Abstract
Multi‐targeted agents represent the next generation of targeted therapies for solid tumors, and patients with acquired resistance to EGFR‐tyrosine kinase inhibitors (TKIs) may also benefit from their combination with TKI therapy. Third‐generation targeted drugs, such as osimertinib, are very expensive, thus a more economical solution is required. The aim of this study was to explore the use of apatinib combined with icotinib therapy for primary acquired resistance to icotinib in three patients with advanced pulmonary adenocarcinoma with EGFR mutations. We achieved favorable oncologic outcomes in all three patients, with progression‐free survival of four to six months. Unfortunately, the patients ultimately had to cease combination therapy because of intolerable adverse effects of hand and foot syndrome and oral ulcers. Combination therapy of apatinib with icotinib for primary acquired resistance to icotinib may be an option for patients with advanced pulmonary adenocarcinoma with EGFR mutations, but physicians must also be aware of the side effects caused by such therapy.
Keywords: Acquired resistance, advanced pulmonary adenocarcinoma, apatinib, icotinib, molecular targeted therapy
Introduction
Lung cancer is one of the most common types of cancer and the leading cause of human cancer death worldwide.1 Non‐small cell lung cancer (NSCLC) accounts for 85% of all lung cancer cases. Treatment strategies for NSCLC have evolved to emphasize molecular targeted therapy based on the genomic classification of patients.2 The increase in lung adenocarcinoma incidence, reported at 1.3% annually in Chinese men between 1990 and 2010, is a significant threat to life.3 Lung adenocarcinoma shows a significantly higher rate of gene mutation than squamous cell carcinoma, especially in non‐smoking patients with lung adenocarcinoma.4, 5 Therefore, for patients with lung adenocarcinoma with EGFR mutations, treatment with EGFR‐tyrosine kinase inhibitors (TKIs) plays an increasingly important role. However, tumors that initially respond to EGFR‐TKIs and anaplastic lymphoma kinase inhibitors eventually acquire resistance, which limits the therapeutic success of these targeted agents,6 and third‐generation targeted drugs such as osimertinib are very expensive. It is well known that tumor angiogenesis is one of the hallmarks of cancer and is an essential step in tumor growth and metastasis.7, 8 Apatinib, a small‐molecule (VEGFR‐2) TKI, is presently undergoing phase II/III clinical trials in China for the treatment of many cancer types, such as NSCLC, breast cancer, and hepatocellular carcinoma.9 Multiple trials and clinical studies have shown the potential advantages of combination therapy of EGFR and VEGF inhibitors in patients with advanced NSCLC and acquired resistance to targeted therapies.10, 11, 12 In addition, NSCLC patients with acquired resistance to EGFR‐TKIs are reported to benefit from the combination with TKI therapy by achieving months to years of disease control.13 To our knowledge, however, there are no reports of combination therapy of VEGFR‐2 with EGFR‐TKIs for primary acquired resistance to EGFR‐TKIs in patients with advanced lung adenocarcinoma with EGFR mutations. Herein, we report cases of three such patients with EGFR mutations at exon 19(+) or exons 19 and 21(+), where a partial response was achieved in one patient and stable disease in two. Therapeutic evaluation was made according to Response Evaluation Criteria in Solid Tumors version 1.1.14
Case presentation
Patient I, a 62‐year‐old male smoker, complained of repeated coughing with sputum for three years and bloody sputum for two weeks. Chest enhanced computed tomography (CT) revealed a space‐occupying lesion in the right upper lung apex and double hilum of the pulmonary lymph nodes. He underwent bronchofiberscopy at a local hospital, and the pathology report revealed adenocarcinoma. Radical video‐assisted thoracic surgery of right upper lung cancer was performed under general anesthesia on 17 November 2014. The pathology report at this time revealed the lesion to be a medium to poorly differentiated pulmonary adenocarcinoma, and EGFR examination of paraffin‐embedded slices detected an exon 19 deletion (Table 1). Four months later, whole‐body positron emission tomography‐CT (PET‐CT) showed increased metabolism in the upper right lung mass, and a slowly rising carcinoembryonic antigen (CEA) serum level. Therefore, 125 mg icotinib was administered three times a day (t.i.d.) for two months (April to June 2015) as first‐line therapy. The therapeutic evaluation indicated stable disease; however, the blood CEA level continued to increase. Icotinib combined with radiotherapy 200 cGy × 30 f and icotinib combined with pemetrexed + cisplatinum was administered for six chemotherapy cycles (June 2015 to August 2016). Chest CT then revealed multiple mediastinal lymph nodes, and his serum CEA level was 247.3 ng/mL (Fig 1), indicating progressive disease. Hence, on 7 November 2016 we began oral administration of apatinib 250 mg/d combined with icotinib 125 mg/t.i.d. Chest CT performed in January 2017 indicated stable disease (Fig 1). At this writing, the patient has achieved progression‐free survival (PFS) of six months, but intolerable grade 2–3 foot syndrome (Fig 2) and oral ulcers have emerged.
Table 1.
Baseline characteristics of patients
| Characteristics | Case 1 | Case 2 | Case 3 |
|---|---|---|---|
| Age | 62 | 66 | 61 |
| Gender | Male | Female | Male |
| Smoking | Yes | No | Yes |
| Pathologic type | Medium‐poorly differentiated adenocarcinoma | Adenocarcinoma | Poorly‐differentiated adenocarcinoma |
| Gene type | |||
| EGFR | 19(+) | 19Del(+) | 19Del (+) and 21 L858R (+) |
| ALK | Negative | Negative | Negative |
| ROS‐1 | Negative | Negative | Negative |
| Metastases | Multiple lung and mediastinal node | Multiple lung and mediastinal node and bone | Multiple lung and clavicle node and bone |
| Stage | IV (cT4N2M0) | IV (cT4N2M1b) | IV (cT4N3M1b) |
| Therapy (response) | |||
| First line | Icotinib (SD) | Icotinib (SD) | Icotinib (SD) |
| Second line | Icotinib and Radiotherapy and Chemotherapy (PD) | Icotinib and Apatinib (PR) | Icotinib and Apatinib (SD) |
| Third line | Icotinib and Apatinib (SD) | ||
| CEA (μg/ml) | |||
| Pre‐apatinib | 247.3 | 113.4 | 332.3 |
| Post‐apatinib | 39.4 | 26.9 | |
| PFS (months) | 6 | 4 | 4 |
| Adverse events | |||
| Hypertension | Grade 1 | Grade 1 | |
| Hand‐foot | Grade 2–3 | Grade 1–2 | Grade 2–3 |
| Diarrhea | Grade 1 | ||
| Fatigue | Grade 1 | Grade 1 | Grade 1 |
| Oral ulcers | Grade 1–2 | Grade 2–3 | Grade 1–2 |
| Anorexia | Grade 1 | Grade 2 | |
CEA, carcinoembryonic antigen; PD, progressive disease; PFS, progression‐free survival; PR, partial response; SD, stable disease.
Figure 1.
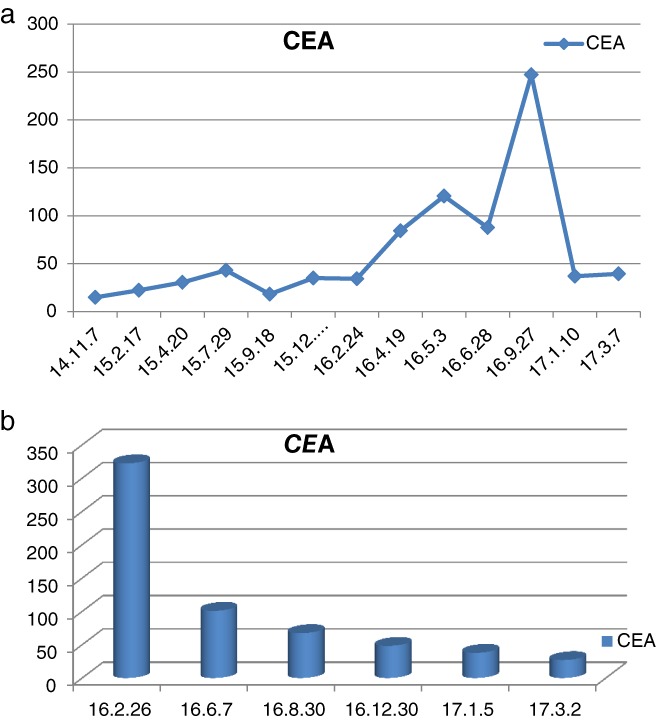
The time course of the carcinoembryonic antigen (CEA) concentrations measured in patients (a) I and (b) III.
Figure 2.
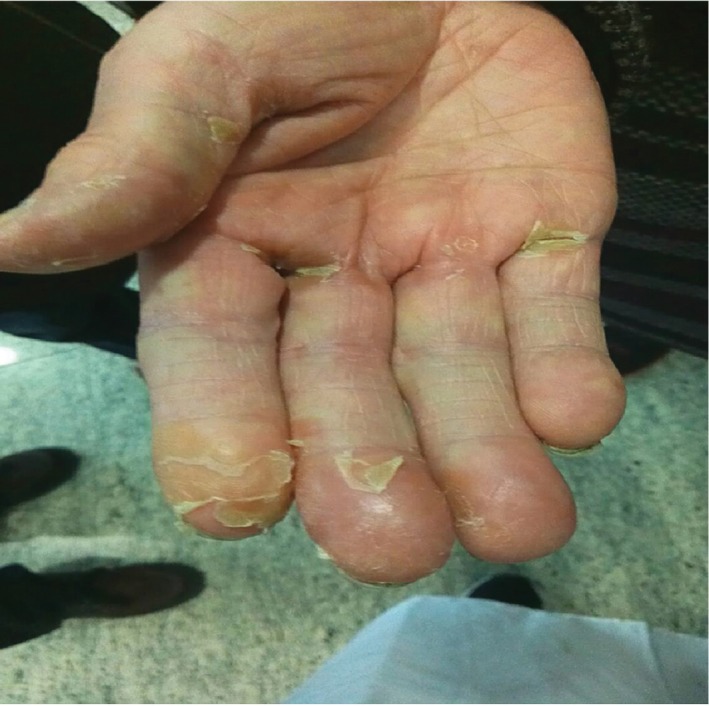
One of three patients developed grade 2–3 hand‐foot syndrome.
Patient II was a 66‐year‐old woman who had never smoked. She underwent CT‐guided percutaneous needle biopsy in November 2015 for a cough with bloody sputum persisting for more than two weeks. The pathology report revealed that the obtained specimen was pulmonary adenocarcinoma, and EGFR examination of paraffin‐embedded slices detected an exon 19 deletion (Table 1). Therefore, icotinib 125 mg t.i.d was administered orally from December 2015. The therapeutic evaluation was stable disease. A whole‐body PET‐CT scan was performed about 10 months later, which showed multiple thoracic bone metastases and the therapeutic evaluation was progressive disease. Therefore, from 1 December 2016 we administered oral doses of apatinib 250 mg/d combined with icotinib 125 mg t.i.d. After more than three months of therapy, chest CT showed a partial response of the lesions (Fig 3). Presently, the patient has achieved PFS of over four months, but intolerable grade 2–3 oral ulcers have developed.
Figure 3.
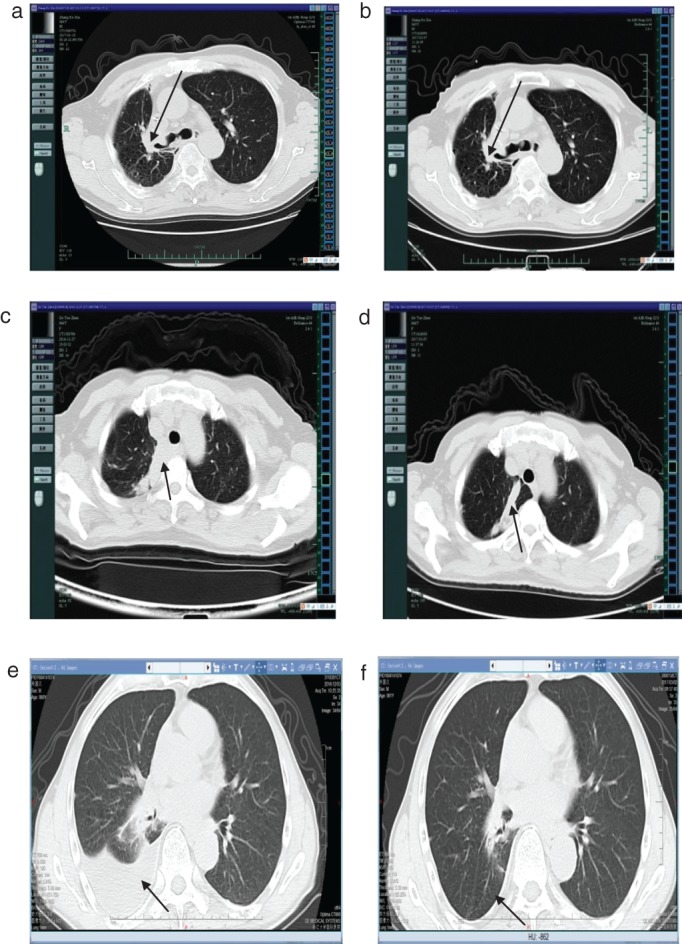
Computed tomographic images from patients I, II, and III show the mass(a,c,e) before apatinib treatment, and (b,d,f) after three months of apatinib therapy, respectively.
Patient III, a 61‐year‐old male heavy smoker complained of a cough with bloody sputum lasting longer than 20 days. A PET‐CT scan showed right lower lung cancer with multiple lung metastases on both sides of the clavicle and mediastinum, along with right hilar lymph node metastasis and multiple bone metastases of stage cT4N3M1b. Bronchofiberscopy was performed at our hospital, and the pathology report showed poorly differentiated cancer. An amplification refractory mutation system EGFR examination of paraffin‐embedded slices detected 19‐Del and 21L858R deletions (Table 1). Icotinib 125 mg/t.i.d was administered as first‐line targeted therapy for seven months (April to November 2016), and the therapeutic evaluation was progressive disease. Chest CT showed malignant pleural effusion and a drastic increase in his serum CEA level (Fig 3). From 8 December 2016, apatinib 250 mg/d combined with icotinib 125 mg t.i.d was administered. A chest CT performed four months later showed rapid control of the malignant pleural effusion and a rapid decrease in his serum CEA level (Figs 1, 3). Hence, the therapeutic evaluation was stable disease. Currently, the patient has achieved PFS of over four months, but as with patient I, intolerable grade 2–3 foot syndrome (Fig 2). and oral ulcers have emerged.
Late in the follow‐up period, it was discovered that suspending the medication for a week could alleviate the side effects. As a result, all patients suspended treatment for a week. However, because of the side effects and economic affordability, apatinib was only used intermittently.
Discussion
Lung cancer has the highest cancer mortality rates worldwide, and incidence increases year by year. NSCLC, usually diagnosed at advanced stage, accounts for > 70% of all lung cancer cases.15 Surgery is an effective means of treatment for early stage lung cancer; however, cancer discovered at advanced stage cannot be treated surgically. Many studies have shown that EGFR‐TKI therapy is effective as first‐line treatment against advanced NSCLC with EGFR mutations. For most advanced NSCLC patients without a genetic driver mutation, platinum‐based doublet chemotherapy is the standard treatment option.16, 17 Compared with traditional first‐line chemotherapy, EGFR‐TKI therapy has achieved statistically significantly longer PFS, a higher objective response rate, numerically longer overall survival, and lower toxicity in patients with advanced NSCLC harboring activated EGFR mutations, breaking the treatment bottleneck experienced with traditional chemotherapeutic drugs.18 Furthermore, published studies have shown that EGFR‐TKIs offer favorable safety, with the vast majority of patients able to tolerate this therapy.19 In the present study of three patients with advanced lung adenocarcinoma and exon 19 or exons 19 and 21 deletions, the lesion was soon controlled after beginning icotinib 125 mg/t.i.d. However, after several months of treatment with this EGFR‐TKI, the tumor cells began to replicate and resist apoptosis, the disease progressed, the lesion size increased, and serum CEA levels underwent a sustained rise, indicating the appearance of acquired resistance,20 and limiting the therapeutic success of these targeted agents. Several randomized, phase III clinical trials of first‐line treatments (including IPASS, WJTOG3405, NEJ002, OPTIMAL, LUXLUNG3, and EURTAC) have revealed that use of EGFR‐TKIs as a first‐line treatment for advanced NSCLC patients with active EGFR gene mutations could achieve PFS of 9.5–13.7 months.21, 22, 23, 24, 25, 26 Moreover, it was reported that NSCLC patients with acquired resistance to EGFR‐TKIs could also benefit from combined therapy with TKIs to achieve months to years of disease control.13
Angiogenesis is a key process for cell growth, especially for tumor growth, development, and metastasis.27 Serum VEGF activates VEGFR‐2, inducing a cascade of different signaling pathways to promote the endothelial cell migration and proliferation necessary for angiogenesis. Studies have revealed that anti‐angiogenesis drugs inhibit the growth of solid tumors, including NSCLC.28 Apatinib (a small molecule drug developed in China in October 2014 that targets the treatment of advanced gastric cancer) strongly inhibits VEGFR‐2 activation, which can suppress endothelial proliferation and ultimately lead to anti‐angiogenesis. Apatinib is confirmed to exert an anti‐tumor effect on various cancers.
Multi‐targeted agents represent the next generation of targeted therapies in solid tumors. Multiple trials and clinical studies have shown the potential advantages of combining EGFR and VEGF inhibitors for patients with advanced NSCLC and acquired resistance to targeted therapies; for example, the median PFS was 16.0 months with erlotinib plus bevacizumab but only 9.7 months with erlotinib alone.10, 11, 29 A report on the safety and pharmacokinetics of apatinib in advanced NSCLC indicated that apatinib could be well tolerated and showed substantial antitumor activity at a dose of 500 or 750 mg once daily.30, 31 However, in accordance with the general condition of our patients and our concern over their intolerance to toxicity, we treated them with targeted therapy comprising daily doses of apatinib at 250 mg/d combined with icotinib at 125 mg t.i.d for 28 days per cycle, with the endpoint of unacceptable toxicity or disease progression. The most frequent adverse effects were hypertension, hand‐foot syndrome, proteinuria, fatigue, anorexia, and elevated aminotransferase level.9 Two of the patients suffered intolerable grade 2–3 foot syndrome and oral ulcers, while the third patient suffered intolerable grade 2–3 oral ulcers.
In summary, the VEGFR‐2 TKI apatinib combined with the first‐generation EGFR‐TKI icotinib provided effective therapeutic action in three patients with advanced pulmonary adenocarcinoma and acquired resistance to icotinib. We treated them with daily doses of apatinib at 250 mg/d combined with icotinib at 125 mg t.i.d, and all three patients achieved long PFS, although they suffered intolerable grade 2–3 foot syndrome and/or oral ulcers. Additional clinical studies are required to clarify the guidelines for clinical treatment with this combination therapy; however, we are convinced that the future for this therapy is promising.
Disclosure
No authors report any conflict of interest.
Acknowledgments
The study was supported by the Key Project of Zhejiang Province Science, Technology Plan12,China (2014C03032), and The National Key Research and Development Program of China (2017YFC0113500).
Contributor Information
Pinghui Xia, Email: 249867563@qq.com.
Jian Hu, Email: dr_hujian@zju.edu.cn.
References
- 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61: 134 (Published erratum appears in) CA Cancer J Clin 2011;61:69–90. [DOI] [PubMed] [Google Scholar]
- 2. Gainor JF, Shaw AT. Novel targets in non‐small cell lung cancer: ROS1 and RET fusions. Oncologist 2013; 18: 865–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cheng I, Le GM, Noone AM et al Lung cancer incidence trends by histology type among Asian American, Native Hawaiian, and Pacific Islander populations in the United States, 1990–2010. Cancer Epidemiol Biomarkers Prev 2014; 23 (11): 2250–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu YL, Zhong WZ, Li LY et al Epidermal growth factor receptor mutations and their correlation with gefitinib therapy in patients with non‐small cell lung cancer: A meta‐analysis based on updated individual patient data from six medical centers in mainland China. J Thorac Oncol 2007; 2: 430–9. [DOI] [PubMed] [Google Scholar]
- 5. Shi Y, Au JS, Thongprasert S et al A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non‐small‐cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol 2014; 9: 154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wieduwilt MJ, Moasser MM. The epidermal growth factor receptor family: Biology driving targeted therapeutics. Cell Mol Life Sci 2008; 65: 1566–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Iman V, Karimian H, Mohan S et al In vitro and in vivo anti‐angiogenic activity of girinimbine isolated from Murraya koenigii . Drug Des Devel Ther 2015; 9: 1281–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fontanella C, Ongaro E, Bolzonello S, Guardascione M, Fasola G, Aprile G. Clinical advances in the development of novel VEGFR2 inhibitors. Ann Transl Med 2014; 2: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang H. Apatinib for molecular targeted therapy in tumor. Drug Des Devel Ther 2015; 9: 6075–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pennell NA, Lynch TJJ. Combined inhibition of the VEGFR and EGFR signaling pathways in the treatment of NSCLC. Oncologist 2009; 14: 399–411. [DOI] [PubMed] [Google Scholar]
- 11. Naumov GN, Nilsson MB, Cascone T et al Combined vascular endothelial growth factor receptor and epidermal growth factor receptor (EGFR) blockade inhibits tumor growth in xenograft models of EGFR inhibitor resistance. Clin Cancer Res 2009; 15: 3484–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tortora G, Ciardiello F, Gasparini G. Combined targeting of EGFR‐dependent and VEGF‐dependent pathways: Rationale, preclinical studies and clinical applications. Nat Clin Pract Oncol 2008; 5: 521–30. [DOI] [PubMed] [Google Scholar]
- 13. Chen Q, Quan Q, Ding L et al Continuation of epidermal growth factor receptor tyrosine kinase inhibitor treatment prolongs disease control in non‐small‐cell lung cancers with acquired resistance to EGFR tyrosine kinase inhibitors. Oncotarget 2015; 6: 24904–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eisenhauer EA, Therasse P, Bogaerts J et al New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–47. [DOI] [PubMed] [Google Scholar]
- 15. Zhi XY, Zou XN, Hu M, Jiang Y, Jia MM, Yang GH. Increased lung cancer mortality rates in the Chinese population from 1973–1975 to 2004–2005: An adverse health effect from exposure to smoking. Cancer 2015; 121 ((Suppl 17)): 3107–12. [DOI] [PubMed] [Google Scholar]
- 16. Xiao HQ, Tian RH, Zhang ZH, Du KQ, Ni YM. Efficacy of pemetrexed plus platinum doublet chemotherapy as first‐line treatment for advanced nonsquamous non‐small‐cell‐lung cancer: A systematic review and meta‐analysis. Onco Targets Ther 2016; 9: 1471–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Manegold C, Schmid‐Bindert G, Pilz LR. Pemetrexed for the treatment of non‐small‐cell lung cancer. Expert Rev Anticancer Ther 2009; 9: 1195–209. [DOI] [PubMed] [Google Scholar]
- 18. Liang JL, Ren XC, Lin Q. Treating advanced non‐small‐cell lung cancer in Chinese patients: Focus on icotinib. Onco Targets Ther 2014; 7: 761–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guan YS, He Q, Li M. Icotinib: Activity and clinical application in Chinese patients with lung cancer. Expert Opin Pharmacother 2014; 15: 717–28. [DOI] [PubMed] [Google Scholar]
- 20. Gao Y, Song P, Li H, Jia H, Zhang B. Elevated serum CEA levels are associated with the explosive progression of lung adenocarcinoma harboring EGFR mutations. BMC Cancer 2017; 17: 484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mok TS, Wu YL, Thongprasert S et al Gefitinib or carboplatin‐paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009; 361: 947–57. [DOI] [PubMed] [Google Scholar]
- 22. Maemondo M, Inoue A, Kobayashi K et al Gefitinib or chemotherapy for non‐small‐cell lung cancer with mutated EGFR. N Engl J Med 2010; 362: 2380–8. [DOI] [PubMed] [Google Scholar]
- 23. Mitsudomi T, Morita S, Yatabe Y et al Gefitinib versus cisplatin plus docetaxel in patients with non‐small‐cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol 2010; 11: 121–8. [DOI] [PubMed] [Google Scholar]
- 24. Zhou C, Wu YL, Chen G et al Erlotinib versus chemotherapy as first‐line treatment for patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (OPTIMAL, CTONG‐0802): A multicentre, open‐label, randomised, phase 3 study. Lancet Oncol 2011; 12: 735–42. [DOI] [PubMed] [Google Scholar]
- 25. Sequist LV, Yang JC, Yamamoto N et al Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013; 31: 3327–34. [DOI] [PubMed] [Google Scholar]
- 26. Rosell R, Carcereny E, Gervais R et al Erlotinib versus standard chemotherapy as first‐line treatment for European patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (EURTAC): A multicentre, open‐label, randomised phase 3 trial. Lancet Oncol 2012; 13: 239–46. [DOI] [PubMed] [Google Scholar]
- 27. Ding L, Li QJ, You KY, Jiang ZM, Yao HR. The use of apatinib in treating nonsmall‐cell lung cancer: Case report and review of literature. Medicine (Baltimore) 2016; 95: e3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lin C, Wang S, Xie W, Zheng R, Gan Y, Chang J. Apatinib inhibits cellular invasion and migration by fusion kinase KIF5B‐RET via suppressing RET/Src signaling pathway. Oncotarget 2016; 7: 59236–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Seto T, Kato T, Nishio M et al Erlotinib alone or with bevacizumab as first‐line therapy in patients with advanced non‐squamous non‐small‐cell lung cancer harbouring EGFR mutations (JO25567): An open‐label, randomised, multicentre, phase 2 study. Lancet Oncol 2014; 15: 1236–44. [DOI] [PubMed] [Google Scholar]
- 30. Fang SC, Zhang HT, Zhang YM, Xie WP. Apatinib as post second‐line therapy in EGFR wild‐type and ALK‐negative advanced lung adenocarcinoma. Onco Targets Ther 2017; 10: 447–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kono T, Hata T, Morita S et al Goshajinkigan oxaliplatin neurotoxicity evaluation (GONE): A phase 2, multicenter, randomized, double‐blind, placebo‐controlled trial of goshajinkigan to prevent oxaliplatin‐induced neuropathy. Cancer Chemother Pharmacol 2013; 72: 1283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]


