anaerobic reactors (CSTRs) | 137 mL CH4 g−1 COD | – | 52% | Mahdy et al. (2016) |
| Chlorella sorokiniana | – | Sonication (200 Watts at 80% amplitude for 10 min) | 30 °C, 42 days | Bench-scale batch | 388 mL CH4 g−1 VS | 13,968 | – | Ayala-Parra et al. (2017) |
| Chlorella sorokiniana | – | – | 30 °C, 42 days | Bench-scale batch | 298 mL CH4 g−1 VS | 10,728 | – | Ayala-Parra et al. (2017) |
| Scenedesmus residues | – | Enzymatic at 50 °C | 37 °C | Batch | 272.8 mL CH4 g−1 VS | 9820.8 | 61.8% | Ramos-Suárez and Carreras (2014) |
| Scenedesmus spp. | – | Rumen microorganisms as inoculum | SRT 100 days | CSTR and anaerobic membrane bioreactor | 214 mL CH4 g−1 COD | – | 70% | Giménez et al. (2017) |
| Chlamydomonas reinhardtii | Batch with white light, nitrogen-limited biomass | 38 °C, 100 rpm, HRT of 20 days | Reactor in semi-continuous mode | 462 mL CH4 g−1 VS days−1 | 16,632 | – | Klassen et al. (2017) | |
| Hydrodictyon reticulatum | – | Enzyme/acid-saccharified | 35 °C | Batch assays | 537.5 mL CH4 g−1 VS | 19,350 | – | Lee et al. (2017) |
COD chemical oxygen demand, HRT hydraulic retention time, SRT sludge retention time
aAssuming a biomass yield of 36 t ha−1 year−1 (Tredici et al. 2015). Calculated value assumes 90% volatile solid content
Microalgae present differences in the structure and composition of the cell wall among species and this composition can affect the biogas production, so this is related to the ease or difficult in which the cell wall is degraded. The resistance of the cell wall is attributed to two polymers, namely, sporopollenin and algaenan (González-Fernández et al. 2012). Strains with no cell wall or protein-based cell could be easily broken; for example, Chlamydomonas reinhardtii is an easily degradable specie, while Scenedesmus obliquus is a difficult degradable species (Mussgnug et al. 2010). These and other differences among microalgae hinder the development of a technology that could be applicable to all species, and perhaps, it would be more convenient to analyze them by groups with similar characteristics. In addition, the design of culture media was investigated: a nitrogen-limited medium was formulated to produce low protein biomass; during anaerobic digestion, the nitrogen-limited biomass was characterized by a stable process with a low concentration of inhibitory compounds and methane productivity of 5.7 times higher compared with the nitrogen-replete biomass (Klassen et al. 2017). This could indicate that the formulation of the culture medium is an important factor that could provide biomass with characteristics that favor biogas production.
On the other hand, a study by Correa et al. (2017) discussed the possible impacts of microalgae production systems on biodiversity. The authors concluded that such systems exert less pressure on biodiversity per unit of fuel generated compared to the first-generation biofuels. This is due to direct or indirect reductions of land-use change, water consumption, or use of pesticides. Therefore, the use of microalgae biomass as a feedstock for biogas production has a great potential as an alternative clean, efficient, and sustainable energy. In addition, other advantages that encompass the use microalgae for biogas production are: low energy consumption, use of residual biomass, wastewater treatment, among others. On the other hand, the disadvantages are that the use of microalgae to obtain energy is economically not feasible due to the high costs of cultivation, harvest, and operation (Jankowska et al. 2017). However, the microalgae are raw material with good energy potential and could reach a high methane yield per hectare (Fig. 1). Therefore, it is necessary to develop new and efficient technologies in the use and feasible and cost-effective cultivation of microalgae.
Macroalgae has been studied to a lesser extent than microalgae biomass for biogas production. Marine macroalgae present a low content of lignin and are a source of feedstock biomass for biogas production, which has been proposed as an alternative for durable energy production (Karray et al. 2016). Tedesco et al. (2014) evaluated the potential of Laminariaceae biomass for biogas production through the application of mechanical pretreatment that enhanced in a 53% methane yield in comparison with untreated samples. Ulva rigida (marine macroalgae) has been used for biogas production utilizing enzymatic pretreatment and reached values of biogas production of 1175 mL g−1 COD (chemical oxygen demand) (Karray et al. 2016).
Another studied macroalgae is Chaetomorpha linum, which was investigated because of its potential use in the co-production of bioethanol and biogas in the biorefinery (Yahmed et al. 2016). The authors observed that digestion of C. linum with an enzyme preparation reached a maximum yield of 0.22 g g−1 dry substrate.
The use of marine macroalgae (mixture of 20% brown and 80% red algae) as a feedstock in an industrial scale biogas plant with co-digestion of chicken manure was evaluated throughout the life cycle, resulting that the co-digestion of algae with chicken manure had a positive impact on the reduction of emissions (Ertem et al. 2017). The authors proposed the possibility of substituting energy crops with macroalgal biomass in bioenergy production to mitigate the negative environmental effects. However, the use of macroalgae in anaerobic digestion could be problematic in the long run due to the potential of high salinity and possible sand accumulation (Laurens et al. 2017). Therefore, it is necessary to carry out more research that allows to overcome these barriers.
Strategies to improve biogas production
Pretreatments
Physiochemical pretreatments
Many factors affect the biodegradability of lignocellulosic biomass, such as crystallinity, grade of polymerization, surface area, solubility, and lignin content (Monlau et al. 2013b). Several studies have evaluated the use of various physical, chemical, and biological pretreatments to get better biodegradation of lignocellulosic biomass and to increase methane production. The choice of pretreatment depending on the characteristics and structure of biomass, and it should increase the formation of biodegradable substrates, without the loss of matter during process. The physical and chemical methods include mechanical, extrusion, steam explosion, liquid hot water, organosolvents, ionic liquids, ozonolysis, among others. Mechanical pretreatment (grinding or milling) has the aim of reducing particulate size for increasing the surface area, reducing crystallinity, and the grade of polymerization (Galbe and Zacchi 2007). Thermal pretreatment consists of heating the biomass to high temperatures depending on the process: for liquid hot water and steam explosion, temperatures of 230 and 260 °C, respectively, can be reached (Monlau et al. 2013b). Liquid hot water consists of heating water at a high temperature and high pressure; this pretreatment was utilized for pretreating sugarcane press mud, reaching the highest yield of methane at 150 °C and 20 min, that represents an increase of 63% compared with untreated substrate. However, at temperatures of 200 and 210 °C, the methane yield was diminished due to the possible formation of refractory compounds (González et al. 2014). Steam explosion is a physicochemical method that consists of the biomass exposed to a steam at high temperature and pressure, and it has been proposed as a cost-effective pretreatment for the degradation of lignocellulosic biomass, but, sometimes, the xylan fraction is partially degraded and inhibitory compounds can be formed during the process (García-Aparicio et al. 2006). Another physical pretreatment is extrusion, which consists of raw biomass that being passed through an extruder with the application of pressure and high temperature (Ravindran and Jaiswal 2016); in this way, the biomass is disrupted.
In the chemical acid pretreatment, the aim is to solubilize hemicellulose by breaking ether bonds in lignin by making the cellulose accessible (Knappert et al. 1981). While the alkaline pretreatment is used to cleave ester bonds in lignin and can be suitable for the treatment of herbaceous plants, because they contain large quantities of alkali–labile lignin phenols (Buranov and Mazza 2008). Koyama et al. (2017a) reported that strong delignification properties of alkaline pretreatment can produce phenolic compounds that can inhibit the anaerobic digestion of lignocellulosic biomass (Koyama et al. 2017a). Furthermore, it has been suggested that dry chemo-mechanical methods increase substrate macroporosity and enhance microbial xylanase activity (Lazuka et al. 2017). Organosolv pretreatment is a method that utilizes organic solvents such as methanol, ethanol, acetone, or ethylene glycol mixed with or without an inorganic catalyst at high temperatures (Ostovareh et al. 2015). The organosolv pretreatment (using ethanol) was successfully used to produce biogas from sweet sorghum stalks with a methane yield of up to 270%, which is the highest compared with the methane yield from untreated substrates (Ostovareh et al. 2015). Other chemical methods are the oxidative pretreatments (H2O2, O3 FeCl3), which are utilized to solubilize lignin and hemicellulose to increase the hydrolysis of cellulose (Monlau et al. 2013b). The ozone method particularly has shown good results in the treatment of different types of biomass like microalgae biomass, where the methane yield was increased up to 66% with respect to biomass without pretreatment (Cardeña et al. 2017). These physical and chemical methods may require expensive and special equipment or high energy input and may produce certain inhibitors [such as 5-hydroxymethylfurfural (HMF)] that could have a negative effect on subsequent process fermentation (Taniguchi et al. 2005). Another disadvantage is that when used temperatures higher than 170 °C, some recalcitrant compounds can be formed in the liquid phase (Monlau et al. 2013a). Moreover, additional treatments, such as a chemical method, are sometimes required to neutralize the pretreated biomass, which can increase the cost of the process. Table 5 shows several pretreatments that have been used. In general, regardless of the biomass utilized, the pretreatments increase the methane yield from 19 to 89%. However, not all the pretreatments were successful, and when the alkaline treatment with calcium hydroxide was used, a decrease of 14% was observed.
Table 5.
Different physiochemical pretreatments applied to the production of biogas
| Biomass | Inoculum | Pretreatment | System | Anaerobic digestion | Degradation of lignin (%) | YieldAP | YieldBP | References |
|---|---|---|---|---|---|---|---|---|
| Agave tequilana bagasse | Anaerobic granular sludge | 2% (w/w) HCl and 2 h at 90 °C | Batch | 32 °C, pH 5 | – | 0.26 L CH4 g−1 COD | – | Arreola-Vargas et al. (2015) |
| Hay Straw |
Sludge | Calcium hydroxide (85 °C) Ammonium (120 °C) |
Batch (40 days) | 35 °C | – | 280 mL CH4 g−1 VS 320 mL CH4 g−1 VS (straw) |
320 mL CH4 g−1 VS 250 mL CH4 g−1 VS |
Fernandes et al. (2009) |
| Corn stover | Effluent of Biogas Plant | Steam explosion (SE) Thermal potassium hydroxide (KOH at 60 °C) |
Batch | 37 °C for 28 days | – | 217.5 mL CH4 g−1 VS 243 mL CH4 g−1 VS |
155.4 mL CH4 g−1 VS | Siddhu et al. (2016) |
| Sunflower stalk | Hydrothermal (180 °C for 1 h) Isopropanol-based organosolvent (160 °C for 30 min + H2SO4) |
Batch | 37 °C for 45 days | Up to 20.9% | 234 mL CH4 g−1 VS 278 mL CH4 g−1 VS |
124 mL CH4 g−1 VS | Hesami et al. (2015) | |
| Wheat straw Sugarcane bagasse |
Sludge wastewater | Alkaline autoclaving | Batch | 35 °C, 30 days | – | 286 mL CH4 g−1 VS 420 mL CH4 g−1 VS |
224 mL CH4 g−1 VS 222 mL CH4 g−1 VS |
Bolado-Rodriguez et al. (2016) |
| Wheat straw | Effluent anaerobic | Urea (1% w/w) | Batch | 35 °C, 120 rpm | – | 305.5 mL CH4 g−1 VS | 210.4 mL CH4 g−1 VS | Yao et al. (2018) |
HRT hydraulic retention time
APAfter pretreatment
BPBefore pretreatment
Biological pretreatments
The biological pretreatment is an alternative technique more environmentally friendly, with a low energy demand (Liu et al. 2014). Biological pretreatment consists of the utilization of pure microorganisms, consortia, or enzymes to increase the biodegradability of lignocellulosic biomass and thus increases the production of biogas. Enzyme pretreatment is a fast method, which can be carried out in a few hours (Table 6), due to the enzymes being smaller than microorganisms. In addition, enzymes have the best mobility, solubility, and the best interactions with the substrate (Romero-Güiza et al. 2016). The biological hydrolysis of cellulose is carried out by enzymes with exogluconase, endogluconase, and β-glucosidase activities, while hemicelluloses hydrolysis requires a lot of enzymes, including endo-xylanase, endo-mannanase, α-glucuronidase, among others (Wei 2016). In enzymatic pretreatment, a significant formation of total phenolic compounds (TPC) was observed; when willow was used, values until of 195 mg L−1 of TCP were reached in a liquid fraction; without reaching inhibitory levels for the process of anaerobic digestion (Schroyen et al. 2015). The authors indicate that the highest TPC values correspond to substrates with higher lignin content. In addition, the authors reported an increase in 24% methane yield from corn stover when enzymatic pretreatment was used compared to untreated corn stover (Table 6). However, enzymatic pretreatment has a higher cost and sometimes requires other pretreatments, such as sterilization, but with the advantage of being a fast method.
Table 6.
Different biological pretreatments applied to the production of biogas
| Biomass | Pretreatment | Degradation of hemicellulose (%) | Degradation of lignin (%) | Anaerobic digestion | Ybiogas | YAP | YBP | References |
|---|---|---|---|---|---|---|---|---|
| Corn stover silage | Fungal pretreatment (Phanerochaete chrysosporium) at 28 °C, 30 days | 32.4 | 22.6 | Batch, 37 °C, 30 days | Close to 500 mL g−1 VS | 265.1 mLCH4 g−1 VS | 215.5 mL CH4 g−1 VS | Liu et al. (2014) |
| Agropyron elongatum | Fungal (Flammulina velutipes) at 28 °C, 28 days | 29 | 35.4 | Batch, 37 °C, pH 7 and around 23 days | 398.1 mL g−1 VS | 169.2 mL CH4 g−1 VS | 125.75 mL CH4 g−1 VS | Lalak et al. (2016) |
| Rice straw | Acid oxalic + enzymatic (cellulase) at 45 °C for 72 h, 200 rpm | – | 63.1 | Batch, 35 °C, pH 7, 45 days | 318.3 mL g−1 rice straw | – | 50.84 mL biogas g−1 VS | Amnuaycheewa et al. (2016) |
| Rice straw | Acid citric + enzymatic (cellulase) at 45 °C for 72 h, 200 rpm | – | 20.8 | Batch, 35 °C, pH 7, 45 days | 322.1 mL g−1 rice straw | – | 50.84 mL biogas g−1 VS | Amnuaycheewa et al. (2016) |
| Corn stover | Enzymatic (laccase and Trametes versicolor) at 30 °C, 6 h | – | – | Batch, 30 days | 238.4 mL CH4 | 191.7 mL CH4 | Schroyen et al. (2015) | |
| Rice straw | Fungal (Pleurotus ostreatus) at 28 °C, 20 days | – | 33.4 | Batch, 37 °C, 20 days | 367 mL g−1 VS | 263 mL CH4 g−1 VS | 127 mL CH4 g−1 VS | Mustafa et al. (2016) |
| Yard trimmings | Fungal (Ceriporiopsis subvermispora) at 28 °C, 30 days | 9.8–16.2 | 14.8–20.2 | Batch, 37 °C, 28 days | – | 34.9–44.6 mLCH4 g−1 VS | 20 mL CH4 g−1 VS | Zhao et al. (2014) |
APAfter pretreatment
BPBefore pretreatment
On the other hand, fungal pretreatment is a low energy method with low chemical requirements, and it decreases the production of undesirable products (Sun and Cheng 2002). This is carried out by fungi such as white, brown, and soft rot. White rot fungi can produce enzymes with high hydrolytic capacity for biodegradation of lignocellulose, such as lignin peroxidase, manganese peroxidase, and lacasse. Pleurotus ostreatus and Trichoderma reesei were used to improve the biodegradability of rice straw; the pretreatment P. ostreatus was most effective at 75% moisture and reached 33.4% of lignin removal and a methane yield of up to 120% higher than the control without pretreatment (Mustafa et al. 2016). Other biological alternatives are the application of an aerobic upstream process using Trichoderma viride, obtaining an increase of up to three times the yield of methane from a mixture of organic waste (Mutschlechner et al. 2015). In addition, other combined pretreatments have been used; for example, cassava peels were evaluated using a combined alkaline and enzymatic pretreatment for bioethanol production followed by biogas production; the combined pretreatment improved the methane yield by up to 56% with respect to the control (Moshi et al. 2015). However, the fungal pretreatment presents some disadvantages, such as a long processing time of up to 30 days, precise conditions of growth, and loss of organic matter as a result of the microbial activity.
Another treatment utilized is ensiling, which is generally used to store wet biomass before being processed (Franco et al. 2016). In the ensilage, the microorganisms transform soluble carbohydrates to lactic acid, acetic acid, propionic acid, and butyric acid. During this process, the pH decreases to values of below 4, inhibiting the growth of microorganisms and favoring conservation of feedstock (Weiland 2010). However, the results also suggest that ensiling can improve methane production under specific conditions (Franco et al. 2016). In this sense, Haag et al. (2015) observed that methane yields from residues of amaranth ensiling were significantly higher than the ones corresponding to amaranth residues without ensiling by up to 31% more. Liu et al. (2014) reported that daily biogas generation and methane yield from corn stover silage were around of twice more than of corn stover. However, the improper handling of silage can lead to a loss of methane production of up to 40% (Zimmer 1980). Indeed, good production performance depends on many factors, such as the type of lignocellulosic biomass, particle size, humidity, environmental conditions, and others. Silage has the additional advantage of having raw material available throughout the year that does not depend on the temporary periods of cultivation.
In addition, bacteria with high hydrolytic capacity are used as biological pretreatment. Several bacteria have been reported to have celluloses synthesis capacity, such as Pseudomonas, Escherichia coli, Salmonella, and others (Zogaj et al. 2001; Ude et al. 2006). Muñoz et al. (2014) evaluated nine bacterial strains with endoglucanase activity for methane production from the biodegradation of microalgae; they observed an increase in the yield of methane of up to 158.7%. Moreover, Poszytek et al. (2016) constructed a microbial consortium with high cellulolytic activity, called MCHCA, for biodegradation of maize silage; they found that biogas production was increased by up to 38%.
Several studies have proposed the use of different combinations of pretreatments to improve methane production. For example, Matsakas et al. (2017) evaluated various pretreatment combinations: organosolv alone, organosolv plus dilute acid, and organosolv combined with dilute acid and cellulolytic enzymes. The authors observed short treatment times, and the highest yield was obtained when cellulolytic enzymes were used. However, it must be taken into account that the more steps involved in a process, the greater the cost of production, making the process economically unattractive and difficult to compete with fossil fuels. Therefore, it is necessary to develop simple, inexpensive, and efficient pretreatments that allow sustainable and economic processes.
The choice of pretreatment will depend on the characteristics of the biomass. Chandra et al. (2012) have indicated that the most suitable substrates for the production of biogas are those with higher contents of carbohydrates, proteins, and fats. In this sense, maize has a high carbohydrate content of around 74.5 g per 100 g (Mejía 2003); to degrade this type of crops with a biological pretreatment such as silage may be sufficient to obtain good yields. In lignocellulosic crops, the most biodegradable components are cellulose and hemicellulose, while lignin is more recalcitrant. There is a wide variety of options to degrade lignocellulosic biomass being the alkaline thermal treatment one of the most recommended as one of the pretreatments which allow to obtain methane yield increased by 63.9% without being necessary to reach very high temperatures (Table 5), and, at the same time, it can be economically feasible. Another pretreatment that presents a good effectiveness and accessible technology is the fungal pretreatment; by means of this treatment, the yield of methane has doubled from rice straw, but require long time of treatment (Table 6).
Bioaugmentation
Bioaugmentation is a feasible strategy that implies the introduction of specific exogenous microorganisms into a microbial community. Bioaugmentation is a strategy used for improving the start-up of a reactor (Ma et al. 2009), improving the performance of the process, or increasing the degradation capacities of a consortium (Goud et al. 2014). Bioaugmentation is an attractive technology that presents various advantages, such as requiring no prior pretreatment, therefore, simplifying the process, and it can allow the development of more economical processes (Wei 2016). Furthermore, bioaugmentation has been utilized to recover reactors that have presented failures due to the accumulation of volatile fatty acids during anaerobic digestion (Town and Dumonceaux 2016), as well as due to the utilization of high load rates.
Regarding the use of bioaugmentation to increase the production of biogas from lignocellulosic biomass, microorganisms have been used alone or in mixtures of microorganisms with high lignocellulosic degradative capacities. Enterobacter ludwigii was introduced in an anaerobic process and increased biogas yield by 47% in comparison to when no E. ludwigii was added externally (Goswami et al. 2016). Moreover, microorganisms with high lignocellulolytic activity, such as Clostridium stercorarium and Bacteroides cellulosolvens, have been used for consortium enrichment and to enhance the degradation of cellulose, hemicelluloses, and lignin in association with a thermal pretreatment (100–150 °C): they reached degradation of 78.2, 89, and 33.7%, respectively, and methane production was increased up to 246%, compared with a process without pretreatment (Hu et al. 2016). Another study reported that routine bioaugmentation with a cellulolytic culture for a treatment of cellulosic waste in the acid phase of a two-phase anaerobic digestion improved the formation of methane by 15% in comparison with a one-time bioaugmentation (Martin-Ryals et al. 2015). However, Ács et al. (2015) suggested that for bioaugmentation, using a single strain added to a microbial community, it is possible to obtain an improvement in biogas production. Methane production from brewery spent grain was improved through bioaugmentation with Pseudobutyrivibrio xylanivorans Mz5T up to 17% with respect to control (Čater et al. 2015).
However, most studies have been carried out in controlled environments, where it is possible to ensure the survival of exogenous microorganisms (El Fantroussi and Agathos 2005). On the other hand, it is possible that exogenous microorganisms added to the system have only a small contribution to process efficiency, due to their metabolic capabilities being insufficient to integrate to the indigenous bacterial population and are, therefore, flushed from the system.
Biohythane
The biological production of hydrogen presents low substrate conversion efficiency; in dark fermentation (Nath and Das 2004), but a significant proportion of volatile fatty acids (VFA) are also formed. VFA produced in this process can be later converted to methane through anaerobic digestion. In this way, it is possible to produce hydrogen and methane in two consecutive stages: in the first stage, hydrolysis and acidogenesis are carried out, producing VFA and hydrogen; in the second stage, the remaining organic material is converted to methane and CO2. This process consists of a two-stage production of hydrogen followed by methane production, which is known as biohythane (Cheng and Liu 2012). Biohythane is a type of clean energy that has advantages such as the reduction of fermentation time and a better control of the process due to the separation of the hydrogen and methane production stages (Liu et al. 2013; Monlau et al 2013b; Si et al. 2016). These two stages present great differences between them in terms of environmental, nutritional, and physiological conditions (Kongjan et al. 2011). The first stage of the process is carried out at a pH range of 5–6 and a hydraulic retention time (HRT) of 1–3 days, while the second step is carried out at a pH range of 7–8 and a HRT of 15–20 days (Mamimin et al. 2015).
Different lignocellulosic substrates have been utilized for obtaining biohythane, such as cornstalk, cattail, sugarcane, among others (Kumari and Das 2016; Si et al. 2016; Nkemka et al. 2015). In addition, different pretreatments have been evaluated to improve biohythane production. For example, it has been proposed to use a system called hydrothermal liquefaction, which has the function of converting biomass in a solid state to a liquid state. Using this method, good efficiency can be obtained with a high organic loading rate (Si et al. 2016). However, the formation of fermentation inhibitors, such as 5-hydroxymethyl furfural and furfural, also occurred during this process. Another alternative that has been suggested is bioaugmentation with an anaerobic fungus in a two-stage system for increased production rates of biohythane from corn silage (Nkemka et al. 2015). Kumari and Das (2016) evaluated the obtaining of hydrogen and methane from sugarcane, utilizing a fungal pretreatment, and results showed an improvement in hydrogen and methane yields of 40.3 and 86.9%, respectively, in comparison with raw sugarcane. The authors attributed the low yield of sugarcane without pretreatment to the crystalline structure and the presence of a lignin barrier, which prevented the interaction of microorganisms with the substrate.
Moreover, a novel system developed recently uses an electrochemical process to capture the CO2 present in biogas and thus generates a mixed biohythane product with a lower content of CO2 (from 40% to less than 15%). In this way, a biogas with a higher heat value of up to 669 kJ mol−1 can be obtained (Huang et al. 2017). The production of methane and hydrogen in two consecutive stages is an promising process, due to could be increase methane yield, enhanced quality of the biogas over, and furthermore, it is environmental friendly.
Future perspectives
The production of biogas through the use of lignocellulosic biomass as a renewable energy source is both sustainable and environmentally friendly. However, this process still has various technological barriers which should be overcome, such as the development of methods to enhance the biodegradation of lignocellulosic biomass to diminish production costs. In this sense, different processes have been proposed to make the substrate more bioavailable for microorganisms. Different chemical, physical, and biological pretreatments have been used, the latter ones of which generate fewer inhibitory byproducts. However, to date, most proposed processes include more than one pretreatment to mineralize lignocellulosic biomass, increasing the number of steps, and, therefore, increasing the cost of biogas production. In this context, the development of an easy, accessible, and economical technology is required.
On the other hand, the yield of methane obtained is largely influenced by the biomass used, with grain crops having the good yield per unit area, but their use may not be sustainable. While it is possible to obtain a high yield of methane from aquatic plants and algae, work must be done to develop a technology that permits large-scale production of these raw material. In addition, some other strategies can also be applied, such as: (a) Development of efficient technologies for cultivation and harvesting of the biomass; (b) the search for new biomass sources that are more biodegradable and that do not compete with the food supply; (c) the utilization of the degradation capacities of microorganisms to metabolize lignocellulose and reduce the number of steps in the process and thus make the process cheaper; (d) the development or adaptation of processes that allow obtaining the maximum possible biomass products, such as methane, hydrogen, electricity (biocell), fertilizers, among others.
Acknowledgements
The present work was supported by the Institute of Ecology (INECOL) (Project 20047/90013). Emir Martínez acknowledges the support from the Mexican Council for Science and Technology (CONACYT) through the CÁTEDRAS program (Research ID 1452, Research Project 596).
Compliance with ethical standards
Conflict of interest
The author declare that there is no conflict of interest regarding the publication of this paper.
References
- Ács N, Bagi Z, Rákhely G, Minárovics J, Nagy K, Kovács KL. Bioaugmentation of biogas production by a hydrogen-producing bacterium. Bioresour Technol. 2015;186:286–293. doi: 10.1016/j.biortech.2015.02.098. [DOI] [PubMed] [Google Scholar]
- Adanikin BA, Ogunwande GA, Adesanwo OO. Evaluation and kinetics of biogas yield from morning glory (Ipomoea aquatica) co-digested with water hyacinth (Eichhornia crassipes) Ecol Eng. 2017;98:98–104. [Google Scholar]
- Akinbile CO, Yusoff MS. Assessing water hyacinth (Eichhornia crassopes) and lettuce (Pistia stratiotes) effectiveness in aquaculture wastewater treatment. Int J Phytorem. 2012;14(3):201–211. doi: 10.1080/15226514.2011.587482. [DOI] [PubMed] [Google Scholar]
- Albano PE, Ruiz TT, Ramos MS, Casero LPJ, Vázquez PFM, Rodriguez MPL, et al. Seed germination and risks of using the invasive plant Eichhornia crassipes (Mart.) Solms-Laub. (water hyacinth) for composting, ovine feeding and biogas production. Acta Bot Gallica. 2015;162(3):203–214. [Google Scholar]
- Ali N, Chaudhary BL, Panwar NL. The fungal pre-treatment of maize cob heart and water hyacinth for enhanced biomethanation. Int J Green Energy. 2014;11(1):40–49. [Google Scholar]
- Amnuaycheewa P, Hengaroonprasan R, Rattanaporn K, Kirdponpattar S, Cheenkachorn K, Sriariyanun M. Enhancing enzymatic hydrolysis and biogas production from rice straw by pretreatment with organic acids. Ind Crops Prod. 2016;87:247–254. [Google Scholar]
- Arreola-Vargas J, Ojeda-Castillo V, Snell-Castro R, Corona-González RI, Alatriste-Mondragón F, Méndez-Acosta HO. Methane production from acid hydrolysates of Agave tequilana bagasse: evaluation of hydrolysis conditions and methane yield. Bioresour Technol. 2015;181:191–199. doi: 10.1016/j.biortech.2015.01.036. [DOI] [PubMed] [Google Scholar]
- Arreola-Vargas J, Flores-Larios A, González-Álvarez V, Corona-González RI, Méndez-Acosta HO. Single and two-stage anaerobic digestion for hydrogen and methane production from acid and enzymatic hydrolysates of Agave tequilana bagasse. Int J Hydrogen Energy. 2016;41(2):897–904. [Google Scholar]
- Ayala-Parra P, Liu Y, Field JA, Sierra-Alvarez R. Nutrient recovery and biogas generation from the anaerobic digestion of waste biomass from algal biofuel production. Renew Energ. 2017;108:410–416. [Google Scholar]
- Bolado-Rodríguez S, Toquero C, Martín-Juárez J, Travaini R, García-Encina PA. Effect of thermal, acid, alkaline and alkaline-peroxide pretreatments on the biochemical methane potential and kinetics of the anaerobic digestion of wheat straw and sugarcane bagasse. Bioresour Technol. 2016;201:182–190. doi: 10.1016/j.biortech.2015.11.047. [DOI] [PubMed] [Google Scholar]
- Börjesson P, Mattiasson B. Biogas as a resource-efficient vehicle fuel. Trends Biotechnol. 2008;26(1):7–13. doi: 10.1016/j.tibtech.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Brennan L, Owende P. Biofuels from microalgae—a review of technologies for production, processing and extractions of biofuels and co-products. Renew Sustain Energy Rev. 2010;14:557–577. [Google Scholar]
- Buranov AU, Mazza G. Lignin in Straw of herbaceous crops. Ind Crops Prod. 2008;28:237–259. [Google Scholar]
- Cardeña R, Moreno G, Bakonyi P, Buitrón G. Enhancement of methane production from various microalgae cultures via novel ozonation pretreatment. Chem Eng J. 2017;307:948–954. [Google Scholar]
- Čater M, Fanedl L, Malovrh Š, Logar RM. Biogas production from brewery spent grain enhanced by bioaugmentation with hydrolytic anaerobic bacteria. Bioresour Technol. 2015;186:261–269. doi: 10.1016/j.biortech.2015.03.029. [DOI] [PubMed] [Google Scholar]
- Chandra R, Takeuchi H, Hasegawa T. Methane production from lignocellulosic agricultural crop wastes: a review in context to second generation of biofuel production. Renew Sustain Energy Rev. 2012;16(3):1462–1476. [Google Scholar]
- Chen M, Smith PM. The US cellulosic biofuels industry: expert views on commercialization drivers and barriers. Biomass Bioenergy. 2017;102:52–61. [Google Scholar]
- Chen CY, Zhao XQ, Yen HW, Ho SH, Cheng CL, Lee DJ, et al. Microalgae-based carbohydrates for biofuel production. Biochem Eng J. 2013;78(15):1–10. [Google Scholar]
- Cheng XY, Liu CZ. Enhanced coproduction of hydrogen and methane from cornstalks by a three-stage anaerobic fermentation process integrated with alkaline hydrolysis. Bioresour Technol. 2012;104:373–379. doi: 10.1016/j.biortech.2011.10.082. [DOI] [PubMed] [Google Scholar]
- Correa DF, Beyer HL, Possingham HP, Thomas-Hall SR, Schenk PM. Biodiversity impacts of bioenergy production: microalgae vs. first generation biofuels. Renew Sustain Energy Rev. 2017;74:1131–1146. [Google Scholar]
- Dai J, Bean B, Brown B, Bruening W, Edwards J, Flowers M, et al. Harvest index and straw yield of five classes of wheat. Biomass Bioenergy. 2016;85:223–227. [Google Scholar]
- DeMarco PM. Reachel Carson’s environmental ethic—a guide for global system decision making. J Cleaner Prod. 2017;140:127–133. [Google Scholar]
- El Fantroussi S, Agathos SN. Is bioaugmentation a feasible strategy for pollutant removal and site remediation? Curr Opin Microbiol. 2005;8(3):268–275. doi: 10.1016/j.mib.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Ertem FC, Neubauer P, Junne S. Environmental life cycle assessment of biogas production from marine macroalgal feedstock for the substitution of energy crops. J Cleaner Prod. 2017;140:977–985. [Google Scholar]
- Fernandes TV, Bos GK, Zeeman G, Sanders JPM, Van Lier JB. Effects of thermo-chemical pre-treatment on anaerobic biodegradability and hydrolysis of lignocellulosic biomass. Bioresour Technol. 2009;100(9):2575–2579. doi: 10.1016/j.biortech.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Mejía D (2003) Maize: Post-Harvest Operation, Food and Agriculture Organization of the United Nations (FAO), AGST
- Franco RT, Buffière P, Bayard R. Ensiling for biogas production: critical parameters. A review. Biomass Bioenergy. 2016;94:94–104. [Google Scholar]
- Galbe M, Zacchi G. Biofuels. Berlin Heidelberg: Springer; 2007. Pretreatment of lignocellulosic materials for efficient bioethanol production; pp. 41–65. [DOI] [PubMed] [Google Scholar]
- Gao J, Chen L, Yan Z, Wang L. Effect of ionic liquid pretreatment on the composition, structure and biogas production of water hyacinth (Eichhornia crassipes) Bioresour Technol. 2013;132:361–364. doi: 10.1016/j.biortech.2012.10.136. [DOI] [PubMed] [Google Scholar]
- García-Aparicio MP, Ballesteros I, González A, Oliva JM, Ballesteros M, Negro MJ. Effect of inhibitors released during steam-explosion pretreatment of barley straw on enzymatic hydrolysis. Appl Biochem Biotechnol. 2006;129(1):278–288. doi: 10.1385/abab:129:1:278. [DOI] [PubMed] [Google Scholar]
- Ghimire A, Kumar G, Sivagurunathan P, Shobana S, Saratale GD, et al. Bio-hythane production from microalgae biomass: key challenges and potential opportunities for algal bio-refineries. Bioresour Technol. 2017;241:525–536. doi: 10.1016/j.biortech.2017.05.156. [DOI] [PubMed] [Google Scholar]
- Giménez JB, Aguado D, Bouzas A, Ferrer J, Seco A. Use of rumen microorganisms to boost the anaerobic biodegradability of microalgae. Algal Res. 2017;24:309–316. [Google Scholar]
- Gissén C, Prade T, Kreuger E, Nges IA, Rosenqvist H, Svensson SE, et al. Comparing energy crops for biogas production—yields, energy input and costs in cultivation using digestate and mineral fertilisation. Biomass Bioenergy. 2014;64:199–210. [Google Scholar]
- González LML, Reyes IP, Dewulf J, Budde J, Heiermann M, Vervaeren H. Effect of liquid hot water pre-treatment on sugarcane press mud methane yield. Bioresour Technol. 2014;169:284–290. doi: 10.1016/j.biortech.2014.06.107. [DOI] [PubMed] [Google Scholar]
- González-Fernández C, Sialve B, Bernet N, Steyer JP. Impact of microalgae characteristics on their conversion to biofuel. Part II: focus on biomethane production. Biofuels Bioprod Biorefin. 2012;6(2):205–218. [Google Scholar]
- Goswami R, Mukherjee S, Chakraborty AK, Balachandran S, Babu SPS, Chaudhury S. Optimization of growth determinants of a potent cellulolytic bacterium isolated from lignocellulosic biomass for enhancing biogas production. Clean Technol Environ Policy. 2016;18(5):1565–1583. [Google Scholar]
- Goud RK, Sarkar O, Chiranjeevi P, Mohan SV. Bioaugmentation of potent acidogenic isolates: a strategy for enhancing biohydrogen production at elevated organic load. Bioresour Technol. 2014;165:223–232. doi: 10.1016/j.biortech.2014.03.049. [DOI] [PubMed] [Google Scholar]
- Guo X, Xiao D, Tian K, Yu H. Biomass production and litter decomposition of lakeshore plants in Napahai wetland, Northwestern Yunnan Plateau, China. Acta Ecol Sin. 2013;33(5):1425–1432. [Google Scholar]
- Haag NL, Nägele HJ, Fritz T, Oechsner H. Effects of ensiling treatments on lactic acid production and supplementary methane formation of maize and amaranth—an advanced green biorefining approach. Bioresour Technol. 2015;178:217–225. doi: 10.1016/j.biortech.2014.08.048. [DOI] [PubMed] [Google Scholar]
- Hendriks ATWM, Zeeman G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour Technol. 2009;100:10–18. doi: 10.1016/j.biortech.2008.05.027. [DOI] [PubMed] [Google Scholar]
- Hernández-García H, Olguín EJ, Sánchez-Galván G, Monroy-Hermosillo O. Production of volatile fatty acids during the hydrolysis and acidogenesis of Pistia stratiotes using ruminal fluid. Water Air Soil Pollut. 2015;226(9):317. [Google Scholar]
- Herrmann C, Heiermann M, Idler C. Effects of ensiling, silage additives and storage period on methane formation of biogas crops. Bioresour Technol. 2011;102(8):5153–5161. doi: 10.1016/j.biortech.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Herrmann C, Idle C, Heiermann M. Biogas crops grown in energy crop rotations: linking chemical composition and methane production characteristics. Bioresour Technol. 2016;206:23–35. doi: 10.1016/j.biortech.2016.01.058. [DOI] [PubMed] [Google Scholar]
- Hesami SM, Zilouei H, Karimi K, Asadinezhad A. Enhanced biogas production from sunflower stalks using hydrothermal and organosolv pretreatment. Ind Crops Prod. 2015;76:449–455. [Google Scholar]
- Himanshu H, Voelklein MA, Murphy JD, Grant J, O’Kiely P. Factors controlling headspace pressure in a manual manometric BMP method can be used to produce a methane output comparable to AMPTS. Bioresour Technol. 2017;238:633–642. doi: 10.1016/j.biortech.2017.04.088. [DOI] [PubMed] [Google Scholar]
- Hu Y, Hao X, Wang J, Cao Y. Enhancing anaerobic digestion of lignocellulosic materials in excess sludge by bioaugmentation and pre-treatment. Waste Manage. 2016;49:55–63. doi: 10.1016/j.wasman.2015.12.006. [DOI] [PubMed] [Google Scholar]
- Huang Z, Lu L, Jiang D, Xing D, Ren ZJ. Electrochemical hythane production for renewable energy storage and biogas upgrading. Appl Energy. 2017;187:595–600. [Google Scholar]
- Jankowska E, Sahu AK, Oleskowicz-Popiel P. Biogas from microalgae: review on microalgae’s cultivation, harvesting and pretreatment for anaerobic digestion. Renew Sustain Energy Rev. 2017;75:692–709. [Google Scholar]
- Jiang X, Sommer SG, Christensen KV. A review of the biogas industry in China. Energy Policy. 2011;39:6073–6081. [Google Scholar]
- Jiang X, Song X, Chen Y, Zhang W. Research on biogas production potential of aquatic plants. Renew Energy. 2014;69:97–102. [Google Scholar]
- Karray R, Hamza M, Sayadi S. Production and characterization of enzymatic cocktail produced by Aspergillus niger using green macroalgae as nitrogen source and its application in the pre-treatment for biogas production from Ulva rigida. Bioresour Technol. 2016;216:622–628. doi: 10.1016/j.biortech.2016.05.067. [DOI] [PubMed] [Google Scholar]
- Klassen V, Blifernez-Klassen O, Wibberg D, Winkler A, Kalinowski J, Posten C, Kruse O. Highly efficient methane generation from untreated microalgae biomass. Biotechnol Biofuels. 2017;10(1):186. doi: 10.1186/s13068-017-0871-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimiuk E, Pokój T, Budzyński W, Dubis B. Theoretical and observed biogas production from plant biomass of different fibre contents. Bioresour Technol. 2010;101(24):9527–9535. doi: 10.1016/j.biortech.2010.06.130. [DOI] [PubMed] [Google Scholar]
- Knappert D, Grethlein H, Converse A (1981) Partial acid hydrolysis of poplar wood as a pretreatment for enzymatic hydrolysis. Biotechnol Bioeng Symp; (United States) (vol 11, No. CONF-810554-). Dartmouth Coll., Hanover, NH, pp 67–77
- Kobayashi T, Wu YP, Lu ZJ, Xu KQ. Characterization of anaerobic degradability and kinetics of harvested submerged aquatic weeds used for nutrient phytoremediation. Energies. 2015;8(1):304–318. [Google Scholar]
- Kongjan P, Sompong O, Angelidaki I. Performance and microbial community analysis of two-stage process with extreme thermophilic hydrogen and thermophilic methane production from hydrolysate in UASB reactors. Bioresour Technol. 2011;102(5):4028–4035. doi: 10.1016/j.biortech.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Koyama M, Yamamoto S, Ishikawa K, Ban S, Toda T. Anaerobic digestion of submerged macrophytes: chemical composition and anaerobic digestibility. Ecol Eng. 2014;69:304–309. [Google Scholar]
- Koyama M, Yamamoto S, Ishikawa K, Ban S, Toda T. Inhibition of anaerobic digestion by dissolved lignin derived from alkaline pre-treatment of an aquatic macrophyte. Chem Eng J. 2017;311:55–62. [Google Scholar]
- Koyama M, Watanabe K, Kurosawa N, Ishikawa K, Ban S, Toda T. Effect of alkaline pretreatment on mesophilic and thermophilic anaerobic digestion of a submerged macrophyte: inhibition and recovery against dissolved lignin during semi-continuous operation. Bioresour Technol. 2017;238:666–674. doi: 10.1016/j.biortech.2017.04.046. [DOI] [PubMed] [Google Scholar]
- Kröger M, Müller-Langer F. Review on possible algal-biofuel production processes. Biofuels. 2012;3(3):333–349. [Google Scholar]
- Kumari S, Das D. Biologically pretreated sugarcane top as potential raw material for the enhancement of gaseous energy recovery by two stage byohythane. Bioresour Technol. 2016;218:1090–1097. doi: 10.1016/j.biortech.2016.07.070. [DOI] [PubMed] [Google Scholar]
- Lalak J, Kasprzycka A, Martyniak D, Tys J. Effect of biological pretreatment of Agropyron elongatum ‘BAMAR’ on biogas production by anaerobic digestion. Bioresour Technol. 2016;200:194–200. doi: 10.1016/j.biortech.2015.10.022. [DOI] [PubMed] [Google Scholar]
- Laureano-Perez L, Teymouri F, Alizadeh H, Dale BE. Understanding factors that limit enzymatic hydrolysis of biomass. Appl Biochem Biotechnol. 2005;121:1081–1099. doi: 10.1385/abab:124:1-3:1081. [DOI] [PubMed] [Google Scholar]
- Laurens LM, Chen-Glasser M, McMillan JD. A perspective on renewable bioenergy from photosynthetic algae as feedstock for biofuels and bioproducts. Algal Res. 2017;24:261–264. [Google Scholar]
- Lazuka A, Roland C, Barakat A, Guillon F, O’Donohue M, Hernandez-Raquet G. Ecofriendly lignocellulose pretreatment to enhance the carboxylate production of a rumen-derived microbial consortium. Bioresour Technol. 2017;236:225–233. doi: 10.1016/j.biortech.2017.03.083. [DOI] [PubMed] [Google Scholar]
- Lee K, Chantrasakdakul P, Kim D, Kim JS, Park KY. Biogas productivity of algal residues from bioethanol production. J Mater Cycles Waste Manage. 2017;19(1):235–240. [Google Scholar]
- Liu Z, Zhang C, Lu Y, Wu X, Wang L, Wang L, et al. States and challenges for high-value biohythane production from waste biomass by dark fermentation technology. Bioresour Technol. 2013;135:292–303. doi: 10.1016/j.biortech.2012.10.027. [DOI] [PubMed] [Google Scholar]
- Liu S, Li X, Wu S, He J, Pang C, Deng Y, Dong R. Fungal pretreatment by Phanerochaete chrysosporium for enhancement of biogas production from corn stover silage. Appl Biochem Biotechnol. 2014;174(5):1907–1918. doi: 10.1007/s12010-014-1185-7. [DOI] [PubMed] [Google Scholar]
- Lizasoain J, Trulea A, Gittinger J, Kral I, Piringer G, Schedl A, et al. Corn stover for biogas production: effect of steam explosion pretreatment on the gas yields and on the biodegradation kinetics of the primary structural compounds. Bioresour Technol. 2017;244:949–956. doi: 10.1016/j.biortech.2017.08.042. [DOI] [PubMed] [Google Scholar]
- Ma F, Guo JB, Zhao LJ, Chang CC, Cui D. Application of bioaugmentation to improve the activated sludge system into the contact oxidation system treating petrochemical wastewater. Bioresour Technol. 2009;100(2):597–602. doi: 10.1016/j.biortech.2008.06.066. [DOI] [PubMed] [Google Scholar]
- Mahdy A, Ballesteros M, González-Fernández C. Enzymatic pretreatment of Chlorella vulgaris for biogas production: influence of urban wastewater as a sole nutrient source on macromolecular profile and biocatalyst efficiency. Bioresour Technol. 2016;199:319–325. doi: 10.1016/j.biortech.2015.08.080. [DOI] [PubMed] [Google Scholar]
- Mamimin C, Singkhala A, Kongjan P, Suraraksa B, Prasertsan P, Imai T, Sompong O. Two-stage thermophilic fermentation and mesophilic methanogen process for biohythane production from palm oil mill effluent. Int J Hydrogen Energy. 2015;40(19):6319–6328. [Google Scholar]
- Markou G, Brulé M, Balafoutis A, Kornaros M, Georgakakis D, Papadakis G. Biogas production from energy crops in northern Greece: economics of electricity generation associated with heat recovery in a greenhouse. Clean Technol Environ Policy. 2017;19(4):1147–1167. [Google Scholar]
- Martin-Ryals A, Schideman L, Li P, Wilkinson H, Wagner R. Improving anaerobic digestion of a cellulosic waste via routine bioaugmentation with cellulolytic microorganisms. Bioresour Technol. 2015;189:62–70. doi: 10.1016/j.biortech.2015.03.069. [DOI] [PubMed] [Google Scholar]
- Matsakas L, Nitsos C, Vörös D, Rova U, Christakopoulos P. High-titer methane from organosolv-pretreated spruce and birch. Energies. 2017;10(3):263. [Google Scholar]
- McKendry P. Energy production from biomass (part 1): overview of biomass. Bioresour Technol. 2002;83(1):37–46. doi: 10.1016/s0960-8524(01)00118-3. [DOI] [PubMed] [Google Scholar]
- Mishima D, Kuniki M, Sei K, Soda S, Ike M, Fujita M. Ethanol production from candidate energy crops: water hyacinth (Eichhornia crassipes) and water lettuce (Pistia stratiotes L.) Bioresour Technology. 2008;99(7):2495–2500. doi: 10.1016/j.biortech.2007.04.056. [DOI] [PubMed] [Google Scholar]
- Monlau F, Latrille E, Da Costa AC, Steyer JP, Carrère H. Enhancement of methane production from sunflower oil cakes by dilute acid pretreatment. Appl Energy. 2013;102:1105–1113. [Google Scholar]
- Monlau F, Barakat A, Trably E, Dumas C, Steyer JP, Carrère H. Lignocellulosic materials into biohydrogen and biomethane: impact of structural features and pretreatment. Crit Rev Environ Sci Technol. 2013;43(3):260–322. [Google Scholar]
- Moshi AP, Temu SG, Nges IA, Malmo G, Hosea KM, Elisante E, Mattiasson B. Combined production of bioethanol and biogas from peels of wild cassava Manihot glaziovii. Chem Eng J. 2015;279:297–306. [Google Scholar]
- Muñoz C, Hidalgo C, Zapata M, Jeison D, Riquelme C, Rivas M. Use of cellulolytic marine bacteria for enzymatic pretreatment in microalgal biogas production. Appl Environ Microbiol. 2014;80(14):4199–4206. doi: 10.1128/AEM.00827-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussgnug JH, Klassen V, Schlüter A, Kruse O. Microalgae as substrates for fermentative biogas production in a combined biorefinery concept. J Biotechnol. 2010;150(1):51–56. doi: 10.1016/j.jbiotec.2010.07.030. [DOI] [PubMed] [Google Scholar]
- Mustafa AM, Poulsen TG, Sheng K. Fungal pretreatment of rice straw with Pleurotus ostreatus and Trichoderma reesei to enhance methane production under solid-state anaerobic digestion. Appl Energy. 2016;180:661–671. [Google Scholar]
- Mutschlechner M, Illmer P, Wagner AO. Biological pre-treatment: enhancing biogas production using the highly cellulolytic fungus Trichoderma viride. Waste Manage. 2015;43:98–107. doi: 10.1016/j.wasman.2015.05.011. [DOI] [PubMed] [Google Scholar]
- Naik SN, Goud VV, Rout PK, Dalai AK. Production of first and second generation biofuels: a comprehensive review. Renew Sustain Energy Rev. 2010;14(2):578–597. [Google Scholar]
- Nath K, Das D. Improvement of fermentative hydrogen production: various approaches. Appl Microbiol Biotechnol. 2004;65(5):520–529. doi: 10.1007/s00253-004-1644-0. [DOI] [PubMed] [Google Scholar]
- Nkemka VN, Gilroyed B, Yanke J, Gruninger R, Vedres D, McAllister T, Hao X. Bioaugmentation with an anaerobic fungus in a two-stage process for biohydrogen and biogas production using corn silage and cattail. Bioresour Technol. 2015;185:79–88. doi: 10.1016/j.biortech.2015.02.100. [DOI] [PubMed] [Google Scholar]
- O’Sullivan C, Rounsefell B, Grinham A, Clarke W, Udy J. Anaerobic digestion of harvested aquatic weeds: water hyacinth (Eichhornia crassipes), cabomba (Cabomba Caroliniana) and salvinia (Salvinia molesta) Ecol Eng. 2010;36(10):1459–1468. [Google Scholar]
- Olguín EJ, García-López DA, González-Portela RE, Sánchez-Galván G. Year-round phytofiltration lagoon assessment using Pistia stratiotes within a pilot-plant scale biorefinery. Sci Total Environ. 2017;592:326–333. doi: 10.1016/j.scitotenv.2017.03.067. [DOI] [PubMed] [Google Scholar]
- Ostovareh S, Karimi K, Zamani A. Efficient conversion of sweet sorghum stalks to biogas and ethanol using organosolv pretreatment. Ind Crops Prod. 2015;66:170–177. [Google Scholar]
- Pakarinen A, Maijala P, Stoddard FL, Santanen A, Tuomainen P, Kymäläinen M, Viikari L. Evaluation of annual bioenergy crops in the boreal zone for biogas and ethanol production. Biomass Bioenergy. 2011;35(7):3071–3078. [Google Scholar]
- Poszytek K, Ciezkowska M, Sklodowska A, Drewniak L. Microbial consortium with high cellulolytic activity (MCHCA) for enhanced biogas production. Front Microbiol. 2016;7:324. doi: 10.3389/fmicb.2016.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Suárez JL, Carreras N. Use of microalgae residues for biogas production. Chem Eng J. 2014;242:86–95. [Google Scholar]
- Ravindran R, Jaiswal AK. A comprehensive review on pre-treatment strategy for lignocellulosic food industry waste: challenges and opportunities. Bioresour Technol. 2016;199:92–102. doi: 10.1016/j.biortech.2015.07.106. [DOI] [PubMed] [Google Scholar]
- Roberts DE, Church AG, Cummins SP. Invasion of Egeria into the Hawkesbury-Nepean River, Australia. J Aquat Plant Manage. 1999;37:31–34. [Google Scholar]
- Romero-Güiza MS, Vila J, Mata-Alvarez J, Chimenos JM, Astals S. The role of additives on anaerobic digestion: a review. Renew Sustain Energy Rev. 2016;58:1486–1499. [Google Scholar]
- Sasaki K, Okamoto M, Shirai T, Tsuge Y, Fujino A, Sasaki D, Morita M, Matsuda F, Kikuchi J, Kondo A. Toward the complete utilization of rice straw: methane fermentation and lignin recovery by a combinational process involving mechanical milling, supporting material and nanofiltration. Bioresour Technol. 2016;216:830–837. doi: 10.1016/j.biortech.2016.06.029. [DOI] [PubMed] [Google Scholar]
- Schievano A, D’imporzano G, Orzi V, Colombo G, Maggiore T, Adani F. Biogas from dedicated energy crops in Northern Italy: electric energy generation costs. Gcb Bioenergy. 2015;7(4):899–908. [Google Scholar]
- Schroyen M, Vervaeren H, Vandepitte H, Van Hulle SW, Raes K. Effect of enzymatic pretreatment of various lignocellulosic substrates on production of phenolic compounds and biomethane potential. Bioresour Technol. 2015;192:696–702. doi: 10.1016/j.biortech.2015.06.051. [DOI] [PubMed] [Google Scholar]
- Si BC, Li JM, Zhu ZB, Zhang YH, Lu JW, Shen RX, Zhang C, Xing X-H, Liu Z. Continuous production of biohythane from hydrothermal liquefied cornstalk biomass via two-stage high-rate anaerobic reactors. Biotechnol Biofuels. 2016;9:254. doi: 10.1186/s13068-016-0666-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddhu MAH, Li J, Zhang J, Huang Y, Wang W, Chen C, Liu G. Improve the anaerobic biodegradability by copretreatment of thermal alkali and steam explosion of lignocellulosic waste. BioMed Res Int. 2016 doi: 10.1155/2016/2786598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth BM, Murphy JD, O’Brien CM. What is the energy balance of grass biomethane in Ireland and other temperate northern European climates? Renew Sustain Energy Rev. 2009;13(9):2349–2360. [Google Scholar]
- Stürmer B, Schmid E, Eder MW. Impacts of biogas plant performance factors on total substrate costs. Biomass Bioenergy. 2011;35(4):1552–1560. [Google Scholar]
- Sun Y, Cheng J. Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour Technol. 2002;83:1–11. doi: 10.1016/s0960-8524(01)00212-7. [DOI] [PubMed] [Google Scholar]
- Taniguchi M, Suzuki H, Watanabe D, Sakai K, Hoshino K, Tanaka T. Evaluation of pretreatment with Pleurotus ostreatus for enzymatic hydrolysis of rice straw. J Biosci Bioeng. 2005;100(6):637–643. doi: 10.1263/jbb.100.637. [DOI] [PubMed] [Google Scholar]
- Tasnim F, Iqbal SA, Chowdhury AR. Biogas production from anaerobic co-digestion of cow manure with kitchen waste and Water Hyacinth. Renewable Energy. 2017;109:434–439. [Google Scholar]
- Tedesco S, Barroso TM, Olabi AG. Optimization of mechanical pre-treatment of Laminariaceae spp. biomass-derived biogas. Renew Energy. 2014;62:527–534. [Google Scholar]
- Town JR, Dumonceaux TJ. Laboratory-scale bioaugmentation relieves acetate accumulation and stimulates methane production in stalled anaerobic digesters. Appl Microbiol Biotechnol. 2016;100(2):1009–1017. doi: 10.1007/s00253-015-7058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tredici MR, Bassi N, Prussi M, Biondi N, Rodolfi L, Zittelli GC, Sampietro G. Energy balance of algal biomass production in a 1-ha “Green Wall Panel” plant: how to produce algal biomass in a closed reactor achieving a high net energy ratio. Appl Energy. 2015;154:1103–1111. [Google Scholar]
- Ude S, Arnold DL, Moon CD, Timms-Wilson T, Spiers AJ. Biofilm formation and cellulose expression among diverse environmental Pseudomonas isolates. Environ Microbiol. 2006;8(11):1997–2011. doi: 10.1111/j.1462-2920.2006.01080.x. [DOI] [PubMed] [Google Scholar]
- Valdez-Vazquez I, Acevedo-Benítez JA, Hernández-Santiago C. Distribution and potential of bioenergy resources from agricultural activities in Mexico. Renew Sustain Energy Rev. 2010;14(7):2147–2153. [Google Scholar]
- Voelklein MA, Rusmanis D, Murphy JD. Increased loading rates and specific methane yields facilitated by digesting grass silage at thermophilic rather than mesophilic temperatures. Bioresour Technol. 2016;216:486–493. doi: 10.1016/j.biortech.2016.05.109. [DOI] [PubMed] [Google Scholar]
- WBA (2017) World Bioenergy Association (WBA), 2017. Global Bioenergy Statistics 2017. http://www.worldbioenergy.org/uploads/WBA%20GBS%202017_hq.pdf. Accessed 20 Dec 2017
- Wei S. The application of biotechnology on the enhancing of biogas production from lignocellulosic waste. Appl Microbiol Biotechnol. 2016;100(23):9821–9836. doi: 10.1007/s00253-016-7926-5. [DOI] [PubMed] [Google Scholar]
- Weiland P. Biomass digestion in agriculture: a successful pathway for the energy production and waste treatment in Germany. Eng Life Sci. 2006;6(3):302–309. [Google Scholar]
- Weiland P. Biogas production: current state and perspectives. Appl Microbiol Biotechnol. 2010;85(4):849–860. doi: 10.1007/s00253-009-2246-7. [DOI] [PubMed] [Google Scholar]
- Weller NA, Childers DL, Turnbull L, Upham RF. Aridland constructed treatment wetlands I: macrophyte productivity, community composition, and nitrogen uptake. Ecol Eng. 2016;97:649–657. [Google Scholar]
- Yadav D, Barbora L, Bora D, Mitra S, Rangan L, Mahanta P. An assessment of duckweed as a potential lignocellulosic feedstock for biogas production. Int Biodeterior Biodegrad. 2017;119:253–259. [Google Scholar]
- Yahmed NB, Jmel MA, Alaya MB, Bouallagui H, Marzouki MN, Smaali I. A biorefinery concept using the green macroalgae Chaetomorpha linum for the coproduction of bioethanol and biogas. Energy Convers Manage. 2016;119:257–265. [Google Scholar]
- Yao Y, Bergeron AD, Davaritouchaee M. Methane recovery from anaerobic digestion of urea-pretreated wheat straw. Renew Energy. 2018;115:39–148. [Google Scholar]
- Yen HW, Brune DE. Anaerobic co-digestion of algal sludge and waste paper to produce methane. Bioresour Technol. 2007;98(1):130–134. doi: 10.1016/j.biortech.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Zhao J, Ge X, Vasco-Correa J, Li Y. Fungal pretreatment of unsterilized yard trimmings for enhanced methane production by solid-state anaerobic digestion. Bioresour Technol. 2014;158:248–252. doi: 10.1016/j.biortech.2014.02.029. [DOI] [PubMed] [Google Scholar]
- Zimmer E. Efficient silage systems. In: Thomas C, editor. Forage conserv. 80’s. Brighton: Bristish Grassland Society; 1980. pp. 186–197. [Google Scholar]
- Zogaj X, Nimtz M, Rohde M, Bokranz W, Römling U. The multicellular morphotypes of Salmonella typhimurium and Escherichia coli produce cellulose as the second component of the extracellular matrix. Mol Microbiol. 2001;39(6):1452–1463. doi: 10.1046/j.1365-2958.2001.02337.x. [DOI] [PubMed] [Google Scholar]