Abstract
Purpose
To examine the associations between sentinel lymph node biopsy (SLNB) and complications among older patients who underwent breast-conserving surgery (BCS) for ductal carcinoma in situ (DCIS).
Methods
We identified women from the Surveillance, Epidemiology, and End Results–Medicare dataset aged 67–94 years diagnosed during 1998–2011 with DCIS who underwent BCS as initial treatment. We assessed incidence of complications, including lymphedema, wound infection, seroma, or pain, within 9 months of diagnosis. We used Mahalanobis matching and generalized linear models to estimate the associations between SLNB and complications.
Results
Our sample consisted of 15,515 beneficiaries, 2409 (15.5%) of whom received SLNB. Overall, 16.8% of women who received SLNB had complications, compared with 11.3% of women who did not receive SLNB (p < 0.001). Use of SLNB was associated with subsequent mastectomy but not radiotherapy. Multivariate analyses of the matched sample showed that, compared with no SLNB, SLNB use was significantly associated with incidence of any complication [adjusted odds ratio (AOR) 1.39; 99% confidence interval (CI) 1.18–1.63], lymphedema (AOR 4.45; 99% CI 2.27–8.75), wound infection (AOR 1.24; 99% CI 1.00–1.54), seroma (AOR 1.40; 99% CI 1.03–1.91), and pain (AOR 1.31; 99% CI 1.04–1.65). Sensitivity analyses excluding patients who underwent mastectomy yielded qualitatively similar results regarding the associations between SLNB and complications.
Conclusions
Among older women with DCIS who received BCS, SLNB use was associated with higher risks of short-term complications. These findings support consensus guidelines recommending against SLNB for this population and provide empirical information for patients.
Electronic supplementary material
The online version of this article (10.1245/s10434-018-6410-0) contains supplementary material, which is available to authorized users.
With increased use of screening mammography, the incidence of ductal carcinoma in situ (DCIS) has increased dramatically over the past four decades.1,2 Approximately 55,000 new cases of DCIS occur among US women each year.3 Fortunately, breast cancer mortality in patients with pure DCIS remains low, with a reported 10-year cancer-specific survival rate of > 97%.4 Treatment of DCIS can include surgery [mastectomy or breast-conserving surgery (BCS)], axillary evaluation, radiotherapy (RT), and endocrine therapy.5 Because DCIS itself is rarely fatal, determining the optimal clinical approach to treat DCIS while minimizing complications and side effects is a research priority identified by the Institute of Medicine and the Patient-Centered Outcomes Research Institute.6,7
Sentinel lymph node biopsy (SLNB) for DCIS management is an area where clinical management can vary. Consensus guidelines, such as those published by the National Comprehensive Cancer Network and the American Society of Clinical Oncology,8,9 recommend against SLNB in women with DCIS undergoing BCS. However, nearly 17% of patients undergoing BCS for DCIS underwent SLNB.10 Furthermore, the findings that SLNB increased from 7.2% in 1998 to 39.4% in 2011 in the USA raised concerns about compliance with these national guidelines.11 A 2015 survey in the UK also revealed that surgeons’ view on indications for SLNB differed from national guidelines.12 In this procedure, the first axillary node or nodes to drain the breast are identified, removed, and histologically examined. SLNB has replaced axillary lymph node dissection (ALND) for patients with invasive breast cancer and a clinically negative axilla. While less invasive than ALND, SLNB still carries a risk of acute and long-term complications, including lymphedema, wound infection, seroma formation, and pain.13 Prior literature evaluating side effects of SLNB was generally limited to the comparison between SLNB and ALND, and to patients with early-stage invasive breast cancer.14,15 Additionally, some evidence from analyses of invasive breast cancer suggested that older age and advanced stage are associated with increased risk of side effects after surgery.16,17 However, it is unclear whether SLNB (compared with no SLNB) increases side effects among older patients with DCIS. A recent analysis of nearly 7000 DCIS patients in Sweden found that receipt of SLNB was not associated with decreased risk of breast cancer mortality.18 Given the current lack of evidence for SLNB benefit, it is important to determine SLNB-related complications in order to inform treatment decision-making.
This study aimed to examine the association between SLNB and acute complications among female Medicare beneficiaries with DCIS. Older women with DCIS represent a unique group of patients who have very favorable prognosis. While the risks associated with SLNB among women undergoing BCS may be small, we hypothesized that they would be higher than risks without SLNB. We anticipated that SLNB might be associated with more aggressive treatments that also have side effects, such as mastectomy or RT; thus, we controlled for these treatments to determine the independent associations between SLNB and acute complications. Determining higher rates of complications associated with SLNB might potentially discourage SLNB use for patients with DCIS.
Methods
Data Source and Study Population
Using the Surveillance, Epidemiology, and End Results (SEER)–Medicare database, we conducted a retrospective cohort study of older female patients diagnosed at age 67–94 years with in situ breast tumors between 1/1/1998 and 12/31/2011.19,20 We used SEER ICD-O-3 behavior and histology codes to identify patients with DCIS (in situ tumor which is consistent with ductal origin, see Online Appendix Table 1). We only included women who received BCS as first breast surgery in the first 6 months after DCIS diagnosis. Patients were excluded if they were male, their diagnosis occurred only according to death certificate or autopsy, or their income or education by zip code was unknown. Women with SLNB were included in our study if SLNB occurred at any point during their treatment (whether at time of initial lumpectomy, as a separate procedure, or at time of a subsequent mastectomy). The Yale Human Investigation Committee determined that this study did not directly involve human subjects.
Exposure and Outcome Ascertainment
We identified SLNB according to the Healthcare Common Procedure Coding System codes 38500, 38525, 38790, 38792, 38900, 78195, A9520, G8878 in the first 6 months after DCIS diagnosis based on prior literature21–25 as well as suggestions from clinicians on our team. Within 9 months from diagnosis we assessed the development of short-term outcomes of lymphedema, wound infection, seroma, or pain (see Online Appendix Table 1). For lymphedema, we included this diagnosis on durable medical equipment claims, as well as the inpatient, outpatient or physician claims assessed for the other short-term outcomes.
Covariate Selection
Patient characteristics included age at diagnosis, race, marital status, year of DCIS diagnosis, SEER registry, metro status of residence, and comorbidity.26 We assessed Elixhauser comorbidity27 and created a disability indicator.28,29 SEER–Medicare also provides census-based estimates of income and education. Tumor characteristics included grade, size, laterality, and estrogen receptor (ER) and progesterone receptor (PR) status, as reported by SEER. We identified flu vaccine, physician visit, and hospitalization as indicators of interaction with the healthcare system in the 3–24 months prior to DCIS diagnosis. Other variables included use of preoperative breast magnetic resonance imaging (MRI), surgeon volume, and receipt of RT. We assessed receipt of mastectomy with and without SLNB after initial BCS through 6 months after DCIS diagnosis.
Statistical Analysis
We used Mahalanobis matching to adjust for baseline characteristics and account for potential treatment selection bias, where those who receive SLNB might be systematically different from those who do not.30,31 Matching was based on the calculated Mahalanobis distance, including age, tumor grade, tumor size, hormone receptor status, year of diagnosis (in 2-year groupings), SEER registry, and geographic region. Matches were assigned by choosing the two best non-SLNB patient matches for each SLNB patient; when two or more SLNB patients matched the same control (that is, had Mahalanobis distance minimized by the same control), one was randomly selected as a match, with this process reiterated until nearly all SLNB patients had two matched controls. We assessed balance diagnostics by comparing prevalence of baseline characteristics using absolute standardized differences (expressed as percentage).32 Prior research has suggested that two-to-one matching can improve precision,33 and standardized difference ≥ 10 indicates meaningful imbalance in the baseline covariate.34
We applied generalized linear models to the Mahalanobis matched cohort to estimate the associations of SLNB and complications. In each model, we accounted for the nesting effects within matched groups. We also adjusted for subsequent mastectomy within 180 days after initial BCS, prior MRI, and RT status. The primary outcomes we used in the regression model were occurrence of any complication and occurrence of each individual complication (lymphedema, wound infection, seroma, and pain). To account for multiple comparisons, statistical significance was determined by p value lower than 0.01. All statistical analyses in this section were performed using STATA 14 (College Station, TX) and SAS 9.4 (SAS Institute, Cary, NC).
Results
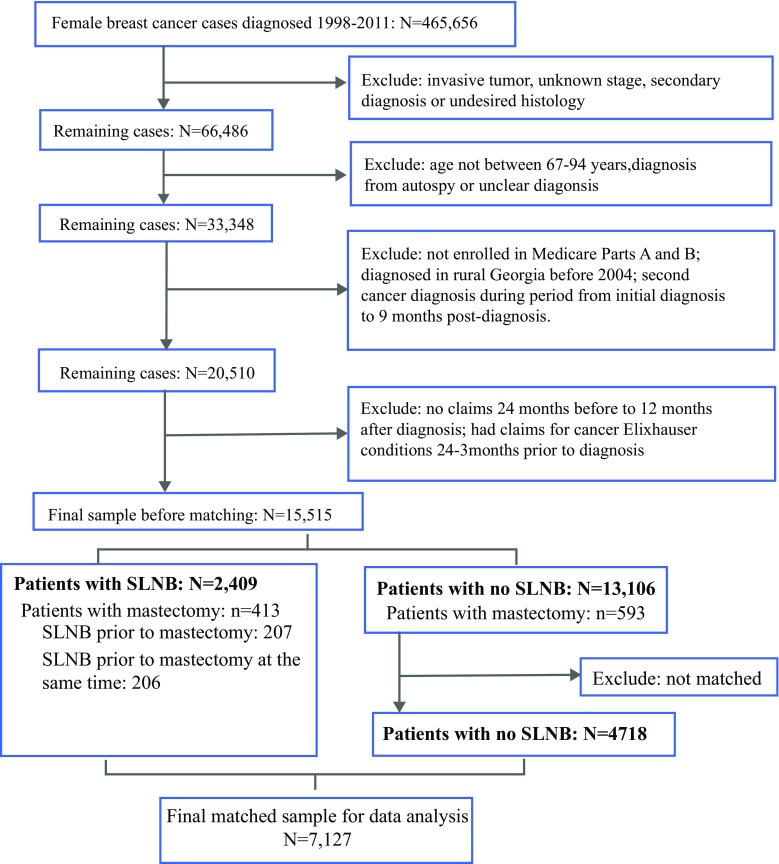
The sample consisted of 15,515 women with DCIS (mean age 75.0 years), including 2409 (15.5%) who underwent SLNB and 4718 matched controls. Detailed cohort creation is shown in Fig. 1. Women who underwent SLNB tended to be younger, White, and married, have fewer comorbidities, and be diagnosed in later years (p < 0.001 for all except p = 0.025 for comorbidity; Table 1). Women who received surgery from surgeons with larger volume were less likely to undergo SLNB (p = 0.014). Tumor characteristics, such as high grade, DCIS tumor size > 2 cm, ER-positive DCIS, and comedonecrosis, were associated with the likelihood of undergoing SLNB (p < 0.001 for all). All 2409 women undergoing SLNB were successfully matched with 4718 non-SLNB controls (2309 women had two controls, 100 women had one control). After matching, baseline characteristics were well balanced between those who underwent SLNB and those who did not, with standardized differences less than 10. Detailed characteristics are reported in Online Appendix Table 2.
Fig. 1.
Cohort selection diagram; SLNB sentinel lymph node biopsy
Table 1.
Selected patient characteristics before and after Mahalanobis matching
| Before match | After match | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No SLNB | SLNB | SDa | No SLNB | SLNB | SD | |||||
| N | % | N | % | % | N | % | N | % | % | |
| Total sample | 13,106 | 84 | 2409 | 16 | N/A | 4718 | 66 | 2409 | 34 | N/A |
| Age (years) | ||||||||||
| 67–69 | 2571 | 20 | 573 | 24 | − 10.1 | 981 | 21 | 573 | 24 | − 7.2 |
| 70–74 | 4104 | 31 | 783 | 33 | − 2.6 | 1592 | 34 | 783 | 33 | 2.6 |
| 75–79 | 3367 | 26 | 614 | 25 | 0.5 | 1263 | 27 | 614 | 25 | 2.9 |
| 80–84 | 2110 | 16 | 333 | 14 | 6.4 | 670 | 14 | 333 | 14 | 1.1 |
| 85+ | 954 | 7 | 106 | 4 | 12.3 | 212 | 4 | 106 | 4 | 0.5 |
| Race | ||||||||||
| White | 11,369 | 87 | 2148 | 89 | − 7.4 | 4099 | 87 | 2148 | 89 | − 7.0 |
| Black | 1032 | 8 | 170 | 7 | 3.1 | 386 | 8 | 170 | 7 | 4.2 |
| Other | 705 | 5 | 91 | 4 | 7.7 | 233 | 5 | 91 | 4 | 5.7 |
| Hispanic ethnicity | ||||||||||
| Yes | 545 | 4 | 135 | 6 | − 6.7 | 221 | 5 | 135 | 6 | − 4.2 |
| No | 12,561 | 96 | 2274 | 94 | 6.7 | 4497 | 95 | 2274 | 94 | 4.2 |
| Marital status | ||||||||||
| Married | 6211 | 47 | 1227 | 51 | − 7.1 | 2286 | 48 | 1227 | 51 | − 5.0 |
| Unmarried | 6204 | 47 | 1085 | 45 | 4.6 | 2196 | 47 | 1085 | 45 | 3.0 |
| Other | 691 | 5 | 97 | 4 | 5.9 | 236 | 5 | 97 | 4 | 4.7 |
| Grade | ||||||||||
| Well differentiated | 2048 | 16 | 254 | 11 | 15.1 | 498 | 11 | 254 | 11 | 0.0 |
| Moderately differentiated | 4456 | 34 | 629 | 26 | 17.3 | 1371 | 29 | 629 | 26 | 6.6 |
| Poorly differentiated | 2925 | 22 | 802 | 33 | − 24.7 | 1449 | 31 | 802 | 33 | − 5.5 |
| Undifferentiated | 1070 | 8 | 321 | 13 | − 16.7 | 601 | 13 | 321 | 13 | − 1.7 |
| Unknown | 2607 | 20 | 403 | 17 | 8.2 | 799 | 17 | 403 | 17 | 0.6 |
| Tumor size | ||||||||||
| < 2.0 cm | 7402 | 56 | 1264 | 52 | 8.1 | 2552 | 54 | 1264 | 52 | 3.2 |
| 2.0 to ≤ 5.0 cm | 1512 | 12 | 434 | 18 | − 18.3 | 749 | 16 | 434 | 18 | − 5.7 |
| > 5.0 cm | 179 | 1 | 89 | 4 | − 14.9 | 149 | 3 | 89 | 4 | − 2.9 |
| Missing | 4013 | 31 | 622 | 26 | 10.7 | 1268 | 27 | 622 | 26 | 2.4 |
| Hormone receptors | ||||||||||
| ER– and PR– | 923 | 7 | 341 | 14 | − 23.3 | 631 | 13 | 341 | 14 | − 2.3 |
| ER+ or PR+ | 5839 | 45 | 1329 | 55 | − 21.4 | 2608 | 55 | 1329 | 55 | 0.2 |
| Missing | 6344 | 48 | 739 | 31 | 36.9 | 1479 | 31 | 739 | 31 | 1.5 |
| Comedonecrosis | ||||||||||
| Yes | 1246 | 10 | 297 | 12 | − 9.1 | 524 | 11 | 297 | 12 | − 3.8 |
| No | 11,860 | 90 | 2112 | 88 | 9.1 | 4194 | 89 | 2112 | 88 | 3.8 |
| Disability | ||||||||||
| Yes | 410 | 3 | 67 | 3 | 2.0 | 129 | 3 | 67 | 3 | − 0.3 |
| No | 12,696 | 97 | 2342 | 97 | − 2.0 | 4589 | 97 | 2342 | 97 | 0.3 |
| Elixhauser comorbidity | ||||||||||
| None | 6321 | 48 | 1219 | 51 | − 4.7 | 2229 | 47 | 1219 | 51 | − 6.7 |
| 1 to 2 | 5177 | 40 | 935 | 39 | 1.4 | 1914 | 41 | 935 | 39 | 3.6 |
| 3 or more | 1608 | 12 | 255 | 11 | 5.3 | 575 | 12 | 255 | 11 | 5.0 |
| Surgeon volumeb | ||||||||||
| 1 | 6243 | 48 | 1140 | 47 | 0.6 | 2182 | 46 | 1140 | 47 | − 2.2 |
| 2 | 3114 | 24 | 578 | 24 | − 0.5 | 1099 | 23 | 578 | 24 | − 1.6 |
| 3 | 1645 | 13 | 329 | 14 | − 3.3 | 596 | 13 | 329 | 14 | − 3.0 |
| 4+ | 1865 | 14 | 300 | 12 | 5.2 | 727 | 15 | 300 | 12 | 8.5 |
| Not assigned | 239 | 2 | 62 | 3 | − 5.1 | 114 | 2 | 62 | 3 | − 1.0 |
| Year of diagnosis | ||||||||||
| 1998–1999 | 1039 | 8 | 30 | 1 | 32.4 | 63 | 1 | 30 | 1 | 0.8 |
| 2000–2001 | 1959 | 15 | 140 | 6 | 30.3 | 286 | 6 | 140 | 6 | 1.1 |
| 2002–2003 | 2011 | 15 | 255 | 11 | 14.2 | 520 | 11 | 255 | 11 | 1.4 |
| 2004–2005 | 2163 | 17 | 431 | 18 | − 3.7 | 863 | 18 | 431 | 18 | 1.0 |
| 2006–2007 | 2021 | 15 | 508 | 21 | − 14.7 | 986 | 21 | 508 | 21 | − 0.5 |
| 2008–2009 | 2007 | 15 | 539 | 22 | − 18.1 | 1048 | 22 | 539 | 22 | − 0.4 |
| 2010–2011 | 1906 | 15 | 506 | 21 | − 17.0 | 952 | 20 | 506 | 21 | − 2.0 |
| Geographic region | ||||||||||
| Midwest | 1980 | 15 | 296 | 12 | 8.2 | 583 | 12 | 296 | 12 | 0.2 |
| Northeast | 3217 | 25 | 488 | 20 | 10.3 | 979 | 21 | 488 | 20 | 1.2 |
| South | 2408 | 18 | 588 | 24 | − 14.8 | 1141 | 24 | 588 | 24 | − 0.5 |
| West | 5501 | 42 | 1037 | 43 | − 2.2 | 2015 | 43 | 1037 | 43 | − 0.7 |
SLNB sentinel lymph node biopsy
aSD refers to standardized difference, which is a statistic that evaluates the balance of matched cohorts. Standardized difference below 10% indicates balance on the variable
bSurgeon volume reflects women who saw a provider who performed BCS on X number of women in our sample for the year of this woman’s surgery
Treatment Received
While BCS was the initial surgery in all patients, 1006 went on to receive completion mastectomy. In the matched cohort, women who underwent SLNB were more likely to receive subsequent mastectomy within 6 months compared with women who did not undergo SLNB (17.1 versus 5.0%, p < 0.001; Table 2). Women who underwent SLNB were also more likely to have preoperative MRI examination (20.5 versus 11.5%, p < 0.001). There was no statistically significant difference between the two groups in terms of RT receipt (58.5 versus 59.6%, p = 0.48). Of the 413 patients who had SLNB and completion mastectomy, 206 women (49.9%) underwent SLNB at time of completion mastectomy and 207 women (50.1%) underwent SLNB prior to mastectomy.
Table 2.
Treatment received in the sample before and after matching
| Table 2 Before matching | Table 2 After matching | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No SLNB N = 13,106 |
SLNB N = 2409 |
χ 2 | No SLNB N = 4718 |
SLNB N = 2409 |
χ 2 | |||||
| N | % | N | % | p value | N | % | N | % | p value | |
| Preoperative MRI | < 0.001 | < 0.001 | ||||||||
| Yes | 1072 | 8.2 | 495 | 20.5 | 542 | 11.5 | 495 | 20.5 | ||
| No | 12,034 | 91.8 | 1914 | 79.5 | 4176 | 88.5 | 1914 | 79.5 | ||
| Mastectomy | < 0.001 | < 0.001 | ||||||||
| Yes | 593 | 4.5 | 413 | 17.1 | 237 | 5.0 | 413 | 17.1 | ||
| No | 12,513 | 95.5 | 1996 | 82.9 | 4481 | 95.0 | 1996 | 82.9 | ||
| Radiotherapy | 0.001 | 0.48 | ||||||||
| Yes | 7237 | 55.2 | 1416 | 58.8 | 2814 | 59.6 | 1416 | 58.8 | ||
| No | 5869 | 44.8 | 993 | 41.2 | 1904 | 40.4 | 993 | 41.2 | ||
SLNB sentinel lymph node biopsy, MRI magnetic resonance imaging
Acute Complications
In the matched sample, SLNB was associated with increased risk of acute complications (Table 3). Occurrence of any complication was 16.8% in the SLNB group and 11.3% in the non-SLNB group (p < 0.001). Multivariate models revealed that SLNB use was independently associated with increased risk of complications (Table 4). Women who underwent SLNB had significantly higher risk of any complication [adjusted odds ratio (AOR) 1.39; 99% confidence interval (CI) 1.18–1.63]. Specifically, SLNB use was associated with each complication, including lymphedema (AOR 4.45; 99% CI 2.27–8.75), wound infection (AOR 1.24; 99% CI 1.00–1.54), seroma (AOR 1.40; 99% CI 1.03–1.91), and pain (AOR 1.31; 99% CI 1.04–1.65). Mastectomy was associated with increased risk of any complication (AOR 1.36; 99% CI 1.05–1.77), wound infection (AOR 1.55; 99% CI 1.12–2.45), and seroma (AOR 2.51; 99% CI 1.60–3.92). Prior MRI use and RT were not significantly associated with acute complications. Sensitivity analyses using the sample before matching and including or excluding patients who underwent mastectomy reached qualitatively similar results regarding the associations between SLNB and complications.
Table 3.
Unadjusted side effects in the sample before and after matching
| Table 2 Before matching | Table 2 After matching | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No SLNB N = 13,106 |
SLNB N = 2409 |
χ 2 | No SLNB N = 4718 |
SLNB N = 2409 |
χ 2 | |||||
| N | % | N | % | p value | N | % | N | % | p value | |
| Any of below | 1476 | 11.3 | 404 | 16.8 | < 0.001 | 534 | 11.3 | 404 | 16.8 | < 0.001 |
| Lymphedema | 57 | 0.4 | 60 | 2.5 | < 0.001 | 23 | 0.5 | 60 | 2.5 | < 0.001 |
| Wound infection | 1290 | 9.8 | 296 | 12.3 | < 0.001 | 453 | 9.6 | 296 | 12.3 | < 0.001 |
| Seroma | 421 | 3.2 | 153 | 6.4 | < 0.001 | 179 | 3.8 | 153 | 6.4 | < 0.001 |
| Pain | 1060 | 8.1 | 237 | 9.8 | 0.004 | 365 | 7.7 | 237 | 9.8 | 0.003 |
SLNB sentinel lymph node biopsy
Table 4.
Generalized models for the association of sentinel lymph node biopsy (SLNB) with specified outcome
| Model description | Any complication | Lymphedema | Wound infection | Seroma | Pain |
|---|---|---|---|---|---|
| SLNB | |||||
| No | REF | REF | REF | REF | REF |
| Yes | 1.39 (1.18–1.63) | 4.45 (2.27–8.75) | 1.24 (1.00–1.54) | 1.40 (1.03–1.91) | 1.31 (1.04–1.65) |
| Mastectomy after BCS | |||||
| No | REF | REF | REF | REF | REF |
| Yes | 1.36 (1.05–1.77) | 2.17 (0.98–4.82) | 1.55 (1.12–2.15) | 2.51 (1.60–3.92) | 0.88 (0.58–1.33) |
| Radiotherapy | |||||
| No | REF | REF | REF | REF | REF |
| Yes | 0.85 (0.85–1.31) | 0.86 (0.44–1.68) | 0.81 (0.65–1.02) | 1.07 (0.76–1.51) | 0.85 (0.67–1.07) |
| Prior MRI | |||||
| No | REF | REF | REF | REF | REF |
| Yes | 1.05 (0.72–1.00) | 1.30 (0.64–2.61) | 0.94 (0.70–1.25) | 1.82 (1.27–2.60) | 1.06 (0.78–1.44) |
Accounting for matching and adjusting for treatment received. Adjusted odds ratio (99% confidence interval)
MRI magnetic resonance imaging, BCS breast-conserving surgery
Discussion
Among women with DCIS who received BCS as initial surgery, use of SLNB was significantly associated with increased risk of complications, including lymphedema, wound infection, seroma, and pain. Given the lack of evidence that SLNB improves long-term outcomes for patients with DCIS,35 our finding of increased risk of SLNB-related complications should further discourage its routine use in these patients.
Our study advances current knowledge about SLNB use in DCIS patients in several important ways. First, we compared use of SLNB versus no SLNB in real-world practice. Existing studies, generally performed as part of a clinical trial or at individual institutions, have demonstrated that SLNB for invasive breast cancer was less likely to lead to side effects compared with ALND, a more invasive procedure.36 For instance, a 2015 review showed that the incidence of lymphedema after ALND was 22.3% and the incidence after SLNB was 6.3%.36 While the incidence of SLNB-related complications is generally acceptable, population-level data on side effects of SLNB and comparisons between SLNB and no SLNB, specifically among patients with DCIS, are lacking. This study, to the best of the authors’ knowledge, is the first to fill this evidence gap. Our findings reveal that approximately one out of six patients who underwent BCS plus SLNB would experience complications compared with one out of nine patients who underwent BCS without SLNB. The latter cohort of patients who experienced complications without axillary surgery merit further study. Our findings support the consensus guidelines that SLNB should not be routinely used for DCIS patients who undergo BCS.
Second, the odds ratio of lymphedema attributed to SLNB use is relatively large, estimated at 4.41. This information is important because both the risk of developing lymphedema and the associated symptoms often persist over the course of a woman’s lifetime and therefore have a great impact on quality of life.37,38 We found that the incidence of lymphedema among women who underwent SLNB was only 2.5%, relatively low compared with prior literature.36 We limited our study to patients with DCIS, which has low likelihood of nodal involvement. The sentinel node procedure includes removal of not only “hot” or “blue” nodes, but also any clinically suspicious nodes. It may be that, during SLNB for DCIS, the clinical suspicion of the surgeon is lower, leading to resection of fewer lymph nodes in this setting, and a resultant lower rate of lymphedema than seen in other series. Additionally, we used claims data to identify lymphedema occurrence; such an approach might only capture severe lymphedema and thus underestimate the incidence. Nevertheless, our results indicate that the incidence of having a claim for lymphedema (0.5%) was very low for patients who did not undergo SLNB, and use of SLNB is a strong predictor for this complication.
Analyzing data from over 600 acute-care hospitals throughout the USA, a prior study found that 16.7% of patients who received BCS for DCIS underwent SLNB and surgeons who had low patient volume were more likely to perform SLNB.10 Building upon this study, our findings suggest that, in addition to low surgeon volume, tumor characteristics, including grade, size, ER status, and comedonecrosis, are also associated with SLNB use. Furthermore, we found that patients who underwent SLNB were more likely to receive preoperative MRI and subsequent mastectomy, reflecting the fact that physicians who are more aggressive with respect to axillary surgery may also be more aggressive with respect to imaging and extent of breast surgery. It is also possible that less experienced surgeons tended to use more aggressive treatments such as SLNB and mastectomy. While SLNB use at time of mastectomy for patients with DCIS might be appropriate, the proportion was quite low in our cohort. In fact, more than 90% of SLNB use was performed outside of current recommendations, either before mastectomy or without undergoing mastectomy. These findings are important, particularly in the context of current controversies regarding overdiagnosis and overtreatment of DCIS patients.39,40 Future programs targeting low-volume physicians with practice improvement interventions may improve DCIS care quality and reduce inappropriate care for patients with DCIS.
Our study, however, has some limitations. First, we only examined short-term SLNB-related complications. Future research examining long-term side effects, recurrence, and survival attributed to SLNB is needed. Second, our study was limited to older population; thus, our results should not be generalized to younger population. While our population comprised beneficiaries enrolled in Medicare fee-for-service programs, we would be surprised if the harm attributed to SLNB differed among Medicare Part C beneficiaries. Third, we used medical claims to capture side effects, including pain, which are subject to estimation errors. Finally, our study is not a randomized trial. While we applied a Mahalanobis matching method to reduce selection bias, we were unable to control for unobserved factors. For instance, obesity is associated with lymphedema,16 yet we do not have data of individual body mass index.
In conclusion, SLNB use led to higher rate of short-term side effects among women undergoing BCS for DCIS. Given a lack of evidence that SLNB use decreases recurrence or improves breast cancer survival for patients with DCIS, our results indicate that using SLNB to detect nodal involvement may be causing more harm than good for older women with DCIS. These data highlight the need to critically consider the impact of SLNB on patient health outcomes.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
Funding
Dr. Gross receives research support from Johnson & Johnson, Inc., and 21st Century Oncology. Dr. Mougalian receives consulting fees from Eisai, Inc. Dr. Killelea receives consulting fees from Genentech. Drs. Gross and Mougalian are on a grant sponsored by National Comprehensive Cancer Network/Pfizer. These sources of support were not used for any portion of the current manuscript.
Disclosure
None of the other coauthors have conflicts to report.
Footnotes
Electronic supplementary material
The online version of this article (10.1245/s10434-018-6410-0) contains supplementary material, which is available to authorized users.
References
- 1.Virnig BA, Wang SY, Shamilyan T, Kane RL, Tuttle TM. Ductal carcinoma in situ: risk factors and impact of screening. J Natl Cancer Inst Monogr. 2010;41:113–116. doi: 10.1093/jncimonographs/lgq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Cancer Society (2013). Cancer Facts & Figure 2013. www.cancer.org/research/cancerfactsfigures/cancerfactsfigures/cancer-facts-figures-2013. Accessed 20 July 2016
- 3.Virnig BA, Tuttle TM, Shamliyan T, Kane RL. Ductal carcinoma in situ of the breast: a systematic review of incidence, treatment, and outcomes. J Natl Cancer Inst. 2010;102(3):170–178. doi: 10.1093/jnci/djp482. [DOI] [PubMed] [Google Scholar]
- 4.Bijker N, Meijnen P, Peterse JL, et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma-in situ: ten-year results of European Organisation for Research and Treatment of Cancer randomized phase III trial 10853–a study by the EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. J Clin Oncol. 2006;24(21):3381–3387. doi: 10.1200/JCO.2006.06.1366. [DOI] [PubMed] [Google Scholar]
- 5.Baxter NN, Virnig BA, Durham SB, Tuttle TM. Trends in the treatment of ductal carcinoma in situ of the breast. J Natl Cancer Inst. 2004;96(6):443–448. doi: 10.1093/jnci/djh069. [DOI] [PubMed] [Google Scholar]
- 6.Patient-Centered Outcomes Research Institute. PCORI funding announcement: large pragmatic studies to evaluate patient-centered outcomes. 2015. http://www.pcori.org/sites/default/files/PCORI-PFA-2015-Winter-Pragmatic-Studies.pdf. Accessed 14 Sept 2015
- 7.Institute of Medicine. Initial National Priorities for Comparative Effectiveness Research. 2009. http://www.iom.edu/en/Reports/2009/ComparativeEffectivenessResearchPriorities.aspx. Accessed 14 Sept 2015.
- 8.Lyman GH, Giuliano AE, Somerfield MR, et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol. 2005;23(30):7703–7720. doi: 10.1200/JCO.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network (2017). NNCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). 2017. http://www.nccn.org/patients. Accessed 17 July 2017.
- 10.Coromilas EJ, Wright JD, Huang Y, et al. The influence of hospital and surgeon factors on the prevalence of axillary lymph node evaluation in ductal carcinoma in situ. JAMA Oncol. 2015;1(3):323–332. doi: 10.1001/jamaoncol.2015.0389. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell KB, Lin H, Shen Y, et al. DCIS and axillary nodal evaluation: compliance with national guidelines. BMC Surg. 2017;17(1):12. doi: 10.1186/s12893-017-0210-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mannu GS, Bettencourt-Silva JH, Ahmed F, Cunnick G. A nationwide cross-sectional survey of UK breast surgeons’ views on the management of ductal carcinoma in situ. Int J Breast Cancer. 2015;2015:104231. doi: 10.1155/2015/104231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McLaughlin SA, Wright MJ, Morris KT, et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: patient perceptions and precautionary behaviors. J Clin Oncol. 2008;26(32):5220–5226. doi: 10.1200/JCO.2008.16.3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lucci A, McCall LM, Beitsch PD, et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol. 2007;25(24):3657–3663. doi: 10.1200/JCO.2006.07.4062. [DOI] [PubMed] [Google Scholar]
- 15.Purushotham AD, Upponi S, Klevesath MB, et al. Morbidity after sentinel lymph node biopsy in primary breast cancer: results from a randomized controlled trial. J Clin Oncol. 2005;23(19):4312–4321. doi: 10.1200/JCO.2005.03.228. [DOI] [PubMed] [Google Scholar]
- 16.Wilke LG, McCall LM, Posther KE, et al. Surgical complications associated with sentinel lymph node biopsy: results from a prospective international cooperative group trial. Ann Surg Oncol. 2006;13(4):491–500. doi: 10.1245/ASO.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 17.Coriddi M, Khansa I, Stephens J, Miller M, Boehmler J, Tiwari P. Analysis of factors contributing to severity of breast cancer-related lymphedema. Ann Plast Surg. 2015;74(1):22–25. doi: 10.1097/SAP.0b013e31828d7285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wadsten C, Garmo H, Fredriksson I, Sund M, Warnberg F. Risk of death from breast cancer after treatment for ductal carcinoma in situ. Br J Surg. 2017;104(11):1506–1513. doi: 10.1002/bjs.10589. [DOI] [PubMed] [Google Scholar]
- 19.Warren JL, Brown ML, Fay MP, Schussler N, Potosky AL, Riley GF. Costs of treatment for elderly women with early-stage breast cancer in fee-for-service settings. J Clin Oncol. 2002;20(1):307–316. doi: 10.1200/JCO.2002.20.1.307. [DOI] [PubMed] [Google Scholar]
- 20.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8 Suppl):IV-3-18. [DOI] [PubMed]
- 21.Caretta-Weyer H, Greenberg CG, Wilke LG, et al. Impact of the American College of Surgeons Oncology Group (ACOSOG) Z0011 trial on clinical management of the axilla in older breast cancer patients: a SEER-medicare analysis. Ann Surg Oncol. 2013;20(13):4145–4152. doi: 10.1245/s10434-013-3193-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis GB, Peric M, Chan LS, Wong AK, Sener SF. Identifying risk factors for surgical site infections in mastectomy patients using the National Surgical Quality Improvement Program database. Am J Surg. 2013;205(2):194–199. doi: 10.1016/j.amjsurg.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Meyer AM, Reeder-Hayes KE, Liu H, et al. Differential receipt of sentinel lymph node biopsy within practice-based research networks. Med Care. 2013;51(9):812–818. doi: 10.1097/MLR.0b013e31829c8ca4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmocker RK, Caretta-Weyer H, Weiss JM, et al. Determining breast cancer axillary surgery within the surveillance epidemiology and end results-Medicare database. J Surg Oncol. 2014;109(8):756–759. doi: 10.1002/jso.23579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yen TW, Laud PW, Sparapani RA, Nattinger AB. Surgeon specialization and use of sentinel lymph node biopsy for breast cancer. JAMA Surg. 2014;149(2):185–192. doi: 10.1001/jamasurg.2013.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bach PB, Guadagnoli E, Schrag D, Schussler N, Warren JL. Patient demographic and socioeconomic characteristics in the SEER-Medicare database applications and limitations. Med Care. 2002;40(8 Suppl):IV-19-25. [DOI] [PubMed]
- 27.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Davidoff AJ, Gardner LD, Zuckerman IH, Hendrick F, Ke X, Edelman MJ. Validation of disability status, a claims-based measure of functional status for cancer treatment and outcomes studies. Med Care. 2014;52(6):500–510. doi: 10.1097/MLR.0000000000000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davidoff AJ, Zuckerman IH, Pandya N, et al. A novel approach to improve health status measurement in observational claims-based studies of cancer treatment and outcomes. J Geriatr Oncol. 2013;4(2):157–165. doi: 10.1016/j.jgo.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19):2265–2281. doi: 10.1002/(SICI)1097-0258(19981015)17:19<2265::AID-SIM918>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 31.Yu JB, Soulos PR, Herrin J, et al. Proton versus intensity-modulated radiotherapy for prostate cancer: patterns of care and early toxicity. J Natl Cancer Inst. 2013;105(1):25–32. doi: 10.1093/jnci/djs463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Austin PC. Statistical criteria for selecting the optimal number of untreated subjects matched to each treated subject when using many-to-one matching on the propensity score. Am J Epidemiol. 2010;172(9):1092–1097. doi: 10.1093/aje/kwq224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Normand ST, Landrum MB, Guadagnoli E, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54(4):387–398. doi: 10.1016/S0895-4356(00)00321-8. [DOI] [PubMed] [Google Scholar]
- 35.Francis AM, Haugen CE, Grimes LM, et al. Is Sentinel lymph node dissection warranted for patients with a diagnosis of ductal carcinoma in situ? Ann Surg Oncol. 2015;22(13):4270–4279. doi: 10.1245/s10434-015-4547-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaitelman SF, Cromwell KD, Rasmussen JC, et al. Recent progress in the treatment and prevention of cancer-related lymphedema. CA Cancer J Clin. 2015;65(1):55–81. doi: 10.3322/caac.21253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11(3):570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 38.Beckjord EB, Reynolds KA, van Londen GJ, et al. Population-level trends in posttreatment cancer survivors’ concerns and associated receipt of care: results from the 2006 and 2010 LIVESTRONG surveys. J Psychosoc Oncol. 2014;32(2):125–151. doi: 10.1080/07347332.2013.874004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sagara Y, Julia W, Golshan M, Toi M. Paradigm shift toward reducing overtreatment of ductal carcinoma in situ of breast. Front Oncol. 2017;7:192. doi: 10.3389/fonc.2017.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Welch HG, Prorok PC, O’Malley AJ, Kramer BS. Breast-cancer tumor size, overdiagnosis, and mammography screening effectiveness. N Engl J Med. 2016;375(15):1438–1447. doi: 10.1056/NEJMoa1600249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.