Abstract
Background
IL‐37 has been identified as a fundamental inhibitor of inflammatory and immunity responses. It plays a crucial protective role in several cancers, but its anti‐tumor activity and the potential regulatory mechanism of IL‐37 in non‐small cell lung cancer (NSCLC) is largely unclear.
Methods
Enzyme‐linked immunosorbent assay was used to detect plasma IL‐37 expression in NSCLC patients and healthy controls. The NSCLC cell line A549 was cultured with recombinant human IL‐37 or recombinant human IL‐6 protein. A549 invasion and metastasis were detected using Transwell invasion and scratch wound healing assays, respectively. Protein expression of STAT3, pSTAT3, E‐cadherin, vimentin, and N‐cadherin were detected using Western blotting, and messenger RNA expression of STAT3, E‐cadherin, vimentin, and N‐cadherin was assessed in each group using real time PCR.
Results
IL‐37 plasma expression was decreased in NSCLC patients, and the downregulation of IL‐37 was correlated with tumor stage. In vitro, IL‐37 inhibited invasion and migration in A549 cells, while IL‐6 promoted invasion and migration in A549 cells. pSTAT3, vimentin, and N‐cadherin expression was increased. E‐cadherin expression was lower in the IL‐6 group than in the control group; however, the opposite pattern was observed in the IL‐37 + IL‐6 group.
Conclusion
Our results showed that IL‐37 plays an inhibitory role in NSCLC progression, possibly by suppressing STAT3 activation and decreasing epithelial‐to‐mesenchymal transition by inhibiting IL‐6 expression. IL‐37 could serve as a potential novel tumor suppressor in NSCLC.
Keywords: Carcinoma, non‐small‐cell lung cancer, interleukin‐37, IL‐6/STAT3 signaling pathway, epithelial‐to‐mesenchymal transition
Introduction
Lung cancer continues to be the most common cause of cancer‐related death worldwide because of high rates of recurrence and metastasis and the lack of dramatic progress in treatment strategies.1, 2 Non‐small cell lung cancer (NSCLC) accounts for 80–85% of the total number of lung cancer cases and is divided into adenocarcinoma, large‐cell carcinoma, and squamous cell carcinoma.1, 3 Although great progress has been made in early detection, radical surgical resection, and multimodalities of treatment in recent decades, the five‐year overall survival rate of NSCLC is generally < 15%.4, 5 Thus, there is an urgent need to understand invasion and metastatic mechanisms in NSCLC for better individualization of treatment.
Interleukin‐37 (IL‐37), a newly discovered member of the IL‐1 family, was originally defined in 2010 as IL1F7.6 There are five splice variants of IL‐37, IL‐37a–e. Of these, IL‐37b is the most extensively studied IL‐37 isoform because it encodes the longest transcript variant and is widely expressed in a variety of human organs, tissues, and tumor cells.7, 8, 9 IL‐37 has been suggested as a fundamental inhibitor of both innate inflammation and immunity.7, 10, 11 IL‐37 can suppress the expression of multiple pro‐inflammatory factors, including IL‐6, IL‐1α, IL‐1β, and TNF‐α.9, 10 Recently, it has been reported that IL‐37 suppresses the progression of colorectal, renal cell, hepatocellular, and colon cancers, and similar conditions. The underlying mechanisms have been proposed as IL‐6/STAT3 signaling pathway suppression, pSmad3C/P21 tumor‐suppressive signaling, β‐catenin suppression, and CD57+ NK recruitment.12, 13, 14, 15, 16 However, whether IL‐37 also shows anti‐tumor effects in NSCLC remains elusive.
Methods
Patients and peripheral venous blood samples
From July 2016 to September 2017, a total of 40 histologically confirmed NSCLC patients were enrolled in this study. None had undergone any preoperative anticancer treatment prior to sample collection. NSCLC histological typing was based on World Health Organization (WHO) criteria and staging according to Union for International Cancer Control Tumor Node Metastasis (TNM) Classification (7th edition). The clinicopathologic features of NSCLC patients are shown in Table 1. Forty healthy age and gender‐matched individuals served as healthy controls. Fasting peripheral venous blood samples were collected from NSCLC patients and healthy controls. IL‐37 plasma expression in all samples was detected by enzyme‐linked immunosorbent assay (Cloud‐Clone Corp, Houston, TX, USA) in strict accordance with the manufacturer’s instructions. The Research Ethics Committee of the Second Affiliated Hospital of the Medical College of Qingdao University approved this study and written informed consent was obtained from all participants and healthy controls.
Table 1.
Association between plasma IL‐37 expression and clinical characteristics in NSCLC patients (–x ± s, pg/mL)
| Characteristics | N | IL‐37 expression | P |
|---|---|---|---|
| Gender | |||
| Male | 25 | 78.58 ± 27.42 | 0.755 |
| Female | 15 | 81.52 ± 30.48 | |
| Age, year | |||
| ≥ 60 | 18 | 75.78 ± 23.52 | 0.437 |
| < 60 | 22 | 82.87 ± 31.80 | |
| Smoking history | |||
| Yes | 22 | 77.02 ± 26.81 | 0.517 |
| No | 18 | 82.93 ± 30.38 | |
| Pathological type | |||
| Adenocarcinoma | 24 | 77.51 ± 24.83 | 0.767 |
| Squamous carcinoma | 12 | 84.77 ± 37.77 | |
| Large cell carcinoma | 4 | 77.47 ± 16.61 | |
| TNM stage | |||
| I + II | 20 | 89.05 ± 30.49 | 0.034* |
| III + IV | 20 | 70.32 ± 22.91 |
P < 0.05 indicates a significant association between the variables. NSCLC, non‐small cell lung cancer; TNM, tumor node metastasis.
Cell culture and treatments
The human NSCLC A549 cell line was preserved in the biotechnology therapeutic center at our hospital. The A549 cell line was maintained at 37°C in a humidified incubator with an atmosphere of 5% CO2 in Dulbecco’s Modified Eagle Medium: Nutrient Mixture F‐12 (DMEM/F‐12) supplemented with 1% penicillin/streptomycin and 10% fetal bovine serum (Life Technologies, Gaithersburg, MD, USA). Recombinant human IL‐37 (rhIL‐37) and recombinant human IL‐6 (rhIL‐6) protein (R&D Systems, Minneapolis, MN, USA) was added to the medium of A549 cells after culturing.
Transwell invasion assay
The invasive ability of A549 cells was determined using a Transwell system (Corning, New York, NY, USA). Matrigel glue (R&D Systems) was diluted and added to the upper Transwell chambers. Cells were re‐suspended in serum‐free medium and then plated in the upper chambers. Complete medium covered the bottom chambers. After incubation at 37°C for 24 hours, the cells above the membrane were wiped off. The traversed cells that had migrated to the bottom side of the membrane were fixed and stained with 1% crystal violet (Solarbio Life Sciences, Beijing, China) for 3 minutes. Cells that passed through the Matrigel were counted under a microscope at × 200 magnification, and the average number from random visual fields was used.
Scratch wound healing assay
The migration ability of A549 cells was determined by scratch wound healing assay. A549 cells were harvested and 5 × 105 cells were plated in six‐well plates. When the plates yielded cells at about 80% confluence, the monolayer was scraped in a straight line to create a scratch using a 200 μl pipette tip. Debris was then removed using phosphate buffered saline and serum‐free medium was added. Photographs were taken under a microscope at 0 and 24 hours. The migration distance was analyzed using Image J software (NIH, Bethesda, MD, USA). The scratch healing rate was calculated as follows: (scratch width 0 hour ‐ scratch width 24 hours)/scratch width 0 hour × 100%.
Western blotting
Total proteins from cultured cells were lysed with radioimmunoprecipitation assay lysis buffer (Beyotime Biotechnology, Shanghai, China). Protein concentration was assayed using a BCA assay kit (Beyotime Biotechnology). Equal quantities of protein were separated on 10% sodium dodecyl sulfate‐polyacrylamide gel electrophoresis, transferred onto polyvinylidene fluoride membranes (Solarbio Life Sciences), and then reacted with primary antibodies against STAT3, N‐cadherin, p‐STAT3, E‐cadherin, vimentin, and GAPDH (Abcam, Cambridge, UK). Proteins were then washed with tris‐buffered saline plus tween 20 and incubated for 30 minutes with goat anti‐rabbit immunoglobulin G horseradish peroxidase (Beyotime Biotechnology). The bands were visualized using electrochemiluminescent luminous fluid (Solarbio Life Sciences). Photographs were taken using by a Gel Dox XR system (Kodak, Shanghai, China).
RNA extraction and real‐time polymerase chain reaction
Total RNA was extracted from A549 cells using TRIzol reagent (TaKaRa, Dalian, China). Primer sequences are shown in Table 2. Reverse transcription (RT) was performed using an RT Kit (TaKaRa) according to the manufacturer’s instructions and the GeneAmp PCR System 9700 (ABI, Oyster Bay, NY, USA). Real‐time PCR amplification was performed using a Platinum SYBRGreen PCR Kit (Invitrogen, Carlsbad, CA, USA). Quantitative PCR (qPCR) was prepared using the 7500 fast Real‐time PCR system (Applied Biosystems, Foster City, CA, USA). PCR products were verified by melting curve analysis. The relative messenger RNA (mRNA) levels of target genes were calculated using the 2−ΔΔCt method.
Table 2.
Real‐time PCR primer sequences
| Name | Sequences |
|---|---|
| STAT3 | F 5,ACCCACTCCTTGCCAGTTGT3,
R 5,GGCCACTTGATCCCAGGTT3, |
| E‐cadherin | F 5,TGGATAACCAGAATAAAGACCAAGTG3,
R 5,TCCTCCGAAGAAACAGCAAGA3, |
| Vimenin | F 5,GCAGGAGGCAGAAGAATGGTA3,
R 5,GGGACTCATTGGTTCCTTTAAGG3, |
| N‐cadherin | F 5,AAAGAACGCCAGGCCAAAC3,
R 5,GGCATCAGGCTCCACAGTGT3, |
| GAPDH | F 5,GAAGGTCGGAGTCAACGGATTT3,
R 5,CCTGGAAGATGGTGATGGGATT3, |
F, forward primer; R, reverse primer.
Statistical analysis
All statistical analyses were performed using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA), GraphPad Prism software (La Jolla, CA, USA), Adobe Photoshop CS4 (San Jose, CA, USA), and Image J software (NIH). Data are expressed as mean ± standard deviation. Comparisons between the two groups were analyzed using a Student’s t‐test. Comparisons among multiple groups were conducted using one‐way analysis of variance. A P value of < 0.05 was considered statistically significant.
Results
Decreased plasma IL‐37 expression and negative association with progression in non‐small cell lung cancer patients
Serum IL‐37 expression was detected in 40 NSCLC patients and healthy controls using enzyme‐linked immunosorbent assay. The data showed that plasma IL‐37 expression decreased in NSCLC patients compared to healthy controls (P = 0.032) (Fig 1). The relationship between plasma IL‐37 expression in NSCLC patients and clinical characteristics was analyzed. As shown in Table 1, low IL‐37 expression was significantly associated with advanced TNM stage (P = 0.034). However, there was no significant correlation between plasma IL‐37 expression and other clinical characteristics, including gender, age, smoking history, and pathological type.
Figure 1.
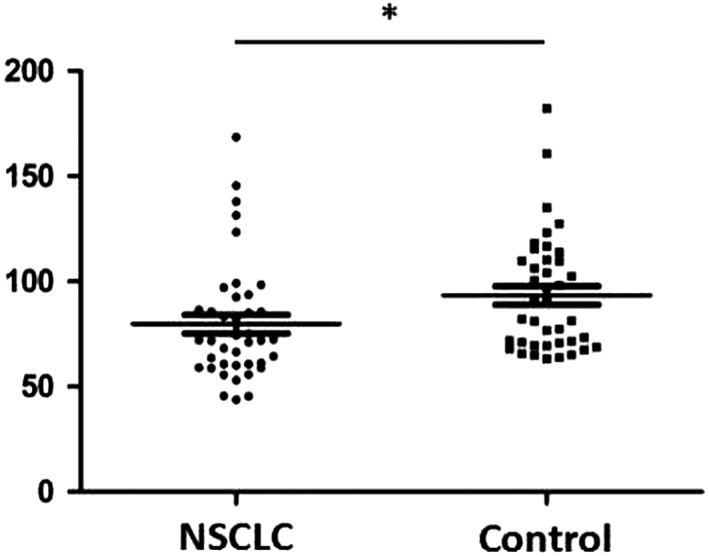
Serum IL‐37 expression in non‐small cell lung cancer (NSCLC) patients and healthy controls (* P < 0.05).
IL‐37 inhibits A549 cell invasion and migration in a dose‐dependent manner
To confirm the role of IL‐37 in NSCLC, cell invasion and migration were detected in a human NSCLC A549 cell line after the administration of different concentrations of rhIL‐37. As shown in Figure 2a, IL‐37 inhibited the invasion of A549 cells in a dose‐dependent manner: the suppression efficiency achieved was (70 ± 4) at 100 ng/mL versus (95 ± 12) in the control group. IL‐37 inhibited the migration of A549 cells in a dose‐dependent manner: the suppression efficiency achieved was (42 ± 1)% at 100 ng/mL versus (51 ± 1)% in the control group (Fig 2b). Thus, the dose of 100 ng/mL rhIL‐37 was used for further study.
Figure 2.
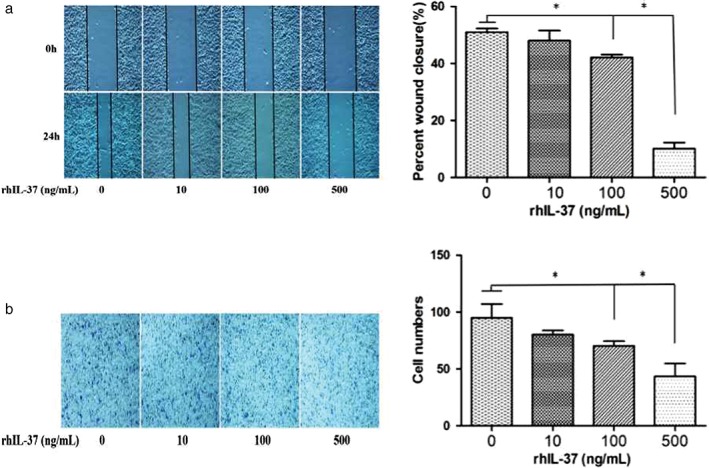
IL‐37 suppresses non‐small cell lung cancer (NSCLC) in a dose‐dependent manner. (a) Scratch wound healing and (b) Transwell invasion assay of A549 cells with different concentrations of rhIL‐37 protein (0, 10, 100, 500 ng/mL). *P < 0.05.
IL‐6 promotes A549 cell invasion and migration in a dose‐dependent manner
As shown in Figure 3, IL‐6 promotes the invasion and migration of A549 cells in a dose‐dependent manner, in which the optimal promotion efficiency occurred at 50 ng/mL. Thus, a dose of 50 ng/mL rhIL‐6 was used for further study.
Figure 3.
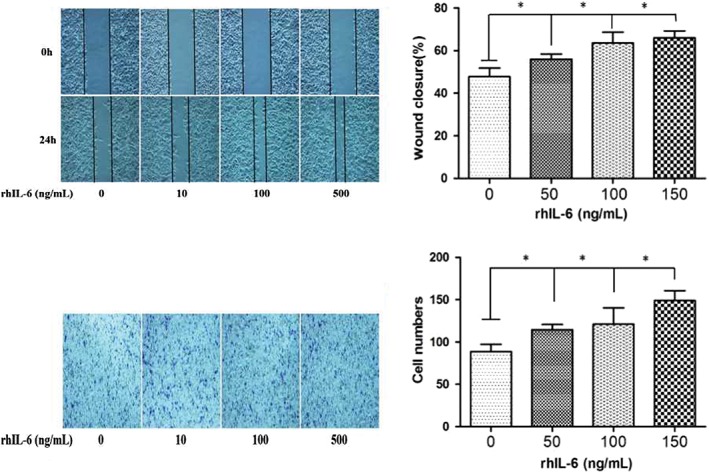
IL‐6 promotes non‐small cell lung cancer (NSCLC) in a dose‐dependent manner. (a) Scratch wound healing and (b) Transwell invasion assay of A549 cells with different concentrations of rhIL‐6 protein (0, 50, 100, 150 ng/mL). * P < 0.05.
IL‐37 modulates the IL‐6/STAT3 signaling pathway in A549 cells
To determine whether the IL‐6/STAT3 signaling pathway is involved in the effects of anti‐invasion and anti‐migration that IL‐37‐mediated in A549 cells, the A549 cells were divided into four groups: control, IL‐37, IL‐6, and IL‐37 + IL‐6. The invasion and migration of A549 cells was detected in each group. IL‐37 inhibited the invasion and migration of A549 cells relative to the control group, while IL‐6 promoted the invasion and migration of A549 cells (Fig 4). IL‐37 downregulated IL‐6‐induced pro‐invasion and pro‐migration in A549 cells. Additionally, STAT3 and pSTAT3 Y705 expression was detected in the four groups. As shown in Figure 5, pSTAT3 protein expression was significantly higher in the IL‐6 group than in the control group. However, pSTAT3 protein expression was markedly lower in the IL‐37 + IL‐6 group than in the IL‐6 group. The results suggest that IL‐37 might inhibit A549 cell invasion and migration via IL‐6/STAT3 signaling pathway.
Figure 4.
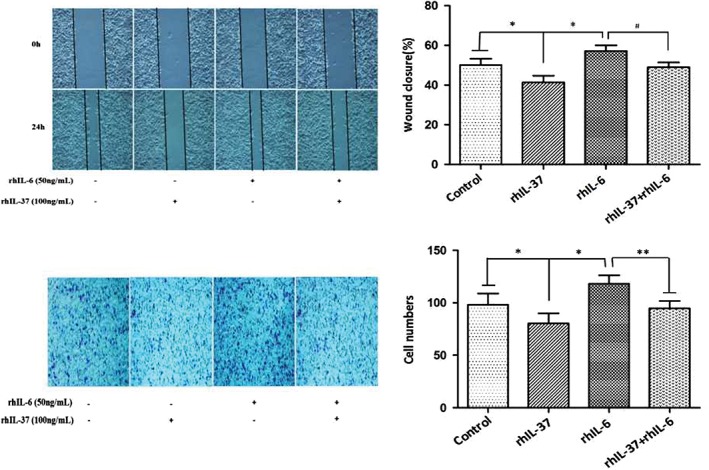
Invasion and metastasis of A549 cells under different treatments. (a) Scratch wound healing and (b) Transwell invasion assay of A549 cells in the four groups. *P < 0.05 versus control group; **P < 0.05 versus IL‐6 group.
Figure 5.
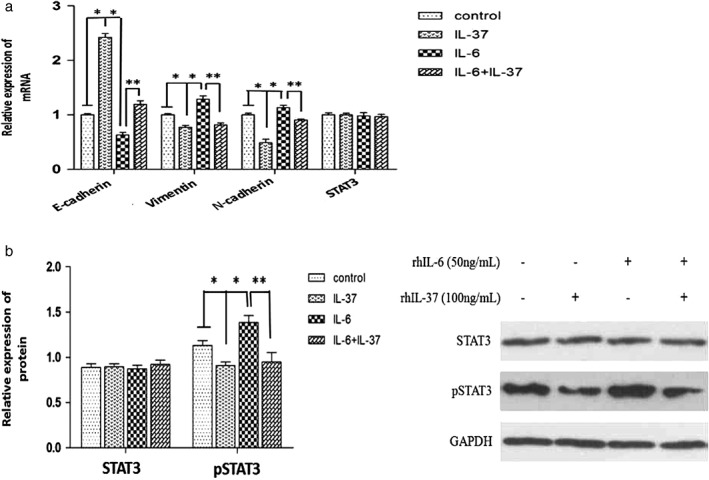
Gene expression in A549 cells under different treatments. (a) STAT3, E‐cadherin, vimentin and N‐cadherin messenger RNA (mRNA) expression in A549 cells was determined by RT‐PCR. (b) STAT3 and pSTAT3 protein expression in A549 cells was determined by Western blot. *P < 0.05 versus control group; ** P < 0.05 versus IL‐6 group.
IL‐37 inhibited epithelial‐to‐mesenchymal transition via the IL‐6/STAT3 signaling pathway
To further explore the potential IL‐37 mechanisms associated with anti‐invasion and anti‐migration in NSCLC, the expression of three epithelial‐to‐mesenchymal transition (EMT)‐related biomarkers (E‐cadherin, vimentin, and N‐cadherin) were detected in the four groups. As shown in Figures 5 and 6, the mRNA and protein expression of epithelial marker E‐cadherin were significantly lower, but the levels of mesenchymal markers vimentin and N‐cadherin were significantly higher in the IL‐6 group than in the control group. However, the mRNA and protein expression of E‐cadherin was significantly increased, while the other two biomarkers were significantly decreased in the IL‐37 + IL‐6 group compared to the IL‐6 group. The results suggest that the inhibition effects of IL‐37 on EMT might be correlated with IL‐6/STAT3 signaling pathways.
Figure 6.
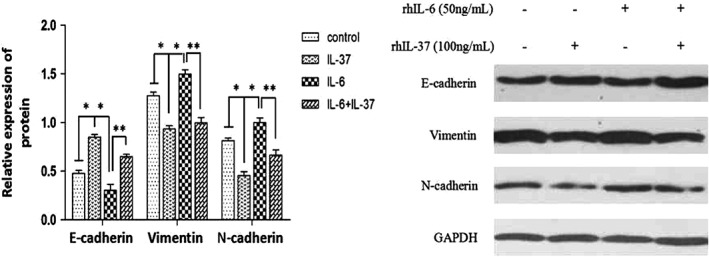
E‐cadherin, vimentin and N‐cadherin protein expression in A549 cells was determined by Western blot. * P < 0.05 versus control group; ** P < 0.05 versus IL‐6 group.
Discussion
Accumulating evidence has shown that IL‐37 has strong anti‐inflammatory and anti‐immune properties.10, 17, 18, 19, 20 Recent studies have indicated that IL‐37 plays a protective role in the development and progression of many types of human cancer.14, 15, 21, 22 However, there is little information regarding whether plasma IL‐37 expression influences the clinical characteristics of NSCLC patients and the mechanisms underlying its anti‐invasive and anti‐metastatic effects in human NSCLC A549 cells. In our study, we showed that plasma IL‐37 expression was significantly decreased in NSCLC patients compared to healthy controls. Further analysis showed that the lack of plasma IL‐37 expression was closely correlated with advanced TNM stage, which suggests that IL‐37 may suppress NSCLC progression.
IL‐6, a pleiotropic cytokine, is involved in inflammation, immune response, and the development of tumors.23, 24, 25 IL‐6 binds to the ligand receptor gp80 and subsequently transfers receptor gp130.26 STAT3 can be activated by phosphorylated tyrosine in gp130 via phosphorylation of tyrosine 705. The IL‐6/STAT3 signaling pathway plays a critical role in promoting the proliferation, invasion, metastasis, immunosuppression, and angiogenesis of tumor cells.25, 27, 28 Previous studies have reported that IL‐37 suppressed cell proliferation, invasion, and metastasis in renal cell carcinoma and human cervical cancer by inhibiting the IL‐6/STAT3 signaling pathway.13, 29
In the present study, our results showed that IL‐37 suppressed and IL‐6 promoted A549 cell invasion and migration, both in a dose‐dependent manner. Therefore, we speculate that the IL‐6/STAT3 signaling pathway may be involved in the effects of IL‐37 on NSCLC cells. Our results showed that pSTAT3 protein expression was significantly higher after culture of A549 cells with IL‐6. However, pSTAT3 protein expression was significantly lower after culturing with IL‐6 + IL‐37 compared to the IL‐6 group. These results suggest that IL‐37 might inhibit the invasion and migration of A549 cells via the IL‐6/STAT3 signaling pathway in NSCLC.
To gain further insight into the role of IL‐37 in NSCLC, we detected the expression of the downstream genes of STAT3. Continuous activation of STAT3 can induce abnormal expression of EMT‐related markers, such as the downregulation of epithelial marker E‐cadherin and the upregulation of interstitial markers E‐cadherin and vimentin that involve the promotion of cell invasion and migration.28, 30, 31 We found that E‐cadherin expression in A549 cells was significantly lower after culture with IL‐6, but significantly increased after culture with IL‐6 + IL‐37. The expression of mesenchymal markers vimentin and N‐cadherin were significantly higher and lower in these groups, respectively. The data indicate that IL‐37 might inhibit the expression of EMT through IL‐6/STAT3 signaling pathways.
In summary, we determined that IL‐37 is downregulated in the serum of NSCLC patients and is negatively correlated with tumor TNM stage. IL‐37 may be able to suppress NSCLC invasion and metastasis or partially suppress the activation of STAT3 and decrease EMT expression by inhibiting IL‐6 expression. Therefore, IL‐37 could function not only as a fundamental inhibitor of innate immunity and inflammatory response, but also as a potential novel tumor suppressor in NSCLC. However, IL‐37 levels in metastatic tissues, and the influence of inhibitory effects of IL‐37 caused by blocking the IL‐6/STAT3 pathway, require further study. The underlying mechanisms by which IL‐37 inhibits IL‐6 expression in NSCLC also remain unclear. NF‐kB has been reported to be a critical regulator of many genes regulating tumor progression and could mediate IL‐6 secretion in cancer.32, 33, 34 Further studies are needed to establish the mechanisms underlying the anti‐tumor activity of IL‐37 in NSCLC.
Disclosure
No authors report any conflict of interest.
Acknowledgments
We sincerely thank all members of the Central Laboratory and Biotherapy Center of Qingdao Central Hospital who provided helpful advice and technical support for our study. We would like to thank LetPub (www/http://letpub.com) for providing linguistic assistance during the preparation of this manuscript.
References
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 2. Wu H, Qiao N, Wang Y et al Association between the telomerase reverse transcriptase (TERT) rs2736098 polymorphism and cancer risk: Evidence from a case‐control study of non‐small‐cell lung cancer and a meta‐analysis. PLoS One 2013; 8: e76372–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu X, Liu Z, Sun M, Liu J, Wang ZX, de W. The long non‐coding RNA HOTAIR indicates a poor prognosis and promotes metastasis in non‐small cell lung cancer. BMC Cancer 2013; 13: 464–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee SH, Suh IB, Lee EJ et al Relationships of coagulation factor XIII activity with cell‐type and stage of non‐small cell lung cancer. Yonsei Med J 2013; 54: 1394–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aberle DR, Adams AM, Berg CD et al Reduced lung‐cancer mortality with low‐dose computed tomographic screening. N Engl J Med 2011; 365: 395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dinarello C, Arend W, Sims J et al IL‐1 family nomenclature. Nat Immunol 2010; 11: 973–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Banchereau J, Pascual V, O’Garra A. From IL‐2 to IL‐37: The expanding spectrum of anti‐inflammatory cytokines. Nat Immunol 2012; 13: 925–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boraschi D, Lucchesi D, Hainzl S et al IL‐37: A new anti‐inflammatory cytokine of the IL‐1 family. Eur Cytokine Netw 2011; 22: 127–47. [DOI] [PubMed] [Google Scholar]
- 9. Yang Y, Zhang ZX, Lian D, Haig A, Bhattacharjee RN, Jevnikar AM. IL‐37 inhibits IL‐18‐induced tubular epithelial cell expression of pro‐inflammatory cytokines and renal ischemia‐reperfusion injury. Kidney Int 2015; 87: 396–408. [DOI] [PubMed] [Google Scholar]
- 10. Nold MF, Noldpetry CA, Zepp JA et al Interleukin 37 is a fundamental inhibitor of innate immunity. Nat Immunol 2010; 11: 1014–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Luo Y, Cai X, Liu S et al Suppression of antigen‐specific adaptive immunity by IL‐37 via induction of tolerogenic dendritic cells. Proc Natl Acad Sci U S A 2014; 111: 15178–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu H, Ren G, Wang T et al Aberrantly expressed Fra‐1 by IL‐6/STAT3 transactivation promotes colorectal cancer aggressiveness through epithelial–mesenchymal transition. Carcinogenesis 2015; 36: 459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jiang Y, Wang Y, Liang L et al IL‐37 mediates the antitumor activity in renal cell carcinoma. Med Oncol 2015; 32: 250–9. [DOI] [PubMed] [Google Scholar]
- 14. Liu R, Tang C, Shen A et al IL‐37 suppresses hepatocellular carcinoma growth by converting pSmad3 signaling from JNK/pSmad3L/c‐Myc oncogenic signaling to pSmad3C/P21 tumor‐suppressive signaling. Oncotarget 2016; 7: 85079–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yan X, Zhao J, Zhang R. Interleukin‐37 mediates the antitumor activity in colon cancer through β‐catenin suppression. Oncotarget 2017; 8: 49064–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhao JJ, Pan QZ, Pan K et al Interleukin‐37 mediates the antitumor activity in hepatocellular carcinoma: Role for CD57+ NK cells. Sci Rep 2014; 4: 5177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gao W, Kumar S, Lotze MT, Hanning C, Robbins PD, Gambotto A. Innate immunity mediated by the cytokine IL‐1 homologue 4 (IL‐1H4/IL‐1F7) induces IL‐12‐dependent adaptive and profound antitumor immunity. J Immunol 2003; 170: 107–13. [DOI] [PubMed] [Google Scholar]
- 18. Mcnamee EN, Masterson JC, Jedlicka P et al From the cover: Interleukin 37 expression protects mice from colitis. Proc Natl Acad Sci U S A 2011; 108: 16711–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ballak DB, van Diepen JA, Moschen AR et al IL‐37 protects against obesity‐induced inflammation and insulin resistance. Nat Commun 2014; 5: 4711–22. [DOI] [PubMed] [Google Scholar]
- 20. Lunding L, Webering S, Vock C et al IL‐37 requires IL‐18Rα and SIGIRR/IL‐1R8 to diminish allergic airway inflammation in mice. Allergy 2015; 70: 366–73. [DOI] [PubMed] [Google Scholar]
- 21. Luo C, Shu Y, Luo J et al Intracellular IL‐37b interacts with Smad3 to suppress multiple signaling pathways and the metastatic phenotype of tumor cells. Oncogene 2017; 36: 2889–99. [DOI] [PubMed] [Google Scholar]
- 22. Abulkhir A, Samarani S, Amre D et al A protective role of IL‐37 in cancer: A new hope for cancer patients. J Leukoc Biol 2017; 101: 395–406. [DOI] [PubMed] [Google Scholar]
- 23. Zarogoulidis P, Yarmus L, Darwiche K et al Interleukin‐6 cytokine: A multifunctional glycoprotein for cancer. Immunome Res 2013; 9: 16535–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yao X, Huang J, Zhong H et al Targeting interleukin‐6 in inflammatory autoimmune diseases and cancers. Pharmacol Ther 2014; 141: 125–39. [DOI] [PubMed] [Google Scholar]
- 25. Lu X, Luo F, Liu Y et al The IL‐6/STAT3 pathway via miR‐21 is involved in the neoplastic and metastatic properties of arsenite‐transformed human keratinocytes. Toxicol Lett 2015; 237: 191–9. [DOI] [PubMed] [Google Scholar]
- 26. Heinrich PC, Behrmann I, Müller‐Newen G, Schaper F, Graeve L. Interleukin‐6‐type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J 1998; 334: 297–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bromberg J, Wang TC. Inflammation and cancer: IL‐6 and STAT3 complete the link. Cancer Cell 2009; 15: 79–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhao Z, Cheng X, Wang Y et al Metformin inhibits the IL‐6‐induced epithelial‐mesenchymal transition and lung adenocarcinoma growth and metastasis. PLoS One 2014; 9: e95884–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang S, An W, Yao Y et al Interleukin 37 expression inhibits STAT3 to suppress the proliferation and invasion of human cervical cancer cells. J Cancer 2015; 6: 962–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Talbot LJ, Bhattacharya SD, Kuo PC. Epithelial‐mesenchymal transition, the tumor microenvironment, and metastatic behavior of epithelial malignancies. Int J Biochem Mol Biol 2012; 3: 117–36. [PMC free article] [PubMed] [Google Scholar]
- 31. Tsai JH, Yang J. Epithelial‐mesenchymal plasticity in carcinoma metastasis. Genes Dev 2013; 27: 2192–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liang S, Chen Z, Jiang G et al Activation of GPER suppresses migration and angiogenesis of triple negative breast cancer via inhibition of NF‐κB/IL‐6 signals. Cancer Lett 2017; 386: 12–23. [DOI] [PubMed] [Google Scholar]
- 33. Xiong S, Wang Y, Li H, Zhang X. Low dose of Bisphenol a activates NF‐κB/IL‐6 signals to increase malignancy of Neuroblastoma cells. Cell Mol Neurobiol 2017; 37: 1095–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang GJ, Shen NJ, Cheng L, Yehan Fang, Huang H, Li KH. Visfatin triggers the in vitro migration of osteosarcoma cells via activation of NF‐κB/IL‐6 signals. Eur J Pharmacol 2016; 791: 322–30. [DOI] [PubMed] [Google Scholar]


