Abstract
The hemodynamic function of multichanneled aortic dissection (MCAD) requires close monitoring and effective management to avoid potentially catastrophic sequelae. This report describes a 47-year-old man who underwent endovascular repair based on findings from four-dimensional (4D) flow magnetic resonance imaging of an MCAD. The acquired 4D flow data revealed complex, bidirectional flow patterns in the false lumens and accelerated blood flow in the compressed true lumen. The collapsed abdominal true lumen expanded unsatisfactorily after primary tear repair, which required further remodeling with bare stents. This case study demonstrates that hemodynamic analysis using 4D flow magnetic resonance imaging can help understand the complex pathologic changes of MCAD.
Multichanneled aortic dissection (MCAD) differs from classic double-channeled aortic dissection (DCAD) and involves the formation of an additional false lumen (FL) in the aortic wall or the flaps. The incidence of MCAD has varied from 4.9% to 9%.1, 2, 3 Currently, treatments for MCAD are derived from studies of classic DCAD. Available guidelines recommend thoracic endovascular aortic repair (TEVAR) in complicated cases.4 In fact, the natural course of MCAD is still unpredictable, especially for those with full true lumen (TL) collapse.5 Regarding TEVAR for MCAD, limited literature is available on how the timing of intervention and treatment strategies may affect patient outcomes and potential complications. Previous studies suggested that the perioperative mortality and morbidity in patients with MCAD were much higher than in patients with DCAD.2, 3 Differences and complexity in flow patterns and hemodynamics may play a crucial role in predicting acute complications and determining the outcomes of TEVAR for MCAD. The purpose of this study was to qualitatively and quantitatively assess the flow in a patient with MCAD using four-dimensional (4D) flow magnetic resonance imaging (MRI) and to determine the outcomes after nonroutine TEVAR. The patient consented to publication of this report.
Case report
A 47-year-old man with a history of hypertension was admitted to the emergency department with severe pain in the chest and back. Apart from medically treated hypertension, the patient had no further comorbidities. An urgent contrast-enhanced computed tomography (CT) scan revealed a type B aortic dissection with three-channeled morphology (Fig 1). The three-channeled type B aortic dissection had a “true-false-false” lumen configuration. The patient was transferred to the intensive care unit, where beta blockers and calcium antagonists were administered and intra-arterial blood pressure was monitored.
Fig 1.
Three-dimensional (3D) computed tomography (CT) reconstruction (A) showed three channels: an inner narrowed true lumen (TL), a middle false lumen (FL), and an outer FL of a “true-false-false” lumen configuration (B). The axial CT image (C) at the primary entry tear showed an additional FL on the same side of the original FL and the compression of the TL. The cleavage plane of the dissection extended into the ostium of the left renal artery and compromised inflow, which caused static ischemia (D).
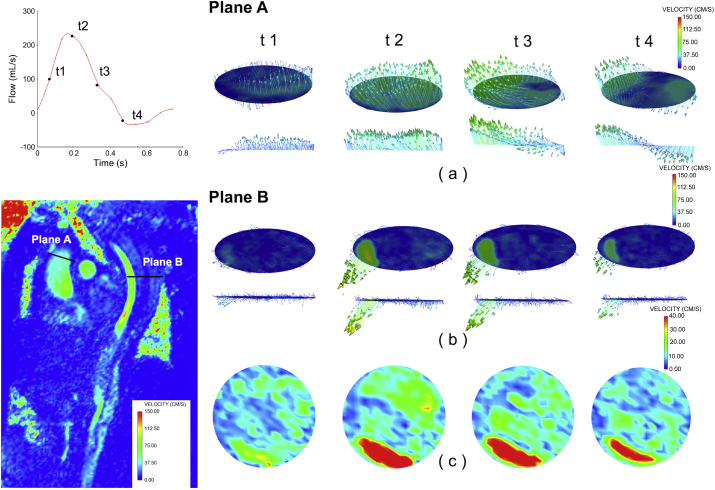
The three-dimensional (3D) volume-rendered CT images (Fig 1, A) and the multiplanar reformations (Fig 1, B-D) allowed a detailed morphologic assessment of the aortic disease. However, no information on hemodynamic variables could be derived from the CT scan. To help choose an effective endovascular strategy, 4D flow MRI was performed on hospital day 10 to visualize and analyze blood flow patterns and thus detect hemodynamically relevant changes in the TL and FLs. The data were acquired with a 3.0T clinical MRI scanner (Magnetom Avanto; Siemens Medical Solutions, Erlangen, Germany) using the following parameters: flip angle, 7 degrees; velocity encoding, 200 cm/s; spatial resolution, 3.1 × 2.3 × 2.5 mm3; and temporal resolution, 40.8 milliseconds. Retrospective electrocardiographic gating was used to synchronize the measurement with the cardiac motion (28 frames/cardiac cycle were reconstructed). The 4D flow MRI preprocessing included noise filtering, correction for eddy currents, and velocity aliasing with custom-built software programmed in MATLAB (MathWorks, Natick, Mass). Measurement planes were manually placed perpendicular to the long axis of the aorta at defined anatomic landmarks in the mid-ascending aorta (Fig 2, Plane A), the proximal thoracic aorta (Figs 2 and 3, Plane B) at the origin of arterial ductus ligament, and the distal thoracic aorta (Fig 3, Plane C). The 3D phase-contrast magnetic resonance angiography was derived from 4D flow MRI data and used to visualize 3D aortic flow patterns by velocity-coded vectors (Fig 2) and to quantitatively assess the flow and mean velocity (Fig 3) at peak flow systole (EnSight; CEI, Apex, NC). In Fig 3, the flow and velocity magnitude were similar in the TL from the proximal (Plane B) to distal dissection (Plane C). The outer FL (FL-1) was characterized by bidirectional flow pattern during late systole and diastole. There was substantial and antegrade flow in the middle FL (FL-2), and the velocities there were not much lower than in FL-1. Notably, even within the same FL, the flow and velocity curves changed considerably from Plane B to Plane C.
Fig 2.
Hemodynamic analysis was performed using four-dimensional (4D) flow magnetic resonance imaging (MRI) data for the through-plane velocity in the ascending aorta (Plane A) and proximal thoracic aorta (Plane B) throughout the systolic period. The through-plane blood flow pattern and velocity in the ascending aorta (a) significantly differed from those in the dissecting aorta (b and c), which showed complex spatial and temporal flow distribution among the three channels. The increasing velocity in the true lumen (TL) was much higher than that of false lumens (FLs), whereas the velocity and direction of the FLs were becoming asynchronous.
Fig 3.
Quantitative comparison between through-plane flow and velocity (mean value of velocity magnitude) at peak systole (t2) in the three-channeled dissecting aorta. The flow and velocity magnitude were shown to be similar in the true lumen (TL) from the proximal (Plane B) to distal dissection (Plane C). Of note, flow and velocity throughout the cardiac cycle showed significant differences between the false lumens (FLs). The outer channel (FL-1) had a bidirectional flow; however, the middle one (FL-2) had only an antegrade flow. There was substantial flow in FL-2, and the velocities there were not much lower than in FL-1. Even within the same FL, the flow and velocity curves throughout the cardiac cycle changed considerably from Plane B to Plane C.
The patient underwent TEVAR without lumbar drains through right femoral artery access on hospital day 14. Angiography showed that all branches originated from the TL, whereas the dissection extended into the ostium of the left renal artery and compromised the inflow. The TL was collapsed in the abdominal aorta, and the abdominal TL expanded slightly after insertion of a stent graft (C-TAG; W. L. Gore & Associates, Flagstaff, Ariz), with an oversizing of 5% to exclude the primary tear and to obliterate the FLs. The proximal part of the stent graft was intended to be deployed up to the left subclavian artery to reduce the radial force on the aortic arch. Subsequently, two more bare stents (22 × 70 mm [Wallstent; Boston Scientific, Marlborough, Mass] and 14 × 100 mm [E-Luminexx; Bard, Murray Hill, NJ]) were deployed into the abdominal aorta to remodel the TL. However, the left renal artery still showed ongoing malperfusion. The patient's recovery was uneventful, and he was discharged 3 days after TEVAR. At 3-month follow-up, the diameter of the proximal descending aorta increased with respect to the one measured before the procedure. However, the follow-up CT scan at 12 months showed shrinkage of the FLs in the thoracic aorta, nearly completed thrombosis in the FLs, expansion of the TL, and perfusion to all visceral arteries (Fig 4).
Fig 4.
Digital subtraction angiography demonstrated abdominal true lumen (TL) with full collapse, and the left renal artery presented with malperfusion (A). Digital subtraction angiography confirmed the three-channeled morphology in the aortic arch (B). The angiogram showed the stent graft deployed with obliteration of the primary false lumens (FLs; C), whereas the abdominal TL failed to expand adequately (D). Abdominal aortography after bare stent deployment showed TL expansion without FL flow (E). Computed tomography (CT) scan (F-H) at 12 months after endovascular repair showed obliteration of the FLs in the thoracic aorta, nearly complete thrombosis of the FLs, expansion of the TL, and perfusion to all visceral arteries.
Discussion
The use of 4D flow MRI enables post hoc time-resolved 3D visualization of blood flow and retrospective quantification at any location in a 3D volume. Its value has been increasingly demonstrated in a variety of cardiovascular diseases for the assessment of blood flow hemodynamics.6, 7 However, understanding hemodynamics from 4D flow MRI data and interpreting its meaning in aortic dissection are particularly challenging.8 Notably, blood flow patterns are strongly influenced by the vessel geometry. Geometrically triggered changes in blood flow characteristics, such as those shown in the TL and FLs as well as in the primary entry and re-entry tears, may serve as useful clinical markers in the evaluation of the severity of the dissection and improve the assessment of its potential risk for future complications.9 Patient-specific analysis using flow visualization and quantification may aid physicians in their preoperative and postoperative assessment of aortic diseases.
However, data on hemodynamic parameters that can be used to estimate blood flow among multiple channels for patients with MCAD are lacking. Previous studies reported the poor prognosis of MCAD compared with DCAD, but the mechanism was unclear.2, 3 Our previous finding suggested that the secondary FL could damage the remaining media of the first FL, which might lead to hemodynamic redistribution and the development of aneurysmal dilation. Moreover, MCAD with TL full collapse was identified as a high-risk factor for hemodynamic instability.5 As shown by the presented images and hemodynamic analysis using 4D flow MRI data, the complex spatial and temporal flow distribution of MCAD was confirmed in either the proximal or distal thoracic aorta. Quantifications of blood flow and velocities, especially in FLs, revealed significant variations throughout the cardiac cycle. The bidirectional flow pattern in FL-1 indicated that flow in the outer FL was strongly influenced by the secondary entry tears, whereas the antegrade flow throughout the cardiac cycle in FL-2 suggested that the primary entry tear might be more important. Stent graft repair of the primary entry tear would result in flow redistribution in FLs for patients with MCAD, at least to some extent, turning multiple channels into two channels. Consequently, the intimal lamellae would undergo continuous movements with each systole and diastole, even after primary entry tear repair. This kept the blood in motion in the FLs, contributing to flow redistribution and preventing thrombosis at this level.10 On the other hand, remolding of the distally collapsed TL after the insertion of bare stents, such as the provisional extension to induce complete attachment (PETTICOAT) technique,11 may contribute to increasing blood flow in the TL and minimizing the flapping movement of dissecting lamellae, thereby reducing retrograde flow into the FLs through secondary or re-entry tears and promoting FL thrombosis.10 At 3 months after the procedure, distal entries with continuous retrograde flow in the proximal FLs might result in the expansion of the FLs. With thrombus formation in the FLs, the decrease of retrograde flow also prompted the shrinkage of the FLs. Although the MRI findings did not influence the choice to proceed with TEVAR, these provided useful information to understand the aortic pathologic changes and to remodel the collapsed TL. Ongoing studies are aimed at identifying the hemodynamic parameters that will help tailor treatment options for patients with MCAD or DCAD.
Conclusions
The 4D flow MRI allows visualization and quantification of hemodynamic patterns in a patient with MCAD. In the future, it may help to better understand complex aortic diseases and guide endovascular strategies.
Footnotes
B.G. and S.P. contributed equally to this work and share co-first authorship.
This work was supported by grants from National Natural Science Foundation of China (81470573, 81371648, 81770474, 81770508) and the Shanghai Committee of Science and Technology, China (16410722900). S.P. is supported by the European Commission within the Horizon 2020 Framework through the MSCA-ITN-ETN European Training Networks (642458). The authors would also like to acknowledge the support of The Royal Society (IE161052), United Kingdom.
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.McReynolds R.A., Shin M.S., Sims R.D. Three-channeled aortic dissection. AJR Am J Roentgenol. 1978;130:549–552. doi: 10.2214/ajr.130.3.549. [DOI] [PubMed] [Google Scholar]
- 2.Ando M., Okita Y., Tagusari O., Kitamura S., Matsuo H. Surgery in three-channeled aortic dissection. A 31-patient review. Jpn J Thorac Cardiovasc Surg. 2000;48:339–343. doi: 10.1007/BF03218153. [DOI] [PubMed] [Google Scholar]
- 3.Sueyoshi E., Nagayama H., Hayashida T., Sakamoto I., Uetani M. Comparison of outcome in aortic dissection with single false lumen versus multiple false lumens: CT assessment. Radiology. 2013;267:368–375. doi: 10.1148/radiol.12121274. [DOI] [PubMed] [Google Scholar]
- 4.Erbel R., Aboyans V., Boileau C., Bossone E., Bartolomeo R.D., Eggebrecht H. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC) Eur Heart J. 2014;35:2873–2926. doi: 10.1093/eurheartj/ehu281. [DOI] [PubMed] [Google Scholar]
- 5.Guo B., Hou K., Guo D., Xu X., Shi Z., Shan Y. Outcomes of thoracic endovascular repair for type B aortic dissection with multichanneled morphology. J Vasc Surg. 2017;66:1007–1017. doi: 10.1016/j.jvs.2016.12.145. [DOI] [PubMed] [Google Scholar]
- 6.Bollache E., van Ooij P., Powell A., Carr J., Markl M., Barker A.J. Comparison of 4D flow and 2D velocity-encoded phase contrast MRI sequences for the evaluation of aortic hemodynamics. Int J Cardiovasc Imaging. 2016;32:1529–1541. doi: 10.1007/s10554-016-0938-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parekh K., Markl M., Rose M., Schnell S., Popescu A., Rigsby C.K. 4D flow MR imaging of the portal venous system: a feasibility study in children. Eur Radiol. 2017;27:832–840. doi: 10.1007/s00330-016-4396-1. [DOI] [PubMed] [Google Scholar]
- 8.Muller-Eschner M., Rengier F., Partovi S., Unterhinninghofen R., Bockler D., Ley S. Tridirectional phase-contrast magnetic resonance velocity mapping depicts severe hemodynamic alterations in a patient with aortic dissection type Stanford B. J Vasc Surg. 2011;54:559–562. doi: 10.1016/j.jvs.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 9.Frydrychowicz A., Stalder A.F., Russe M.F., Bock J., Bauer S., Harloff A. Three-dimensional analysis of segmental wall shear stress in the aorta by flow-sensitive four-dimensional-MRI. J Magn Reson Imaging. 2009;30:77–84. doi: 10.1002/jmri.21790. [DOI] [PubMed] [Google Scholar]
- 10.Melissano G., Bertoglio L., Rinaldi E., Civilini E., Tshomba Y., Kahlberg A. Volume changes in aortic true and false lumen after the “PETTICOAT” procedure for type B aortic dissection. J Vasc Surg. 2012;55:641–651. doi: 10.1016/j.jvs.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 11.Nienaber C.A., Kische S., Zeller T., Rehders T.C., Schneider H., Lorenzen B. Provisional extension to induce complete attachment after stent-graft placement in type B aortic dissection: the PETTICOAT concept. J Endovasc Ther. 2006;13:738–746. doi: 10.1583/06-1923.1. [DOI] [PubMed] [Google Scholar]