Highlights
-
•
Chronic subdural hematoma (CSDH) can be treated by a relatively simple burr hole surgery. Acute subarachnoid hemorrhage (SAH) occurring after surgery for CSDH has been reported as a rare but severe complication. So, we reported this case.
-
•
Acute SAH occurring after surgery for CSDH is a rare but severe complication associated with the postoperative drainage speed. Rapid variation of cerebral blood flow and perioperative parenchymal shift occur due to the speed of the drainage system. Thus, the height of the tube should not be too low, thereby avoiding“too rapid or too excessive postoperative drainage.”
-
•
Slow decompression with closed-system drainage is recommended to avoid rapid dynamic intracranial changes during drainage of a subdural hematoma, including brain shift or restoration of normal perfusion, to prevent devastating complications.
Keywords: Chronic subdural hematoma, Drainage, Subarachnoid hemorrhage
Abstract
Introduction
Chronic subdural hematoma(CSDH) can be treated by a relatively simple burr hole surgery. Acute subarachnoid hemorrhage (SAH) occurring after surgery for CSDH has been reported as a rare but severe complication.
Case report
An 88-year-old female complained of progressive headache and dizziness for one month. A right fronto-temporo-parietal CSDH with a shift in the midline structures and lateral ventricle compression was shown by computed tomography (CT) scans. Closed-system drainage of the hematoma was performed via one burr hole under general anesthesia. Two hours after we began draining the hematoma at the patient’s bedside, the patient complained of headache and exhibited impaired consciousness that progressively degenerated. The drainage bag collected 200 ml of bloody liquid overa short time. A subsequent CT scan revealed SAH and an acute subdural hematoma. A CT angiogram excluded the presence of intracranial aneurysms. The patient died of hypostatic pneumonia after 15 days despite conservative medical management.
Discussion
Relevant literature was reviewed, and we believe that the occurrence of a hematoma in the opposite hemisphere and the hyperperfusion resulted from the rapid drainage of the hematoma, which caused the rupture of weak bridging veins during drainage.
Conclusion
Slow decompression with closed-system drainage is recommended to avoid rapid dynamic intracranial changes during drainage of a subdural hematoma, including brain shift or restoration of normal perfusion,to prevent devastating complications.
1. Introduction
A chronic subdural hematoma (CSDH) can be treated by a relatively simple burr hole surgery, which exhibits a mortality rate of 0.5–4.0%. Elderly individuals and alcoholics are commonly affected by CSDH [[1], [2]]. A CSDH might be found incidentally on brain imaging. Surgical intervention is considered when the mass effect on the surrounding brain tissue results in symptomology. Relatively simple, safe, and effective procedures have been used to manage this condition [3]. Treatment for CSDH is often successful. A burrhole with closed drainage is a simple and effective method that most surgeons prefer. However, some rare but severe complications, such as intracerebral hematoma and intracranial extracerebral hemorrhage, have been reported [[4], [5]]. Here, we report a case of a subarachnoid hemorrhage (SAH) and CSDH occurring immediately after we began draining a CSDH following a burr hole operation. The possible mechanisms of this rare complication are discussed, and the relevant literature is also reviewed. Ourreport is in accordance with the SCARE criteria [6].
2. Case report
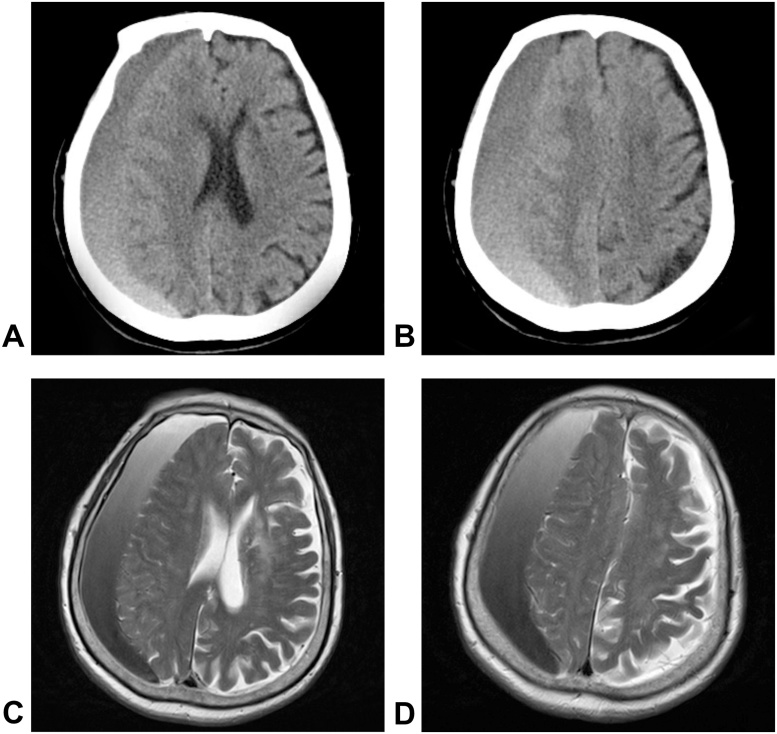
An 88-year-old female was admitted to our hospital with a one-month history of progressive headache and dizziness. She also complained of a walking disability. A neurological examination revealed gait difficulties, and the power in the left lower limb was 3/5 based onthe Medical Research Council grading system. The routine laboratory test results were within normal limits. Routine tests such as chest computed tomography (CT), electrocardiography, and routine blood tests showednormal findings. CT scans and MRI showed a right fronto-temporo-parietal CSDH with a shift of the midline structures and lateral ventricle compression (Fig. 1). Closed-system drainage of the hematoma was performed via one burr hole under general anesthesia. The burr hole was located at the parietal protuberance. After incision of the dura mater, spontaneous outflow and aspiration of the hematoma produced a volume of approximately 220 ml. The subdural compartment was irrigated with Ringer’s solution, and a subdural drainage system was carefully placed.
Fig. 1.
Preoperative images, CT scans and MRI showed a CSDH in the right hemisphere with a shift of the midline structures and lateral ventricle compression. A–B: brain CT images; C–D: brain T2-weighted MRI.
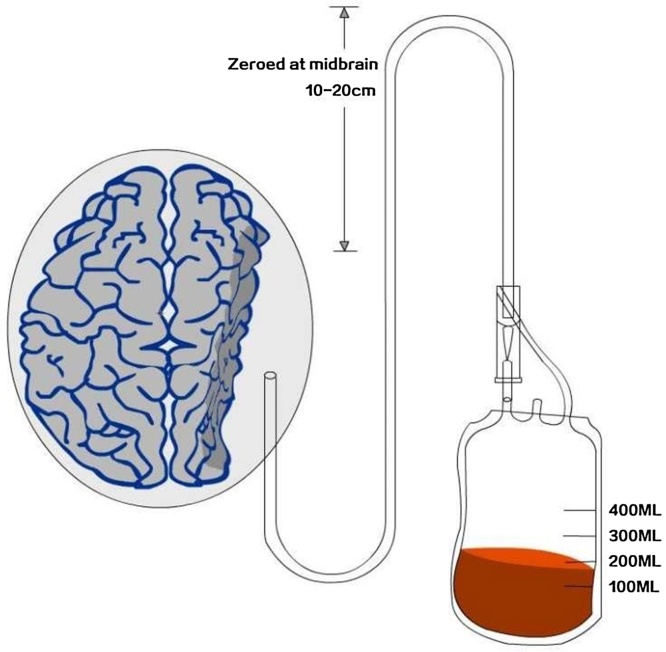
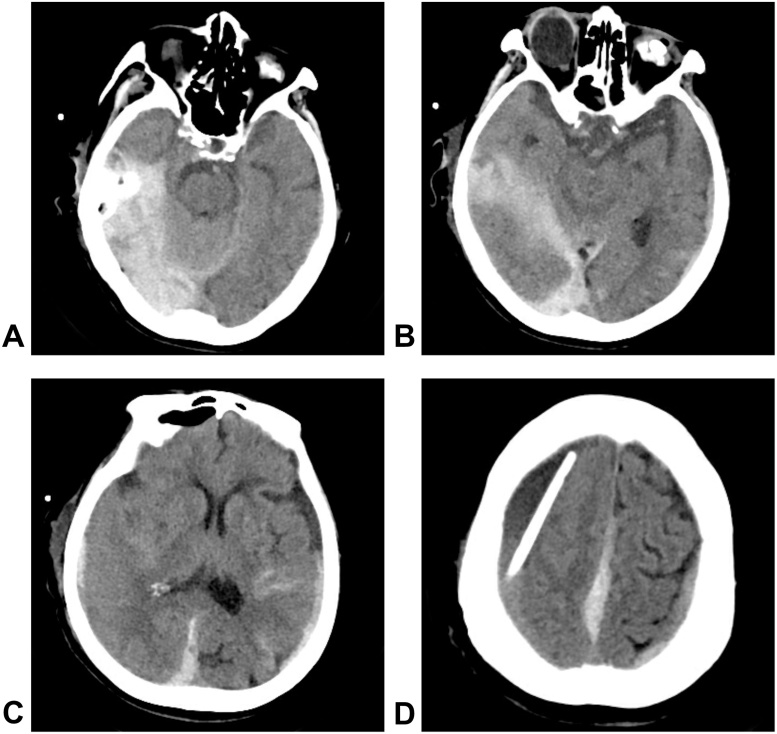
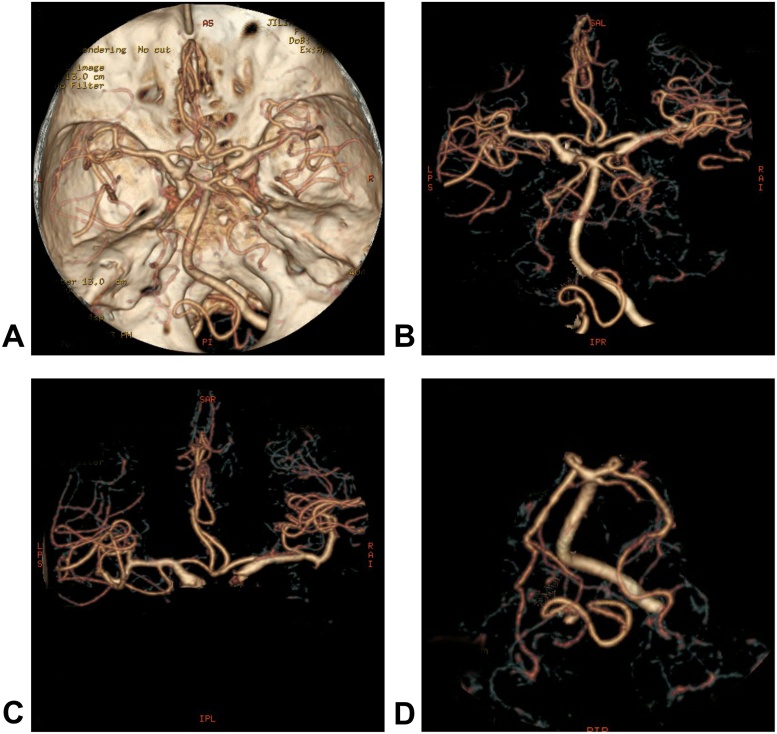
The operation itself was performed uneventfully, and the patient’s blood pressure was well-controlled. The drainage system was initiated when she returned to the ICU ward, and the highest point of the drainage system was 15 cm, relative to the midbrain (Fig. 2). However, 2 h after we began draining the hematoma at the patient’s bedside, the patient complained of a headache and exhibited consciousness functionsthat progressively degenerated. The drainage bag collected 200 ml of bloody liquid. A subsequent CT scan revealed an SAH focused on the tentorium cerebella, a parasagittal acute subdural hematoma and a contralateral acute subdural hematoma. The right lateral ventricle compression was markedly reduced, and the midline structure had generally returned to the correct position compared to the preoperative image (Fig. 3). A postoperative CT angiogram excluded the presence of intracranial aneurysms (Fig. 4). Conservative treatments such as pharmacological sedation, hyperventilation, blood component transfusion, and administration of hypertonic saline, osmotic diuretics, human serum albumin, and systemic antibiotics were administered. A tracheotomy was performed at two days postoperative,but the patient died of hypostatic pneumonia after 14 days despite intensive medical management.
Fig. 2.
Closed-system drainage for CSDH after a burrhole craniostomy.
Fig. 3.
Postoperative CT images, A–C: CT scans revealed an SAH focused on the tentorium cerebella, a parasagittal acute subdural hematoma and a contralateral acute subdural hematoma. The right lateral ventricle compression was markedly reduced, and the midline structure shad generally returned to their original positions. D: The drainage catheter can be seen in the hematoma cavity.
Fig. 4.
Postoperative CT angiography images, A–D: A CT angiogram showed normal arteries and excluded the presence of intracranial aneurysms.
3. Discussion
CSDH is one of the most common neurosurgical pathologies. Complications during the postoperative period, including failure of the brain to re-expand, recurrence of hematoma and tension pneumocephalus, are associated with a poor prognosis [1]. Postoperative hemorrhagic complications, such as intracerebral hematoma, acute subdural hematoma or SAH, are rare but severe. Acutely deteriorated symptoms have been found in CSDH patients within several hours after the initial burrhole craniostomy, and the subsequent CT scans of the brain confirmed diagnoses of various intracranial hemorrhages. In previous reports, postoperative bleeding almost always occurred in a single spot [7]. However, in our case, bleeding occurred in multiple spots, including an acute subdural hematoma and SAH. The location of the hemorrhagesincluded the tentorium cerebella, a parasagittal acute subdural hematoma and a contralateral hematoma. The cause and pathophysiological mechanism of intracranial acute hemorrhage remains unclear, and the mechanism of this occurrence is still a matter of conjecture. Research has revealed that “too rapid or too excessive postoperative drainage” can be the primary and onset cause [8].The height of the tube is the main factor that determines the speed of postoperative drainage. If the tube is placed too low, the drainage speed will be increased and may cause a sharp changeleading toserious pathological events such asincreased intracranial pressure, a shift of midline structures, decreased cerebral blood flow and other related changes [9]. If the drainage system is placed too high, it does not function properly.
Increasing evidenceindicatesthat a sudden variation in cerebral blood flow is a consequence of cerebral decompression after CSDH drainage [[5], [10], [11], [12]]. Ogasawara et al. showed in their single positron emission computed tomogram (SPECT) studies that rapid decompression of CSDH frequently results in transient hyperemia in the cerebral cortex beneath the hematoma postoperatively [13]. Brodersen et al. reported that cerebral blood flow decreases before surgery and then increases after surgery, as shown by intra-arterial 133-Xenon cerebral blood flow studies of CSDH patients [14]. Other Xenon-enhanced CT studies have demonstrated that cerebral blood flow is decreased in CSDH patients, particularly in the ipsilateral frontal region, Rolandic cortical area, putamen and thalamus [[15], [16], [17]]. Koike et al. showed experimentally that leaky bleeding can arise in intraparenchymal brain tissue due to rapid hemodynamic changes. Thus, if cerebral blood flow or cerebral autoregulation is impaired due to long-term compression by a CDSH, and a decrease in intracranial pressure occurs due to drainage of a hematoma, hyperperfusion can arise [18]. A sudden increase in intracranial blood flow, combined with defective vascular autoregulation, caused the intracranial hemorrhage after surgical correction of CSDH. Tatter et al. reported two cases of SAH that might have been caused by rupture of a perforating artery during the procedure used to drain a hematoma [19].
Marcus et al. reported that the less invasive surgical technique of bedside percutaneous subdural tapping and spontaneous hematoma efflux after twist drill craniostomy under local anesthesia produced satisfactory results. In their study, decompression-related intracerebral hemorrhage and acute subdural hematoma were extremely rare complications in patients who underwent percutaneous twist drill craniostomy without drainage [20]. In our case, the patient had a one-month history of progressive exacerbation of symptoms, after which we aspirated 200 ml of hematoma during the surgical procedure, and the drainage bag collected 200 ml of bloody liquid within two hours postoperatively. Thus, we believe that the cerebral blood flow changed dramatically in a short time. It is reasonable to assume that hyperperfusion occurred immediately after the drainage procedure, and the sudden variation in cerebral blood flow, as a consequence of cerebral decompression,caused weaker perforating arteries to rupture and bleed.
Some researchers believe that the factor common to all causes of remote hemorrhage during and after neurosurgical procedures is related to brain deformations, the so-called brain shift, which invariably occurs during all open craniotomy procedures [21]. Kelly et al. were the first group to report brain shift during volumetric stereotaxy when they observed dislocation of small steel balls placed along the surgeon’s line of view [22]. Brain shift produces stretching and transient occlusion of the cortico-dural bridging veins that drain into the peripheral dural sinus. Consequently, venous infarcts occur in the venous drainage territories, and hemorrhagic transformation results when perfusion is re-established within ischemic tissue [21].
Rapid perioperative parenchymal shift could dramatically worsen cerebral venous drainage. A sudden copious drainage of hematoma fluid could lead to perioperative brain shift, which can tear the contralateral or other bridging veins and subsequently result in intracranial hemorrhage [[23], [24]]. The theory of rapid perioperative parenchymal shift causing direct vascular damage fits well with the phenomenon of multiple intracranial hemorrhages in our patient who had a previous unilateral subdural hematoma with a shift of the midline structures. In this case, as revealed in the CT and MR images, the displacement of the brain hemisphere, especially the midline structures, changed markedly after surgery.
Other individual variable factors, such as anti-coagulant and anti-platelet therapy, cerebral amyloid angiopathy, diffuse cerebral atrophy, seizures, failure of cerebral vascular self-regulation and fragile cerebral vessels due to aging, may be important potential risk factors related to acute hemorrhage after a CSDH operation [[25], [26], [27]]. Our patient was 88 years old and had brain atrophy.After the drainage, the failure of cerebral vascular self-regulation and traction of fragile cerebral vessels could have resulted in hemorrhage. Although aggressive non-surgical treatment was administered, the patient died due to hypostatic pneumonia.
Intracranial hemorrhage remote from the site of surgery is an uncommon and poorly understood complication following neurosurgical procedures. The occurrence of postoperative hemorrhage due to chronic subdural hematoma depends mainly on the speed of drainage.Drainage can lead to a rapid shift in intracranial pressure, and brain tissue can decline rapidly, resulting in changes in cerebral blood flow and venous bridge tears, which can cause various types of intracranial hemorrhage. Sudden decompression should be avoided during and after surgery for patients with CSDH who show imaging evidence of severe brain compression due to massive hematoma. The occurrence of hematoma in the opposite hemisphere and the hyperperfusion resulted from the drainage of the hematoma, which caused the rupture of weak cerebral blood vessels. Therefore, slow decompression with controlled re-expansion and closed-system drainage should be used to avoid rapid, dynamic intracranial changes, including brain shift or restoration of normal perfusion during decompression of the brain, and to prevent devastating complications. The drainage speed is determined by the height of the drainage system. Therefore, the highest point of the drainage system should be decreased slowly. This may lead to a slow recovery of cerebral vascular regulation and a slowing of brain shift. We stress that the speed of drainage should be controlled through the height setting during drainage for patients after a CSDH burrhole operation. The patient should be carefully monitored, and postoperative CT scans should be acquired early to detect possible complications.
4. Conclusion
Acute SAH occurring after surgery for CSDH is a rare but severe complication associated with the postoperative drainage speed. Rapid variation of cerebral blood flow and perioperative parenchymal shift occur due tothe speed of the drainage system. Thus, the height of the tube should not be too low, thereby avoiding“too rapid or too excessive postoperative drainage.” The height of the drainage tube after the burr hole procedure should be decreased slowly during drainage to control the drainage velocity and to avoid acute bleeding after the operation and during the drainage period.
Conflict of interest
There is no conflict of interest to be declared
Funding
No source of funding
Ethical approval
Being a Case Report, with written consent our institution doesn’t require formal Ethical Approval. Consent written informed consent was obtained from the patient’s daughter for publication of this case report
Consent
Written informed consent was obtained from the patient’s daughter for publication of this case report and accompanying images. All authors assurance that alterations do not distort scientific meaning.
Author contribution
Guangming Wang: development of the idea and writing of the article
Jinlu Yu: data collection and writing of the final draft
Guarantor
Jinlu Yu
Contributor Information
Guangming Wang, Email: gmwang@jlu.edu.cn.
Jinlu Yu, Email: jlyu@jlu.edu.cn.
References
- 1.Patibandla M.R., Thotakura A.K., Shukla D., Purohit A.K., Addagada G.C., Nukavarapu M. Postoperative hematoma involving brainstem, peduncles, cerebellum, deep subcortical white matter, cerebral hemispheres following chronic subdural hematoma evacuation. Asian J. Neurosurg. 2017;12:259–262. doi: 10.4103/1793-5482.144163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sambasivan M. An overview of chronic subdural hematoma: experience with 2300 cases. Surg. Neurol. 1997;47:418–422. doi: 10.1016/s0090-3019(97)00188-2. [DOI] [PubMed] [Google Scholar]
- 3.Almenawer S.A., Farrokhyar F., Hong C., Alhazzani W., Manoranjan B., Yarascavitch B., Arjmand P., Baronia B., Reddy K., Murty N., Singh S. Chronic subdural hematoma management: a systematic review and meta-analysis of 34,829 patients. Ann. Surg. 2014;259:449–457. doi: 10.1097/SLA.0000000000000255. [DOI] [PubMed] [Google Scholar]
- 4.Shibahashi K., Hoda H., Takasu Y. Contralateral subdural hematoma development following unilateral acute subdural hematoma evacuation. Br. J. Neurosurg. 2016:1–5. doi: 10.1080/02688697.2016.1211251. [DOI] [PubMed] [Google Scholar]
- 5.Rohde V., Graf G., Hassler W. Complications of burr-hole craniostomy and closed-system drainage for chronic subdural hematomas: a retrospective analysis of 376 patients. Neurosurg. Rev. 2002;25:89–94. doi: 10.1007/s101430100182. [DOI] [PubMed] [Google Scholar]
- 6.Agha R.A., Fowler A.J., Saetta A., Barai I., Rajmohan S., Orgill D.P., For the SCARE group The SCARE statement: consensus-based surgical case report guidelines. Int. J. Surg. 2016;27:187–189. doi: 10.1016/j.ijsu.2016.01.094. [DOI] [PubMed] [Google Scholar]
- 7.Corrivetti F., Moschettoni L., Lunardi P. Isolated oculomotor nerve palsy as presenting symptom of bilateral chronic subdural hematomas: two consecutive case report and review of the literature. World Neurosurg. 2016;88(686) doi: 10.1016/j.wneu.2015.11.012. e689–612. [DOI] [PubMed] [Google Scholar]
- 8.Harada K., Ohtsuru K., Nakayama K., Takagi S., Shigemori M., Tokunaga T., Sugita Y., Torigoe R. Contralateral development of acute subdural hematoma following surgery for chronic subdural hematoma–case report. Neurol. Med. Chir. 1992;32:969–971. doi: 10.2176/nmc.32.969. [DOI] [PubMed] [Google Scholar]
- 9.Xu C., Chen S., Yuan L., Jing Y. Burr-hole irrigation with closed-system drainage for the treatment of chronic subdural hematoma: a meta-analysis. Neurol. Med. Chir. 2016;56:62–68. doi: 10.2176/nmc.ra.2015-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gelabert-Gonzalez M., Garcia-Allutn A., Fernandez-Villa J.M. Intracerebral hemorrhage following chronic subdural hematoma evacuation: report of two cases. Neurocirugia. 2003;14:537–538. doi: 10.1016/s1130-1473(03)70515-6. [DOI] [PubMed] [Google Scholar]
- 11.Missori P., Salvati M., Polli F.M., Conserva V., Delfini R. Intraparenchymal haemorrhage after evacuation of chronic subdural haematoma. Report of three cases and review of the literature. Br. J. Neurosurg. 2002;16:63–66. doi: 10.1080/026886902753512637. [DOI] [PubMed] [Google Scholar]
- 12.Moussaoui A., Amor M., Kabbaj S., Maazouzi W. Spontaneous intracerebral haemorrhage following evacuation of chronic subdural haematoma. Ann. Fr. Anesth. Reanim. 2006;25:468–469. doi: 10.1016/j.annfar.2005.11.007. in French. [DOI] [PubMed] [Google Scholar]
- 13.Ogasawara K., Koshu K., Yoshimoto T., Ogawa A. Transient hyperemia immediately after rapid decompression of chronic subdural hematoma. Neurosurgery. 1999;45:484–489. doi: 10.1097/00006123-199909000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Brodersen P., Gjerris F. Regional cerebral blood flow in patients with chronic subdural hematomas. Acta Neurol. Scand. 1975;51:233–239. doi: 10.1111/j.1600-0404.1975.tb07604.x. [DOI] [PubMed] [Google Scholar]
- 15.Ikeda K., Ito H., Yamashita J. Relation of regional cerebral blood flow to hemiparesis in chronic subdural hematoma. Surg Neurol. 1990;33:87–95. doi: 10.1016/0090-3019(90)90017-j. [DOI] [PubMed] [Google Scholar]
- 16.Inao S., Kawai T., Kabeya R., Sugimoto T., Yamamoto M., Hata N., Isobe T., Yoshida J. Relation between brain displacement and local cerebral blood flow in patients with chronic subdural haematoma. J. Neurol. Neurosurg. Psychiatry. 2001;71:741–746. doi: 10.1136/jnnp.71.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanaka A., Yoshinaga S., Kimura M. Xenon-enhanced computed tomographic measurement of cerebral blood flow in patients with chronic subdural hematomas. Neurosurgery. 1990;27:554–561. doi: 10.1097/00006123-199010000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Koike J. Permeability change of cerebral vessels in acute hypertension and external decompression. Neurol. Med. Chir. 1983;23:325–335. doi: 10.2176/nmc.23.325. in Japanese. [DOI] [PubMed] [Google Scholar]
- 19.Tatter S.B., Buonanno F.S., Ogilvy C.S. Acute lacunar stroke in association with angiogram-negative subarachnoid hemorrhage. Mechanistic implications of two cases. Stroke. 1995;26:891–895. doi: 10.1161/01.str.26.5.891. [DOI] [PubMed] [Google Scholar]
- 20.Reinges M.H., Hasselberg I., Rohde V., Kuker W., Gilsbach J.M. Prospective analysis of bedside percutaneous subdural tapping for the treatment of chronic subdural haematoma in adults. J. Neurol. Neurosurg. Psychiatry. 2000;69:40–47. doi: 10.1136/jnnp.69.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morandi X., Haegelen C., Henaux P.L., Riffaud L. Brain shift is central to the pathogenesis of intracerebral haemorrhage remote from the site of the initial neurosurgical procedure. Med. Hypoth. 2006;67:856–859. doi: 10.1016/j.mehy.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 22.Kelly P.J., Kall B.A., Goerss S., Earnest F., IV Computer-assisted stereotaxic laser resection of intra-axial brain neoplasms. J. Neurosurg. 1986;64:427–439. doi: 10.3171/jns.1986.64.3.0427. [DOI] [PubMed] [Google Scholar]
- 23.Sun H.L., Chang C.J., Hsieh C.T. Contralateral acute subdural hematoma occurring after evacuation of subdural hematoma with coexistent contralateral subdural hygroma. Neurosciences. 2014;19:229–232. [PMC free article] [PubMed] [Google Scholar]
- 24.Moon K.S., Lee J.K., Kim T.S., Jung S., Kim J.H., Kim S.H., Kang S.S. Contralateral acute subdural hematoma occurring after removal of calcified chronic subdural hematoma. J. Clin. Neurosci. 2007;14:283–286. doi: 10.1016/j.jocn.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 25.Miyazaki T., Matsumoto Y., Ohta F., Daisu M., Moritake K. A case of unknown origin subarachnoid hemorrhage immediately following drainage for chronic subdural hematoma. Kurume Med. J. 2004;51:163–167. doi: 10.2739/kurumemedj.51.163. [DOI] [PubMed] [Google Scholar]
- 26.Sato M., Nakano M., Asari J., Watanabe K. Intracerebral haemorrhage during surgery for chronic subdural haematoma. J. Clin. Neurosci. 2007;14:81–83. doi: 10.1016/j.jocn.2005.12.044. [DOI] [PubMed] [Google Scholar]
- 27.Grunwald F., Menzel C., Pavics L., Bauer J., Hufnagel A., Reichmann K., Sakowski R., Elger C.E., Biersack H.J. Ictal and interictal brain SPECT imaging in epilepsy using technetium-99m-ECD. J. Nucl. Med. 1994;35:1896–1901. [PubMed] [Google Scholar]