Abstract
Human epididymis protein 4 (HE4) is an important clinical biomarker used for the detection of epithelial ovarian cancer (EOC). While much is known about the predictive power of HE4 clinically, less has been reported regarding its molecular role in the progression of EOC. A deeper understanding of HE4’s mechanistic functions may help contribute to the development of novel targeted therapies. Thus far, it has been difficult to recommend HE4 as a therapeutic target owing to the fact that its role in the progression of EOC has not been extensively evaluated. This review summarizes what is collectively known about HE4 signaling and how it functions to promote tumorigenesis, chemoresistance, and metastasis in EOC, with the goal of providing valuable insights that will have the potential to aide in the development of new HE4-targeted therapies.
Keywords: human epididymis protein 4, epithelial ovarian cancer, tumorigenesis, chemoresistance, metastasis
Introduction
Approximately 22,280 new cases of epithelial ovarian cancer (EOC) are diagnosed each year, resulting in 14,240 deaths annually in the United States (1). The 5-year survival rate for stage III ovarian cancer is only 39% (1). These dire statistics are due to the fact that the disease is frequently detected at an advanced stage, which drastically impacts overall patient survival. Initially, many patients respond well to first-line therapy that includes cytoreduction surgery and platinum-based treatment. However, many patients experience a chemoresistant recurrence within the first 2 years following treatment (2). Therefore, there is an urgent need for tools to aid in the early diagnosis of ovarian cancer when the disease is fundamentally curable, as well as improved treatment options for later stage disease.
Human epididymis protein 4 (HE4) is a secretory protein that is member of the whey acidic protein domain family, bearing a conserved motif found in a number a protease inhibitors (3). HE4 was initially suggested to be involved in the innate immune defense of multiple epithelia and has also been found to function in epithelial host defense (4). In ovarian tissue, HE4 is highly overexpressed in EOC compared normal tissue (5, 6). Clinically, HE4 has been identified as a novel therapeutic biomarker for EOC and has also proven useful in detection of recurrent disease (7) Serum HE4 level predicts EOC with equal sensitivity to the established biomarker CA125 and is less likely to be elevated in benign disease (5). A multicenter study led by our institution established the FDA-approved Risk of Ovarian Malignancy Algorithm (ROMA), which combines menopausal status and serum levels of both HE4 and CA125 to detect and monitor EOC. ROMA demonstrates improved sensitivity and specificity over the Risk of Malignancy Index that uses CA125 alone as a serum based biomarker (6). Recently, it has been reported that HE4 can be detected in EOC patient urine, indicating the possibility that it may be utilized as a non-invasive biomarker (8).
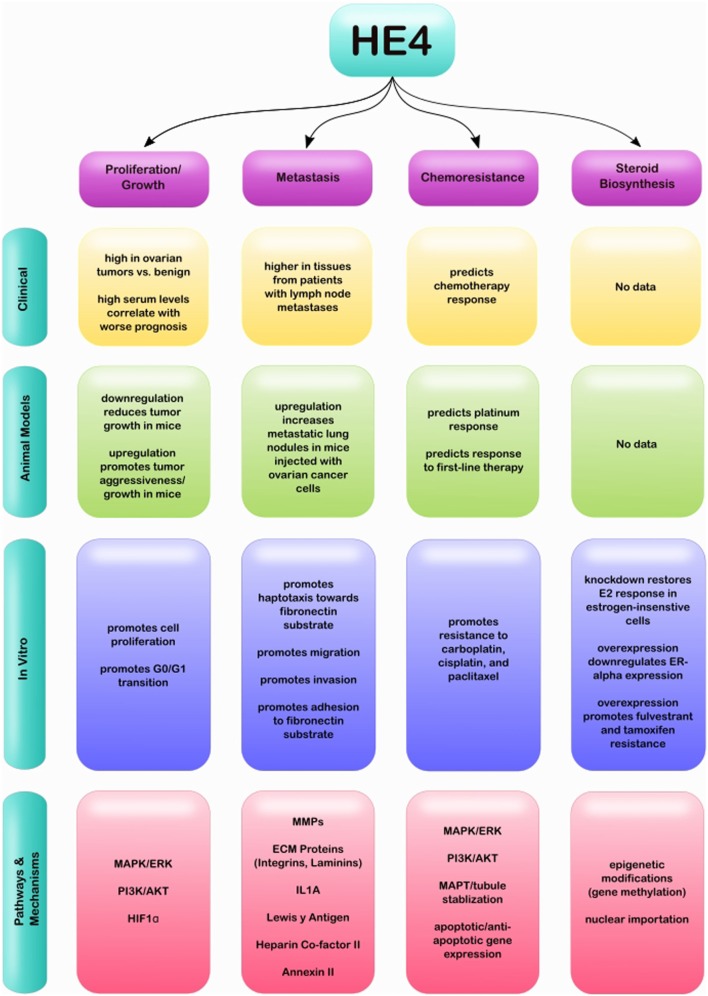
While HE4 has been well studied in the clinical setting, less is known regarding its specific molecular and biological roles in EOC. Several studies have investigated its effect on gene expression in EOC cells, as well as on events associated with aggressive disease. This review will summarize HE4’s effect on cell proliferation and tumor growth; invasion, migration, and adhesion; chemoresistance; and steroid biosynthesis (Figure 1). Each section will detail associated pathways and factors that are reported to be involved in these HE4-mediated effects, with the goal of revealing common themes in signaling pathways affected by HE4 and exposing gaps in our knowledge of HE4 molecular and biological functions.
Figure 1.
Graphical representation of clinical, in vivo and in vitro studies completed relating to HE4 and EOC, as well as associated pathways and mechanisms.
Cell Proliferation and Tumor Growth
Within the past 5 years, a handful of in vitro and in vivo studies have begun to examine HE4’s role in proliferation and tumor growth in EOC. A study by Wang et al. examined the role of HE4 in cell proliferation and found that cells treated with recombinant HE4 formed a statistically greater number of colonies compared with control treated cells (9). Furthermore, cells stimulated with recombinant HE4 exhibited greater cell viability compared with respective controls. In another study by Zhu et al. (10), proliferation rate in two different HE4-overexpressing cell lines was significantly higher than in the control cells. Likewise, Zhu et al. (11) and Lee et al. (12) determined that when HE4 was ablated via shRNA, cell proliferation decreased accordingly. Kong et al. report conflicting results, stating that HE4 inhibits proliferation in ovarian cells (13); however, no other studies support these claims, necessitating further explanation to understand the implications of their results.
Several in vitro studies suggest that HE4 promotes proliferation through its involvement in cell cycle regulation (11). Silencing of HE4 causes G0/G1 cell cycle arrest and blocks the transition from the G1 to the S phase of the cell cycle. Conversely, when cells are stimulated with recombinant HE4, the number of cells in the G2/M phase is increased, while the number of cells in the G0/G1 phase is reduced (9). These results indicate that HE4 may mediate the cell cycle by promoting the G0/G1 transition. In addition, in vivo tumorigenicity studies using HE4 knockdown clones revealed a marked inhibition in the growth of ovarian tumors in nude mice (14), while injection of HE4-overexpressing cells led to more aggressive tumor growth and an overall higher tumor volume compared with controls (10, 15). Taken together, results from numerous in vitro and in vivo studies provide compelling evidence that HE4 plays a role in cell proliferation and the promotion of tumorigenesis. A full list of factors associated with HE4-mediated cell proliferation and tumor growth can be found in Table 1A and is outlined in greater detail below.
Table 1.
Summary of factors associated with human epididymis protein 4 (HE4) in epithelial ovarian cancer (EOC).
| Gene symbol | Description | Association |
|---|---|---|
| (A) Cell proliferation and tumor growth | ||
| AKT | Protein kinase B | |
| HIF1α | Hypoxia-inducible factor-1 alpha | |
| ERK | Extracellular signal-regulated kinase | |
| CHUK | Conserved helix–loop–helix ubiquitous kinase |
|
| GADD45A | Growth arrest and DNA-damage-inducible protein GADD45 alpha | |
| IL1A | Interleukin-1 alpha | |
| RPS6KA1 | Ribosomal protein S6 kinase alpha 1 | |
| HSPA1B | Heat shock 70 kDa protein 1B, | |
| DUSP1 | Dual specificity protein phosphatase 1 | |
| JUND | Transcription factor JunD | |
| EGF/EGFR | Epidermal growth factor/epidermal growth factor receptor | |
| VEGF | Vascular endothelial growth factor |
|
| INS | Insulin | |
| (B) Invasion, migration, and adhesion | ||
| MMP-9 | Matrix metallopeptidase 9 |
|
| MMP-2 | Matrix metallopeptidase 9 | |
| CTSB | Cathepsin B | |
| IL1A | Interleukin-1 alpha | |
| ITGβ5 | Integrin β5 | |
| SDC1 | Syndecan 1 |
|
| COL1A1 | Collagen type 1 alpha 1 | |
| DAG1 | Dystroglycan 1 | |
| LAMB3 | Laminin-β3 |
|
| LAMC2 | Laminin-γ2 | |
| GREM1 | Gremlin 1 | |
| TNC | Tenascin C | |
| SERPIND2 | Serine peptidase inhibitor member 2 | |
| LAMA3 | Laminin 332 |
|
| Lewis y antigen | ||
| SERPIN D1 | Heparin cofactor II | |
| ANXA2 | Annexin II | |
| LAMB2 | Laminin subunit beta-2 |
|
| MKN2 | MAP kinase-interacting serine/threonine-protein kinase 2 | |
| (C) Chemoresistance | ||
| EGR1 | Early growth response protein 1 |
|
| p38 | p38 mitogen-activated protein kinase |
|
| BCL2 | B-cell lymphoma 2 |
|
| BAX | bcl-2-like protein 4 |
|
| MAPT | Microtubule-associated protein tau |
|
| SEPT3 | Septin 3 | |
| TUBB | β-Tubulin |
|
| ERK | Extracellular signal-regulated kinase |
|
| AKT | Protein kinase B | |
| (D) Steroid biosynthesis | ||
| FOXA2 | Forkhead box protein A2 |
|
| SQLE | Squalene monooygenase |
|
| DHCR7 | Dehydrocholesterol reductase | |
| NSDHL | Sterol-4-alpha-carboxylate-3 dehydrogenase | |
| 5-MC | 5-Methylcytosine |
|
| ESR1 | Estrogen/estrogen receptor | |
Associated Pathways and Factors—Cell Proliferation and Tumor Growth
Human epididymis protein 4 has been connected to several oncogenic signaling cascades that play key roles in ovarian cancer progression, including the PI3K/AKT pathway, HIF1α, and ERK/mitogen-activated protein kinase (MAPK) signaling. Evidence of HE4’s effect on activation of each of these pathways is discussed below.
Protein Kinase B Signaling
AKT has been established as a strong promoter of tumorigenesis, and the PI3K/AKT pathway is one of the most commonly hyperactivated pathways in many types of human cancers (16). Its diverse signaling regulates proliferation, growth, survival, motility, angiogenesis, and glucose metabolism (17). HE4-overexpressing OVCAR3 ovarian cancer cells were found to have a marked increase in activation of protein kinase B (AKT) compared with control cells, while HE4 knockdown in OVCAR3 cells reduced AKT activation (12). Moreover, it was found that HE4-overexpressing SKOV3 clones had naturally higher gene levels of AKT3 compared with the null-vector control (18), bolstering the claim that HE4 affects the PI3K/AKT pathway.
Hypoxia-Inducible Factor-1 Alpha (HIF1α)
Adaptation of malignant cells to hypoxic conditions is a key step in the promotion of tumorigenesis and angiogenesis (19–21), a process that is regulated by the transcription factor HIF1α. Co-immunoprecipitation revealed an interaction between HIF1α and HE4 in HE4-overexpressing SKOV3 xenografts. There was also strong colocalization of HE4 and HIF1α in SKOV3 ovarian xenograft tissue. In addition, when SKOV3 cells were treated with HIF1α siRNA or 2-methoxyestradiol (a HIF1α inhibitor), there was a marked decrease in HE4 protein levels (15). It is important to note that 2-methoxyestradiol is not a specific HIF1α inhibitor as it primarily causes the depolymerization of microtubules, which in turn prevents HIF1α expression (22). Thus, the specificity of the effect of HIF1α inhibition on HE4 levels may require further investigation. Although the exact mechanism and significance of the HE4-HIF1α interaction is not understood, this evidence suggests that HE4 could play a role in regulating HIF1α functions in angiogenesis.
MAPK Signaling
The MAPK pathway is composed of a family of conserved kinases that mediate essential cellular processes such as migration, growth, proliferation, differentiation, and apoptosis (23). The extracellular signal-regulated kinase (ERK) pathway is the best characterized of all MAPK pathways and is deregulated in approximately one-third of all cancers. Several studies have shown activation of ERK in response to HE4 treatment or overexpression, or suppression of ERK phosphorylation in response to HE4 knockdown (11, 12, 18). Using microarray analysis, Zhu et al. determined that seven genes involved in the MAPK pathway (CHUK, GADD45A, IL1A, RPS6KA1, HSPA1B, DUSP1, and JUND) were differentially regulated in response to HE4 overexpression in ES-2 cells (10).
Activation of the MAPK/ERK pathway occurs through EGF binding of its membrane bound receptor, EGFR (24). Using co-immunoprecipitation studies in SKOV3 cells, Moore et al. found that HE4 interacts with EGFR, with a greater degree of immunoprecipitation seen in HE4-overexpressing clones than wild-type cells (15). Furthermore, ovarian xenograft tissue showed colocalization of HE4 and EGFR. In addition, when SKOV3 and OVCAR8 cells were stimulated with growth factors EGF, VEGF, and Insulin, nuclear localization of HE4 was significantly increased. Finally, when EGF was repressed by the small molecule inhibitor Iressa, relative intensity of HE4 staining was decreased in ovarian cancer cell lines. Collectively, these results provide several layers of evidence that HE4 is tied to growth factor signaling and the MAPK/ERK pathway, although further research is needed to elucidate the precise mechanisms involved.
HE4’s Role in Proliferation in Other Cancers
Human epididymis protein 4 has been investigated as a putative biomarker in endometrial (25–39), lung (40–52), breast (53, 54), pancreatic (55, 56), and gastric cancer (57). While the majority of these studies examine the value of HE4 as a clinical biomarker for detecting and monitoring disease, one study investigated the molecular mechanisms of HE4 in pancreatic and endometrial cancer. Lu et al. stimulated both pancreatic and endometrial cancer cell lines with recombinant HE4 and found that cell viability, cell growth, and DNA synthesis was increased prominently in both cancer types (56). They also report that HE4 upregulates gene expression of proliferating cell nuclear antigen (PCNA) and downregulates p21 in both cancer cell lines in a dose dependent manner. PCNA, which is expressed in the late G1/S phase of the cell cycle, is required for DNA repair, replication, cell proliferation, and cell cycle progression (58), while p21 is an important effector of tumor suppressor pathways by promoting cell cycle arrest. Specifically, p21 is able to facilitate p53-dependent G1 growth arrest (59). Therefore, results from this study highlight HE4’s role in proliferation in both pancreatic and endometrial cancer and lend support to similar evidence from studies published on EOC.
Invasion, Migration, and Adhesion
Several studies have associated HE4 with metastatic properties, including invasion, migration, and adhesion of ovarian cancer cells. Lu et al. found that adhesion to a fibronectin substrate was twofold greater in SKOV3 cells overexpressing HE4 than in mock cells. In addition, a transwell migration assay demonstrated that the HE4-overexpressing clones had a 1.8-fold greater migration capacity than mock transfected cells. By contrast, immunofluorescence analysis showed that HE4 knockout clones displayed inhibited cell-spreading ability in a statistically significant fashion compared with respective controls. Furthermore, cell invasion, proliferation, and migration were significantly decreased in these clones (14). In agreement with this study, Ribeiro et al. also found that OVCAR8 ovarian cells treated with recombinant HE4 exhibited 2.07-fold greater invasion capacity and 1.29-fold greater adhesion to a fibronectin matrix compared with untreated controls. Interestingly, there was no change in adhesion to collagen I, IV, laminin I, and fibrinogen matrices, suggesting that HE4 has a specific effect on fibronectin adhesion. Haptotaxis toward a fibronectin substrate also was increased in the ovarian cancer cells treated with recombinant HE4 by 1.72-fold (60).
Zhu et al. used wound healing and transwell invasion assays to show that HE4-overexpressing ES-2 and CaOV3 cells possess enhanced cell migration and invasion capacities. In addition, in vivo tail vein injection of HE4-overexpressing ES-2 cells into nude mice resulted in significantly more metastatic lung nodules than mock transfected cells (10). Using the same ovarian cancer cell lines, Zhuang et al. report the importance of HE4 interaction with annexin II (ANXA2) to promote invasion and migration in vitro and metastasis in vivo (61). Finally, Zou et al. found that knockdown of HE4 in SKOV3.ip1 cells inhibited migration and invasion (62). Taken together, these studies strongly suggest that HE4 plays a prominent role in the promotion of ovarian cancer metastasis. A full list of factors associated with HE4-mediated invasion, migration, and adhesion can be found in Table 1B and is outlined in greater detail below.
Associated Pathways and Factors—Invasion, Migration, and Adhesion
Human epididymis protein 4 appears to interact with numerous molecular pathways that promote metastasis in ovarian cancer. However, it is still not entirely known how HE4 affects signaling pathways and gene expression signatures to promote invasion, migration, and adhesion of ovarian cancer cells. Following is a summary of HE4-mediated molecular pathways that are involved in metastatic events in EOC.
Matrix Metalloproteinases (MMPs)
Human epididymis protein 4 has been associated with MMPs MMP-9 and MMP-2, and Cathepsin B. MMPs are a family of zinc-dependent endopeptidases that are vital for the remodeling of the extracellular matrix (63). They are expressed in almost all types of cancers and are responsible for stimulating angiogenesis, tumor growth, and metastasis (64, 65). Cathepsin B is a lysosomal cysteine protease that has been linked to cancer progression (66), specifically in signaling pathways related to angiogenesis (67). In addition, it can promote MMP activity by degrading MMP inhibitors (68). Interestingly, silencing of HE4 in ovarian cancer cells led to a decrease in protein levels of MMP-9, MMP-2, and Cathepsin B, suggesting these factors may be involved in HE4-mediated tumor promoting effects (11).
Interleukin-1 Alpha (IL1A)
Interleukin-1 alpha is a pro-inflammatory cytokine that is involved in angiogenesis and metastasis. ILIA can directly stimulate the synthesis of VEGF (69) and fibroblastic pro matrix metallic proteinase I (70, 71). IL1A causes resistance to EGFR inhibitors in both colon and head and neck cancers (72, 73). IL1A was also found to be differentially expressed in three separate microarray studies involving HE4. In two microarrays, IL1A levels positively associated with HE4 levels (10, 74), while in one study their levels were inversely associated (18). While there may be some ambiguity as to how HE4 and IL1A are mechanistically linked, the consistent connection between IL1A with HE4 merits further investigation.
Extracellular Matrix Proteins
Integrins are a family of transmembrane proteins that are vital to ECM adhesion and play important roles in wound healing as well as the pathogenesis of cancer (75–77). Integrin β5 (ITGβ5) gene expression was differentially regulated by HE4 in ES-2 and CaOV3 cells, which was confirmed by positive correlation of ITGB5 and HE4 staining in paraffin embedded ovarian tissue samples (10). This finding suggests that integrin signaling is one mechanism by which HE4 can promote increased adhesion of ovarian cancer cells. However, further research is needed to clarify the mechanisms involved.
In addition to ITGβ5, three other genes related to ECM modeling—syndecan 1 (SDC1), collagen type 1 alpha 1 (COL1A1), and dystroglycan 1 (DAG1)—were more highly expressed in cells overexpressing HE4 and were downregulated in cells with HE4 knockdown (10). SDC1, also known as CD138, is an essential cell surface adhesion molecule that is responsible for maintaining cell morphology and interactions within the surrounding microenvironment (78). Loss of SCD1 in cancer cells is associated with reduced ECM adhesion and enhanced invasion and cell motility (79). Another ECM gene found to be affected by HE4 expression levels, COL1A1, is a crucial component of the ECM as it supports cartilage, bone, and tendon tissues in the body and also functions to maintain the rigidity and elasticity of tissues (80, 81). COL1A1 plays an important role in cancer, since tumor cells that express COL1A1 are able to dissociate from their surrounding stromal components, which is essential for tumor growth (81). The final ECM gene found to be affected by HE4 is DAG1, which is a cell adhesion molecule that plays a key role basement membrane assembly (82), muscle integrity (83), and the maintenance of basolateral cell adhesion in numerous epithelial tissues (84). Loss of DAG1 is associated with cancer progression (85). Taken together, these results show that HE4 is strongly interconnected with ECM related proteins, specifically those involved in the ITGβ5 signaling pathway.
Our lab has also determined that HE4 regulates several components of the extracellular matrix (60). We performed microarray analyses comparing untreated OVCAR8 wild-type cells to recombinant HE4 treated cells, and OVCAR8 cells overexpressing HE4 to null-vector control cells. Serpin peptidase inhibitor, member 2 (SERPINB2), gremlin 1 (GREM1), laminin-β3 (LAMB3), laminin-γ2 (LAMC2), fibroblast growth factor 5 (FGF5), and tenascin C (TNC) were all found to be significantly upregulated upon treatment with recombinant HE4. These genes encode for extracellular matrix proteins that promote cell migration and adhesion (60). Specifically, we found that HE4 upregulates LAMC2 and LAMB3 proteins in a time-dependent manner, and this increase of both factors in turn leads to an increase in laminin-332 levels (60). Laminin-332, a heterotrimer composed of LAMC2, LAMB3, and LAMA2, is an important component of the basement membrane in epithelial tissue. Abnormal increases in its levels have been shown to promote increased invasion in cancers (86). Further evidence suggested involvement of the FAK pathway in these events. In addition, activation of matriptase, a serine protease responsible for cleaving laminin-332 in its β chain and regulating its effects on metastatic properties, increased upon in vitro exposure to recombinant HE4 (60). This study provides compelling evidence that HE4 is involved in basement membrane invasion and adhesion.
Lewis y Antigen
Human epididymis protein 4 undergoes glycosylation before it is secreted by ovarian cells (87), prompting Zhuang et al. to examine the relationship between HE4 glycosylation status and metastatic properties. Lewis y antigen is a glycosyl antigen that is overexpressed in ovarian cancer and has been associated with chemoresistance and poor prognosis (88–97). They determined that Lewis y antigen was present in HE4 from benign and malignant ovarian tissues, in vitro cancer cells, and culture medium. HE4 from ovarian cancer samples contained higher levels of Lewis y antigen than HE4 from benign tissues, and their expression co-localized in ovarian cancer tissue (98). Furthermore, when Lewis y antigen was over expressed, it promoted HE4-mediated invasion and metastasis in ovarian cancer cell lines. Conversely, when Lewis y antigen was blocked, the invasive and metastatic properties of HE4 were significantly decreased (99). Interestingly, overexpression of Lewis y antigen increased tyrosine phosphorylation of EGFR and HER/neu, which promoted cell proliferation through the PI3K/Akt and Raf/MEK/MAPK pathways (100). Thus, it appears that Lewis y antigen and HE4 affect similar signaling pathways that promote tumor growth and malignancy (101). Taken together, these results show that Lewis y antigen could be a potential therapeutic target to decrease HE4 function in the treatment of EOC.
Heparin Cofactor II (HCII)
SERPIND1 encodes for the protein HCII, which is a serum glycoprotein and protease inhibitor (102). A study in non-small cell lung cancer (NSCLC) showed that HCII promotes cell motility, invasion, and filopodium dynamics through the PI3K/AKT pathway. High HCII expression in NSCLC tissue correlated to an increased recurrence rate and shorter overall survival (103). Furthermore, its levels were upregulated in metastatic brain cell lines compared with non-metastatic parental lines, suggesting an involvement of SERPIND1 in metastatic functions (104). Results from a microarray study by Zhu et al. showed that SERPIND1 was upregulated in HE4-overexpressing cells and conversely downregulated in HE4 knockdown cells. These results were validated via qPCR and immunohistochemistry. In addition, they found that 37/50 ovarian cancer samples showed positive expression of both SERPIND1 and HE4, and Spearman correlation analysis confirmed that HE4 and SERPIND1 were positively correlated. Finally, Kaplan–Meier analysis revealed that patients with high levels of HE4 and SERPIND1 had a worse prognosis (74). While these data strongly suggest a connection between HE4 and SERPIND1, which may be related to their roles in promoting ovarian cancer metastasis, further study of the association between these two proteins is required.
Annexin II
Annexin II is a calcium-dependent, phospholipid-binding protein that is overexpressed in a variety of cancers and is involved in angiogenesis, proliferation, apoptosis, cell migration, invasion, and adhesion (105). High levels of Annexin II activate MAPK signaling, which in turn promotes tumor proliferation (106), invasion (107), and metastasis (108). Zhuang et al. employed mass spectrometry and co-immunoprecipitation to identify Annexin II (ANXA2) as a strong HE4 interacting partner (61). This binding promoted invasion and metastasis in ES-2 and CaOV3 ovarian cancer cells. HE4 and ANXA2 gene expression levels were found to be co-dependent, and examination of EOC tissue revealed that both HE4 and Annexin II levels were increased in malignant phenotypes compared with benign and normal ovarian tissues. Both proteins were also more highly expressed in tissues from patients with lymph node metastases than those without. Downregulation of HE4 was found to decrease expression of MKNK2 (MAP kinase-interacting serine/threonine-protein kinase 2) and LAMB2 (laminin, beta-2), two factors associated with MAPK and focal adhesion signaling pathways. When HE4 protein was supplemented, this effect was reversed. Collectively, these results show that HE4 interaction with Annexin II to activate MAPK and focal adhesion signaling is one mechanism by which HE4 may promote ovarian cancer metastasis.
Chemoresistance
Several studies show that HE4 is associated with chemoresistance clinically. The addition of HE4 serum levels in the ROMA score better predicts platinum resistance in patients than CA125 alone (15). Angioli et al. found that HE4 was able to predict chemotherapy response in EOC patients undergoing first-line therapy (109). In addition, higher levels of serum HE4 are reported in women who are resistant to first-line chemotherapy (110). Finally, higher HE4 levels inversely correlate with clinical outcome (111), optimal cytoreduction (112), progression free survival (113), and overall survival (15, 113). While the mechanism underlying HE4’s contribution to chemoresistance has not been established fully, a few studies have begun to delineate HE4’s role in this process. A full list of factors associated with HE4-mediated chemoresistance can be found in Table 1C and is outlined in detail below.
Associated Pathways and Factors—Chemoresistance
Antiapoptotic Gene Expression
A study performed in our lab by Ribeiro et al. determined that HE4 overexpression promotes collateral chemoresistance to both cisplatin and paclitaxel in SKOV3 and OVCAR8 cells (18). Conversely, CRISPR/Cas9 mediated knockdown of HE4 in SKOV3 cells overexpressing HE4 partially reversed their chemoresistance. Microarray analysis revealed suppression of cisplatin-induced early growth response 1 (EGR1) gene expression in HE4-overexpressing SKOV3 cells compared with null vector-transfected cells (18). EGR1 is a transcription factor that regulates apoptosis, proliferation, and differentiation through regulating expression of genes such as p53, BCL2, PTEN, IGF2, PDGF, VEGF, TGFB1, and TNF (114, 115). EGR1 expression is influenced by MAPK signaling, including phospho-ERK and phospho-p38 (115). Ribeiro et al. found that p38 was strongly activated in SKOV3 null vector-transfected cells treated with cisplatin, while its activation was suppressed in HE4-overexpressing clones (18), suggesting that HE4-mediated chemoresistance may involve MAPK signaling.
Similarly, a study by Wang et al. showed that HE4 represses carboplatin-induced apoptosis in vitro. Recombinant HE4 caused an increase in expression of antiapoptotic protein B-cell lymphoma 2 (BCL-2) and a decrease in expression of pro-apoptotic Bax (Bcl-2 associated X protein) in SKOV3 cells treated with carboplatin (9). This decrease in the Bax/Bcl-2 ratio, in addition to the suppression of EGR1 when HE4 is overexpressed, may contribute to the overall decrease in pro-apoptotic factors that leads to chemoresistance in EOC.
Microtubule Stabilization
Microtubule-associated protein tau, which has been associated with paclitaxel resistance in ovarian (116), breast (117), and gastric cancer (118), was upregulated in SKOV3 cells overexpressing HE4 compared with null-vector cells (18). In addition, HE4-overexpressing cells were found to express significantly higher levels of SEPT3 (Septin 3) mRNA compared with null-vector controls (18). Septins are a family of conserved GTP binding proteins that are associated with microtubules and actin filaments and have an important role in cytoskeletal organization (119). Furthermore, recombinant HE4 treatment of SKOV3 cells increased β-tubulin levels, indicating that HE4 might promote microtubule stability, leading to paclitaxel resistance.
Kinase Signaling Pathways
Human epididymis protein 4 knockdown has also been shown to lead to a reduction in cell growth and the resensitization of ovarian cancer cells to both cisplatin and paclitaxel (12). Lee et al. found that this effect was due to corresponding decreases of ERK and AKT in HE4 knockouts. Activation of these pathways suppresses apoptotic signaling in tumors, suggesting that HE4’s regulation of these pathways may be an important mechanism of chemoresistance (120).
Steroid Biosynthesis
Evidence suggests an association between sex steroids and EOC pathogenesis, which is explained by processes that take place during the menstrual cycle. The ovarian surface epithelium (OSE) plays a critical role in ovulation and postovulatory wound repair. During the menstrual cycle, the OSE proliferates during the pro-estrus/estrus transition. After, ovulation the proliferation rate decreases (121). It is hypothesized that when the OSE is repeatedly exposed to high doses of luteinizing hormone and follicle stimulating hormone during the menstrual cycle, this can promote cell proliferation and increase the likelihood of tumor growth over time (121). Furthermore, epidemiological data have suggested that ovarian cancer progression, pathogenesis, and etiology are highly dependent on the activity of estrogens (121), and numerous experimental studies have demonstrated the promotive effect of estrogens on ovarian tumors in mice and human EOC cell lines (122). However, activation of diverse oncogenic pathways in EOC may lead to the eventual downregulation of ERα levels and the overall decrease in ERα related signaling in ovarian cancers, rendering them resistant to anti-estrogen therapies (122). Some evidence exists that HE4 may be involved in this process by regulating steroid signaling in EOC. A full list of factors associated with HE4-mediated steroid biosynthesis can be found in Table 1D and is outlined in detail below.
Steroid Biosynthesis Gene Expression
Two separate microarray pathway analyses identified steroid biosynthesis as a pathway affected by HE4 (10, 74). Important genes that were differentially expressed between HE4-overexpressing and HE4 knockdown cell lines were Forkhead box protein A2 (FOXA2) (74), squalene monooygenase (SQLE), 7-dehydrocholesterol reductase (DHCR7), 24-dehydrocholesterol (DHCR24), and sterol-4-alpha-carboxylate-3-dehydrogenase (NSDHL) (10). FOXA2, a transcription factor required for normal metabolism (123), promotes cell proliferation, maintains cancer stem cells, and is associated with a higher rate of relapse in triple-negative breast cancer (124).
Another gene differentially regulated by HE4, SQLE, is an enzyme required in the later stages of cholesterol synthesis (125). Out of 22 cancer types, SQLE copy number-driven gene expression was highest in breast, ovarian and colorectal cancer (125). Also affected by HE4 levels was DHCR7, one of the terminal enzymes involved in the production of cholesterol from 7-dehydrocholesterol (7DHC). DHCR7 was found to be an important regulatory determinate between cholesterol and vitamin synthesis, as cholesterol is able to accelerate the proteasomal degradation of DHCR7, which can result in the accumulation of 7DHC and an increased production of vitamin D (126). DHCR24, which was also affected by modulation of HE4 levels, is another enzyme in the cholesterol biosynthesis pathway (127). It interacts physically and functionally with DHCR7 (128) and has a number of different cellular functions including anti-inflammatory and antiapoptotic functions, as well as regulation of oxidative stress and cell differentiation (129). DHCR24 has also been proposed to be involved in tumor progression, as its deregulation has been linked to prostate, ovarian, and urothelial carcinomas (127).
Finally, NSDHL is also involved in cholesterol biosynthesis and produces metabolites that are essential in the conversion of squalene to cholesterol (130). Interestingly, NSLD1 was found to have a role in the control of signaling, vesicular trafficking, and degradation of EGFR and its dimerization partners ERBB2 and ERBB3. A study by Sukhanova et al. showed that NSLD1 knockout in vivo leads to a reduction in EGFR activation (131). The results from these microarrays show that modulating HE4 levels results in differential expression of several genes involved in steroid biosynthesis—especially cholesterol—suggesting that HE4 may affect tumor metabolism and ultimately contribute to tumorigenesis.
Estrogen Signaling
In support of the above described pathway analyses, two other studies have shown that HE4 interacts with steroid signaling, specifically estrogen signaling. Lokich et al. showed that ERα expression was reduced in HE4-overexpressing SKOV3 cells, resulting in increased resistance to tamoxifen and fulvestrant compared with wild-type cells (132). 5-Methylcytosine (5-MC), a methylated form of the DNA base cytosine, is one of the most prominently identified epigenetic modifications, and can cause suppression of ERα gene expression. Deregulation of DNA methylation can result in abnormal gene expression and tumorigenesis (133, 134). Lokich et al. found that 5-MC was readily detected in SKOV3 wild-type and null-vector cells but not in HE4-overexpressing clones, suggesting that HE4 overexpression may have an effect on epigenetic modifications (132). However, methylation of the ERα gene was not specifically examined in this study. It is unclear whether HE4 overexpression would promote increased methylation at the ERα promoter region (even with the presence of global demethylation), which would be expected given the reported suppression of ERα in this study.
Interestingly, Chen et al. reported that when HO8910 ovarian cancer cells were stimulated with estradiol (E2), there was an increase in the expression of HE4 at the mRNA and protein level. This effect was not observed in estrogen-insensitive SKOV3 cells; however, when HE4 was knocked down in SKOV3 cells, their proliferative response to estrogen was restored (135). Collectively with the results shown by Lokich et al, this study suggests that HE4 works to suppress estrogen signaling in ovarian cancer cells, which can contribute to resistance to anti-estrogen therapies. Conversely, it appears that estradiol promotes HE4 expression in estrogen-responsive cells, which could indicate a role for HE4 in the initial tumor promoting effects of estrogen. Further clarification of the effect of HE4 on estrogen signaling may be useful in improving implementation of anti-estrogen based therapies.
Conclusion
Ovarian cancer is an extremely deadly disease owing to the fact that patients are typically diagnosed at a late stage. Initially, patients respond well to frontline platinum therapy; however, a majority of tumors recur, and the initial chemosensitivity eventually gives way to a broad chemoresistance (136). Available detection methods have improved in recent years with the discovery of HE4 as a diagnostic and prognostic biomarker. However, there has yet to be a breakthrough targeted therapy to combat EOC. While PARP inhibitors are used in the maintenance setting for all patients, this therapy has most significantly benefited BRCA-positive patients, who comprise only 20–25% of patients (137, 138). In addition, inhibitors of immune checkpoints, such as programmed death ligand-1 have demonstrated modest benefit in clinical trials for ovarian cancer (139). Therefore, there is still a crucial need for novel targeted EOC treatments.
Although HE4 is well established as a clinical biomarker for ovarian cancer, it has been largely understudied for its therapeutic targeting potential. However, ongoing research continues to support that HE4 is profoundly involved in the pathogenesis of EOC. The individual studies mentioned in this review provide evidence that HE4 promotes EOC progression through pathways associated with cell proliferation, tumor growth, metastasis, chemoresistance, and steroid biosynthesis. These pathways, along with specific genes that have been shown to be associated with HE4, are summarized in Table 1. This compilation of HE4 regulated factors and pathways will serve as a starting point for scientists to further elucidate specific mechanisms by which HE4 ultimately drives tumorigenesis. In addition, a comprehensive summary of clinical, in vivo, and in vitro studies related to each facet of EOC progression and HE4 can be seen in Figure 1. This diagram highlights the progress that has been made to establish HE4 as an attractive therapeutic target, while simultaneously denoting areas of research that are still lacking. The results discussed here suggest that inhibition of HE4 via a neutralizing antibody or small molecule inhibitor could provide viable treatment options for patients in dire need of more effective therapies.
Author Contributions
NJ, CC, and JR contributed conceptually to this review. All the authors reviewed and approved final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Paul DiSilvestro MD, Richard Moore, MD, and Rakesh Singh, PhD for their support and collaboration.
References
- 1.Cancer facts fig. 2016. Am Cancer Soc (2016) 1:1–66. [Google Scholar]
- 2.Westin SN, Herzog TJ, Coleman RL. Investigational agents in development for the treatment of ovarian cancer. Invest New Drugs (2013) 31:213–29. 10.1007/s10637-012-9837-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bingle L, Singleton V, Bingle CD. The putative ovarian tumour marker gene HE4 (WFDC2), is expressed in normal tissues and undergoes complex alternative splicing to yield multiple protein isoforms. Oncogene (2002) 21:2768–73. 10.1038/sj.onc.1205363 [DOI] [PubMed] [Google Scholar]
- 4.Bingle L, Cross SS, High AS, Wallace WA, Rassl D, Yuan G, et al. WFDC2 (HE4): a potential role in the innate immunity of the oral cavity and respiratory tract and the development of adenocarcinomas of the lung. Respir Res (2006) 7:61. 10.1186/1465-9921-7-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heliström I, Raycraft J, Hayden-Ledbetter M, Ledbetter JA, Schummer M, McIntosh M, et al. The HE4 (WFDC2) protein is a biomarker for ovarian carcinoma. Cancer Res (2003) 63:3695–700. [PubMed] [Google Scholar]
- 6.Moore RG, McMeekin DS, Brown AK, DiSilvestro P, Miller MC, Allard WJ, et al. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol (2009) 112:40–6. 10.1016/j.ygyno.2008.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piovano E, Attamante L, Macchi C, Cavallero C, Romagnolo C, Maggino T, et al. The role of HE4 in ovarian cancer follow-up: a review. Int J Gynecol Cancer (2014) 24:1359–65. 10.1097/IGC.0000000000000218 [DOI] [PubMed] [Google Scholar]
- 8.Sandow JJ, Rainczuk A, Infusini G, Makanji M, Bilandzic M, Wilson AL, et al. Discovery and validation of novel protein biomarkers in ovarian cancer patient urine. Proteomics Clin Appl (2018) 9:1700135. 10.1002/prca.201700135 [DOI] [PubMed] [Google Scholar]
- 9.Wang H, Zhu L, Gao J, Hu Z, Lin B. Promotive role of recombinant HE4 protein in proliferation and carboplatin resistance in ovarian cancer cells. Oncol Rep (2015) 33:403–12. 10.3892/or.2014.3549 [DOI] [PubMed] [Google Scholar]
- 10.Zhu L, Zhuang H, Wang H, Tan M, Schwab CL, Deng L, et al. Overexpression of HE4 (human epididymis protein 4) enhances proliferation, invasion and metastasis of ovarian cancer. Oncotarget (2015) 7:729–44. Available from: http://www.impactjournals.com/oncotarget/index.php?journal=oncotarget&page=article&op=view&path%5B%5D=6327&path%5B%5D=21088 (Accessed: December 14, 2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu YF, Gao GL, Tang SB, Zhang ZD, Huang QS. Effect of WFDC 2 silencing on the proliferation, motility and invasion of human serous ovarian cancer cells in vitro. Asian Pac J Trop Med (2013) 6:265–72. 10.1016/S1995-7645(13)60055-3 [DOI] [PubMed] [Google Scholar]
- 12.Lee S, Choi S, Lee Y, Chung D, Hong S, Park N. Role of human epididymis protein 4 in chemoresistance and prognosis of epithelial ovarian cancer. J Obstet Gynaecol Res (2017) 43:220–7. 10.1111/jog.13181 [DOI] [PubMed] [Google Scholar]
- 13.Kong X, Chang X, Cheng H, Ma R, Ye X, Cui H. Human epididymis protein 4 inhibits proliferation of human ovarian cancer cells via the mitogen-activated protein kinase and phosphoinositide 3-kinase/AKT pathways. Int J Gynecol Cancer (2014) 24:427–36. 10.1097/IGC.0000000000000078 [DOI] [PubMed] [Google Scholar]
- 14.Lu R, Sun X, Xiao R, Zhou L, Gao X, Guo L. Human epididymis protein 4 (HE4) plays a key role in ovarian cancer cell adhesion and motility. Biochem Biophys Res Commun (2012) 419:274–80. 10.1016/j.bbrc.2012.02.008 [DOI] [PubMed] [Google Scholar]
- 15.Moore RG, Hill EK, Horan T, Yano N, Kim K, MacLaughlan S, et al. HE4 (WFDC2) gene overexpression promotes ovarian tumor growth. Sci Rep (2014) 4:3574. 10.1038/srep03574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crowell JA, Steele VE, Fay JR. Targeting the AKT protein kinase for cancer chemoprevention. Mol Cancer Ther (2007) 6:2139–48. 10.1158/1535-7163.MCT-07-0120 [DOI] [PubMed] [Google Scholar]
- 17.Testa JR, Tsichlis PN. AKT signaling in normal and malignant cells. Oncogene (2005) 24:7391–3. 10.1038/sj.onc.1209100 [DOI] [PubMed] [Google Scholar]
- 18.Ribeiro JR, Schorl C, Yano N, Romano N, Kim KK, Singh RK, et al. HE4 promotes collateral resistance to cisplatin and paclitaxel in ovarian cancer cells. J Ovarian Res (2016) 9:28. 10.1186/s13048-016-0240-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature (1999) 399:271–5. 10.1038/20459 [DOI] [PubMed] [Google Scholar]
- 20.Dang CV, Semenza GL. Oncogenic alterations of metabolism. Trends Biochem Sci (1999) 24:68–72. 10.1016/S0968-0004(98)01344-9 [DOI] [PubMed] [Google Scholar]
- 21.Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, et al. Overexpression of hypoxia-inducible factor 1α in common human cancers and their metastases. Cancer Res (1999) 59:5830–5. [PubMed] [Google Scholar]
- 22.Diaz-Gonzalez JA, Russell J, Rouzaut A, Gil-Bazo I, Montuenga L. Targeting hypoxia and angiogenesis through HIF-1α inhibition. Cancer Biol Ther (2005) 4:1055–62. 10.4161/cbt.4.10.2195 [DOI] [PubMed] [Google Scholar]
- 23.Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene (2007) 26:3279–90. 10.1038/sj.onc.1210421 [DOI] [PubMed] [Google Scholar]
- 24.Schulze WX, Deng L, Mann M. Phosphotyrosine interactome of the ErbB-receptor kinase family. Mol Syst Biol (2005) 1:E1–13. 10.1038/msb4100012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bian J, Sun X, Li B, Ming L. Clinical significance of serum HE4, CA125, CA724, and CA19-9 in patients with endometrial cancer. Technol Cancer Res Treat (2016) 16:435–9. 10.1177/1533034616666644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gasiorowska E, Magnowska M, Izycka N, Warchol W, Nowak-Markwitz E. The role of HE4 in differentiating benign and malignant endometrial pathology. Ginekol Pol (2016) 87:260–4. 10.17772/gp/62356 [DOI] [PubMed] [Google Scholar]
- 27.Li J, Chen H, Mariani A, Chen D, Klatt E, Podratz K, et al. HE4 (WFDC2) promotes tumor growth in endometrial cancer cell lines. Int J Mol Sci (2013) 14:6026–43. 10.3390/ijms14036026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Angioli R, Capriglione S, Scaletta G, Aloisi A, Miranda A, De Cicco Nardone C, et al. The role of HE4 in endometrial cancer recurrence: how to choose the optimal follow-up program. Tumour Biol (2016) 37:4973–8. 10.1007/s13277-015-4324-z [DOI] [PubMed] [Google Scholar]
- 29.Minář L, Klabenešová I, Jandáková E. [The importance of HE4 in differential diagnosis of endometrial cancer]. Ces Gynekol (2015) 80:256–63. [PubMed] [Google Scholar]
- 30.Brennan DJ, Hackethal A, Mann KP, Mutz-Dehbalaie I, Fiegl H, Marth C, et al. Serum HE4 detects recurrent endometrial cancer in patients undergoing routine clinical surveillance. BMC Cancer (2015) 15:33. 10.1186/s12885-015-1028-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Capriglione S, Plotti F, Miranda A, Ricciardi R, Scaletta G, Alosi A, et al. Utility of tumor marker HE4 as prognostic factor in endometrial cancer: a single-center controlled study. Tumour Biol (2015) 36:4156. 10.1007/s13277-015-3049-3 [DOI] [PubMed] [Google Scholar]
- 32.Antonsen SL, Høgdall E, Christensen IJ, Lydolph M, Tabor A, Loft Jakobsen A, et al. HE4 and CA125 levels in the preoperative assessment of endometrial cancer patients: a prospective multicenter study (ENDOMET). Acta Obstet Gynecol Scand (2013) 92:1313–22. 10.1111/aogs.12235 [DOI] [PubMed] [Google Scholar]
- 33.Presl J, Novotny Z, Topolcan O, Vlasak P, Kucera R, Fushsova R, et al. CA125 and HE4 levels in a Czech female population diagnosed with endometrial cancer in preoperative management. Anticancer Res (2014) 34:327–31. [PubMed] [Google Scholar]
- 34.Brennan DJ, Hackethal A, Metcalf AM, Coward J, Ferguson K, Oehler MK, et al. Serum HE4 as a prognostic marker in endometrial cancer – a population based study. Gynecol Oncol (2014) 132:159–65. 10.1016/j.ygyno.2013.10.036 [DOI] [PubMed] [Google Scholar]
- 35.Saarelainen SK, Peltonen N, Lehtimäki T, Perheentupa A, Vuento MH, Mäenpää JU. Predictive value of serum human epididymis protein 4 and cancer antigen 125 concentrations in endometrial carcinoma. Am J Obstet Gynecol (2013) 209:142.e1–6. 10.1016/j.ajog.2013.04.014 [DOI] [PubMed] [Google Scholar]
- 36.Prueksaritanond N, Cheanpracha P, Yanaranop M. Association of serum HE4 with primary tumor diameter and depth of myometrial invasion in endometrial cancer patients at Rajavithi hospital. Asian Pac J Cancer Prev (2016) 17:1489–92. 10.7314/APJCP.2016.17.3.1489 [DOI] [PubMed] [Google Scholar]
- 37.Kalogera E, Scholler N, Powless C, Weaver A, Drapkin R, Li J, et al. Correlation of serum HE4 with tumor size and myometrial invasion in endometrial cancer. Gynecol Oncol (2012) 124:270–5. 10.1016/j.ygyno.2011.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zamani N, Modares Gilani M, Zamani F, Zamani MH. Utility of pelvic MRI and tumor markers HE4 and CA125 to predict depth of myometrial invasion and cervical involvement in endometrial cancer. J Family Reprod Health (2015) 9:177–83. [PMC free article] [PubMed] [Google Scholar]
- 39.Bie Y, Zhang Z. Diagnostic value of serum HE4 in endometrial cancer: a meta-analysis. World J Surg Oncol (2014) 12:169. 10.1186/1477-7819-12-169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoon HI, Kwon OR, Kang KN, Shin YS, Shin HS, Yeon EH, et al. Diagnostic value combining tumor and inflammatory markers in lung cancer. J Cancer Prev (2016) 21:187–93. 10.15430/JCP.2016.21.4.302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeng Q, Liu M, Zhou N, Liu L, Song X. Serum human epididymis protein 4 (HE4) may be a better tumor marker in early lung cancer. Clin Chim Acta (2016) 455:102–6. 10.1016/j.cca.2016.02.002 [DOI] [PubMed] [Google Scholar]
- 42.Tang QF, Zhou ZW, Ji HB, Pan WH, Sun MZ. Value of serum marker HE4 in pulmonary carcinoma diagnosis. Int J Clin Exp Med (2015) 8:19014–21. [PMC free article] [PubMed] [Google Scholar]
- 43.Li BT, Lou E, Hsu M, Yu HA, Naidoo J, Zauderer MG, et al. Serum biomarkers associated with clinical outcomes fail to predict brain metastases in patients with stage IV non-small cell lung cancers. PLoS One (2016) 11(1):e0146063. 10.1371/journal.pcne.0146063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lan WG, Hao YZ, Xu DH, Wang P, Zhou YL, Ma LB. Serum human epididymis protein 4 is associated with the treatment response of concurrent chemoradiotherapy and prognosis in patients with locally advanced non-small cell lung cancer. Clin Transl Oncol (2016) 18:375–80. 10.1007/s12094-015-1375-y [DOI] [PubMed] [Google Scholar]
- 45.Lamy P-J, Plassot C, Pujol J-L. Serum HE4: an independent prognostic factor in non-small cell lung cancer. PLoS One (2015) 10:e0128836. 10.1371/journal.pone.0128836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma Q, Wang Q, Zhong DS. Advances of human epididymis protein 4 in lung cancer. Chin J Lung Cancer (2015) 18:184–6. 10.3779/j.issn.1009-3413.2015.03.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang Y, Wang C, Lv B, Ma G, Wang L. Expression level of serum human epididymis 4 and its prognostic significance in human non-small cell lung cancer. Int J Clin Exp Med (2014) 7:5568–72. [PMC free article] [PubMed] [Google Scholar]
- 48.Lou E, Johnson M, Sima C, Gonzalez-Espinoza R, Fleisher M, Kris MG, et al. Serum biomarkers for assessing histology and outcomes in patients with metastatic lung cancer. Cancer Biomark (2014) 14:207–14. 10.3233/CBM-140399 [DOI] [PubMed] [Google Scholar]
- 49.Yamashita S, Tokuishi K, Moroga T, Yamamoto S, Ohbo K, Miyahara S, et al. Serum level of HE4 is closely associated with pulmonary adenocarcinoma progression. Tumour Biol (2012) 33:2365–70. 10.1007/s13277-012-0499-8 [DOI] [PubMed] [Google Scholar]
- 50.Yamashita SI, Tokuishi K, Hashimoto T, Moroga T, Kamei M, Ono K, et al. Prognostic significance of HE4 expression in pulmonary adenocarcinoma. Tumour Biol (2011) 32:265–71. 10.1007/s13277-010-0118-5 [DOI] [PubMed] [Google Scholar]
- 51.Iwahori K, Suzuki H, Kishi Y, Fujii Y, Uehara R, Okamoto N, et al. Serum HE4 as a diagnostic and prognostic marker for lung cancer. Tumour Biol (2012) 33:1141–9. 10.1007/s13277-012-0356-9 [DOI] [PubMed] [Google Scholar]
- 52.Tokuishi K, Yamashita SI, Ohbo K, Kawahara K. Splice variant HE4-V3 expression is associated with favorable prognosis in pulmonary adenocarcinoma. Tumour Biol (2012) 33:103–9. 10.1007/s13277-011-0252-8 [DOI] [PubMed] [Google Scholar]
- 53.Gündüz UR, Gunaldi M, Isiksacan N, Gündüz S, Okuturlar Y, Kocoglu H. A new marker for breast cancer diagnosis, human epididymis protein 4: a preliminary study. Mol Clin Oncol (2016) 5:355–60. 10.3892/mco.2016.919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Durur-Karakaya A, Durur-Subasi I, Karaman A, Akcay MN, Palabiyik SS, Erdemchi B, et al. The use of breast magnetic resonance imaging parameters to identify possible signaling pathways of a serum biomarker, HE4. J Comput Assist Tomogr (2016) 40:436–41. 10.1097/RCT.0000000000000390 [DOI] [PubMed] [Google Scholar]
- 55.Huang T, Jiang SW, Qin L, Senkowski C, Lyle C, Terry K, et al. Expression and diagnostic value of HE4 in pancreatic adenocarcinoma. Int J Mol Sci (2015) 16:2956–70. 10.3390/ijms16022956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu Q, Chen H, Senkowski C, Wang J, Wang X, Brower S, et al. Recombinant HE4 protein promotes proliferation of pancreatic and endometrial cancer cell lines. Oncol Rep (2015) 35 Available from: http://www.ncbi.nlm.nih.gov/pubmed/26497244 (Accessed: December 15, 2017). [DOI] [PubMed] [Google Scholar]
- 57.Guo YD, Wang JH, Lu H, Li XN, Song WW, Zhang XD, et al. The human epididymis protein 4 acts as a prognostic factor and promotes progression of gastric cancer. Tumour Biol (2015) 36:2457–64. 10.1007/s13277-014-2858-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thomas H, Nasim MM, Sarraf CE, Alison MR, Love S, Lambert HE, et al. Proliferating cell nuclear antigen (PCNA) immunostaining – a prognostic factor in ovarian cancer? Br J Cancer (1995) 71:357–62. 10.1038/bjc.1995.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abbas T, Dutta A. P21 in cancer: intricate networks and multiple activities. Nat Rev Cancer (2009) 9:400–14. 10.1038/nrc2657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ribeiro JR, Gaudet HM, Kahn M, Schorl C, James NE, Oliver MT, et al. Human epididymis protein 4 promotes events associated with metastatic ovarian cancer via regulation of the extracelluar matrix. Front Oncol (2018) 7:332. 10.3389/fonc.2017.00332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhuang H, Tan M, Liu J, Hu Z, Liu D, Gao J, et al. Human epididymis protein 4 in association with Annexin II promotes invasion and metastasis of ovarian cancer cells. Mol Cancer (2014) 13:1–14. 10.1186/1476-4598-13-243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zou S-L, Chang X-H, Ye X, Cheng H-Y, Cheng Y-X, Tang Z-J, et al. Effect of human epididymis protein 4 gene silencing on the malignant phenotype in ovarian cancer. Chin Med J (Engl) (2011) 124:3133–40. [PubMed] [Google Scholar]
- 63.Cathcart J, Pulkoski-Gross A, Cao J. Targeting matrix metalloproteinases in cancer: bringing new life to old ideas. Genes Dis (2015) 2:26–34. 10.1016/j.gendis.2014.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature (2004) 432:332–7. 10.1038/nature03096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer (2006) 6:392–401. 10.1038/nrc1877 [DOI] [PubMed] [Google Scholar]
- 66.Aggarwal N, Sloane BF. Cathepsin B: multiple roles in cancer. Proteomics Clin Appl (2014) 8:427–37. 10.1002/prca.201300105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lakka SS, Gondi CS, Rao JS. Proteases and glioma angiogenesis. Brain Pathol (2005) 15:327–41. 10.1111/j.1750-3639.2005.tb00118.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gondi CS, Rao JS. Cathepsin B as a cancer target. Expert Opin Ther Targets (2013) 17:281–91. 10.1517/14728222.2013.740461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dabkeviciene D, Sasnauskiene A, Leman E, Kvietkauskaite R, Daugelaviciene N, Stankevicius V, et al. MTHPC-mediated photodynamic treatment up-regulates the cytokines VEGF and IL-1alpha. Photochem Photobiol (2012) 88:432–9. 10.1111/j.1751-1097.2011.01062.x [DOI] [PubMed] [Google Scholar]
- 70.Ma J, Sawai H, Matsuo Y, Ochi N, Yasuda A, Takahashi H, et al. Interleukin-1α enhances angiogenesis and is associated with liver metastatic potential in human gastric cancer cell lines. J Surg Res (2008) 148:197–204. 10.1016/j.jss.2007.08.014 [DOI] [PubMed] [Google Scholar]
- 71.Löffek S, Zigrino P, Angel P, Anwald B, Krieg T, Mauch C. High invasive melanoma cells induce matrix metalloproteinase-1 synthesis in fibroblasts by interleukin-1alpha and basic fibroblast growth factor-mediated mechanisms. J Invest Dermatol (2005) 124:638–43. 10.1111/j.0022-202X.2005.23629.x [DOI] [PubMed] [Google Scholar]
- 72.Gelfo V, Rodia MT, Pucci M, Dall’Ora M, Santi S, Solmi R, et al. A module of inflammatory cytokines defines resistance of colorectal cancer to EGFR inhibitors. Oncotarget (2016) 7 Available from: http://www.ncbi.nlm.nih.gov/pubmed/27708224 (Accessed: January 3, 2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stanam A, Gibson-Corley KN, Love-Homan L, Ihejirika N, Simons AL. Interleukin-1 blockade overcomes erlotinib resistance in head and neck squamous cell carcinoma. Oncotarget (2016) 7 Available from: http://www.ncbi.nlm.nih.gov/pubmed/27738319 (Accessed: January 3, 2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhu L, Guo Q, Jin S, Feng H, Zhuang H, Liu C, et al. Analysis of the gene expression profile in response to human epididymis protein 4 in epithelial ovarian cancer cells. Oncol Rep (2016) 36:1592–604. 10.3892/or.2016.4926 [DOI] [PubMed] [Google Scholar]
- 75.Giancotti FG, Ruoslahti E. Integrin signaling. Science (1999) 285:1028–33. 10.1126/science.285.5430.1028 [DOI] [PubMed] [Google Scholar]
- 76.Duperret EK, Dahal A, Ridky T. Focal-adhesion-independent integrin-αv regulation of FAK and c-Myc is necessary for 3D skin formation and tumor invasion. J Cell Sci (2015) 128:3997–4013. 10.1242/jcs.175539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer (2010) 10:9–22. 10.1038/nrc2748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Akl MR, Nagpal P, Ayoub N, Prabhu S, Gilksman M, Tai B, et al. Molecular and clinical profiles of syndecan-1 in solid and hematological cancer for prognosis and precision medicine. Oncotarget (2015) 6:28693–715. 10.18632/oncotarget.4981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Teng YHF, Aquino RS, Park PW. Molecular functions of syndecan-1 in disease. Matrix Biol (2012) 31:3–16. 10.1016/j.matbio.2011.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bou-Gharios G, Ponticos M, Rajkumar V, Abraham D. Extra-cellular matrix in vascular networks. Cell Prolif (2004) 37:207–20. 10.1111/j.1365-2184.2004.00306.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Karsenty G, Park RW. Regulation of type I collagen genes expression. Int Rev Immunol (1995) 12:177–85. 10.3109/08830189509056711 [DOI] [PubMed] [Google Scholar]
- 82.Matthew G, Mitchell A, Down JM, Jacobs LA, Hamdy FC, Eaton C, et al. Nuclear targeting of dystroglycan promotes the expression of androgen regulated transcription factors in prostate cancer. Sci Rep (2013) 3:2792. 10.1038/srep02792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ervasti JM, Ohlendieck K, Kahl SD, Gaver MG, Campbell KP. Deficiency of glycoprotein component of the dystrophin complex in dystrophic muscle deficiency of a glycoprotein component of the dystrophin complex in dystrophic muscle. Nature (1990) 345:315–9. 10.1038/345315a0 [DOI] [PubMed] [Google Scholar]
- 84.Durbeej M, Larsson E, Ibraghimov-Beskrovnaya O, Roberds SL, Campbell KP, Ekblom P. Non-muscle alpha-dystroglycan is involved in epithelial development. J Cell Biol (1995) 130:79–91. 10.1083/jcb.130.1.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cross SS, Lippitt J, Mitchell A, Hollingsbury F, Balasubramanian SP, Reed MWR, et al. Expression of beta-dystroglycan is reduced or absent in many human carcinomas. Histopathology (2008) 53:561–6. 10.1111/j.1365-2559.2008.03157.x [DOI] [PubMed] [Google Scholar]
- 86.Tsuruta D, Kobayashi H, Imanishi H, Sugawara K, Ishii M, Jones JCR. Laminin-332-integrin interaction: a target for cancer therapy? Curr Med Chem (2008) 15:1968–75. 10.2174/092986708785132834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Drapkin R, Von Horsten HH, Lin Y, Mok SC, Crum CP, Welch WR, et al. Human epididymis protein 4 (HE4) is a secreted glycoprotein that is overexpressed by serous and endometrioid ovarian carcinomas. Cancer Res (2005) 65:2162–9. 10.1158/0008-5472.CAN-04-3924 [DOI] [PubMed] [Google Scholar]
- 88.Yin BW, Finstad CL, Kitamura K, Federici MG, Welshinger M, Kudryashov V, et al. Serological and immunochemical analysis of Lewis y (Ley) blood group antigen expression in epithelial ovarian cancer. Int J Cancer (1996) 65:406–12. [DOI] [PubMed] [Google Scholar]
- 89.Li Q, Liu S, Lin B, Yan L, Wang Y. Expression and correlation of Lewis y antigen and integrins a5 and b1 in ovarian serous and mucinous carcinoma. Int J Gynecol Cancer (2010) 20:1482–9. [DOI] [PubMed] [Google Scholar]
- 90.Sabbatini PJ, Kudryashiv V, Ragupath G, Danishefsky SJ, Livingston PO, Bornmann W, et al. Immunization of ovarian cancer patients with a synthetic Lewis(y)-protein conjugate vaccine: a phase 1 trial. Int J Cancer (2000) 87:79–85. [DOI] [PubMed] [Google Scholar]
- 91.Smaletz O, Diz MD, do Carmo CC, Sabbaga J, Cunha-Junior GF, Azevedo S, et al. A phase II trial with anti-Lewis-Y monoclonal antibody (hu3S193) for the treatment of platinum resistant/refractory ovarian, fallopian tube and primary peritoneal carcinoma. Gynecol Oncol (2015) 138:272–7. 10.1016/j.ygyno.2015.05.023 [DOI] [PubMed] [Google Scholar]
- 92.Gao J, Hu Z, Liu J, Liu D, Wang Y, Cai M, et al. Expression of CD147 and Lewis y antigen in ovarian cancer and their relationship to drug resistance. Med Oncol (2014) 31:920. 10.1007/s12032-014-0920-9 [DOI] [PubMed] [Google Scholar]
- 93.Gao J, Hu Z, Liu D, Liu J, Liu C, Hou R, et al. Expression of Lewis y antigen and integrin αv, β3 in ovarian cancer and their relationship with chemotherapeutic drug resistance. J Exp Clin Cancer Res (2013) 32: 36 Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3699420&tool=pmcentrez&rendertype=abstract (Accessed: January 6, 2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang D, Gao J, Zhu L, Hu Z, Hou R, Liu S, et al. Chemoresistance is associated with MUC1 and lewis y antigen expression in ovarian epithelial cancers. Int J Mol Sci (2013) 14:11024–33. 10.3390/ijms140611024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hu Z, Gao J, Zhang D, Liu Q, Yan L, Gao L, et al. High expression of Lewis y antigen and CD44 is correlated with resistance to chemotherapy in epithelial ovarian cancers. PLoS One (2013) 8:e57250. 10.1371/journal.pone.0057250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hu Z, Gao S, Gao J, Hou R, Liu C, Liu J, et al. Elevated levels of Lewis Y and integrin α5β1 correlate with chemotherapeutic drug resistance in epithelial ovarian carcinoma. Int J Mol Sci (2012) 13:15588–600. 10.3390/ijms131215588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang F, Liu J, Lin B, Liu Q, Zhao Y, Zhu L, et al. Increase in docetaxel-resistance of ovarian carcinoma-derived RMG-1 cells with enhanced expression of Lewis Y antigen. Int J Mol Sci (2011) 12:7323–34. 10.3390/ijms12117323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhuang H, Gao J, Hu Z, Liu J, Liu D, Lin B. Co-expression of Lewis y antigen with human epididymis protein 4 in ovarian epithelial carcinoma. PLoS One (2013) 8:e68994. 10.1371/journal.pone.0068994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhuang H, Hu Z, Tan M, Zhu L, Liu J, Liu D, et al. Overexpression of Lewis y antigen promotes human epididymis protein 4-mediated invasion and metastasis of ovarian cancer cells. Biochimie (2014) 105:91–8. 10.1016/j.biochi.2014.06.022 [DOI] [PubMed] [Google Scholar]
- 100.Liu J-J, Lin B, Hao Y-Y, Li F-F, Liu D-W, Qi Y, et al. Lewis(y) antigen stimulates the growth of ovarian cancer cells via regulation of the epidermal growth factor receptor pathway. Oncol Rep (2010) 23:833–41. [PubMed] [Google Scholar]
- 101.Burotto M, Chiou VL, Lee JM, Kohn EC. The MAPK pathway across different malignancies: a new perspective. Cancer (2014) 120:3446–56. 10.1002/cncr.28864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tollefsen DM. Heparin cofactor II modulates the response to vascular injury. Arterioscler Thromb Vasc Biol (2007) 27:454–60. 10.1161/01.ATV.0000256471.22437.88 [DOI] [PubMed] [Google Scholar]
- 103.Liao W-Y, Ho C-C, Hou H-H, Hsu T-H, Tsai M-F, Chen K-Y, et al. Heparin co-factor II enhances cell motility and promotes metastasis in non-small cell lung cancer. J Pathol (2015) 235:50–64. 10.1002/path.4421 [DOI] [PubMed] [Google Scholar]
- 104.Valiente M, Obenauf AC, Jin X, Chen Q, Zhang XHF, Lee DJ, et al. Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell (2014) 156:1002–16. 10.1016/j.cell.2014.01.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lokman NA, Ween MP, Oehler MK, Ricciardelli C. The role of annexin A2 in tumorigenesis and cancer progression. Cancer Microenviron (2011) 4:199–208. 10.1007/s12307-011-0064-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ortiz-Zapater E, Peiró S, Roda O, Corominas JM, Aguilar S, Ampurdané C, et al. Tissue plasminogen activator induces pancreatic cancer cell proliferation by a non-catalytic mechanism that requires extracellular signal-regulated kinase 1/2 activation through epidermal growth factor receptor and annexin A2. Am J Pathol (2007) 170:1573–84. 10.2353/ajpath.2007.060850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Delys L, Detours V, Franc B, Thomas G, Bogdanova T, Tronko M, et al. Gene expression and the biological phenotype of papillary thyroid carcinomas. Oncogene (2007) 26:7894–903. 10.1038/sj.onc.1210588 [DOI] [PubMed] [Google Scholar]
- 108.Zhang Y, Zhou Z-H, Bugge TH, Wahl LM. Urokinase-type plasminogen activator stimulation of monocyte matrix metalloproteinase-1 production is mediated by plasmin-dependent signaling through annexin A2 and inhibited by inactive plasmin. J Immunol (2007) 179:3297–304. 10.4049/jimmunol.179.5.3297 [DOI] [PubMed] [Google Scholar]
- 109.Angioli R, Capriglione S, Aloisi A, Guzzo F, Luvero D, Miranda A, et al. Can HE4 predict platinum response during first-line chemotherapy in ovarian cancer? Tumour Biol (2014) 35:7009–15. 10.1007/s13277-014-1836-x [DOI] [PubMed] [Google Scholar]
- 110.Chudecka-Głaz AM, Cymbaluk-Płoska AA, Menkiszak JL, Sompolska-Rzechuła AM, Tołoczko-Grabarek AI, Rzepka-Górska IA. Serum HE4, CA125, YKL-40, bcl-2, cathepsin-L and prediction optimal debulking surgery, response to chemotherapy in ovarian cancer. J Ovarian Res (2014) 7:62. 10.1186/1757-2215-7-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gonzalez VD, Samusik N, Chen TJ, Savig ES, Aghaeepour N, Quigley DA, et al. Commonly occurring cell subsets in high-grade serous ovarian tumors identified by single-cell mass cytometry. Cell Rep (2018) 22:1875–88. 10.1016/j.celrep.2018.01.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Angioli R, Plotti F, Capriglione S, Aloisi A, Montera R, Luvero D, et al. Can the preoperative HE4 level predict optimal cytoreduction in patients with advanced ovarian carcinoma? Gynecol Oncol (2013) 128:579–83. 10.1016/j.ygyno.2012.11.040 [DOI] [PubMed] [Google Scholar]
- 113.Yuan C, Li R, Yan S, Kong B. Prognostic value of HE4 in patients with ovarian cancer. Clin Chem Lab Med (2018). 10.1515/cclm-2017-1176 [DOI] [PubMed] [Google Scholar]
- 114.Zhao DY, Jacobs KM, Hallahan DE, Thotala D. Silencing Egr1 attenuates radiation-induced apoptosis in normal tissues while killing cancer cells and delaying tumor growth. Mol Cancer Ther (2015) 1535–7163. 10.1158/1535-7163.MCT-14-1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Manente AG, Pinton G, Tavian D, Lopez-Rodas G, Brunelli E, Moro L. Coordinated sumoylation and ubiquitination modulate EGF induced EGR1 expression and stability. PLoS One (2011) 6:e25676. 10.1371/journal.pone.0025676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gurler H, Yu Y, Choi J, Kajdacsy-Balla AA, Barbolina MV. Three-dimensional collagen type i matrix up-regulates nuclear isoforms of the microtubule associated protein tau implicated in resistance to paclitaxel therapy in ovarian carcinoma. Int J Mol Sci (2015) 16:3419–33. 10.3390/ijms16023419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang K, Deng QT, Liao N, Zhang GC, Liu YH, Xu FP, et al. Tau expression correlated with breast cancer sensitivity to taxanes-based neoadjuvant chemotherapy. Tumour Biol (2013) 34:33–8. 10.1007/s13277-012-0507-z [DOI] [PubMed] [Google Scholar]
- 118.Wu H, Huang M, Lu M, Zhu W, Shu Y, Cao P, et al. Regulation of microtubule-associated protein tau (MAPT) by miR-34c-5p determines the chemosensitivity of gastric cancer to paclitaxel. Cancer Chemother Pharmacol (2013) 71:1159–71. 10.1007/s00280-013-2108-y [DOI] [PubMed] [Google Scholar]
- 119.Russell SEH, Hall PA. Do septins have a role in cancer? Br J Cancer (2005) 93:499–503. 10.1038/sj.bjc.6602753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cantley LC. The phosphoinositide 3-kinase pathway. Science (2002) 296:1655–7. 10.1126/science.296.5573.1655 [DOI] [PubMed] [Google Scholar]
- 121.Mungenast F, Thalhammer T. Estrogen biosynthesis and action in ovarian cancer. Front Endocrinol (2014) 5 10.3389/fendo.2014.00192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ribeiro JR, Freiman RN. Estrogen signaling crosstalk: implications for endocrine resistance in ovarian cancer. J Steroid Biochem Mol Biol (2014) 143:160–73. 10.1016/j.jsbmb.2014.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wolfrum C, Shih DQ, Kuwajima S, Norris AW, Kahn CR, Stoffel M. Role of Foxa-2 in adipocyte metabolism and differentiation. J Clin Invest (2003) 112:345–56. 10.1172/JCI18698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Perez-Balaguer A, Ortiz-Martinez F, Garcia-Martinez A, Pomares-Navarro C, Lerma E, Peiró G. FOXA2 mRNA expression is associated with relapse in patients with triple-negative/basal-like breast carcinoma. Breast Cancer Res Treat (2015) 153:465–74. 10.1007/s10549-015-3553-6 [DOI] [PubMed] [Google Scholar]
- 125.Brown DF, Caffa I, Cirmena G, Piras D, Garuti A, Gallo M, et al. Squalene epoxidase is a bona fide oncogene by amplification with clinical relevance in breast cancer. Sci Rep (2016) 6:19435. 10.1038/srep19435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Prabhu AV, Luu W, Sharpe LJ, Brown AJ. Cholesterol-mediated degradation of 7-dehydrocholesterol reductase switches the balance from cholesterol to vitamin D synthesis. J Biol Chem (2016) 291:8363–76. 10.1074/jbc.M115.699546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dai M, Zhu XL, Liu F, Xu QY, Jiang SH, Yang XM, et al. Cholesterol synthetase DHCR24 induced by insulin aggravates cancer invasion and progesterone resistance in endometrial carcinoma. Sci Rep (2017) 7:41404. 10.1038/srep41404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Luu W, Hart-Smith G, Sharpe LJ, Brown AJ. The terminal enzymes of cholesterol synthesis, DHCR24 and DHCR7, interact physically and functionally. J Lipid Res (2015) 56:888–97. 10.1194/jlr.M056986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zerenturk EJ, Sharpe LJ, Ikonen E, Brown AJ. Desmosterol and DHCR24: unexpected new directions for a terminal step in cholesterol synthesis. Prog Lipid Res (2013) 52:666–80. 10.1016/j.plipres.2013.09.002 [DOI] [PubMed] [Google Scholar]
- 130.Gabitiva L, Restifo D, Gorin A, Manocha K, Handorf E, Yang DH, et al. Endogenous sterol metabolites regulate growth of EGFR/KRAS-dependent tumors via LXR. Cell Rep (2015) 12:1927–38. 10.1016/j.celrep.2015.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sukhanova A, Gorin A, Serebriiskii IG, Gabitova L, Zheng H, Restifo D, et al. Targeting C4-demethylating genes in the cholesterol pathway sensitizes cancer cells to EGF receptor inhibitors via increased EGF receptor degradation. Cancer Discov (2013) 3:96–112. 10.1158/2159-8290.CD-12-0031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lokich E, Singh RK, Han A, Romano N, Yano N, Kim K, et al. HE4 expression is associated with hormonal elements and mediated by importin-dependent nuclear translocation. Sci Rep (2014) 4:5500. 10.1038/srep05500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Robertson KD. DNA methylation and human disease. Nat Rev Genet (2005) 6:597–610. 10.1038/nrg1655 [DOI] [PubMed] [Google Scholar]
- 134.Bird A. DNA methylation patterns and epigenetic memory. Genes Dev (2002) 16:6–21. 10.1101/gad.947102 [DOI] [PubMed] [Google Scholar]
- 135.Chen Y, Wang S, Liu T, Wu Y, Li J, Li M. WAP four-disulfide core domain protein 2 gene(WFDC2) is a target of estrogen in ovarian cancer cells. J Ovarian Res (2016) 9:10. 10.1186/s13048-015-0210-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cottrill H, Cason S, Caroen S, Oronsky B, Donaldson E. A recurrent platinum refractory ovarian cancer patient with a partial response after RRx-001 resensitization to platinum doublet. J Investig Med High Impact Case Rep (2018) 6:2324709618760080. 10.1177/2324709618760080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liu G, Yang D, Sun Y, Shmulevich I, Xue F, Sood AK, et al. Differing clinical impact of BRCA1 and BRCA2 mutations in serous ovarian cancer. Pharmacogenomics (2012) 13:1523–35. 10.2217/pgs.12.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Earl H, Molica S, Rutkowski P. Spotlight on landmark oncology trials: the latest evidence and novel trial designs. BMC Med (2017) 15:111. 10.1186/s12916-017-0884-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gaillard SL, Secord AA, Monk B. The role of immune checkpoint inhibition in the treatment of ovarian cancer. Gynecol Oncol Res Pract (2016) 3:11. 10.1186/s40661-016-0033-6 [DOI] [PMC free article] [PubMed] [Google Scholar]