Highlights
-
•
Granular cell tumors of the abdominal wall are rare.
-
•
Mesenchymal tumors may have similar findings on diagnostic imaging studies.
-
•
Laparoscopic-endoscopic cooperative surgery may useful to resect gastric submucosal tumors.
Abbreviations: GIST, gastrointestinal stromal tumor; FNA, fine needle aspiration; SUV, standardized uptake value; CT, computed tomography; MRI, magnetic resonance imaging; 18F-FDG-PET, 18F-fluorodeoxyglucose positron emission tomography
Keywords: Granular cell tumor, Gastric leiomyoma, Laparoscopic-endoscopic cooperative surgery, Case report
Abstract
Introduction
Gastric leiomyomas are benign mesenchymal tumors, comprising about 2.5% of gastric neoplasms, which can be difficult to differentiate from gastrointestinal stromal tumors which have malignant potential. Granular cell tumors in the abdominal wall are also rare. Since mesenchymal tumors are difficult to diagnose by imaging, further studies are needed to establish the diagnosis.
Presentation of case
A 60-year-old asymptomatic woman underwent routine upper endoscopy and was found to have a gastric submucosal lesion. Computed tomography scan also showed an abdominal wall mass. The appearance of both lesions on imaging studies were similar, but it was unclear if the two lesions had the same origin. Endoscopic ultrasound-guided fine needle aspiration biopsy of the gastric lesion was insufficient to establish the diagnosis. Laparoscopic-endoscopic cooperative resection of the gastric lesion and ultrasound-guided core-needle biopsy of the abdominal wall mass enabled pathological diagnosis of both lesions.
Discussion
Diagnostic imaging findings of these two lesions were similar. Histologic and immunohistochemical studies are essential to establish a definitive diagnosis. Laparoscopic-endoscopic cooperative surgery may be an effective minimally invasive approach, allowing both pathological diagnosis and complete resection of a gastric submucosal tumor, especially when endoscopic-ultrasound guided fine needle aspiration or biopsy fails to make the diagnosis.
Conclusion
Laparoscopic-endoscopic cooperative surgery can be an effective minimally invasive approach to resect some lesions. This is first report of the patient with a synchronous gastric leiomyoma and an intramuscular granular cell tumor in the abdominal wall.
1. Introduction
Gastric leiomyomas represent approximately 2.5% of all gastric neoplasms [1], while gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the stomach [2]. Gastric leiomyomas share many characteristics with GISTs, although GISTs have malignant potential [[3], [4]]. Diagnosis of a gastric leiomyoma by imaging studies is often difficult [[1], [5]]. Endoscopically obtained biopsy specimens may be insufficient to establish the diagnosis due to the thick overlying mucosa [6]. Fine-needle aspiration obtained with or without endoscopic ultrasound guidance may be adequate to establish the pathological diagnosis of a submucosal tumor [6]. The diagnostic yield of aspiration biopsies of gastric lesions is not high [7]. Granular cell tumors are rare and typically originate from the head, neck and tongue [8]. Overall, <1% of all granular cell tumors are malignant [9]. The abdominal wall is an extremely rare site for granular cell tumors [10].
We report a patient with a gastric leiomyoma and an intramuscular abdominal wall granular cell tumor which showed similar features on diagnostic imaging. Laparoscopic-endoscopic cooperative surgery enabled both establishing a pathological diagnosis and resection of the gastric submucosal tumor [11]. This work is reported in line with the SCARE criteria [12].
2. Presentation of case
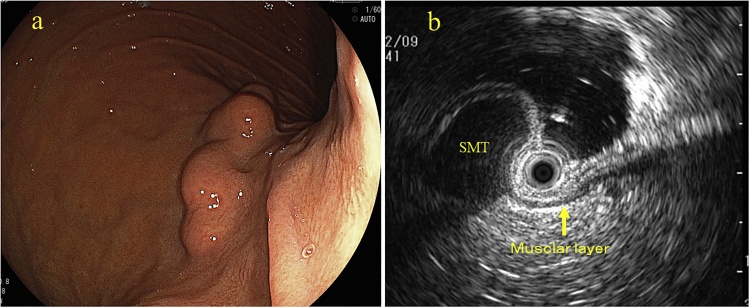
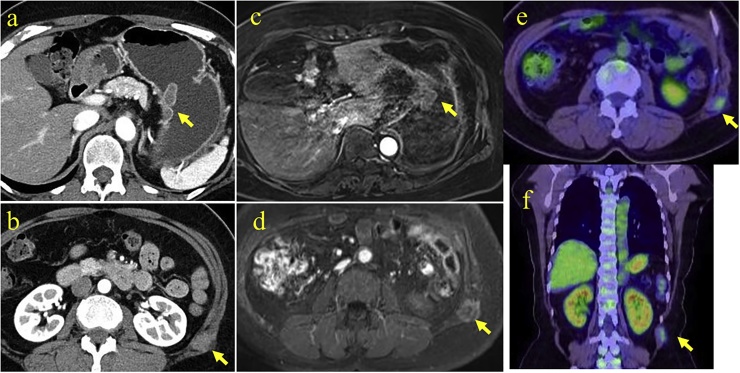
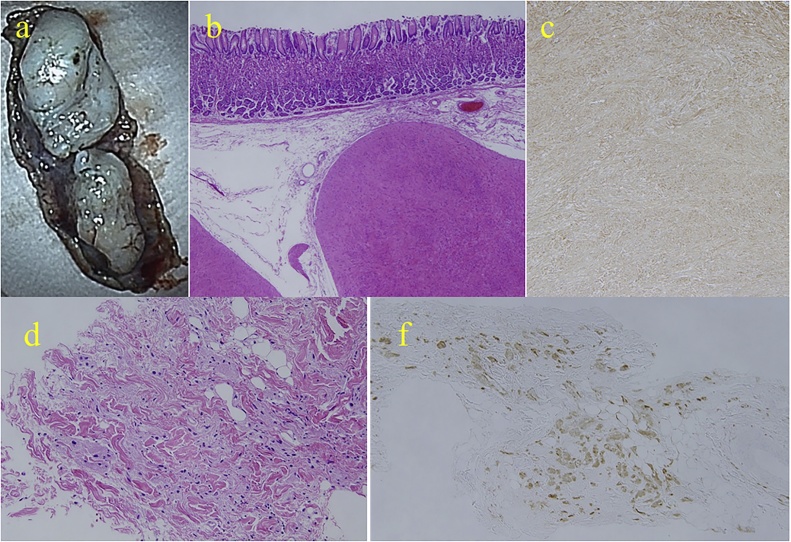
An asymptomatic 60-year-old Japanese woman with no significant past medical history underwent routine upper endoscopy, which revealed a 30 mm submucosal lesion on the posterior wall of the proximal stomach (Fig. 1a). Endoscopic ultrasound showed a homogeneous low echo lesion in the muscularis of the gastric wall (Fig. 1b). Endoscopic ultrasound-guided fine-needle aspiration biopsy was performed twice, but was insufficient to establish a diagnosis. An incidental soft tissue mass was found in the anterior abdominal wall by contrast-enhanced computed tomography (CT) scan, and further evaluated with magnetic resonance imaging (MRI). CT scan revealed poorly enhancing lesions in the stomach (Fig. 2a) and anterior abdominal wall (Fig. 2b). Axial T2 – weighted images on MRI scan showed a homogeneous low signal intensity and enhanced T1-weighted image showed homogeneous moderate enhancement of both the gastric (Fig. 2c) and abdominal wall lesions (Fig. 2d). Fluorine-18 fluorodeoxyglucose positron emission tomography (18F-FDG-PET) CT revealed 18F-FDG uptake only in the abdominal wall tumor with a maximum standardized uptake value (SUVmax) of 1.92 (Fig. 2e, f). It was considered that the gastric submucosal tumor and abdominal wall tumor may be of similar origin, possibly with malignant potential. Laparoscopic-endoscopic cooperative surgery was used to resect the gastric submucosal tumor. Ultrasound-guided core-needle biopsy of the abdominal wall tumor was performed. The gastric submucosal tumor was composed of two white solid areas, each about 20 mm (Fig. 3a). Histopathologic examination showed elongated fusiform cells with abundant eosinophilic cytoplasm proliferating in a palisading manner (Fig. 3b) with few mitoses. Immunohistochemically, α-smooth muscle actin was positive in the gastric lesion (Fig. 3c) but C-kit and CD34 were negative. Histopathologically, the ultrasound-guided core needle biopsy of the abdominal wall lesion revealed sheets of polygonal cells with eosinophilic, granular cytoplasm and vesicular nuclei (Fig. 3d), and S-100 was positive (Fig. 3f). C-kit, CD34, MyoD1 and Myogenin were negative. These results confirmed that the gastric submucosal lesion was a leiomyoma and the abdominal wall mass was a granular cell tumor. The patient had an uneventful hospital course and was discharged. Her abdominal wall granular cell tumor has been followed for 12 months with no evidence of recurrence.
Fig. 1.
Upper gastrointestinal endoscopy showed a 30 mm submucosal polypoid lesion on the posterior wall of the proximal stomach (a). Endoscopic-ultrasound showed that the submucosal tumor was homogenous with an echogenicity similar to that of the normal muscularis (arrow) (b).
Fig. 2.
Contrast-enhanced computed tomography (CT) scan revealed poorly enhanced tumors in the stomach (a) and the anterior abdominal wall (b) (arrows). Axial enhanced T1-weighted image on magnetic resonance imaging showed similarly homogeneous moderate enhancement in both gastric (c) and abdominal wall lesions (d) (arrows). 18F-fluorodeoxyglucose positron emission tomography CT scan revealed 18FDG uptake only in the abdominal wall lesion with a maximum standardized uptake value of 1.92 (e, f) (arrows).
Fig. 3.
(a) The gastric lesion was resected with laparoscopic-endoscopic cooperative surgery and showed two solid tumors, 23 and 22 mm in diameter. (b) Histopathological findings established the diagnosis of gastric leiomyomata, with spindle-shaped cells arranged in an interlacing and palisading pattern with few mitoses (Hematoxylin-Eosin stain, 40×). (c) Immunostaining revealed that the cells were positive for α-smooth muscle actin (100×). (d) Core needle biopsy of the abdominal wall tumor revealed polygonal cells with eosinophilic, granular cytoplasm and vesicular nuclei spreading in the muscle and adipose tissue (Hematoxylin-Eosin stain, 100×). (f) The cells stained positive for S-100 (100×).
3. Discussion
Gastric submucosal tumors are often asymptomatic, and are usually discovered incidentally during routine upper gastrointestinal endoscopy [4]. Gastric leiomyomas are a rare type of gastrointestinal mesenchymal tumor that arise most commonly in the muscularis propria or muscularis mucosae of the stomach [13], which they have in common with GISTs, making them hard to differentiate using diagnostic imaging studies such as contrast MRI, CT scan or endoscopic ultrasound [5]. GISTs generally have malignant potential, including the development of metastases [4]. The main issues in asymptomatic patients with gastric submucosal tumors is to determine whether or not the tumors have malignant potential [4] and the necessity for resection.
Granular cell tumors are mesenchymal benign lesions which comprise about 0.5% of all soft tissue tumors [14]. The malignant potential of granular cell tumors is thought to be quite low [9]. Common sites for granular cell tumors include the tongue (40%), breast (15%), respiratory tract (10%) and esophagus (2%) [15], but rarely originate in the abdominal wall10. In this patient with a gastric leiomyoma and intramuscular abdominal wall granular cell tumor, the diagnostic imaging studies of both lesions appeared similar, suggesting that the two lesions might be related. Endoscopic ultrasound-guided fine needle aspiration biopsy of the gastric lesion was performed twice but the specimens were inadequate to establish the diagnosis.
The best radiographic modality for the characterization of granular cell tumors is reported to be MRI [16]. Benign granular cell tumors are classically iso-intense or brighter than muscle on T1-weighted sequences. On T2-weighted sequences, the signal from the central portion of the lesion is classically iso-intense with muscle or suppressed fat. However, these findings are similar to the gastric submucosal tumor in this patient. Malignant granular cell tumors have been described with invasion of adjacent structures [17] and the border of the abdominal wall tumor in this patient was also unclear on MRI imaging.
Esophageal leiomyomas generally have lower 18F-FDG uptake [18], although 18F-FDG uptake in gastric leiomyomas has not been well investigated [1]. 18F-FDG uptake in this patient was negative in the gastric submucosal tumor although the abdominal wall tumor showed low uptake at 1.92 SUVmax. The PET-CT scan suggested that these two tumors may have different origins. Histologic and immunohistochemical studies were essential, since we could not eliminate the possibility of a malignancy.
The location of gastric submucosal tumors may be difficult to determine laparoscopically, because of their small size or intraluminal growth pattern [11]. Laparoscopic-endoscopic cooperative surgery is a particularly good approach to evaluate a mass growing in an intra-luminal area such as gastric submucosal tumors [19] and is less invasive than open resection. The diagnostic yield of endoscopic ultrasound-guided fine needle aspiration or biopsy for gastric submucosal tumors was reported to range from 34 to 79% [7]. Laparoscopic-endoscopic cooperative surgery was performed in this patient for to establish the pathological diagnosis, since fine needle aspiration did not provide an adequate sample.
Immnohistochemically, leiomyomas are generally positive for α-smooth muscle actin and desmin, and are negative for C-kit and CD34 [20]. Granular cell tumors are derived from Schwann cells of peripheral nerve fibers [10]. The expression of markers such as S-100 [20] and neuron-specific enolase demonstrates the histogenetic derivation of granular cell tumors from Schwann cells. The features of malignant granular cell tumors include spindle-cell morphology, necrosis, prominent nucleoli, an increased nuclear-to-cytoplasmic ratio, nuclear pleomorphism and an increased rate of mitosis [8]. To be diagnosed as a malignant granular cell tumor, at least three of these features must be met [8]. Histologic and immunohistochemical studies of the resected gastric submucosal tumor obtained by laparoscopic-endoscopic cooperative surgery and of the abdominal wall lesion using a core needle biopsy confirmed that the gastric submucosal tumor was a leiomyoma and the abdominal wall tumor was a benign granular cell tumor.
Based on the studies obtained, the gastric leiomyoma and the abdominal wall granular cell tumor in this patient are not associated. These two rare mesenchymal tumors were incidentally synchronous in this patient. Laparoscopic-endoscopic cooperative surgery was useful to establish the pathological diagnosis and also allowed a curative resection of the gastric submucosal tumor.
4. Conclusion
In summary, we report two synchronous rare lesions in a 60-year-old woman, including a leiomyoma of the stomach and an abdominal wall granular cell tumor. Diagnostic imaging studies of the two lesions appeared similar, which suggested a similar origin. Laparoscopic-endoscopic cooperative surgery was useful for both establishing the diagnosis and allowing complete resection of gastric submucosal tumor.
Conflicts of interest
The authors declare no conflicts of interests regarding the publication of this paper.
Funding
All authors have no funding regarding this paper.
Ethical approval
The ethical approval for this paper has been exempted by our committee of Jichi medical university hospital, because this is a case report.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Author contribution
All authors in this manuscript contributed to the interpretation of data, and drafting and writing of this manuscript. SS, CY, SM, HF and YH were engaged in patient’s care in her hospital coarse including surgery and endoscopy under the supervision of AL, JK and NS. AL helped in drafting the manuscript and interpretation of data. DM was engaged in pathological and immunohistochmical studies. All authors have read and approved this manuscript for publication.
Guarantor
Dr. Sata, who is the president of Jichi medical university hospital, is the Guarantor.
References
- 1.Hirose Y., Kaida H., Kawahara A., Kurata S., Ishibashi M., Abe T. (1)(8)F-FDG PET/CT and contrast enhanced CT in differential diagnosis between leiomyoma and gastrointestinal stromal tumor. Hell J. Nucl. Med. 2015;18(3):257–260. doi: 10.1967/s002449910308. [DOI] [PubMed] [Google Scholar]
- 2.Gagne A., Sazonova O., Marceau S., Perigny M., Joubert P. A foregut duplication cyst of the stomach in association with a gastrointestinal stromal tumor and a leiomyoma: a case eeport. Case Rep. Pathol. 2016;2016:1537240. doi: 10.1155/2016/1537240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Min Y.W., Park H.N., Min B.H., Choi D., Kim K.M., Kim S. Preoperative predictive factors for gastrointestinal stromal tumors: analysis of 375 surgically resected gastric subepithelial tumors. J. Gastrointest. Surg. 2015;19(4):631–638. doi: 10.1007/s11605-014-2708-9. [DOI] [PubMed] [Google Scholar]
- 4.Kim G.H., Park D.Y., Kim S., Kim D.H., Kim D.H., Choi C.W. Is it possible to differentiate gastric GISTs from gastric leiomyomas by EUS? World J. Gastroenterol. 2009;15(27):3376. doi: 10.3748/wjg.15.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang H.K., Kim Y.H., Lee Y.J., Park J.H., Kim J.Y., Lee K.H. Leiomyomas in the gastric cardia: CT findings and differentiation from gastrointestinal stromal tumors. Eur. J. Radiol. 2015;84(9):1694–1700. doi: 10.1016/j.ejrad.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 6.Niimi K., Goto O., Kawakubo K., Nakai Y., Minatsuki C., Asada-Hirayama I. Endoscopic ultrasound-guided fine-needle aspiration skill acquisition of gastrointestinal submucosal tumor by trainee endoscopists: a pilot study. Endosc. Ultrasound. 2016;5(3):157–164. doi: 10.4103/2303-9027.183970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polkowski M., Bergman J.J. Endoscopic ultrasonography-guided biopsy for submucosal tumors: needless needling? Endoscopy. 2010;42(4):324–326. doi: 10.1055/s-0029-1244070. [DOI] [PubMed] [Google Scholar]
- 8.Wang L., Zhu F., Shi H., Lou S., Shen W. Diagnosis of a granular cell tumour at the abdominal wall using fine needle aspiration cytology and histology: case report. J. Int. Med. Res. 2015;43(4):592–596. doi: 10.1177/0300060515583079. [DOI] [PubMed] [Google Scholar]
- 9.Lara M. Castillo, Herrera A. Martinez, Cardoso R. Torrejon, Lopez D.M. Lubian. Granular cell tumor in breast: a case report. Breast Cancer. 2017;9:245–248. doi: 10.2147/BCTT.S131446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panunzi A., D'Orazi V., Toni F., Coppola G.A., D'Alessandro V., Pontone S. Unexpected granular cell tumor in abdominal wall: case report and literature review. Tumori. 2012;98(1):e18–21. doi: 10.1177/030089161209800132. [DOI] [PubMed] [Google Scholar]
- 11.Kang W.M., Yu JC Ma ZQ, Zhao Z.R., Meng Q.B., Ye X. Laparoscopic-endoscopic cooperative surgery for gastric submucosal tumors. World J. Gastroenterol. 2013;19(34):5720–5726. doi: 10.3748/wjg.v19.i34.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agha R.A., Fowler A.J., Saeta A., Barai I., Rajmohan S., Orgill D.P. The SCARE statement: consensus-based surgical case report guidelines. Int. J. Surg. 2016;34:180–186. doi: 10.1016/j.ijsu.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 13.Morgan B.K., Compton C., Talbert M., Gallagher W.J., Wood W.C. Benign smooth muscle tumors of the gastrointestinal tract. A 24-year experience. Ann. Surg. 1990;211(1):63–66. doi: 10.1097/00000658-199001000-00011. PMID:2294846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toi P., Siddaraju N., Basu D. Fine-needle aspiration cytology of granular cell tumor: a report of two cases. J. Cytol. 2013;30(3):195–197. doi: 10.4103/0970-9371.117641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Z., Mira J.L., Vu H. Diagnosis of malignant granular cell tumor by fine needle aspiration cytology. Acta Cytol. 2001;45(6):1011–1021. doi: 10.1159/000328347. [DOI] [PubMed] [Google Scholar]
- 16.Behzatoglu K., Bahadir B. Malignant granular cell tumor with unusual histological features. Pathol. Int. 2007;57(2):115–119. doi: 10.1111/j.1440-1827.2006.02066.x. [DOI] [PubMed] [Google Scholar]
- 17.Blacksin M.F., White L.M., Hameed M., Kandel R., Patterson F.R., Benevenia J. Granular cell tumor of the extremity: magnetic resonance imaging characteristics with pathologic correlation. Skeletal Radiol. 2005;34(10):625–631. doi: 10.1007/s00256-005-0925-8. [DOI] [PubMed] [Google Scholar]
- 18.Winant A.J., Gollub M.J., Shia J., Antonescu C., Bains M.S., Levine M.S. Imaging and clinicopathologic features of esophageal gastrointestinal stromal tumors. AJR Am. J. Roentgenol. 2014;203(2):306–314. doi: 10.2214/AJR.13.11841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aoba T., Kato T., Hiramatsu K., Shibata Y., Yoshihara M., Yamaguchi N. A case of gastric glomus tumor resection using laparoscopy endoscopy cooperative surgery (LECS) Int. J. Surg. Case Rep. 2017;42:204–207. doi: 10.1016/j.ijscr.2017.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bosman Fred T., Ralph F.C., Hruban H., Neil D. 4th edition. International Agency for Research on Cancer Lyon; 2010. Theise. WHO Classification of Tumours of the Digestive System. [Google Scholar]