Abstract
Stenotrophomonas maltophilia is an opportunistic Gram-negative pathogen with increasing incidence in clinical settings. The most critical aspect of S. maltophilia is its frequent resistance to a majority of the antibiotics of clinical use. Quorum Sensing (QS) systems coordinate bacterial populations and act as major regulatory mechanisms of pathogenesis in both pure cultures and poly-microbial communities. Disruption of QS systems, a phenomenon known as Quorum Quenching (QQ), represents a new promising paradigm for the design of novel antimicrobial strategies. In this context, we review the main advances in the field of QS in S. maltophilia by paying special attention to Diffusible Signal Factor (DSF) signaling, Acyl Homoserine Lactone (AHL) responses and the controversial Ax21 system. Advances in the DSF system include regulatory aspects of DSF synthesis and perception by both rpf-1 and rpf-2 variant systems, as well as their reciprocal communication. Interaction via DSF of S. maltophilia with unrelated organisms including bacteria, yeast and plants is also considered. Finally, an overview of the different QQ mechanisms involving S. maltophilia as quencher and as object of quenching is presented, revealing the potential of this species for use in QQ applications. This review provides a comprehensive snapshot of the interconnected QS network that S. maltophilia uses to sense and respond to its surrounding biotic or abiotic environment. Understanding such cooperative and competitive communication mechanisms is essential for the design of effective anti QS strategies.
Keywords: multi-drug resistance, quorum sensing, quorum quenching, nosocomial infections, antimicrobial resistance
Introduction
Stenotrophomonas maltophilia is a ubiquitous multidrug resistant Gram-negative bacterium that has emerged as an important nosocomial pathogen (Brooke, 2012; Adegoke et al., 2017) and stands as one of the most common lung pathogens in cystic fibrosis patients (Amin and Waters, 2014). The most important natural reservoir of this microorganism is thought to be the rhizosphere (Berg et al., 2005; Ryan et al., 2009), a highly competitive niche that facilitates the acquisition by bacteria of antimicrobial-resistance genes (Berg et al., 2005) and favors the establishment of communication networks between neighboring organisms (Bais et al., 2006). The result of this competitive coevolution appears to have a strong impact when translated to clinical environments.
Bacterial cells can communicate through the production and detection of signal molecules, a mechanism known as quorum sensing (QS) (Waters and Bassler, 2005; Papenfort and Bassler, 2016). Through cell-to-cell communication, bacterial populations synchronize gene expression and globally respond to changes in the environment, also during infection (Rutherford and Bassler, 2012). QS communication may also connect different bacterial species and even members of different kingdoms (Lowery et al., 2008). On the other end, the disruption of exogenous QS, a phenomenon termed Quorum Quenching (QQ), constitutes a varied and widespread protection mechanism exploited by bacterial competitors and by host defenses in case of infection (Dong et al., 2007). Indeed, interrupting bacterial QS strongly impairs bacterial pathogenic capacity (Kalia and Purohit, 2011).
Several different QS signals and QQ mechanisms have been identified in the last decades, significantly expanding our knowledge on bacterial communication (Kleerebezem et al., 1997; Dong et al., 2007; Deng et al., 2011; Kalia and Purohit, 2011; Ryan et al., 2015; Papenfort and Bassler, 2016; Zhou et al., 2017). Here, we review recent advances in the characterisation of the QS network of S. maltophilia, focusing on the two variants regulating the diffusible signal factor (DSF) system, as well as the QQ mechanisms in which this microorganism is involved. We also discuss the role of N-acyl homoserine lactone (AHL) signaling molecules and the controversial Ax21 system in the QS network of this species. Overall, this review provides a comprehensive picture of the signaling network that interconnects S. maltophilia with its surrounding environment.
DSF-quorum sensing in S. maltophilia
So far, the most studied QS system in S. maltophilia is that based on the DSF fatty acid (FA) signal cis-11-methyl-2-dodecenoic acid, originally described in Xanthomonas campestris pv. campestris (Xcc) (Barber et al., 1997). As a Xanthomonadales member and differently than the unrelated DSF-like-producing bacteria Burkholderia cenocepacia and Pseudomonas aeruginosa, S. maltophilia governs DSF communication through the genes co-localized in the rpf (regulation of pathogenicity factors) cluster (Huedo et al., 2015). Genes within this cluster include key enzymes of DSF synthesis, perception and signal transduction and are organized in two adjacent operons that are convergently transcribed. One operon is composed by the genes encoding for the FA ligase RpfB and the synthase RpfF, while the opposite operon encodes for a two-component system including the sensor kinase RpfC and the cytoplasmic regulator RpfG (Fouhy et al., 2007; Huedo et al., 2014b). Unlike Xanthomonas sp. and similar to Xylella fastidiosa, the rpf cluster in S. maltophilia does not encode for the transmembrane protein RpfH (Huedo et al., 2014b).
Two rpf cluster variants in S. maltophilia
A distinctive feature of the DSF system in S. maltophilia is the presence of two rpf cluster variants that produce and sense DSF signals distinctly and regulate important biological processes (Huedo et al., 2014b). Two initial studies investigating the relation between genotypic and phenotypic traits of S. maltophilia isolates suggested that a significant group of strains lacked the rpfF gene (Pompilio et al., 2011; Zhuo et al., 2014). Later, a population study focused on DSF-QS revealed that, unlike the other Xanthomonadales, S. maltophilia presents two rpfF variants (named rpfF-1 and rpfF-2) and that primers designed to PCR-amplify the rpfF gene didn't recognize the rpfF-2 variant (Huedo et al., 2014b). More recently, the existence of the two rpfF alleles in S. maltophilia clinical and environmental isolates has been further validated by a population genomic analysis (Lira et al., 2017). The two rpfF variants differ, in particular, in the sequence encoding for the N-terminal 108 residues (Huedo et al., 2014b). Taking all the published data together (Huedo et al., 2014b; Lira et al., 2017) and assuming that the rpfF− isolates from Pompilio et al. (2011) and Zhuo et al. (2014) belong to the rpfF-2 variant, the rpfF-1 variant has been so far identified in 98 isolates (55.5%), while rpfF-2 has been detected in 81 isolates (44.5%).
Investigation of the rpf cluster in the two rpfF variant strains showed that the sensor RpfC presents two variants as well, with a fixed association between the rpfF variant and its cognate rpfC, meaning that all strains harboring rpfF-1 necessarily carry the rpfC-1 variant and likewise for the rpfFC-2 pair (Huedo et al., 2014b). Besides differences in amino-acid sequence, the two RpfC variants vary in length and secondary structure. RpfC-1 displays 10 trans-membrane regions (TMR) in the N-terminal region that are highly related to the RpfC-RpfH complex constituting the DSF sensor domain in Xcc (Slater et al., 2000; Huedo et al., 2014b). On the contrary, RpfC-2 lacks 5 of these TMRs as in Xylella fastidiosa (Xf) RpfC (Chatterjee et al., 2008; Huedo et al., 2014b). Differences between the rpf cluster variants strongly affect DSF synthesis, perception, and regulation of biological processes in S. maltophilia.
rpf-1 and rpf-2 strains distinctly synthesize and sense DSF signals
Remarkably, while rpf-1 strains display evident DSF production in standard growth conditions, rpf-2 isolates require extra copies of rpfF-2 or the absence of the sensor/repressor component RpfC-2 to achieve detectable levels of DSF (Huedo et al., 2014b). The mechanistic aspects of DSF synthesis and perception in S. maltophilia rpf-1 seem to be similar to those reported for the model organism Xcc. Both microorganisms synthesize cis-11-methyl-2-dodecenoic acid as the main DSF signal (He and Zhang, 2008; Huedo et al., 2014b). Xcc RpfF produces additional DSF signals including cis-2-dodecenoic acid, cis-11-methyldodeca-2,5-dienoic acid, and cis-10-methyl-2-dodecenoic acid (Deng et al., 2015, 2016; Zhou et al., 2015). The production of seven derivatives of the cis-11-methyl-2-dodecenoic acid by one S. maltophilia strain (WR-C) had been also reported (Huang and Lee Wong, 2007). More recently, however, the canonical cis-11-methyl-2-dodecenoic acid was the only unsaturated FA signal identified in culture supernatants of the S. maltophilia strains E77 (RpfF-1) and M30 (RpfF-2) (Huedo et al., 2014a,b) (Table 1).
Table 1.
Stenotrophomonas maltophilia strains in which the diffusible signal factor (DSF) quorum sensing (QS) system has been investigated.
| Strain | Origin | RpfF variant | DSF molecules | Biological processes | References |
|---|---|---|---|---|---|
| K279a | Clinical (blood infection) | 1 | cis-11-Methyl-2-dodecenoic acid (DSF) | Motility; Protease production; Lipopolysaccharide synthesis; Antimicrobial resistance; OMV production; Virulence | Fouhy et al., 2007; Devos et al., 2015 |
| WR-C | Environmental (septic tank) | NA* | cis-11-Methyl-2-dodecenoic acid (DSF); Δ2-tridecenoic acid; 11-methyl-dodecanoic acid; 10-methyl-dodeccanoic acid; Δ2-12-methyl-tridecenoic acid; Δ2-tetradecenoic acid; Δ2-12-methyl-tetradecenoic acid; Δ2-13-methyl-tetradecenoic acid | Motility | Huang and Lee Wong, 2007 |
| E77 | Clinical (sputum) | 1 | cis-11-Methyl-2-dodecenoic acid (DSF) | Motility; Biofilm; Virulence | Huedo et al., 2014b, 2015 |
| M30 | Clinical (decubitus ulcer) | 2 | cis-11-Methyl-2-dodecenoic acid (DSF) | Virulence | Huedo et al., 2014b, 2015 |
| R551-3 | Environmental (endophyte of Populus trichocarpa) | 1 | cis-11-Methyl-2-dodecenoic acid (DSF) | Promote seed germination and plant growth | Alavi et al., 2013 |
NA*, Genomic data is not available.
As reported for the DSF synthases of B. cenocepacia (Bi et al., 2012) and Xcc (Zhou et al., 2015), both the RpfF-1 and RpfF-2 proteins from S. maltophilia appear to have a double acyl-ACP dehydratase and thioesterase activity that catalyze the conversion of (R)-3-hydroxy-11-methyl-dodecanoyl-ACP to DSF in two steps (Huedo et al., 2015). In addition, the thioesterase activity of all RpfF proteins seems to be nonspecific, cleaving a variety of medium- and long-chain acyl-ACP substrates and thus generating free FAs that are then released to the extracellular environment (Bi et al., 2012; Huedo et al., 2015; Zhou et al., 2015). In S. maltophilia the major free FA released by this thioesterase activity is the 13-methyltetradecanoic acid (iso-15:0), which is also the most abundant FA in the phospholipids of both Xanthomonas sp. (Vauterin et al., 1996) and S. maltophilia (Kim et al., 2010). Surprisingly, iso-15:0 is actually considered a biomarker phospholipid FA for the Gram-positive group (Kaur et al., 2005) and seems to be present only in Gram-negative bacteria displaying DSF communication. Interestingly, DSF and iso-15:0 are generated through the same biosynthetic pathway (Heath and Rock, 2002), which suggests a potential connection between DSF production and membrane synthesis (Huedo et al., 2015).
In line with this observations, the presence of iso-15:0 in the medium appears to modulate DSF production in rpf-1 strains, perhaps because the intact RpfC-1 sensor (10 TMR) is able to detect this FA, thus liberating free active RpfF-1 capable of subsequent DSF synthesis (Huedo et al., 2015). Several other environmental factors modulate DSF synthesis in rpf-1 strains. For example, while rich media and 28°C seem to be the optimal culture conditions to achieve high amounts of DSF in the supernatant (Huedo et al., 2015), iron restriction has been found to induce DSF production through the Fur system in strain K279a (García et al., 2015).
Contrary to rpf-1 strains, DSF synthesis in strains harboring the rpf-2 allele seems to be permanently repressed under wild-type conditions. Nevertheless, the presence of exogenous DSF triggers DSF production in these strains (Huedo et al., 2015; Figure 1). These findings suggest that rpf-2 strains require a stoichiometric unbalance (RpfF-2>RpfC-2) for DSF production and that the 5-TMR sensor component of RpfC-2 is much more specific than RpfC-1, enabling free-active RpfF-2 only upon detection of DSF itself.
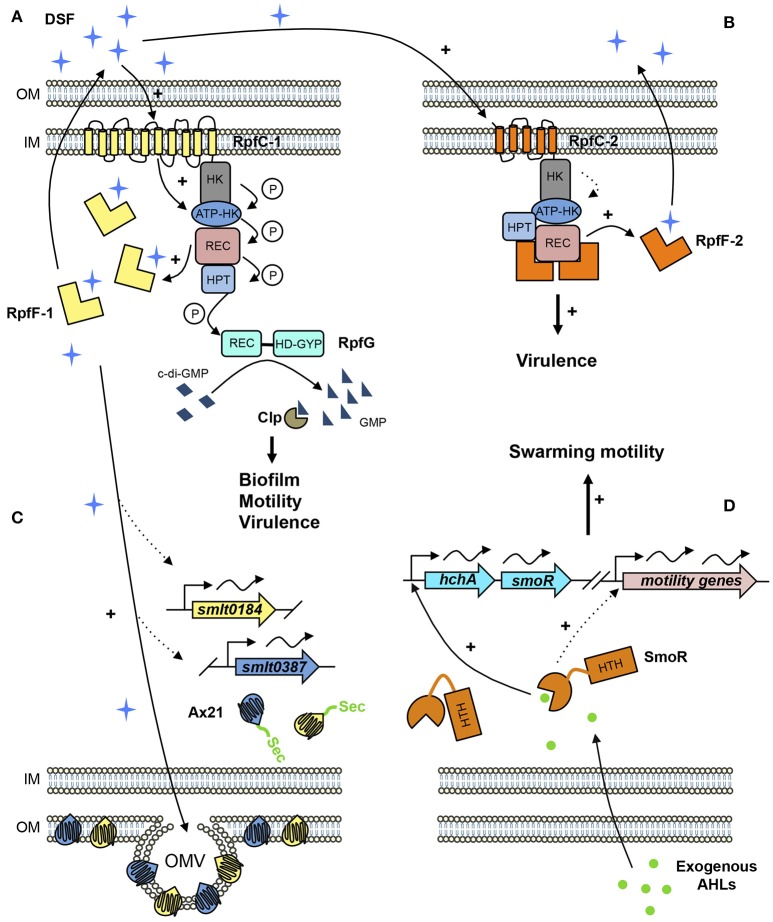
Figure 1.
Proposed QS signaling network in S. maltophilia. (A) In rpf-1 strains, RpfC-1 (including 10 TMR) stimulates RpfF-1 basal activity—that increases with cell density—and synthesizes DSF (cis-11-Methyl-2-dodecenoic acid) that accumulates in the extracellular environment. Once DSF concentration reaches a critical threshold, RpfC-1 senses DSF, and induces a phosphorylation cascade throughout its cytoplasmic domains ending in the response regulator RpfG, which degrades cyclic diguanylate monophosphate (c-di-GMP) to GMP activating the transcriptional regulator Clp that stimulates expression of genes involved in biofilm formation, motility, and virulence. (B) In rpf-2 strains, RpfC-2 (5 TMR) permanently represses RpfF-2, resulting in no DSF detection in axenic conditions. DSF produced by neighboring bacteria (e.g., rpf-1 strain) is sensed by RpfC-2 allowing free-active RpfF-2 and subsequent DSF synthesis. (C) DSF also stimulates the production of outer membrane vesicles (OMV) containing high amounts of the two Ax21 proteins (Smlt0184 and Smlt0387). Both Ax21 proteins present a signal peptide that is processed by the general secretory (Sec) system. (D) Exogenous AHL signals, specifically C8-HSL and oxo-C8-HSL, are sensed by the LuxR solo SmoR (Smlt1839), annotated as “LuxR chaperone HchA-associated,” activating the transcription of its own operon and promoting swarming motility. Dotted lines represent predicted or supposed interactions on the basis of reported experimental evidences. Protein domains are abbreviated as follows. HK, Histidine kinase domain; REC, Receiver domain; HPT, Histidine phosphotransferase domain; HD-GYP, Phosphodiesterase domain containing an additional GYP motif; HTH, Helix-Turn-Helix domain.
Biological processes regulated by DSF in rpf-1 and rpf-2 strains
Deletion of rpfF-1 in the S. maltophilia clinical strain E77 resulted in altered biofilm formation, reduced bacterial motility and reduced virulence in the Caenorhabditis elegans and zebrafish models of infection (Huedo et al., 2014b). In the clinical model strain K279a (rpfF-1), interruption of the rpfF gene also resulted in decreased antibiotic resistance and protease secretion, and an altered lipopolysaccharide (LPS) (Fouhy et al., 2007). In the environmental strain WR-C, DSF-derivative signals stimulate flagella-independent motility (Huang and Lee Wong, 2007) and deletion of rpfF or rpfB decrease the expression of the ferric citrate receptor FecA (Huang and Wong, 2007). Recently, DSF produced by strain 44/33 has been shown to contribute to outer membrane vesicle (OMV) secretion (Devos et al., 2015; Table 1).
On the contrary, mutation of rpfF-2 does not significantly alter biofilm formation, bacterial motility or virulence in the clinical strain M30 (Table 1). This results are in line with the fact that the RpfF-2 variant seems to be permanently repressed (Huedo et al., 2014b). Nevertheless, when the rpf-1 and rpf-2 subpopulations cohabit, both DSF production and virulence capacity of the whole population are enhanced (Huedo et al., 2015; Figure 1). This suggests that rpf-2 strains have evolved as a receptor group, in terms of DSF communication, displaying a lethargic DSF-deficient phenotype under axenic conditions until the presence of DSF-producing bacteria (e.g., Xcc or S. maltophilia rpf-1 variant) triggers reciprocal DSF communication. This behavior evokes to some extend the P. aeruginosa “social cheaters”—spontaneous lasR mutants that take advantage of the intact QS-regulation of their neighboring bacteria (Sandoz et al., 2007). Clearly, further research is required to better understand the intriguing role of the DSF system in the rpf-2 S. maltophilia subpopulation, including the specific advantages and disadvantages of this particular behavior.
DSF-mediated communication of S. maltophilia with distant organisms
S. maltophilia has been shown to interact, via DSF production, with unrelated bacteria, yeast, and even plants. In particular, DSF produced by S. maltophilia K279a is detected by P. aeruginosa through the sensor kinase PA1396, modulating biofilm formation and antibiotic resistance (Ryan et al., 2008) as well as virulence and persistence in lungs of cystic fibrosis patients (Twomey et al., 2012). Likewise, synthesis of DSF by the strain K279a affects planktonic and biofilm growth of Candida albicans and inhibits its morphological transition (de Rossi et al., 2014). Finally, DSF produced by the environmental strain R551-3 causes a positive effect on plant germination and growth of rapeseed (Alavi et al., 2013) (Table 1).
AHL-based quorum sensing
N-acyl homoserine lactone (AHL) QS is the most studied and widespread communication system in Gram-negative bacteria (Papenfort and Bassler, 2016). Typically, AHL signals are produced by LuxI-type synthases and sensed by LuxR-type transcriptional regulators (Ng and Bassler, 2009; LaSarre and Federle, 2013).
AHL synthesis in Stenotrophomonas species
It has been shown that S. maltophilia does not produce detectable levels of AHLs (Zhu et al., 2001; Veselova et al., 2003), reinforced by the lack of homologs to known AHL LuxI-family synthase genes in publicly available genomes. Nevertheless, AHL activity has been detected in some Stenotrophomonas sp. isolated from sediments of wastewater treatment systems (Valle et al., 2004; Hu et al., 2016) and activated sludge (Tan et al., 2014, 2015). Besides the Stenotrophomonas genus, AHL-activity has also been detected in other Xanthomonadaceae including Thermomonas (Ishizaki et al., 2017), Lysobacter (Tan et al., 2015) and Xanthomonas sp. (Veselova et al., 2003). Given the elevated genomic diversity of the genus Stenotrophomonas, future identification of more AHL-producing isolates or the existence of a novel LuxI-family synthase cannot be ruled out.
AHL response in S. maltophilia
LuxR solos are typical AHL-regulators lacking its cognate LuxI and are widely spread throughout bacterial genomes, including Xanthomonadaceae species (Subramoni and Venturi, 2009; Hudaiberdiev et al., 2015). The genome of S. maltophilia strain K279 encodes for 15 putative LuxR solos from which only SmoR presents the typical N-terminal AHL-binding domain and the C-terminal helix-turn-helix (HTH) DNA-binding domain (Martínez et al., 2015). In vitro AHL-binding assays confirmed that SmoR from strain E77 binds to AHL signal oxo-C8-HSL, regulating swarming motility. The S. maltophilia E77 parental strain but not its derivative ΔsmoR mutant strongly stimulates swarming motility in the presence of a P. aeruginosa supernatant (containing high levels of AHLs including oxo-C8-HSL), indicating that SmoR senses AHL signals produced by neighboring bacteria (Martínez et al., 2015) (Figure 1). The role of the other LuxR solos in S. maltophilia is yet to be elucidated.
The proposed quorum-sensing factor Ax21
The small protein Ax21 (activator of XA21-mediated immunity in plants) was proposed to serve as a new QS mechanism in Xanthomonadaceae (Lee et al., 2009; Han et al., 2011; McCarthy et al., 2011; Ronald, 2011). However, after almost 10 years of research on the Ax21 protein, we are practically at the starting point, since the key studies proposing its function have been placed in doubt (Han et al., 2013; Lee et al., 2013; Bahar et al., 2014; McCarthy et al., 2017).
What appears to apply to S. maltophilia, based on two independent proteomic analyses, is that Ax21 is an outer membrane protein secreted in association with OMVs (Devos et al., 2015; Ferrer-Navarro et al., 2016). Interestingly, it has been found that the relative levels of the two Ax21 paralogs (K279a locus tags Smlt0184 and Smlt0387) in some S. maltophilia strains seem to correlate with their virulence potential (Ferrer-Navarro et al., 2013, 2016), and that the increase in OMV-associated secretion of Ax21 proteins is somehow regulated by the DSF-QS system (Devos et al., 2015) (Figure 1). Based on the evidences reported so far, we believe that Ax21 cannot be considered a QS system component itself. However, the link between DSF signaling, OMV production and Ax21 secretion, as well as the implication of this regulatory pathway on the virulence ability of S. maltophilia, should be further investigated.
Quorum quenching involving S. maltophilia
The most studied QQ mechanisms are those disrupting AHL signaling (Wang et al., 2004), although QQ has been described for almost all QS systems including DSF (Newman et al., 2008; Defoirdt, 2017). Despite quenching of DSF-QS in S. maltophilia has not yet been reported, this species exhibits an interesting behavior in terms of QQ. It has been shown that the FA cis-9-octadecenoic acid synthesized by S. maltophilia strain BJ01 displays QQ of AHL signals resulting in antibiofilm activity on P. aeruginosa (Singh et al., 2013). AHL-QQ activity against 3-oxo-C12-HSL has been also observed in several Stenotrophomonas sp. and S. maltophilia isolates from activated sludge samples (Tan et al., 2015). Another study on activated sludge samples reported that one isolate from the genus Stenotrophomonas was able to degrade the C10-HSL signal (Ochiai et al., 2013). Endophytic isolates of S. maltophilia have been also shown to degrade 3-hydroxy palmitic acid methyl ester (3OH-PAME), the main QS signal of Ralstonia solanacearum (Achari and Ramesh, 2015). On the other side, detection, and response to AHL signals by S. maltophilia can be disrupted by the lactonase AiiA from Bacillus subtilis (Pan et al., 2008), resulting in non-swarming stimulation (Martínez et al., 2015).
Regarding the quenching of DSF-QS, S. maltophilia strain E77 grown in LB medium containing 5 μM of synthetic octadecanoic acid (18:0) reduces DSF production to undetectable levels (Huedo et al., 2015). Moreover, plant-associated bacterial species and particularly Pseudomonas spp. are able to rapidly degrade DSF molecules of Xcc (Newman et al., 2008), a mechanism that may apply against S. maltophilia DSF signals. Finally, DSF produced by S. maltophilia K279a inhibits the yeast-to-hyphal transition of Candida albicans, most probably by acting as antagonist of the C. albicans signal farnesoic acid, a DSF homolog (de Rossi et al., 2014).
In summary, S. maltophilia appears as a species with potential QQ applications. However, QQ mechanisms disrupting S. maltophilia signaling have never been reported.
Concluding remarks and future perspectives
Research conducted during last years has significantly improved our understanding of cell-to-cell signaling processes in S. maltophilia but, at the same time, has aroused new questions and hypothesis.
The mechanistic processes of the DSF-QS system in the rpf-1 subpopulation seem highly similar to those reported for the DSF model organism Xcc. However, more efforts should be addressed to investigate the molecular basis of DSF-QS in the rpf-2 group (45% of isolates) in order to uncover the biological significance of this particular variant.
The sensing and quenching response of S. maltophilia to exogenous AHL signals suggests that this bacterium has evolved in close contact with AHL-producing bacteria. Given the high phenotypic and genotypic heterogeneity among isolates from the genus Stenotrophomonas and considering that AHL-producing isolates of Stenotrophomonas spp. have been already reported, the existence of S. maltophilia strains producing AHLs cannot be discarded and should be further investigated.
S. maltophilia clearly interacts with the organisms conforming its environment. Examples of cooperation via DSF are divers and include the stimulation of seed germination and growth of the rapeseed, but also an increment of biofilm formation and antibiotic resistance of P. aeruginosa in the lungs. However, in most known cases S. maltophilia appears to exert a negative effect on its competitors' QS systems. This is because S. maltophilia isolates possess an extraordinary array of QQ mechanisms including production of FAs with quenching activities as well as degradation of AHL and PAME signals.
Given the increasing incidence of multi-resistant isolates of S. maltophilia in clinical settings, new antimicrobial strategies should be explored. Exogenous mechanisms quenching DSF communication in S. maltophilia have not yet been investigated and may represent a promising approach to overcome bacterial multidrug resistance. With the knowledge on the DSF system increasing and particularly since the determination of the structure of the synthase RpfF and the sensor RpfC, designing and testing compounds with antagonist activity against these key QS components could provide further opportunities for the development of novel combination therapies with antibiotics.
Comprehensively, S. maltophilia appears to be extraordinarily well connected to its environment and to take part in inter-species communication by synthesizing sensing and degrading a wide range of signaling molecules, therefore actively participating in the decisions taken by the whole community.
Author contributions
PH, XC, and DY conceptually designed the article and authored the first draft. XD, IG, and DY provided academic input and expertise, and finished critical revision of the article. All authors have approved the final version.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the Spanish MICINN (BIO2015-66674-R) and the Catalan AGAUR (2014-SGR-1280).
References
- Achari G. A., Ramesh R. (2015). Characterization of bacteria degrading 3-hydroxy palmitic acid methyl ester (3OH-PAME), a quorum sensing molecule of Ralstonia solanacearum. Lett. Appl. Microbiol. 60, 447–455. 10.1111/lam.12389 [DOI] [PubMed] [Google Scholar]
- Adegoke A. A., Stenström T. A., Okoh A. I. (2017). Stenotrophomonas maltophilia as an emerging ubiquitous pathogen: looking beyond contemporary antibiotic therapy. Front. Microbiol. 8:2276. 10.3389/fmicb.2017.02276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alavi P., Müller H., Cardinale M., Zachow C., Sánchez M. B., Martínez J. L., et al. (2013). The DSF quorum sensing system controls the positive influence of Stenotrophomonas maltophilia on plants. PLoS ONE 8:e67103. 10.1371/journal.pone.0067103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin R., Waters V. (2014). Antibiotic treatment for Stenotrophomonas maltophilia in people with cystic fibrosis. Cochrane Database Syst. Rev. CD009249. 10.1002/14651858.CD009249.pub4 [DOI] [PubMed] [Google Scholar]
- Bahar O., Pruitt R., Luu D. D., Schwessinger B., Daudi A., Liu F., et al. (2014). The Xanthomonas Ax21 protein is processed by the general secretory system and is secreted in association with outer membrane vesicles. PeerJ 2:e242. 10.7717/peerj.242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bais H. P., Weir T. L., Perry L. G., Gilroy S., Vivanco J. M. (2006). The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 57, 233–266. 10.1146/annurev.arplant.57.032905.105159 [DOI] [PubMed] [Google Scholar]
- Barber C. E., Tang J. L., Feng J. X., Pan M. Q., Wilson T. J., Slater H., et al. (1997). A novel regulatory system required for pathogenicity of Xanthomonas campestris is mediated by a small diffusible signal molecule. Mol. Microbiol. 24, 555–566. 10.1046/j.1365-2958.1997.3721736.x [DOI] [PubMed] [Google Scholar]
- Berg G., Eberl L., Hartmann A. (2005). The rhizosphere as a reservoir for opportunistic human pathogenic bacteria. Environ. Microbiol. 7, 1673–1685. 10.1111/j.1462-2920.2005.00891.x [DOI] [PubMed] [Google Scholar]
- Bi H., Christensen Q. H., Feng Y., Wang H., Cronan J. E. (2012). The Burkholderia cenocepacia BDSF quorum sensing fatty acid is synthesized by a bifunctional crotonase homologue having both dehydratase and thioesterase activities. Mol. Microbiol. 83, 840–855. 10.1111/j.1365-2958.2012.07968.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooke J. S. (2012). Stenotrophomonas maltophilia: an emerging global opportunistic pathogen. Clin. Microbiol. Rev. 25, 2–41. 10.1128/CMR.00019-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S., Wistrom C., Lindow S. E. (2008). A cell–cell signaling sensor is required for virulence and insect transmission of Xylella fastidiosa. Proc. Natl. Acad. Sci.U.S.A. 105, 2670–2675. 10.1073/pnas.0712236105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defoirdt T. (2017). Quorum-sensing systems as targets for antivirulence therapy. Trends Microbiol. 26, 313–328. 10.1016/j.tim.2017.10.005 [DOI] [PubMed] [Google Scholar]
- Deng Y., Liu X., Wu J., Lee J., Chen S., Cheng Y., et al. (2015). The host plant metabolite glucose is the precursor of diffusible signal factor (DSF) family signals in Xanthomonas campestris. Appl. Environ. Microbiol. 81, 2861–2868. 10.1128/AEM.03813-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y., Wu J., Tao F., Zhang L. H. (2011). Listening to a new language: DSF-based quorum sensing in gram-negative bacteria. Chem. Rev. 111, 160–173. 10.1021/cr100354f [DOI] [PubMed] [Google Scholar]
- Deng Y., Wu J., Yin W., Li P., Zhou J., Chen S., et al. (2016). Diffusible signal factor family signals provide a fitness advantage to Xanthomonas campestris pv. campestris in interspecies competition. Environ. Microbiol. 18, 1534–1545. 10.1111/1462-2920.13244 [DOI] [PubMed] [Google Scholar]
- de Rossi B. P., García C., Alcaraz E., Franco M. (2014). Stenotrophomonas maltophilia interferes via the DSF-mediated quorum sensing system with Candida albicans filamentation and its planktonic and biofilm modes of growth. Rev. Argent. Microbiol. 46, 288–297. 10.1016/S0325-7541(14)70084-7 [DOI] [PubMed] [Google Scholar]
- Devos S., Van Oudenhove L., Stremersch S., Van Putte W., De Rycke R., Van Driessche G., et al. (2015). The effect of imipenem and diffusible signaling factors on the secretion of outer membrane vesicles and associated Ax21 proteins in Stenotrophomonas maltophilia. Front. Microbiol. 6:298. 10.3389/fmicb.2015.00298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y. H., Wang L. H., Zhang L. H. (2007). Quorum-quenching microbial infections: mechanisms and implications. Philos. Trans. R. Soc. B Biol. Sci. 362, 1201–1211. 10.1098/rstb.2007.2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer-Navarro M., Planell R., Yero D., Mongiardini E., Torrent G., Huedo P., et al. (2013). Abundance of the quorum-sensing factor Ax21 in four strains of Stenotrophomonas maltophilia correlates with mortality rate in a new zebrafish model of infection. PLoS ONE 8:e67207. 10.1371/journal.pone.0067207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer-Navarro M., Torrent G., Mongiardini E., Conchillo-Solé O., Gibert I., Daura X. (2016). Proteomic analysis of outer membrane proteins and vesicles of a clinical isolate and a collection strain of Stenotrophomonas maltophilia. J. Proteomics 142, 122–129. 10.1016/j.jprot.2016.05.001 [DOI] [PubMed] [Google Scholar]
- Fouhy Y., Scanlon K., Schouest K., Spillane C., Crossman L., Avison M. B., et al. (2007). Diffusible signal factor-dependent cell-cell signaling and virulence in the nosocomial pathogen Stenotrophomonas maltophilia. J. Bacteriol. 189, 4964–4968. 10.1128/JB.00310-07 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- García C. A., Alcaraz E. S., Franco M. A., Passerini de Rossi B. N. (2015). Iron is a signal for Stenotrophomonas maltophilia biofilm formation, oxidative stress response, OMPs expression, and virulence. Front. Microbiol. 6:926. 10.3389/fmicb.2015.00926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S. W., Sriariyanun M., Lee S.-W., Sharma M., Bahar O., Bower Z., et al. (2011). Small protein-mediated quorum sensing in a Gram-negative bacterium. PLoS ONE 6:e29192. 10.1371/journal.pone.0029192 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Han S.-W., Sriariyanun M., Lee S.-W., Sharma M., Bahar O., Bower Z., et al. (2013). Retraction: small protein-mediated quorum sensing in a gram-negative bacterium. PLOS ONE 6:e29192. 10.1371/annotation/880a72e1-9cf3-45a9-bf1c-c74ccb73fd35 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- He Y.-W., Zhang L.-H. (2008). Quorum sensing and virulence regulation in Xanthomonas campestris. FEMS Microbiol. Rev. 32, 842–857. 10.1111/j.1574-6976.2008.00120.x [DOI] [PubMed] [Google Scholar]
- Heath R. J., Rock C. O. (2002). The Claisen condensation in biology. Nat. Prod. Rep. 19, 581–596. 10.1039/b110221b [DOI] [PubMed] [Google Scholar]
- Hu H., He J., Liu J., Yu H., Tang J., Zhang J. (2016). Role of N-acyl-homoserine lactone (AHL) based quorum sensing on biofilm formation on packing media in wastewater treatment process. RSC Adv. 6, 11128–11139. 10.1039/C5RA23466B [DOI] [Google Scholar]
- Huang T.-P., Lee Wong A. C. (2007). Extracellular fatty acids facilitate flagella-independent translocation by Stenotrophomonas maltophilia. Res. Microbiol. 158, 702–711. 10.1016/j.resmic.2007.09.002 [DOI] [PubMed] [Google Scholar]
- Huang T.-P., Wong A. C. L. (2007). A cyclic AMP receptor protein-regulated cell-cell communication system mediates expression of a FecA homologue in Stenotrophomonas maltophilia. Appl. Environ. Microbiol. 73, 5034–5040. 10.1128/AEM.00366-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudaiberdiev S., Choudhary K. S., Vera Alvarez R., Gelencsér Z., Ligeti B., Lamba D., et al. (2015). Census of solo LuxR genes in prokaryotic genomes. Front. Cell. Infect. Microbiol. 5:20. 10.3389/fcimb.2015.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huedo P., Conchillo-Solé Ó., Yero D., Martínez-Servat S., Daura X., Gibert I. (2014a). Draft genome sequence of Stenotrophomonas maltophilia strain M30, isolated from a chronic pressure ulcer in an elderly patient. Genome Announc. 2:e00576-14. 10.1128/genomeA.00576-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huedo P., Yero D., Martínez-Servat S., Estibariz I., Planell R., Martínez P., et al. (2014b). Two different rpf clusters distributed among a population of Stenotrophomonas maltophilia clinical strains display differential diffusible signal factor production and virulence regulation. J. Bacteriol. 196, 2431–2442. 10.1128/JB.01540-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huedo P., Yero D., Martínez-Servat S., Ruyra À., Roher N., Daura X., et al. (2015). Decoding the genetic and functional diversity of the DSF quorum-sensing system in Stenotrophomonas maltophilia. Front. Microbiol. 6:761. 10.3389/fmicb.2015.00761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki S., Sugiyama R., Okabe S. (2017). Membrane fouling induced by AHL-mediated soluble microbial product (SMP) formation by fouling-causing bacteria co-cultured with fouling-enhancing bacteria. Sci. Rep. 7:8482. 10.1038/s41598-017-09023-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia V. C., Purohit H. J. (2011). Quenching the quorum sensing system: potential antibacterial drug targets. Crit. Rev. Microbiol. 37, 121–140. 10.3109/1040841X.2010.532479 [DOI] [PubMed] [Google Scholar]
- Kaur A., Chaudhary A., Kaur A., Choudhary R., Kaushik R. (2005). Phospholipid fatty acid: a bioindicator of environment monitoring and assessment in soil ecosystem. Curr. Sci. 89, 1103–1112. [Google Scholar]
- Kim H.-B., Srinivasan S., Sathiyaraj G., Quan L.-H., Kim S.-H., Bui T. P. N., et al. (2010). Stenotrophomonas ginsengisoli sp. nov., isolated from a ginseng field. Int. J. Syst. Evol. Microbiol. 60, 1522–1526. 10.1099/ijs.0.014662-0 [DOI] [PubMed] [Google Scholar]
- Kleerebezem M., Quadri L. E., Kuipers O. P., de Vos W. M. (1997). Quorum sensing by peptide pheromones and two-component signal-transduction systems in Gram-positive bacteria. Mol. Microbiol. 24, 895–904. 10.1046/j.1365-2958.1997.4251782.x [DOI] [PubMed] [Google Scholar]
- LaSarre B., Federle M. J. (2013). Exploiting quorum sensing to confuse bacterial pathogens. Microbiol. Mol. Biol. Rev. 77, 73–111. 10.1128/MMBR.00046-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.-W., Han S.-W., Sririyanum M., Park C.-J., Seo Y.-S., Ronald P. C. (2009). A type I-secreted, sulfated peptide triggers XA21-mediated innate immunity. Science 326, 850–853. 10.1126/science.1173438 [DOI] [PubMed] [Google Scholar]
- Lee S.-W., Han S.-W., Sririyanum M., Park C.-J., Seo Y.-S., Ronald P. C. (2013). Retraction. A type I-secreted, sulfated peptide triggers XA21-mediated innate immunity. Science 342:191 10.1126/science.342.6155.191-a [DOI] [PubMed] [Google Scholar]
- Lira F., Berg G., Martínez J. L. (2017). Double-face meets the bacterial world: the opportunistic pathogen Stenotrophomonas maltophilia. Front. Microbiol. 8:2190. 10.3389/fmicb.2017.02190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery C. A., Dickerson T. J., Janda K. D. (2008). Interspecies and interkingdom communication mediated by bacterial quorum sensing. Chem. Soc. Rev. 37, 1337–1346. 10.1039/b702781h [DOI] [PubMed] [Google Scholar]
- Martínez P., Huedo P., Martinez-Servat S., Planell R., Ferrer-Navarro M., Daura X., et al. (2015). Stenotrophomonas maltophilia responds to exogenous AHL signals through the LuxR solo SmoR (Smlt1839). Front. Cell. Infect. Microbiol. 5:41 10.3389/fcimb.2015.00041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy Y., Dow J. M., Ryan R. P. (2011). The Ax21 protein is a cell-cell signal that regulates virulence in the nosocomial pathogen Stenotrophomonas maltophilia. J. Bacteriol. 193, 6375–6378. 10.1128/JB.05949-11 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- McCarthy Y., Dow J. M., Ryan R. P. (2017). Retraction for McCarthy et al., “The Ax21 protein is a cell-cell signal that regulates virulence in the nosocomial pathogen Stenotrophomonas maltophilia.” J. Bacteriol. 199:e00156–17. 10.1128/JB.00156-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman K. L., Chatterjee S., Ho K. A., Lindow S. E. (2008). Virulence of plant pathogenic bacteria attenuated by degradation of fatty acid cell-to-cell signaling factors. Mol. Plant Microbe Interact. 21, 326–334. 10.1094/MPMI-21-3-0326 [DOI] [PubMed] [Google Scholar]
- Ng W.-L., Bassler B. L. (2009). Bacterial quorum-sensing network architectures. Annu. Rev. Genet. 43, 197–222. 10.1146/annurev-genet-102108-134304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochiai S., Morohoshi T., Kurabeishi A., Shinozaki M., Fujita H., Sawada I., et al. (2013). Production and degradation of N-acylhomoserine lactone quorum sensing signal molecules in bacteria isolated from activated sludge. Biosci. Biotechnol. Biochem. 77, 2436–2440. 10.1271/bbb.130553 [DOI] [PubMed] [Google Scholar]
- Pan J., Huang T., Yao F., Huang Z., Powell C. A., Qiu S., et al. (2008). Expression and characterization of aiiA gene from Bacillus subtilis BS-1. Microbiol. Res. 163, 711–716. 10.1016/j.micres.2007.12.002 [DOI] [PubMed] [Google Scholar]
- Papenfort K., Bassler B. L. (2016). Quorum sensing signal–response systems in Gram-negative bacteria. Nat. Rev. Microbiol. 14:576 10.1038/nrmicro.2016.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompilio A., Pomponio S., Crocetta V., Gherardi G., Verginelli F., Fiscarelli E., et al. (2011). Phenotypic and genotypic characterization of Stenotrophomonas maltophilia isolates from patients with cystic fibrosis: Genome diversity, biofilm formation, and virulence. BMC Microbiol. 11:159 10.1186/1471-2180-11-159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald P. C. (2011). Small protein-mediated quorum sensing in a gram-negative bacterium: novel targets for control of infectious disease. Discov. Med. 12, 461–470. [PubMed] [Google Scholar]
- Rutherford S. T., Bassler B. L. (2012). Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harb. Perspect. Med. 2:a012427. 10.1101/cshperspect.a012427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan R. P., An S., Allan J. H., McCarthy Y., Dow J. M. (2015). The DSF family of cell–cell signals: an expanding class of bacterial virulence regulators. PLoS Pathog. 11:e1004986. 10.1371/journal.ppat.1004986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan R. P., Fouhy Y., Garcia B. F., Watt S. A., Niehaus K., Yang L., et al. (2008). Interspecies signalling via the Stenotrophomonas maltophilia diffusible signal factor influences biofilm formation and polymyxin tolerance in Pseudomonas aeruginosa. Mol. Microbiol. 68, 75–86. 10.1111/j.1365-2958.2008.06132.x [DOI] [PubMed] [Google Scholar]
- Ryan R. P., Monchy S., Cardinale M., Taghavi S., Crossman L., Avison M. B., et al. (2009). The versatility and adaptation of bacteria from the genus Stenotrophomonas. Nat. Rev. Microbiol. 7:514. 10.1038/nrmicro2163 [DOI] [PubMed] [Google Scholar]
- Sandoz K. M., Mitzimberg S. M., Schuster M. (2007). Social cheating in Pseudomonas aeruginosa quorum sensing. Proc. Natl. Acad. Sci. U.S.A. 104, 15876–15881. 10.1073/pnas.0705653104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V. K., Kavita K., Prabhakaran R., Jha B. (2013). Cis-9-octadecenoic acid from the rhizospheric bacterium Stenotrophomonas maltophilia BJ01 shows quorum quenching and anti-biofilm activities. Biofouling 29, 855–867. 10.1080/08927014.2013.807914 [DOI] [PubMed] [Google Scholar]
- Slater H., Alvarez-Morales A., Barber C. E., Daniels M. J., Dow J. M. (2000). A two-component system involving an HD-GYP domain protein links cell–cell signalling to pathogenicity gene expression in Xanthomonas campestris. Mol. Microbiol. 38, 986–1003. 10.1046/j.1365-2958.2000.02196.x [DOI] [PubMed] [Google Scholar]
- Subramoni S., Venturi V. (2009). LuxR-family “solos”: bachelor sensors/regulators of signalling molecules. Microbiol. Read. Engl. 155, 1377–1385. 10.1099/mic.0.026849-0 [DOI] [PubMed] [Google Scholar]
- Tan C. H., Koh K. S., Xie C., Tay M., Zhou Y., Williams R., et al. (2014). The role of quorum sensing signalling in EPS production and the assembly of a sludge community into aerobic granules. ISME J. 8, 1186–1197. 10.1038/ismej.2013.240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan C. H., Koh K. S., Xie C., Zhang J., Tan X. H., Lee G. P., et al. (2015). Community quorum sensing signalling and quenching: microbial granular biofilm assembly. Npj Biofilms Microbiomes 1:15006. 10.1038/npjbiofilms.2015.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twomey K. B., O'Connell O. J., McCarthy Y., Dow J. M., O'Toole G. A., Plant B. J., et al. (2012). Bacterial cis-2-unsaturated fatty acids found in the cystic fibrosis airway modulate virulence and persistence of Pseudomonas aeruginosa. ISME J. 6, 939–950. 10.1038/ismej.2011.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle A., Bailey M. J., Whiteley A. S., Manefield M. (2004). N-acyl-l-homoserine lactones (AHLs) affect microbial community composition and function in activated sludge. Environ. Microbiol. 6, 424–433. 10.1111/j.1462-2920.2004.00581.x [DOI] [PubMed] [Google Scholar]
- Vauterin L., Yang P., Swings J. (1996). Utilization of fatty acid methyl esters for the differentiation of new xanthomonas species. Int. J. Syst. Bacteriol. 46, 298–304. 10.1099/00207713-46-1-298 [DOI] [Google Scholar]
- Veselova M., Kholmeckaya M., Klein S., Voronina E., Lipasova V., Metlitskaya A., et al. (2003). Production of N-acylhomoserine lactone signal molecules by gram-negative soil-borne and plant-associated bacteria. Folia Microbiol. 48, 794–798. 10.1007/BF02931516 [DOI] [PubMed] [Google Scholar]
- Wang L.-H., Weng L.-X., Dong Y.-H., Zhang L.-H. (2004). Specificity and enzyme kinetics of the quorum-quenching N-Acyl homoserine lactone lactonase (AHL-lactonase). J. Biol. Chem. 279, 13645–13651. 10.1074/jbc.M311194200 [DOI] [PubMed] [Google Scholar]
- Waters C. M., Bassler B. L. (2005). Quorum sensing: cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 21, 319–346. 10.1146/annurev.cellbio.21.012704.131001 [DOI] [PubMed] [Google Scholar]
- Zhou L., Yu Y., Chen X., Diab A. A., Ruan L., He J., et al. (2015). The multiple DSF-family QS signals are synthesized from carbohydrate and branched-chain amino acids via the FAS elongation cycle. Sci. Rep. 5:13294. 10.1038/srep13294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Zhang L.-H., Cámara M., He Y.-W. (2017). The DSF family of quorum sensing signals: diversity, biosynthesis, and turnover. Trends Microbiol. 25, 293–303. 10.1016/j.tim.2016.11.013 [DOI] [PubMed] [Google Scholar]
- Zhu H., Thuruthyil S. J., Willcox M. D. (2001). Production of N-acyl homoserine lactones by Gram-negative bacteria isolated from contact lens wearers. Clin. Experiment. Ophthalmol. 29, 150–152. 10.1046/j.1442-9071.2001.00397.x [DOI] [PubMed] [Google Scholar]
- Zhuo C., Zhao Q., Xiao S. (2014). The Impact of spgM, rpfF, rmlA gene distribution on biofilm formation in Stenotrophomonas maltophilia. PLoS ONE 9:e108409. 10.1371/journal.pone.0108409 [DOI] [PMC free article] [PubMed] [Google Scholar]