Abstract
Virus families have evolved different strategies for genome uncoating, which are also followed by recombinant vectors. Vectors derived from adeno-associated viruses (AAV) are considered as leading delivery tools for in vivo gene transfer, and in particular gene therapy. Using a combination of atomic force microscopy (AFM), biochemical experiments, and physical modeling, we investigated here the physical properties and stability of AAV vector particles. We first compared the morphological properties of AAV vectors derived from two different serotypes (AAV8 and AAV9). Furthermore, we triggered ssDNA uncoating by incubating vector particles to increasing controlled temperatures. Our analyses, performed at the single-particle level, indicate that genome release can occur in vitro via two alternative pathways: either the capsid remains intact and ejects linearly the ssDNA molecule, or the capsid is ruptured, leaving ssDNA in a compact entangled conformation. The analysis of the length distributions of ejected genomes further revealed a two-step ejection behavior. We propose a kinetic model aimed at quantitatively describing the evolution of capsids and genomes along the different pathways, as a function of time and temperature. This model allows quantifying the relative stability of AAV8 and AAV9 particles.
Electronic supplementary material
The online version of this article (10.1007/s10867-018-9488-5) contains supplementary material, which is available to authorized users.
Keywords: Capsid disassembly, Atomic force microscopy, Stochastic forces, Genome uncoating
Introduction
Adeno-associated virus (AAV) is a nonpathogenic parvovirus that has emerged as one of the most popular tools for in vivo gene transfer in particular gene therapy [1]. AAV vectors have capacity to transduce many different cell types in vivo leading to stable transgene expression in post-mitotic tissues. AAV vector particles are composed of a recombinant DNA genome (vector genome), generally single stranded (ss), packaged into an 18 to 25-nm capsid containing 60 capsid monomers arranged with T = 1 icosahedral symmetry. The capsid can be derived from several natural AAV serotypes, or from engineered capsids [2, 3]. Recent clinical trials with AAV vectors not only confirmed vector safety for intravenous as well as local vector administration but also demonstrated therapeutic effects [1]. Transduction efficiency of AAV depends on successful completion of a multistep infection process [4]. After binding to the cell receptor, AAV particles are endocytosed and transported toward the cell nucleus. AAV particles then enter the nucleus, through a not-yet-defined mechanism, where the ssDNA genome is released from the capsid, a process termed uncoating, and converted into a double-stranded (ds) DNA molecule. It is therefore conceivable to state that efficiency of cell transduction is at least depending on efficiency of vector internalization, including nuclear delivery and efficiency of uncoating in the nucleus [5, 6]. However, despite numerous attempts, mechanisms of AAV vectors uncoating is far from being understood. The genome-freeing process could either be the result of capsid disassembly and/or of a controlled ejection of the viral DNA from intact or partially disrupted capsid structure. The purpose of the present work was therefore to gain better insight into the genome uncoating process by investigating the physical properties of AAV vector particles and in particular their stability. Up to now, only a few studies have been conducted on physical properties of AAV vectors [7–11]. In particular, studies indicated that AAV capsid stability may vary according to the serotype and to the nature (ss or ds) and length of vector DNA that is packaged inside [7–9]. However, it is still unclear how these physical measures relate to the biological properties of the particles.
Using atomic force microscopy, biochemical tools, and physical modeling, we here investigated the morphology and thermodynamic stability of AAV vector particles, derived from two different natural serotypes, at the single-particle level. In particular, we quantified vector genome uncoating induced upon incubation of AAV particles at increasing temperatures. Testing particle stability upon temperature increase is quite natural, due to the inherent stochasticity within molecular self-assemblies. The thermal stability of AAV particles has been previously examined by using differential scanning calorimetry (DSC) [9]. In this work, we go beyond these ensemble measurements by using AFM to visualize the disassembly of AAV vector particles at the single-object level. Since viral capsid self-assemblyis a stochastic process, the temperature controls the stabitiliy of the assembly. At physiological temperature (37 °C), viral capsids are stable because the amplitude of stochastic forces is not strong enough to destabilize them. Within the classical description of stochastic effects, this stability is quantified by the typical lifetime of intact particles (i.e., the time needed for the molecular assembly to disassemble spontaneously), which is large compared to observation time scales for stable conformations [12]. Increasing the temperature is expected to reduce the time scale associated to capsid destabilization processes, like those taking place during genome uncoating. From an experimental point of view, the measurement of thermal stability can therefore produce quantitative information on the energetic barriers associated to stable particles at a physiological temperature.
In this study, we applied these analyses to AAV8 and AAV9-derived capsids, two relatively distant AAV serotypes derived from non-human primates and humans, respectively, which are extensively used for in vivo gene transfer, in particular in the liver or the central nervous system [2].
We show here that the analysis of AAV particle thermal stability associated to measurement of genome release allows to define two possible models of AAV vector genome ejection in vitro. We additionally show that both morphology and thermal stability can be used to discriminate between these two AAV serotypes, further suggesting a possible correlation between these two physical parameters with their biological behavior in vivo.
Methods
AAV vector production and purification
Stocks of AAV vector particles were generated by calcium phosphate transfection of HEK-293 cells as described previously [13]. The vector plasmid used in all of these experiments, AAVCMVeGFP, included the enhanced green fluorescence cDNA under the control of the cytomegalovirus promoter. The total length of the vector was 3769 bases, including the inverted terminal repeats. The helper plasmids used for production were pDG8 (a kind gift from P. Moullier, INSERM U649, France) for AAV8 vectors, and pXX6 [14], and pAAV2/9 (a kind gift from X. Xiao, University of Pennsylvania, USA). Vector particles were purified by two cesium chloride gradient ultracentrifugations and titered by dot blot and quantitative PCR (qPCR). AAV vector titers ranged between 1.1012 and 1.1013 genome-containing particles/ml (gp/ml).
Native dot blot
For the native dot blot analysis, samples were diluted in PBS and transferred to a nitrocellulose membrane using a vacuum blotter. After saturation, the membranes were incubated overnight at 4 °C with the B1 antibody (diluted 1/5 in blocking solution) [15]. After several PBS washing steps, a horseradish peroxidase-conjugated anti-mouse antibody (Sigma) was applied to the membrane at a 1/10000 dilution for 1 h at room temperature. Finally, the membranes were incubated for 5 min with an enhanced chemiluminescence reagent (SuperSignal® West Dura Extended Duration Chemiluminescent Substrate, Thermo Scientific) and exposed to an autoradiography film.
Sample preparation for AFM imaging
Vector stock solution was diluted in PBS (pH = 7.2) (to reach an approximate concentration of 0.5 to 1 × 1011 gp/ml) and then complemented with 5 mM of MgCl2 in order to favor vector particle and/or DNA adhesion on the mica surface.
In temperature-destabilization experiments, AAV vector particles were initially diluted in PBS (pH = 7.2), before incubation at defined temperatures (ranging from room temperature to 80 °C) for a given time (typically 15 min). Then the reaction was quenched for a few seconds on ice followed by a twofold dilution in water to reduce PBS monovalent ion concentration. Finally, as for the standard sample, 5 mM of MgCl2 was added and the vector solution was deposited onto a freshly cleaved mica surface for 5 min before rinsing with 1 ml of milliQ-Ultrapure© water and gently drying by nitrogen flow.
AFM imaging
The samples were imaged using a Nanoscope V Multimode 8 atomic force microscope Bruker, Billerica MA (USA). The images were obtained in Tapping Mode in air using TESP300 tips (resonant frequency ~300 kHz) or in PeakForce Mode in air at scanning rates of 1 to 2 Hz over scan areas of 0.5 to 3 μm wide. Imaging force during our measurements was limited to 100–120 pN.
AFM image analysis (see Supplementary Information)
The AFM images have been analyzed automatically using a homemade Matlab program allowing to extract morphological parameters for the detected objects [16]. In particular, each particle has been characterized by its height and its equivalent diameter. This last quantity was defined as the in-plane diameter of the particle at half of the maximal height (Fig. S2).
The DNA length distribution was obtained using a second home-made Matlab script used to remove the capsids from the image (height thresholding) and skeletonize the DNA fragments (Fig. S3) to measure their length distribution as a function of temperature.
To remove the DNA present as compact secondary structured molecule, the area/length parameter was restricted to an accepted range corresponding to an equivalent linear molecule diameter as measured on the images (7 to 15 nm).
Physical modeling of genome uncoating
As mentioned in the Introduction, genome uncoating is a stochastic process: following a biochemical trigger usually attributed to micro-environment conditions, viral vector particles undergo a conformational change leading to the liberation of nucleic acid in a molecular configuration compatible with further processing of the vector genome. From an energetic point of view, this conformational change is associated to a transition from a metastable state toward a stable state of lower energy. Following the classical description of rate theory [12], the transition between the two states, labeled arbitrarily A (metastable state) and C (stable state), is realized by crossing an energetic barrier localized at a reaction coordinate B. This is possible at a given temperature only if thermal energy is high enough. Increasing temperature in a metastable system allows therefore to speed up the transition toward the stable state. This is quantitatively demonstrated by estimating the mean first passage time to go from A to B. The temperature dependence of this average transition time can be obtained using the classical result of Kramer [12]:
| 1 |
where U′′(A) and U′′(B) are the curvature of the energy at the metastable state A and barrier’s top B, respectively, ∆U = U(B) − U(A) is the barrier height, T is the temperature in Kelvin, and k is the Boltzmann constant. The inverse of the mean first passage time is usually interpreted as the transition rate between metastable state A and stable state C within kinetic models.
In the particular case of genome uncoating, we learned from experiments that heating samples results mainly in two morphologies: either ssDNA is linearly ejected and the capsid is intact (at least up to a maximal temperature), or the capsid is disassembled, leaving folded or entangled ssDNA. Among the particles that eject linearly their genome, we quantified the ssDNA length distribution. Within this distribution, we were able to extract two sub-populations: particles that eject a small amount of ssDNA (linear length smaller than 670 nm) and particles with larger lengths (> 670 nm). By analyzing a large number of images, we were able to quantify the populations corresponding to these four states: closed capsids “C0”, ruptured capsids “C1” with entangled ssDNA, and intact capsids with a small length of ssDNA ejected “P0” and with a large length “P1”. These observations can be modeled at the simplest level using the pathway depicted in Fig. 5a with three irreversible transitions between the four states. The classical kinetic equations associated to this pathway are written as
| 2 |
where the constants ki are associated to each transition according to the pathway of Fig. 5a. The time-dependent evolution of these populations was obtained analytically by solving the previous equations leading to these formulas:
Fig. 5.
Kinetic model for AAV genome uncoating. a Uncoating pathway, showing the transitions among particle populations. Intact particles (C0) are destabilized either through translocation events (P0 and P1), or through capsid rupture (C1). The temperature-dependent rates are obtained using Kramer’s formula. b Relative population of P0 as function of reduced temperature (rescaled by thermal energy at T0 = 25 °C) at t = 15 min. Experimental data with a 10% error are represented in blue (the number is the temperature in Celsius degrees). The red curve is the best fit with the model proposed in a, providing ∆Ucp = ∆Ucc ≅ 20kT0 and ∆Upp ≅ 21kT0. The dashed orange curves represent spreading of the model around optimal values of parameters, for ∆Ucp = ∆Ucc = ∆Upp =20 kT0 (lower curve) and 22 kT0(upper curve). c Relative population of P0 as function of time at T = 65 °C. Experimental data with a 10% error are represented in blue (the number is the time in minutes). The parameters of b are used to compare the model with data, with identical legend for red and orange curves. The vertical lines represent the mean first passage time (τcp in dashed green lines and τpp in dashed blue lines) computed from the optimal values of the parameters
| 3 |
with PC0(0) the population of intact and closed capsids at the initial time. The comparison of this model with experimental data is discussed in the following section. In particular, all rates are assumed to follow Kramers law (see Eq. 1): . The constants Ai contain information on the second derivative of the energetic landscape at the bottom and the top of the barrier.
Results
Morphological characterization of AAV8 and AAV9 particles
Large-scale AFM imaging, typically in the range of 3 × 3 μm2 of AAV8 or AAV9 particles at room temperature (T = 25 °C) can be seen in Fig. 1a and b. AAV capsids are well dispersed on the mica surface and a single type of particle can be observed as detected by their apparent height. From several tens of such images, the statistical analysis performed using our Matlab script allowed us to isolate single AAV vector particles (Fig. S2 and [16]), and to extract several morphological parameters such as the equivalent diameter D (i.e., the diameter at half height of the AFM height profile) and the maximum height h. The results are represented as a 2D histogram with the two previous parameters (Fig. 1c and d). On such height/diameter maps, the color represents the probability to observe a particle with such D and h values. The projection of 2D histogram on each axis results in a more standard probability density distribution of the particle height (Fig. 1e and f) or diameter (Fig. 1g). Unlike the equivalent diameter, the maximum height can be directly compared for the two serotypes, as there is no effect of convolution by the tip: AAV8 is 15.9 ± 0.05 nm in height while AAV9 is slightly higher with 17.7 ± 0.05 nm. This slight difference in height could result either from different capsid structures and electrostatic charging, resulting in different adhesion properties as mica is also charged (negatively) or from different elastic parameters. We could expect a reduction of height upon adsorption under similar adhesion conditions to be associated to a decrease in stiffness [17] but it has been shown recently that such a difference in capsid adhesion could also produce a height difference without modifying the capsid stiffness [18].
Fig. 1.
Morphological analysis at thermodynamic equilibrium of AAV8 and AAV9 particles. AFM images of a AAV8 and b AAV9 capsids. 2D map of height and equivalent diameter for c AAV8 (N = 787) and d AAV9 (N = 633) capsids. The projections of the 2D maps were used to compare AAV8 and AAV9 (respectively e and f) height and g diameter distributions. No statistically significant difference on the equivalent diameter is observed, whereas the AAV9 capsid appears slightly higher than that of AAV8
Analysis of AAV8 thermodynamic stability
To analyze the effect of temperature on capsid stability, AAV8 vector particles were heated to distinct temperatures for 15 min (suppl. Fig. S1) and then deposited on the mica surface and imaged with AFM (Fig. 2). Below a temperature of 50 °C we observed exclusively intact capsids. By increasing the temperature to 60 °C, a limited number of ssDNA molecules were visible surrounding capsids showing no further evidence of rupture, and the state was therefore labeled as “intact”. At 65 and 70 °C, both free ssDNA (completely ejected) and ssDNA linked to capsids were observed. At higher temperatures (> 75 °C), intact capsids could not be detected anymore. However, ssDNA filaments were observed. The large range of temperature probed (37–75 °C) highlights the remarkable stability of AAV vectors.
Fig. 2.
AFM imaging of heat-induced AAV8 particles destabilization. Typical AFM images for different heating conditions: a T = 50 °C, b T = 60 °C, c T = 65 °C, d T = 70 °C, e T = 75 °C and f T = 80 °C. Each image is shown twice: on the right, a color scale of 25 nm is used to see the viral capsids, on the left, a 5-nm color scale is chosen to visualize the objects near the surface (the ssDNA width is below 1 nm)
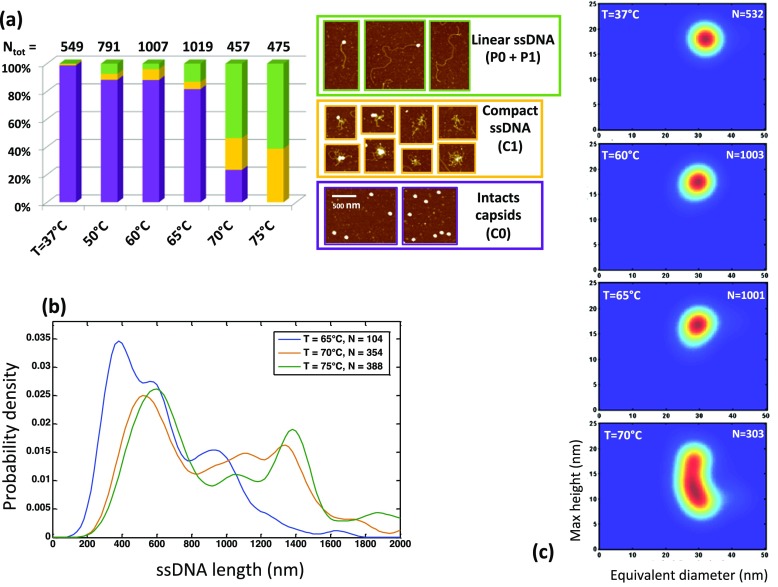
By analyzing hundreds of AFM images, we were able to distinguish in a first step three populations (Fig. 3a): intact capsids (labeled C0 following the model defined in the section 2.6 of methods), capsids linked with apparent linear ssDNA molecule (populations P0 and P1), and ruptured capsids with entangled ssDNA (population C1). We performed a ‘manual’ counting of all the objects that could be identified on AFM images to sort them in these three defined categories. For each experimental condition, we kept the same parameters of dilution and deposition and also analyzed the same number of objects in order to obtain comparable data sets in term of statistics. Quantitative measurements on AAV8 particles confirmed our first observations about the ssDNA ejection as a function of the temperature changes (Fig. 3a): at 70 °C, the proportion of intact capsids drastically decreased, and above this temperature, free DNA was observed on the surface.
Fig. 3.
DNA ejection from AAV8 particles as a function of temperature. a Statistical analysis of population distributions for AAV8 as a function of temperature. Three types of viral objects are defined for population counting: population C0 of intact particles (purple), population (P0 + P1) of particles with linearly ejected ssDNA (green), population C1 of ruptured capsids with entangled ssDNA (yellow). The total number of particles and/or molecules is indicated for each condition. b ssDNA length distribution is plotted at three temperatures. ssDNA length that corresponds to the full AAV DNA molecule (3.7 kb) is around 1400 nm. c Morphology of destabilized AAV8 particles as shown by 2D histogram representing the height as a function of the equivalent diameter probability under various temperatures
In order to quantify more precisely the population of intact capsids with linearly ejected ssDNA, we developed an automated Matlab script that could extract only ssDNA molecules from the image in order to measure their length (Fig. S3). In Fig. 3b we compared the ssDNA length distributions for three temperatures (T = 60, 65, and 75 °C). The analysis indicates that at lower temperature, the population of intact capsids with small ssDNA filaments (L ~ 200 nm) is dominant. When the temperature increases, more ssDNA is ejected from the capsid with full-length ssDNA molecules appearing at T = 70 °C.
To correlate the information about DNA ejection with the morphological data on destabilized capsids, we plotted a 2D histogram with particle height and equivalent diameter (Fig. 3c) throughout the heating process. A small deformation of the capsid started at 65 °C. As the colors of this map represent the probability, we can see that at T = 70 °C, a large portion of AAV8 capsids seems to be much lower (h~ 10 nm) than their usual height (h~ 17 nm). This correlates with the observation of ejected full-length DNA visible on the surface (Fig. 3b). Therefore, thermally induced ssDNA release is associated with a change in particle morphology.
Comparison with AAV9
We next analyzed the behavior of AAV9 vector accordingly. As described previously, four populations of capsids (C0, C1, P0, and P1) were detected (Fig. 4a and c). In contrast to AAV8, AAV9 vector particles started to eject some ssDNA filaments already at T = 37 °C. Nevertheless, most particles were still intact at 70 °C, arguing for a higher thermal stability of this serotype as compared to AAV8 (Fig. 3a). Remarkably, even at 75 °C, genome ejection from AAV9 particles was not complete. To confirm these observations, biochemical analyses of AAV8 and AAV9 capsids were performed by native dot blot (Fig. 4d). In this assay, capsid disassembly is indicated by antibody binding to a capsid epitope, localized inside the intact capsid that becomes externalized following capsid disassembly [15]. As indicated in Fig. 4d, and in line with our AFM measurements, AAV9 capsids are disassembled at a temperature nearly 10 °C higher than that required for AAV8 particles, allowing to conclude to a superior thermal stability of AAV9.
Fig. 4.
Comparison of thermal stability of AAV8 and AAV9 particles. a Typical AFM images as a function of temperature as indicated for AAV8 (top) and AAV9 (bottom). Statistical analysis of population distributions under temperature variations for b AAV8 and c AAV9 particles. d Native dot-blot analysis of AAV8 and AAV9 capsids incubated at different temperatures. After heating at the indicated temperature, AAV particles were bound on the membrane under non-denaturing conditions and incubated with the B1 antibody, which recognizes the internal part of the three VP capsid proteins [15]. A signal is present only when following capsid disassembly
Discussion
Since the capsid protects viral and vector genomes, genomes need to be released to allow transcription to take place. Thus, uncoating is a major rate-limiting step in the transduction process [5, 6], however the mechanisms underlying this process remain obscure. Parvoviral capsid, and in particular AAV, are known to be extremely stable against thermal and pH stress, leading to the hypothesis that uncoating is maybe more likely achieved by controlled genome ejection rather than by complete capsid disassembly [19, 20]. Here, we used AFM to measure the thermal stability of AAV capsids derived from serotypes 8 and 9. The measurements of AAV vector particle stability upon temperature increase is rationalized following a kinetic model presented in the Section 2. According to this model, intact particles are considered to be in a metastable state (labeled “C0”). Destabilization of capsids is associated with transitions from the initial metastable state toward a partially disassembled state, thanks to thermal fluctuations. Experimental observations strongly suggest that destabilization occurs either by linear ejection of ssDNA (“Pi” states), or by capsid rupture (“C1” state) (Fig. 5a). Interestingly, a previous study performed on capsid derived from the Minute Virus of Mice (MVM) and B19, two parvoviruses distantly related to AAV, exposed to high temperatures, suggested that genome ejection mainly occurs without complete capsid disassembly [21]. Our study rather suggests that, at least in the case of AAV vector particles, another mechanism involving complete capsid disassembly may exist. It remains to be verified whether this is unique to vector particles or also observed with AAV capsids containing the wild-type viral genome.
In our study, among the population of linearly ejecting capsids, we can distinguish between conformations associated with ejection of short and well-defined amount of ssDNA (“P0”), from those with an almost complete ejection of the genome (“P1”). For sake of simplicity, we chose half of the complete DNA length (670 nm) as a threshold. Thanks to our automated script measuring the length of ejected DNA, we could quantify the P0 and P1 populations, as well as the two other populations, C0 and C1. The kinetic model associated to the irreversible transitions among these populations is solved analytically (see Section 2). In particular, it provides time-dependent populations, as a function of model parameters. Within the simplest form of the model, these correspond to the transition rates kcp(T), kpp(T), and kcc(T). The comparison of experimental and theoretical relative population P0/(P0 + P1) as a function of temperature at imposed time t = 15 min for AAV8 provides an estimation of the parameter values. Using a combination of standard non-linear regression and Kramer’s formula for the transition rates , the optimal barrier heights for all three transitions are found to be of the same order of magnitude (Fig. 5b): ∆Ucp = 20.1kT0, ∆Ucc = 20.1kT0, and ∆Upp = 21.1kT0, with kT0 the thermal energy at room temperature T0 = 25 °C. Note that the Ai factor provides information on the curvature of the energetic potential, both in the metastable state and at the barrier’s top. For sake of simplicity, a common value assumed for this parameter was Acp = Acc = App ≅ 9.8 104s−1. In order to address further the quality of these parameters, we tested neighboring values of parameters and observed the deviation obtained from the optimal parameters. It turns out that a rather narrow range of energetic values (between ∆Ui = 20kT0 and ∆Ui = 22kT0) is able to describe quantitatively experimental values of populations and their maximal estimated deviation (10% error), as it is shown with the orange curves in Fig. 5b. The order of magnitude of 20kT0 for the energetic barrier of the first two transitions (C0 toward C1, and C0 toward P0) is consistent with a typical capsomere binding energy within capsids [22–24]. This implies that the rate-limiting step in order to trigger capsid disassembly is the removal of one capsomere, even in the case of linearly ejecting DNA. The last transition from P0 to P1 corresponds to a classical translocation process. Our measurements suggest that the energetic barrier associated to this transition is also of order 20kT0. For a pure translocation process, the energetic barrier is of entropic origin and it is expected to be smaller (between 1 and 10kT0) [25]. Our experimental value is likely to reflect either ssDNA adhesion within the capsid or the presence of secondary structure in ssDNA, therefore raising in both cases the height of the energetic barrier.
These energetic parameters can be used further to predict true kinetics of AAV8 particles destabilization at fixed temperature. We compared the prediction of the model with the experimental measurement of the kinetics at T = 65°C. We observe (Fig. 5c) that although the agreement between theoretical and experimental data is not complete, the order of magnitude of a typical time scale associated to intact capsid destabilization is correct. More precisely, the inverse of the transition rate, which gives the so-called mean first passage time (MFPT) within Kramers’ rate theory, provides an estimation of this time. For AAV8, the MFPT for ssDNA ejection and capsid rupture at T = 65 °C is τcp = τcc ≅ 480s ≅ 8 min. This can also be interpreted as the typical lifetime of intact capsids at this temperature.
Finally, we analyzed the data obtained on AAV9 particles within the kinetic model. The optimal parameters for the model are now Acp = Acc = App ≅ 18 s−1 and ∆Ucp = ∆Ucc = ∆Upp ≅ 11.4kT0. The barrier is much smaller, but the typical transition trial frequency Ai is also reduced, resulting in an overall lowering of the transition rate. The parameters of the model allowing to describe quantitatively the population P0/(P0 + P1) can then be used in order to compute the MFPT at T = 65 °C. Its value is now τcp = τcc = τpp ≅ 1234s ≅ 20 min. We observe therefore that the typical lifetime of AAV9 particles is almost twice that of AAV8.
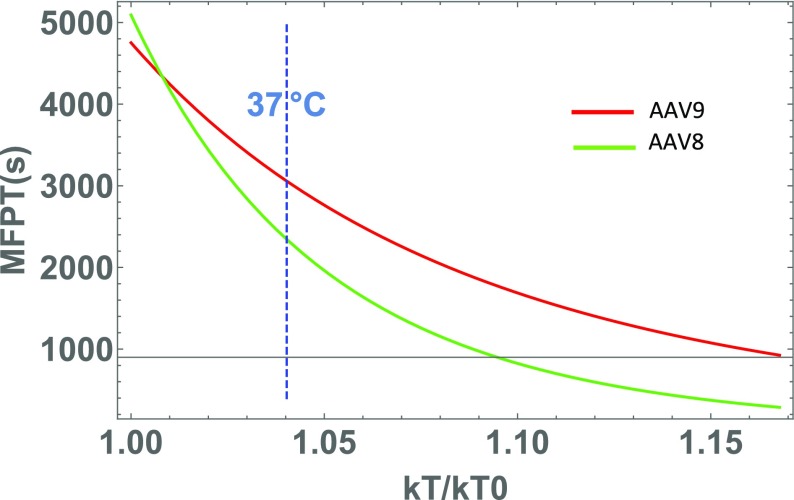
Thanks to Kramer’s formula, the MFPT for both serotypes can be extrapolated for a large range of temperatures (Fig. 6). This shows that its value increases as temperature decreases. More precisely, at 60 °C, the lifetime of AAV9 particles is roughly three times larger than the one of AAV8, while the model predicts that at T = 37 °C, this ratio drops to 1.2. We conclude that heating the particles allow to enhance their different destabilization.
Fig. 6.
Mean first passage time as a function of temperature comparison for AAV8 and AAV9 particles. The optimal parameters of the model are used to compute the mean first passage time (MFPT) according to Kramer’s formula for AAV8 (green) and AAV9 (red). The typical lifetime of AAV9 particles is larger than that of AAV8, expect at low temperatures
Conclusions
Within the present study, we investigated the morphology and thermal stability of two AAV vector particles, derived from two natural serotypes, at the single-particle level. We demonstrated that both parameters allowed to distinguish the two serotypes. We observed typically that there is a height increase for AAV9 as compared with AAV8, and that thermally induced genome release is more efficient for AAV8 than for AAV9. For both serotypes, using AFM images in order to monitor thermally induced AAV uncoating allowed to decipher two typical pathways for capsid disassembly: ssDNA is either linearly ejected from an almost intact capsid, or the capsid is completely disassembled and releases ssDNA molecules in a highly entangled conformation. Furthermore, by analyzing the length distribution of the linearly ejected ssDNA molecules, we showed that the translocation process leading to genome uncoating occurs in two steps: a small length of ssDNA is first ejected, and final ejection is hindered by an energetic barrier. If the temperature is high enough, the second energetic barrier is crossed, allowing full ejection. The typical values for energetic barriers found within our model are consistent with typical capsomere–capsomere binding energies, therefore validating our approach to quantify the stability of intact particles at room temperature.
Altogether, these results indicate that measuring capsid disassembly and genome ejection by AFM represents an interesting alternative to quantify typical disassembly pathways and energetic barriers associated to these processes. From the biological standpoint, this study provides for the first time insight into the two possible mechanisms leading to genome ejection in vivo. Further investigations will be required to determine the relevance of these mechanisms in vivo, in particular their cell specificity and their consequences on vector transduction efficiency. Understanding these mechanisms will be also essential to further improve vector development, in particular by capsid engineering [3].
Electronic supplementary material
Supplemental Figure 1 Protocol for the analysis of the AAV response to changes in temperature. Heating of AAV capsids is done for a fixed time at various temperatures ranging from RT to 80 °C before quenching on ice. Mg2+ ions are necessary for DNA binding onto mica, they are added after the heating process as divalent ions are expected to have an effect on capsid stability. The solution is incubated for 5 min on the mica to favor electrostatic adsorption of both DNA and AAV virions. Supplemental Fig. 2: AFM topographic image of AAV8 particles and automated image analysis. (a) raw AFM topographic image of AAV8 capsids, (b) binary image resulting from height and area thresholding, and (c) final topographic image after a few more steps including fractal parameter selection. The further measure of diameter, height or asymmetry on several hundreds of capsids analyzed one by one provides a quantitative morphological characterization of AAV. Supplemental Fig. 3: Automated image analysis of the DNA molecules ejected from the destabilized virions. Using a home-made Matlab script, we can remove the capsids from the image (height thresholding) and skeletonize the DNA fragments in order to measure their length distribution as a function of temperature. Each image with its number corresponds to a different step in the analysis procedure. (a) Initial AFM topographic image. (b) The capsids are removed by applying a height threshold that keep only pixels below 2 nm. (c) Image obtained after the virion has been removed and following by erosion of 1 to 2 pixels around the holes created by capsid removal (height color has been changed). (d) Binary image of DNA only obtained by applying a noise threshold (0.2 nm). (e) The skeleton of DNA filaments can be extracted from the binary image by using morphological tools (erosion). (f) Final topographic image corresponds to image in (c) where the DNA skeleton path has been overlapped in red, as it can clearly been distinguished in the image zoom. Supplemental Fig. 4: AFM imaging of AAV9 virion destabilization induced by heating. Typical AFM images for different heating conditions (a) T = 50 °C, (b) T = 60 °C, (c) T = 65 °C, (d) T = 70 °C, (e) T = 75 °C and (f) T = 80 °C. Each image is shown twice: on the right a color scale of 25 nm is used to see the viral capsids, on the left a 5 nm color scale is chosen to explore what is happening near the surface (ss-DNA width is below 1 nm). At T = 50 °C (a) opened capsids are already observed as DNA is visible on the surface. By increasing the temperature (b), (c) and (d), some filaments can still be observed around the capsids. At higher temperatures (e), both free DNA and DNA linked to capsids exist. At 80 °C mostly free DNA is detected on the surface (f). (PDF 5983 kb)
Acknowledgments
We would like to thank Federico Mingozzi for helpful discussions. This work was supported by Institut National de la Santé et de la Recherche Médicale (INSERM), Centre National de la Recherche Scientifique (CNRS; PEPS MPI). It was also funded by grants from the Ecole Normale Supérieure (ENS) de Lyon (to CFM and AS) and Association Française contre les Myopathies (AFM) to AS, HB, and CFM.
Authors’ contributions statement
JB, AR, AS, and CFM conceived and designed the experiments. JB, AF, AR, LG, and AL performed the experiments. JB, AF, AR, MC, HB, AS, and CFM analyzed the data. MC, AS, CFM wrote the paper. .
Funding
The authors declare no competing financial interests.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Julien Bernaud and Axel Rossi contributed equally to this work.
Electronic supplementary material
The online version of this article (10.1007/s10867-018-9488-5) contains supplementary material, which is available to authorized users.
Contributor Information
Martin Castelnovo, Email: martin.castelnovo@ens-lyon.fr.
Anna Salvetti, Email: anna.salvetti@inserm.fr.
Cendrine Faivre-Moskalenko, Email: cendrine.moskalenko@ens-lyon.fr.
References
- 1.Mingozzi F, High KA. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat. Rev. Genet. 2011;12(5):341–355. doi: 10.1038/nrg2988. [DOI] [PubMed] [Google Scholar]
- 2.Asokan A, Schaffer DV, Samulski RJ. The AAV vector toolkit: poised at the clinical crossroads. Mol. Ther. : J. Am. Soc. Gene Ther. 2012;20(4):699–708. doi: 10.1038/mt.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buning H, Huber A, Zhang L, Meumann N, Hacker U. Engineering the AAV capsid to optimize vector–host interactions. Curr. Opin. Pharmacol. 2015;24:94–104. doi: 10.1016/j.coph.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Nonnenmacher M, Weber T. Intracellular transport of recombinant adeno-associated virus vectors. Gene Ther. 2012;19(6):649–658. doi: 10.1038/gt.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hauck B, Zhao W, High C, Xiao W. Intracellular processing, not single-stranded DNA accumulation, is crucial for recombinant adeno-associated virus transduction. J. Virol. 2004;78:13678–13686. doi: 10.1128/JVI.78.24.13678-13686.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas CE, Storm TA, Huang Z, Kay MA. Rapid uncoating of vector genomes is the key to efficient liver transduction with pseudotyped adeno-associated virus vectors. J. Virol. 2004;78(6):3110–3122. doi: 10.1128/JVI.78.6.3110-3122.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennett A, Patel S, Mietzsch M, Jose A, Lins-Austin B, Yu JC, Bothner B, McKenna R, Agbandje-McKenna M. Thermal stability as a determinant of AAV serotype identity. Mol. Ther. Methods Clin. Dev. 2017;6:171–182. doi: 10.1016/j.omtm.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horowitz ED, Rahman KS, Bower BD, Dismuke DJ, Falvo MR, Griffith JD, Harvey SC, Asokan A. Biophysical and ultrastructural characterization of adeno-associated virus capsid uncoating and genome release. J. Virol. 2013;87(6):2994–3002. doi: 10.1128/JVI.03017-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rayaprolu V, Kruse S, Kant R, Venkatakrishnan B, Movahed N, Brooke D, Lins B, Bennett A, Potter T, McKenna R, Agbandje-McKenna M, Bothner B. Comparative analysis of adeno-associated virus capsid stability and dynamics. J. Virol. 2013;87(24):13150–13160. doi: 10.1128/JVI.01415-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Venkatakrishnan B, Yarbrough J, Domsic J, Bennett A, Bothner B, Kozyreva OG, Samulski RJ, Muzyczka N, McKenna R, Agbandje-McKenna M. Structure and dynamics of adeno-associated virus serotype 1 VP1-unique N-terminal domain and its role in capsid trafficking. J. Virol. 2013;87(9):4974–4984. doi: 10.1128/JVI.02524-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeng, C., Moller-Tank, S., Asokan, A., Dragnea, B.: Probing the link among genomic cargo, contact mechanics, and Nanoindentation in recombinant adeno-associated virus 2. J. Phys. Chem. B 121(8), 1843–1853 (2017a). 10.1021/acs.jpcb.6b10131 [DOI] [PMC free article] [PubMed]
- 12.Van Kampen, N.G.: Stochastic processes in physics and chemistry, 3rd edition. North-Holland (2007)
- 13.Salvetti A, Oreve S, Chadeuf G, Favre D, Cherel Y, Champion-Arnaud P, David-Ameline J, Moullier P. Factors influencing recombinant adeno-associated virus production. Hum. Gene Ther. 1998;9(5):695–706. doi: 10.1089/hum.1998.9.5-695. [DOI] [PubMed] [Google Scholar]
- 14.Xiao X, Li J, Samulski RJ. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J. Virol. 1998;72(3):2224–2232. doi: 10.1128/jvi.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wobus CE, Hugle-Dorr B, Girod A, Petersen G, Hallek M, Kleinschmidt JA. Monoclonal antibodies against the adeno-associated virus type 2 (AAV-2) capsid: epitope mapping and identification of capsid domains involved in AAV-2-cell interaction and neutralization of AAV-2 infection. J. Virol. 2000;74(19):9281–9293. doi: 10.1128/JVI.74.19.9281-9293.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faivre-Moskalenko C, Bernaud J, Thomas A, Tartour K, Beck Y, Iazykov M, Danial J, Lourdin M, Muriaux D, Castelnovo M. RNA control of HIV-1 particle size polydispersity. PLoS One. 2014;9(1):e83874. doi: 10.1371/journal.pone.0083874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Komura S, Tamura K, Kato T. Buckling of spherical shells adhering onto a rigid substrate. Eur. Phys. J. E. Soft Matter. 2005;18(3):343–358. doi: 10.1140/epje/e2005-00038-5. [DOI] [PubMed] [Google Scholar]
- 18.Zeng C, Hernando-Perez M, Dragnea B, Ma X, van der Schoot P, Zandi R. Contact mechanics of a small icosahedral virus. Phys. Rev. Lett. 2017;119(3):038102. doi: 10.1103/PhysRevLett.119.038102. [DOI] [PubMed] [Google Scholar]
- 19.Bleker S, Sonntag F, Kleinschmidt JA. Mutational analysis of narrow pores at the fivefold symmetry axes of adeno-associated virus type 2 capsids reveals a dual role in genome packaging and activation of phospholipase A2 activity. J. Virol. 2005;79(4):2528–2540. doi: 10.1128/JVI.79.4.2528-2540.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kronenberg S, Bottcher B, von der Lieth CW, Bleker S, Kleinschmidt JA. A conformational change in the adeno-associated virus type 2 capsid leads to the exposure of hidden VP1 N termini. J. Virol. 2005;79(9):5296–5303. doi: 10.1128/JVI.79.9.5296-5303.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ros C, Baltzer C, Mani B, Kempf C. Parvovirus uncoating in vitro reveals a mechanism of DNA release without capsid disassembly and striking differences in encapsidated DNA stability. Virology. 2006;345(1):137–147. doi: 10.1016/j.virol.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 22.Luque A, Zandi R, Reguera D. Optimal architectures of elongated viruses. Proc. Natl. Acad. Sci. U. S. A. 2010;107(12):5323–5328. doi: 10.1073/pnas.0915122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reddy VS, Giesing HA, Morton RT, Kumar A, Post CB, Brooks CL, 3rd, Johnson JE. Energetics of quasiequivalence: computational analysis of protein–protein interactions in icosahedral viruses. Biophys. J. 1998;74(1):546–558. doi: 10.1016/S0006-3495(98)77813-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zandi R, Reguera D. Mechanical properties of viral capsids. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2005;72(2 Pt 1):021917. doi: 10.1103/PhysRevE.72.021917. [DOI] [PubMed] [Google Scholar]
- 25.Muthukumar M. Polymer escape through a nanopore. J. Chem. Phys. 2003;118:5174. doi: 10.1063/1.1553753. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 Protocol for the analysis of the AAV response to changes in temperature. Heating of AAV capsids is done for a fixed time at various temperatures ranging from RT to 80 °C before quenching on ice. Mg2+ ions are necessary for DNA binding onto mica, they are added after the heating process as divalent ions are expected to have an effect on capsid stability. The solution is incubated for 5 min on the mica to favor electrostatic adsorption of both DNA and AAV virions. Supplemental Fig. 2: AFM topographic image of AAV8 particles and automated image analysis. (a) raw AFM topographic image of AAV8 capsids, (b) binary image resulting from height and area thresholding, and (c) final topographic image after a few more steps including fractal parameter selection. The further measure of diameter, height or asymmetry on several hundreds of capsids analyzed one by one provides a quantitative morphological characterization of AAV. Supplemental Fig. 3: Automated image analysis of the DNA molecules ejected from the destabilized virions. Using a home-made Matlab script, we can remove the capsids from the image (height thresholding) and skeletonize the DNA fragments in order to measure their length distribution as a function of temperature. Each image with its number corresponds to a different step in the analysis procedure. (a) Initial AFM topographic image. (b) The capsids are removed by applying a height threshold that keep only pixels below 2 nm. (c) Image obtained after the virion has been removed and following by erosion of 1 to 2 pixels around the holes created by capsid removal (height color has been changed). (d) Binary image of DNA only obtained by applying a noise threshold (0.2 nm). (e) The skeleton of DNA filaments can be extracted from the binary image by using morphological tools (erosion). (f) Final topographic image corresponds to image in (c) where the DNA skeleton path has been overlapped in red, as it can clearly been distinguished in the image zoom. Supplemental Fig. 4: AFM imaging of AAV9 virion destabilization induced by heating. Typical AFM images for different heating conditions (a) T = 50 °C, (b) T = 60 °C, (c) T = 65 °C, (d) T = 70 °C, (e) T = 75 °C and (f) T = 80 °C. Each image is shown twice: on the right a color scale of 25 nm is used to see the viral capsids, on the left a 5 nm color scale is chosen to explore what is happening near the surface (ss-DNA width is below 1 nm). At T = 50 °C (a) opened capsids are already observed as DNA is visible on the surface. By increasing the temperature (b), (c) and (d), some filaments can still be observed around the capsids. At higher temperatures (e), both free DNA and DNA linked to capsids exist. At 80 °C mostly free DNA is detected on the surface (f). (PDF 5983 kb)