Abstract
Mycobacterium marinum (M. marinum) is a slowly growing nontuberculous mycobacterium. The incidence of M. marinum infections in Denmark is unknown. We conducted a retrospective nationwide study including all culture confirmed cases of M. marinum from 2004 to 2017 in Denmark. All available medical records were reviewed. Demographics, clinical characteristics, and treatment regiments were analyzed. Fifty-five patients were identified, 40 (72.7%) were men with a median age of 50 years. Aquatic exposure was reported by 48 (90.6%) of the patients. Site of infection was upper extremities in 49 (92.5%) patients and 49 (92.5%) had superficial infection. The median time from symptom presentation to diagnosis was 194 days. All patients received antibiotics. Median time of treatment duration among all patients was 112 days. Treatment outcome was classified as improved in 40 (75%), improved with sequela in 4 (7.6%) patients and only 3 patients (3.8%) were classified as failed. Infection with M. marinum is rare and there is a long delay from symptom manifestation to diagnosis. The infection is predominantly related to aquatic exposure. M. marinum should be a differential diagnose in patients with slow-developing cutaneous elements and relevant exposure. Treatment outcomes are overall good and severe sequela are rare.
Introduction
Mycobacterium marinum (M. marinum) is a slowly growing nontuberculous mycobacterium, which was first isolated from marine fish. M. marinum is found in non-disinfected salt- and freshwater reservoirs such as swimming pools and fish tanks1,2. The bacteria may cause infections in humans when contaminated water or infected fish or seafood come in contact with skin lesions3.
Most infections with M. marinum are superficial skin infections characterized by granuloma and lymphangitis also known as “fish tank granuloma” or “swimming pool granuloma”; however, the infection may spread to deeper tissue causing tendinitis, arthritis and osteomyelitis3.
The incidence of M. marinum infections in Denmark is unknown. In France in 1996–1998, the incidence was reported to be 0.04 per 100 000 person years4. Recent studies from The United States reported an increase in incidence of cutaneous nontuberculous mycobacterial infections and M. marinum infections in particular1,5.
The treatment of M. marinum infection is long-term antibiotic treatment and in the more severe cases surgery may be needed1,5,6. The optimal duration of antibiotic treatment is not known; as little as four weeks of antibiotic treatment has been reported as successful but often the treatment duration will be extended to several months6–15.
This study was conducted to provide information regarding the incidence, clinical features, treatment regimens and clinical outcome of patients infected with M. marinum infections in Denmark.
Methods
We conducted a nationwide retrospective study in Denmark from January 1st, 2004 to May 31st, 2017.
All patients with a positive culture of M. marinum were included. Patients were followed from the time of registration of microbiological material at International Reference Laboratory of Mycobacteriology until the end of treatment, lost to follow-up, or May 31st, 2017, whichever came first.
The International Reference Laboratory of Mycobacteriology (IRLM) at Statens Serum Institut (SSI) is the only laboratory which performs all cultures, culture-based drug susceptibility testing, and molecular typing of M. tuberculosis complex and nontuberculous mycobacteria in Denmark16.
Cultures were performed in Mycobacterium Growth Indicator Tube system (MGIT; Becton Dickinson, Sparks, MD) and on Loewenstein- Jensen (LJ) incubated at 35 °C. For skin specimens and specimens for which M. marinum culture was requested, a supplemental LJ tube was inoculated and incubated in 33 °C. M. marinum was identified with nucleic acid techniques varying over the study period. Microscopy for acid-fast bacilli (AFB) using auramine-rhodamine stain were performed on all specimens.
We retrieved data from the laboratory register at IRLM on patients with positive M. marinum culture in the study period. M. marinum cases were cross-linked with administrative and medical registers using the unique Danish civil registration number, which is assigned to all residents of Denmark at birth or after residing legally in Denmark for 3 months. Medical records were reviewed for the following variables: age, sex, immunocompromised status (HIV, organ transplantation, chronic corticosteroid use or other immunosuppressive drug use, or other immunocompromised condition (i.e. inflammatory bowel disease, rheumatoid arthritis)), history of aquatic exposure and type, localization and severity of infection (cutaneous or subcutaneous infections, ulcerations, noduli, lymphangitis, tenosynovitis, arthritis, and osteomyelitis), time of onset of symptoms, diagnostic methods, any radiographic findings, perioperative findings, antibiotic treatment (type, duration, and cause of treatment change as well as having received empiric antibiotics prior to diagnosis), outcome and date of last registered contact.
The time to diagnosis was defined as the time from onset of symptoms to diagnosis of M. marinum infection by microscopy or culture.
Infection severity was classified into four clinical categories17,18: localized superficial cutaneous lesions (type I), nodular lymphangitis or with nodules, abscesses and granulomas (type II), deeper infections with or without skin involvement (type III), and disseminated infection (type IV).
In accordance with previous definitions, four treatment outcomes were defined: (a) “improved” if no recurrence of disease after cessation of antibiotic treatment, (b) “improved with morbidity” if sign of improvement but without return of full function of the affected limb, (c) “failed” if there was recurrence of disease after cessation of antibiotic treatment or persistence of disease despite ongoing therapy at time of last contact, and (d) “lost to follow-up” if no documentation of outcome or treatment in medical records7.
The initial antibiotic treatment regimen was defined as the first treatment regimen that was prescribed, while backbone antibiotic regimen was defined as the regimen used for the majority of the treatment period7.
Surgery was defined as having any surgery besides biopsy or aspiration.
Ethics
This study was approved by the Danish Data Protection Agency (Jnr. 16/13130 and 2012-54-0100) and the Danish Health Authority (Jnr 3-3013-1640/1). In accordance with Danish law, observational studies performed in Denmark do not need approval from the Medical Ethics Committee or written consent from participants. All analyses are presented anonymously.
Analyses
All cases were included in the incidence rate analyses, but only cases with available medical records were used in the following analyses.
Continuous variables were described using medians with interquartile range (IQR), and categorical variables were described using frequency counts and percentages.
When appropriate, differences between categories were analyzed using the t test for qualitative values. Comparisons were made using the Student’s t-test, and a p-value of <0.05 was considered statistically significant.
The yearly population of Denmark on July 1 came from Statistics Denmark19. The population was defined as contributing to 1 person year per resident per year in the incidence rate analyses. The crude annual incidence rates were calculated as the number of incident cases per 100 000 person years with the corresponding 95% confidence intervals (95% CI).
The statistical analyses were performed using Stata® (StataCorp., College Station, TX, USA, version 15.0).
Results
From January 1st, 2004 to May 31st, 2017, we identified 55 patients with culture verified M. marinum infections. No medical records were available for 2 patients, both treated in non-hospital settings.
The incidence rates are shown in Fig. 1. The incidence rate during 2004 to 2009 was 0.04–0.06 per 100 000 person-years, from 2010 to 2016 the incidence rate was 0.05–0.13 per 100 000 person-years.
Figure 1.
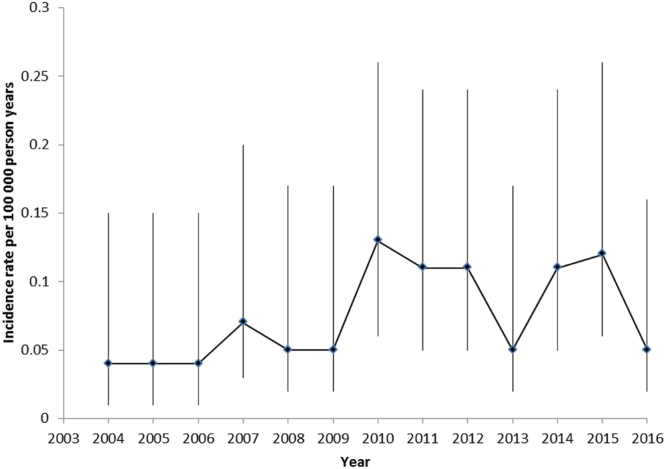
Incidence rate per 100 000 person years of M. marinum infection in Denmark with 95% confidence intervals. The population was defined as contributing to 1 person year per resident per year in the incidence rate analyses.
In total, 38 (71.7%) of the patients were men and the median age was 49 years (IQR: 36–59). The general patient characteristics, comorbidity and clinical findings are listed in Table 1. Almost all patients had a history of aquatic exposure (90.6%), the most common exposure came from maintenance of fish tanks. For the remaining 5 patients, the exposure was unknown. Type I infection was the most frequent (66%) and finger/hand (88.7%) was the most common site of infection.
Table 1.
Characteristics and diagnostic methods used in patients with M. marinum infection in Denmark.
| Variables | Number (%) |
|---|---|
| Total | 53a |
| Men | 38 (71.7) |
| Age in years: median | 49 (IQR: 36–59) |
| Immunocompromised | 9 (17.0) |
| History of aquatic exposure: | |
| Total | 48 (90.6) |
| Fish tank | 44 (83.0)b |
| Fishing | 3 (5.7) |
| Handled fish/seafood | 2 (3.8) |
| Swimming/diving | 1 (1.9) |
| Severity of infection: | |
| Type I | 35 (66.0) |
| Type II | 14 (26.4) |
| Type III | 2 (3.8) |
| Type IV | 2 (3.8) |
| Localization of infection: | |
| fFinger/hand | 47 (88.7) |
| iInvolved arm | 17 (32.1)c |
| lLeg | 3 (5.7) |
| Involved joints | 2 (3.8)d |
| Diagnostic methods: | |
| Microbiological: | |
| Acid-fast bacilli stain positive | 7 (13.2) |
| Culture from biopsy or aspiration or material from surgical procedures | 53 (100) |
| Pathology examination: | |
| Granulomatous inflammation | 35 (66.0)e |
| No granulomatous inflammation or positive acid-fast bacilli stain | 4 (7.6) |
| Imaging performed: | |
| X-ray only | 5 (9.4) |
| MRI | 3 (5.7)f |
| PET-CT | 3 (5.7)g |
| None | 43 (81.1) |
aTwo patients with missing medical records were left out of the analyses.
bTwo patients reported exposure to fish tank and fishing.
cFifteen patients had involvement of finger/hand and arm.
dOne patient had involvement of joint and finger/hand and arm.
eForty-two patients had histological examination performed.
fThree patients had X-ray and MRI performed.
gThree patients had X-ray and PET-CT performed, one patient had X-ray, MRI and PET-CT performed.
The median time from symptom presentation to diagnosis was 194 days (IQR: 80–548). A total of 21 patients received empiric antibiotic therapy, 9 patients received a regimen which was effective against M. marinum infection, 6 patient received beta-lactam antibiotics, and 1 patient received dapsone. For the remaining patients, information regarding the empiric antibiotic regimen was not accessible.
The incubation period could not be assessed since time of initial infection was unknown for the majority of the patients.
Treatment regimens and duration
The treatment regimens and median duration are listed in Table 2.
Table 2.
Treatment of patients with M. marinum infection in Denmark.
| Total | Number (%) |
|---|---|
| 53 | |
| Backbone antibiotic regimen a,b | |
| One drug | 25 (48.1) |
| Two drugs | 18 (34.6) |
| Three drugs | 8 (15.4) |
| Four drugs | 1 (1.9) |
| Treatment duration in days, median (IQR): | |
| Overall: | 112 (64–203) |
| Type I | 120 (83–143) |
| Type II | 74 (30–180) |
| Type III | 284 (284–284) |
| Type IV | 145 (50–240) |
| Regimens including: | |
| Rifampicin and ethambutol | 176 (112–284) |
| Clarithromycin and ethambutol | 123 (87–244) |
| Rifampicin, clarithromycin and ethambutol | 176 (112–244) |
| Outcome: | |
| Improved | 40 (75.5) |
| Improved with morbidity | 4 (7.5) |
| Failed | 3 (5.7) |
| Lost to follow up | 6 (11.3) |
| Outcome: “improved” for one drug regimen (n = 27) including: | |
| Tetracyclines (n = 16)c | 14 (87.5)d |
| Macrolides (n = 6)e | 6 (100.0) |
| Outcome: “improved” for 2–4 drug regimen (n = 27) includingf | |
| Rifampicin and ethambutol (n = 11) | 7 (63.6) |
| Clarithromycin and ethambutol (n = 15) | 9 (60.0) |
| Rifampicin, clarithromycin and ethambutol (n = 9) | 5 (55.6) |
| NOT including clarithromycin and ethambutol (n = 12) | 9 (75.0) |
aThe antibiotic regimen used for the majority of the treatment period.
bOne patient received multiple combinations of antibiotics; backbone therapy could not be assessed.
cTen patients received doxycycline and 6 patients received tetracycline.
dTwo patients were lost to follow up.
eFive patients received clarithromycin and one patient received azithromycin.
fClarithromycin was the only macrolide used in the 2–4 drug regimens.
All patients were treated with antibiotics either as monotherapy (45.3%) or as a combination of up to four antibiotics. Only 6 patients (11.3%) needed surgery performed.
All patients were treated by specialists in dermatology, infectious diseases, or pulmonary diseases. Only 11 patients (20%) were treated by dermatologists in non-hospital settings; 10 of these patients had type I infection and one patient had type II infection. All patients treated in non-hospital settings received a one-drug regimen, and median treatment duration was 80 days (IQR: 42–113). The most frequent drug of choice was doxycycline (36.4%) and median treatment duration was 91 days (IQR: 61–126). Outcome was classified as improved for 10 patients and 1 patient was classified as failed.
The majority of patients treated in hospital settings was diagnosed with type I infection (61.9%). Of those with type I infection, 10 patients (38.5%) received a one-drug treatment. Among all patients treated in hospital settings, 18 (43.9%) received a two-drug backbone regime and the overall median treatment duration was 123 days (IQR 84–244). The most commonly used combination in the 2, 3, and 4 drug backbone regimes were rifampicin and clarithromycin (38.9%).
Overall superficial infection (type I and type II) accounted for 92.5% of the cases, with a median treatment time of 110 days (IQR: 64–183). Fifty-one percent received a one drug regime and doxycycline was the most common drug prescribed (40%). The median treatment duration was 87 days (IQR: 42–130).
Among patients with deep tissue infection (type III and type IV), median time of treatment was 240 days (IQR: 50–284). They all received a treatment regime of at least 2 drugs, clarithromycin and ethambutol were included in all these treatment regimes.
Treatment outcome
The majority of the patients (75.5%) were classified as improved at treatment termination. Four patients were classified as improved with morbidity, two of these patients had type IV infection, and one patient had a finger amputated. The other patient received immunosuppressive therapy and had extensive scar tissue related to the sites of infection. The last two patients had type I infection; one of these patients was diagnosed with inflammatory bowel disease and received immunosuppressive therapy. At treatment termination, both patients were described having moderate scar tissue related to the site of infection.
Only 3 patients (5.7%) were classified as failed. In this group, two patients were diagnosed with rheumatoid arthritis and received immunosuppressive therapy at time of diagnosis. The first patient was treated for a type III infection for more than 5 years, treatment was discontinued several times, but the patient quickly showed signs of relapse, the patient was still receiving treatment as the study was concluded. The second patient was diagnosed with type I infection and was treated for 897 days but had signs of relapse at follow-up. The last patient had type II infection, was not immunocompromised, received a one drug regimen for 15 days and at the last visit the lesions were described as slightly improved and still visible.
Discussion
In this nationwide study, we reported 55 cases of culture confirmed M. marinum infections during a 13-year period. Infections with M. marinum are rare and related to aquatic exposure in Denmark. There is a long delay in diagnosis, but treatment outcome is overall good. However, severe manifestation of infection may be correlated with long treatment duration and need of surgery and sequelae.
More than 90% of the patients reported aquatic exposure. This is in consensus with the fact that M. marinum has been isolated from marine animals, fresh and saltwater2. The most common risk factor identified was fish tank maintenance, yet the incidence of M. marinum infections among owners of fish tanks is unknown. The last survey in Denmark regarding pet owners was completed in 2000 and reported that 80.000 families own fish tanks20 this is less than 2% of families in Denmark. This indicates that the incidence of M. marinum infection among fish tank owners in Denmark is very low. However, there has been reported an increase in number of families (10%) owning fish tanks in other countries2, which has been correlated to an increase in incidence of M. marinum infections during the last decade1. Yet, we were not able to identify a significant increase in the incidence rate of M. marinum infections in Denmark.
One patient in our study reported swimming pool as the sole known exposure. Before chlorination of swimming pools, several outbreaks of M. marinum infections caused by swimming in pools were reported in the literature21,22. Today, this is an unusual mode of transmission. Outbreaks of M. marinum have been reported in fish farms8 and fish markets during the last decade6. In our study, there were no clustered reports of M. marinum cases, hence no suspicion of an outbreak.
The majority of the patients in this study reported location of infection corresponding to upper extremities (92.5%). This correlates with the most common exposure: maintenance of fish tanks, hence finger/hands are the most common exposed areas. Several previous studies have likewise reported upper extremities as the most common infection site1,8,10–14,23,24.
Median time from symptom onset to diagnosis in this patient group was 194 days, earlier studies have reported great variance in delay of diagnosis (median 35–105 days)1,6,24. Both patient delay and doctor’s delay contributed to the delay of diagnosis. The majority of patients had had symptoms for weeks to months before consulting a doctor. The doctor’s delay could be attributed to the rare nature of M. marinum infection and the fact that cutaneous elements can resemble a staphylococcus infection. M.marinum will typically be detected with a mycobacterial culture performed at a lower temperature than usual. Unless the necessary information was lacking, all specimens from skin or from patients who had a suspected M. marinum infection were also cultured at a lower temperature. However, a delay in diagnosis was not significantly related to a poorer outcome in this study.
Our study consisted of patients with culture confirmed M. marinum infection, but only 13.2% had a positive AFB microscopy. Previous studies have also reported a low occurrence of positive AFB microscopy among patients with M. marinum infection (0–39%)6,8,11,12,14,24,25, indicating that a negative AFB microscopy cannot rule out the diagnosis of M. marinum infection and must be accompanied by culture.
Only 19% of patients had imagining performed. However more than 90% of our patients had type I or type II infection, which is compatible with superficial cutaneous infection. Therefore, in the majority of these cases there will not be an indication for imaging. Previous studies have shown a similar distribution of the severity of infection, hence M. marinum infection is more commonly a superficial infection that is limited to the skin. Most deep tissue infections with M. marinum have been reported as case studies in older and or immunocompromised patients1,18,26–28. This correlates with our results that only 4 patients (8%) had deep tissue infection and 2 of these patients received immunosuppressive therapy.
The results of this study revealed that 24 (45%) of the patients received a one-drug regimen. All of these patients had superficial cutaneous infection (type I: 80%, type II: 20%). Reported outcome was good; 88% of these patients were classified as improved and only one patient was classified as treatment failure at last contact. The remaining two patients in the group treated with a one-drug regimen were lost to follow-up.
Infections with M. marinum are rare, consequently there have not been any trials comparing treatment regimens and the optimal combination of antibiotics and treatment duration remains unknown. In Denmark there is no national guideline regarding treatment of M. marinum infections. But according to the guidelines of the American Thoracic Society and the Infectious Disease Society of America, a combination of clarithromycin and ethambutol, with the addition of rifampicin for deep structure infection is preferred, and a one drug regimen is discouraged29. However, earlier studies have described a successful outcome with a single drug regimen for superficial cutaneous infection8,10,15. One-hundred percent of the patients treated with a 3 or 4 drug backbone regime received a combination of rifampicin, clarithromycin and ethambutol, however of the patients receiving a 2 drug backbone regimen only a third received clarithromycin and ethambutol. Notably, the outcome was not significant better in the group receiving clarithromycin and ethambutol. In our study, all patients with type III or type IV infection were treated with a combination of two drugs or more, including clarithromycin and ethambutol. When the study was completed, two of these patients were still on treatment. Both patients had been diagnosed with rheumatoid arthritis and received immunosuppressive therapy and had been treated for M. marinum infection for more than 2 years. Furthermore, all patients on immunosuppressive therapy were treated longer than patients who did not receive immunosuppressive therapy. However, the overall outcome was good. In total, 66.6% of these patients were described as improved at treatment termination. This supports earlier studies, suggesting that immunocompromised patients may need a prolonged multi-drug regimen when diagnosed with a M. marinum infection30.
The strengths of this study are the nationwide design and all cases are culture confirmed. In only 2 cases the patients’ medical records could not be obtained.
Our study has inherent limitations: the true incidence of M. marinum infections might be greater than estimated in this study, as we included only patients with culture-confirmed M. marinum infections and patients treated based on a clinical diagnosis were left out.
Conclusion
M. marinum infections are rare and there is a long delay from symptom manifestation to diagnosis. The infection is highly related to aquatic exposure, which emphasizes that M. marinum should be a differential diagnose in patients with slow-developing cutaneous elements and relevant exposure. Prolonged therapy may be needed in patients receiving immunosuppressive therapy and severe sequelae are rare but often related to deep tissue infection.
Author Contributions
I.K.H. and I.S.J. have concepted and designed the study. I.S.J. has supervised the study. I.K.H., K.M., A.B.A. and C.W. have collected hospital data and E.S. provided the mycobacterial laboratory data. I.K.H., I.S.J. and M.K. have made the data analyses. I.K.H. has drafted the manuscript and all authors read, contributed, and approved the final manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Johnson MG, Stout JE. Twenty-eight cases of Mycobacterium marinum infection: retrospective case series and literature review. Infection. 2015;43:655–662. doi: 10.1007/s15010-015-0776-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aubry, A., Mougari, F., Reibel, F. & Cambau, E. Mycobacterium marinum. Microbiol Spectr5, 10.1128/microbiolspec.TNMI7-0038-2016 (2017). [DOI] [PMC free article] [PubMed]
- 3.Jernigan JA, Farr BM. Incubation period and sources of exposure for cutaneous Mycobacterium marinum infection: case report and review of the literature. Clin Infect Dis. 2000;31:439–443. doi: 10.1086/313972. [DOI] [PubMed] [Google Scholar]
- 4.Aubry A, Chosidow O, Caumes E, Robert J, Cambau E. Sixty-three cases of Mycobacterium marinum infection: clinical features, treatment, and antibiotic susceptibility of causative isolates. Arch Intern Med. 2002;162:1746–1752. doi: 10.1001/archinte.162.15.1746. [DOI] [PubMed] [Google Scholar]
- 5.Wentworth AB, Drage LA, Wengenack NL, Wilson JW, Lohse CM. Increased incidence of cutaneous nontuberculous mycobacterial infection, 1980 to 2009: a population-based study. Mayo Clin Proc. 2013;88:38–45. doi: 10.1016/j.mayocp.2012.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sia TY, et al. Clinical and Pathological Evaluation of Mycobacterium marinum Group Skin Infections Associated With Fish Markets in New York City. Clin Infect Dis. 2016;62:590–595. doi: 10.1093/cid/civ937. [DOI] [PubMed] [Google Scholar]
- 7.Johnson, M. G. & Stout, J. E. Twenty-eight cases of Mycobacterium marinum infection: retrospective case series and literature review. Infection, 10.1007/s15010-015-0776-8 (2015). [DOI] [PMC free article] [PubMed]
- 8.Feng Y, et al. Outbreak of a cutaneous Mycobacterium marinum infection in Jiangsu Haian, China. Diagn Microbiol Infect Dis. 2011;71:267–272. doi: 10.1016/j.diagmicrobio.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Eberst E, et al. Epidemiological, clinical, and therapeutic pattern of Mycobacterium marinum infection: a retrospective series of 35 cases from southern France. J Am Acad Dermatol. 2012;66:e15–16. doi: 10.1016/j.jaad.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 10.Ang P, Rattana-Apiromyakij N, Goh CL. Retrospective study of Mycobacterium marinum skin infections. Int J Dermatol. 2000;39:343–347. doi: 10.1046/j.1365-4362.2000.00916.x. [DOI] [PubMed] [Google Scholar]
- 11.Lewis FM, Marsh BJ, von Reyn CF. Fish tank exposure and cutaneous infections due to Mycobacterium marinum: tuberculin skin testing, treatment, and prevention. Clin Infect Dis. 2003;37:390–397. doi: 10.1086/376628. [DOI] [PubMed] [Google Scholar]
- 12.Wu TS, et al. Fish tank granuloma caused by Mycobacterium marinum. PLoS One. 2012;7:e41296. doi: 10.1371/journal.pone.0041296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casal M, Casal MM. & Spanish Group of, M. Multicenter study of incidence of Mycobacterium marinum in humans in Spain. Int J Tuberc Lung Dis. 2001;5:197–199. [PubMed] [Google Scholar]
- 14.Dodiuk-Gad R, et al. Nontuberculous mycobacterial infections of the skin: A retrospective study of 25 cases. J Am Acad Dermatol. 2007;57:413–420. doi: 10.1016/j.jaad.2007.01.042. [DOI] [PubMed] [Google Scholar]
- 15.Veraldi S, Cuka E, Nazzaro G. Treatment of sporotrichoid fish tank granuloma with pulsed clarithromycin. Dermatology. 2014;229:83–87. doi: 10.1159/000362199. [DOI] [PubMed] [Google Scholar]
- 16.Hermansen TS, Ravn P, Svensson E, Lillebaek T. Nontuberculous mycobacteria in Denmark, incidence and clinical importance during the last quarter-century. Sci Rep. 2017;7:6696. doi: 10.1038/s41598-017-06931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hurst LC, Amadio PC, Badalamente MA, Ellstein JL, Dattwyler RJ. Mycobacterium marinum infections of the hand. The Journal of hand surgery. 1987;12:428–435. doi: 10.1016/S0363-5023(87)80018-7. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen HH, Fadul N, Ashraf MS, Siraj DS. Osteomyelitis Infection of Mycobacterium marinum: A Case Report and Literature Review. Case Rep Infect Dis. 2015;2015:905920. doi: 10.1155/2015/905920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Statistics_Denmark. (Statistics Denmark - https://www.statistikbanken.dk/statbank5a/default.asp?w=1440, 2018).
- 20.Statistics_Denmark. In Landbrug Vol. 2000 (ed B Nielsen) (Statistics Denmark - http://www.dst.dk/pukora/epub/Nyt/2000/NR499.pdf, 2000).
- 21.Mollohan CS, Romer MS. Public health significance of swimming pool granuloma. Am J Public Health Nations Health. 1961;51:883–891. doi: 10.2105/AJPH.51.6.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linell F, Norden A. Mycobacterium balnei, a new acid-fast bacillus occurring in swimming pools and capable of producing skin lesions in humans. Acta Tuberc Scand Suppl. 1954;33:1–84. [PubMed] [Google Scholar]
- 23.Abbas O, et al. Cutaneous non-tuberculous Mycobacterial infections: a clinical and histopathological study of 17 cases from Lebanon. J Eur Acad Dermatol Venereol. 2011;25:33–42. doi: 10.1111/j.1468-3083.2010.03684.x. [DOI] [PubMed] [Google Scholar]
- 24.Edelstein H. Mycobacterium marinum skin infections. Report of 31 cases and review of the literature. Arch Intern Med. 1994;154:1359–1364. doi: 10.1001/archinte.1994.00420120079009. [DOI] [PubMed] [Google Scholar]
- 25.Cheung JP, Fung B, Ip WY, Chow SP. Mycobacterium marinum infection of the hand and wrist. J Orthop Surg (Hong Kong) 2012;20:214–218. doi: 10.1177/230949901202000216. [DOI] [PubMed] [Google Scholar]
- 26.Plana-Veret C, Iranzo-Fernandez P, Peris P, Monegal A, Guanabens N. Invasive Mycobacterium marinum infection. Joint Bone Spine. 2015;82:462. doi: 10.1016/j.jbspin.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 27.Streit M, et al. Disseminated Mycobacterium marinum infection with extensive cutaneous eruption and bacteremia in an immunocompromised patient. Eur J Dermatol. 2006;16:79–83. [PubMed] [Google Scholar]
- 28.Nielsen CT, Andersen AB. Hypercalcemia and renal failure in a case of disseminated Mycobacterium marinum infection. Eur J Intern Med. 2009;20:e29–31. doi: 10.1016/j.ejim.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 29.Griffith DE, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 30.Rallis E, Koumantaki-Mathioudaki E. Treatment of Mycobacterium marinum cutaneous infections. Expert Opin Pharmacother. 2007;8:2965–2978. doi: 10.1517/14656566.8.17.2965. [DOI] [PubMed] [Google Scholar]


