Abstract
Background
In recent years, lung cancer incidence has been increasing; however the impact of different histological types of lung cancer is not yet clear.
Methods
Trends in the lung cancer incidence rate by histological type were examined based on data of 36 658 primary lung cancer patients from West China Hospital between 1995 and 2015.
Results
The most common histological type of lung cancer in our hospital was adenocarcinoma (ADC) in both genders, followed by squamous cell carcinoma (SQCC), and small cell carcinoma (SCLC), which is consistent with general worldwide trends. The proportion of young patients with SCLC showed a downward trend. In the overall population with lung cancer, the number of elderly patients with lung cancer increased significantly, while the proportion of elderly patients increased gradually. The mean age at diagnosis also increased. The number of women with ADC increased sharply in recent years, especially in young patients, and the incidence rate in women is now greater than in men.
Conclusion
Significant increases in the number of patients with ADC and the rate of lung cancer in women over recent years were observed, indicating that research on the pathogenesis of disease in these patients is urgent.
Keywords: Adenocarcinoma, incidence rate, lung cancer, small cell carcinoma, squamous cell carcinoma
Introduction
Lung cancer is one of the most common malignancies worldwide and is the leading cause of death of all types of malignant tumors. In 2012, there were approximately 1 800 000 new cases and 1 590 000 deaths from lung cancer worldwide.1 Statistics from the United States in 2016 revealed that lung cancer accounted for the second highest incidence and the highest mortality in both men and women.2 In China, lung cancer is the leading cause of malignancy and cancer‐related death.3
Smoking is the major risk factor for lung cancer and is closely related to incidence and death rates. GLOBOCAN 2012 cancer statistics indicated that the incidence of lung cancer has declined in some countries that had an early prevalence of tobacco use, but continues to increase in countries that took up tobacco use at a later stage.1 Although smoking is closely associated with lung cancer, many studies have reported an increasing trend in the incidence of lung cancer among non‐smokers, especially in Asia.4
In China, lung cancer incidence is gradually increasing with the prevalence of tobacco use, the acceleration of industrialization, and the aging population. Many studies have reported changes in the histological types of lung cancer, but few have effectively analyzed these characteristics in China.
Therefore, we analyzed the dynamic changes in histological types and clinical characteristics of lung cancer using data of lung cancer patients diagnosed at West China Hospital of Sichuan University between 1995 and 2015. The results of our study provide information for the prevention and treatment of lung cancer in the Sichuan area.
Methods
Subjects
West China Hospital of Sichuan University is the largest national center for the diagnosis and treatment of difficult and critical diseases in western China and is one of the world's largest general hospitals. The West China Hospital was one of the first to establish a Medical Laboratory Center and the first in China to receive American Pathologists Society approval. We collected the data of all lung cancer patients diagnosed by the pathology department at West China Hospital between 1 January 1995 and 12 March 2015. Pathological diagnoses of malignant epithelial tumors were obtained by: pleural effusion (760 patients), sputum (1242), fibrobronchoscope (8956), percutaneous lung puncture biopsy (16 558), and gross surgical specimens (9142).
The study inclusion criteria were: (i) histological diagnosis of malignant epithelial tumors obtained through hydrothorax, sputum, bronchoscopy, percutaneous lung puncture, or gross surgical specimens; and (ii) in cases of differing histological results, diagnosis was based on the gross surgical specimen or biopsy. The exclusion criteria were: (i) cases of recurrence, identified by patient name, hospital number, and other personal data; and (ii) mesenchymal, lymphohistiocytic, or benign tumors, pulmonary metastases, and uncertain source of disease. A total of 36 658 patients were included in the study, and 173 929 were excluded.
Classification
Histological types were classified based on the criteria of the World Health Organization (WHO) Classification of Lung Tumors as: adenocarcinoma (ADC); squamous cell carcinoma (SQCC); small cell lung carcinoma (SCLC); other specified histological type (including adenosquamous, large cell, neuroendocrine, large cell neuroendocrine, combined large cell neuroendocrine, sarcomatoid, pleomorphic, spindle cell, giant cell, lymphoepithelioma‐like, mucoepidermoid, adenoid cystic, epithelial‐myoepithelial, clear cell, undifferentiated, and atypical carcinoid carcinomas, carcinosarcoma, pulmonary blastoma, and carcinoid tumors); unspecified type; poorly differentiated; and double primary lung cancer (DPLC).
A total of 21 years of pathological diagnoses, made using the criteria of the 1981/1999/2004/2015 editions of the WHO Classification of Lung Tumors, were included. With sequential editions, the classification criteria of the four versions became more detailed, with a slight change in the definition of SCLC, a refined classification of ADC, an adjustment of the morphological classification of SQCC, and variations in the classification of some cancers of uncommon types. We considered poorly differentiated carcinoma and DPLC separately because the WHO classification criteria did not classify these two types.
Since the pathological registration system was renewed by West China Hospital in November 2008, some of the age data collected before 2008 were missing. The proportion of missing data varied yearly and fluctuated between 0.08% and 6.6%. Cases with missing data were not included in subgroup analysis of age stratification.
The main data collected included personal information, gender, age, and histological type. Categories were created for cases and populations according to gender, time period, and age group; specifically, the categories included men and women at three‐year periods from 1995–1997 to 2013–2015, and five‐year age groups from 0–4 to 85. Age stratification was defined according to WHO age segmentation: young (≤ 44 years), middle‐aged (45–59), and elderly (≥ 60).
Statistical analysis
We established a database of 210 587 primary lung cancer patients after applying the inclusion and exclusion criteria. The number, proportion, and characteristics of each histological type were statistically analyzed.
The data were analyzed using SPSS version 22.0 (IBM, Armonk, NY, USA), including a descriptive analysis of characteristics for 1995 to 2015. Categorical data were analyzed using chi‐square tests, and measurement using t tests. P values < 0.05 were considered statistically significant.
Results
The total number of patients with lung cancer and the number of patients in each histological group increased yearly from 1995 to 2015. Of 210 587 patients with lung‐related pathological diagnoses, 36 658 primary lung cancer patients were included in the analysis.
Lung cancer incidence by age group
The number of patients in the young group increased from 194 in 1995–1997 to 995 in 2013–2015; during this time, the proportion of young patients initially increased from 13.95% (1995–1997) to 14.7% (2004–2006), then decreased to 8.59% (2013–2015). The difference between 2004–2006 and 1995–1997 was not significant (P = 0.512), but the difference observed between 1995–1997 and 2013–2015 was significant (P < 0.001). Overall, the proportion of young patients tended to decrease, particularly in recent years. The number of patients in the middle‐aged group increased from 604 (1995–1997) to 4213 (2013–2015), but the proportion decreased significantly from 43.42% to 36.38% (P < 0.001). The number of patients in the elderly group increased from 593 (1995–1997) to 6372 (2013–2015), and the proportion increased significantly from 42.63% to 55.03% (P < 0.001) (Fig 1).
Figure 1.
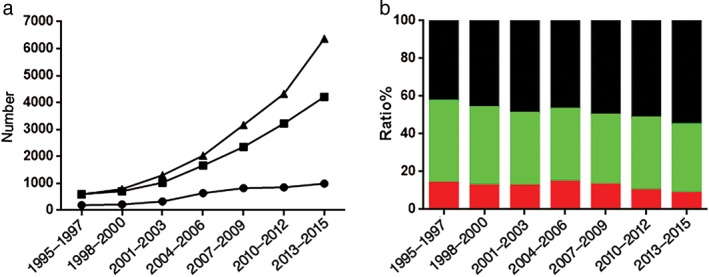
Changing lung cancer trends in all age groups by (a) number ( ) young, (
) young, ( ) middle, and (
) middle, and ( ) old and (b) proportion. (
) old and (b) proportion. ( ) Old, (
) Old, ( ) middle‐aged, and (
) middle‐aged, and ( ) young.
) young.
Highest incidence of lung cancer by age
The median age of patients increased from 57 (1995–1997) to 61 (2013–2015) years. The average age of patients in 1995–1997 compared to 2013–2015 was significantly different (56.12 ± 10.818 vs. 59.84 ± 10.913, respectively; P < 0.001). During 1995–2003, occurrence was highest at age 60–64 years, and in 2004–2006 at 50–54, 55–59, 60–64, and 65–69 years, with no significant differences among them. The highest occurrence during 2007–2012 was at 55–59 years, which was slightly higher than the group aged 60–64. Similar to 1995–2003, the high‐occurrence age group in 2013–2015 was 60–64 years. There was no obvious increase in the age of onset (Fig 2, Table 1). The missing data rate fluctuated between 0.08% and 6.6% during 1995–2008 (6.6%, 3.4%, 2.3%, 5.3%, 2.2%, 3.9%, 5.6%, 2.4%, 0.08%, 0.2%, 0.3%, 0.2%, 0.5%, 0.1% for each year, respectively, and a total of 214 patients).
Figure 2.
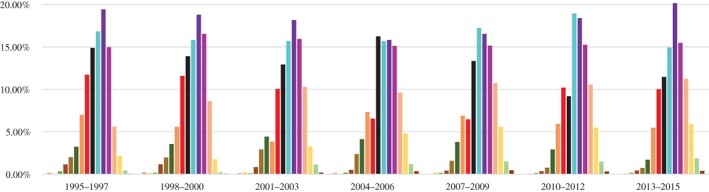
Changes in age groups with a high occurrence of lung cancer. ( ) 0–14, (
) 0–14, ( ) 15–19, (
) 15–19, ( ) 20–24, (
) 20–24, ( ) 25–29, (
) 25–29, ( ) 30–34, (
) 30–34, ( ) 35–39, (
) 35–39, ( ) 40–44, (
) 40–44, ( ) 45–49, (
) 45–49, ( ) 50–54, (
) 50–54, ( ) 55–59, (
) 55–59, ( ) 60–64, (
) 60–64, ( ) 65–69, (
) 65–69, ( ) 70–74, (
) 70–74, ( ) 75–79, (
) 75–79, ( ) 80–84, and (
) 80–84, and ( ) 85+.
) 85+.
Table 1.
Changes in the average and median age of lung cancer
| Year | Patients (n) | Average age | Median age | Minimum age | Maximum age |
|---|---|---|---|---|---|
| 2013–2015 | 11 580 | 59.84 ±10.913 | 61 | 15 | 94 |
| 2010–2012 | 8404 | 59.29 ±10.905 | 60 | 11 | 95 |
| 2007–2009 | 6352 | 58.80 ±11.531 | 59 | 0 | 91 |
| 2004–2006 | 4334 | 57.74 ±11.694 | 58 | 1 | 96 |
| 2001–2003 | 2664 | 57.70 ±11.591 | 59 | 3 | 94 |
| 1998–2000 | 1719 | 56.81 ±10.951 | 58 | 5 | 85 |
| 1995–1997 | 1391 | 56.12 ±10.818 | 57 | 10 | 85 |
Lung cancer incidence in men and women
The number of cases of lung cancer increased in both men and women from 1155 and 291 cases (1995–1997) to 7614 and 3966 cases (2013–2015), respectively. The proportion of female patients increased significantly over time, rising from 20.12% (1995–1997) to 34.25% (2013–2015), whereas that of males decreased significantly from 79.88% to 65.75% (P < 0.001) (Fig 3).
Figure 3.
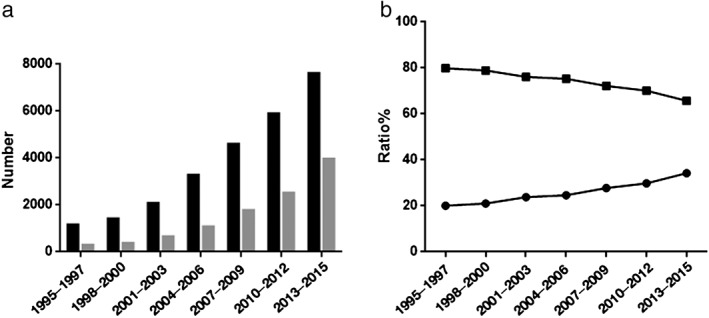
Lung cancer trends in men and women by (a) number ( ) men and (
) men and ( ) women and (b) proportion. (
) women and (b) proportion. ( ) Men, (
) Men, ( ) women.
) women.
Different histological types of lung cancer
At West China Hospital, 1446 cases of lung cancer were diagnosed during 1995–1997 and 11 580 cases during 2013–2015. There was an upward trend in the number of patients with lung cancer over the study period, and an increase in the number of patients with different histological types. There were 375 cases of ADC in 1995–1997, and 6526 in 2013–2015. The proportion of ADC increased from 25.93% (1995–1997) to 56.36% (2013–2015) (P < 0.001). The number of SQCC cases increased from 710 (1995–1997) to 3050 (2013–2015); however, the proportion decreased from 49.1% to 26.34% (P < 0.001). Between 2007 and 2009, the proportion of ADC cases exceeded SQCC, and ADC became the main histologic type. The proportion of SCLC cases decreased from 1995–1997 to 2013–2015 (P = 0.001). SCLC tended to decline in general. The proportion of other specified histological types of lung cancer also increased from 8.23% (1995–1997) to 10.56% (1998–2000) (P = 0.026), then decreased to 3.45% (2013–2015) (P < 0.001). The proportion of uncertain type decreased from 2.28% (1995–1997) to 1.06% (1998–2000) (P = 0.007), increased to 4.65% (2010–2012) (P < 0.001), and finally decreased to 2.73% (2013–2015) (P < 0.001). The difference between 1995–1997 and 2013–2015 was not significant (P = 0.386). Our results suggest that the proportion of uncertain type significantly fluctuated. The proportion of differentiated carcinoma decreased significantly from 3.6% (1995–1997) to 0.65% (2013–2015) (P < 0.001). To some extent, this reflects improvements in pathological diagnostic techniques at West China Hospital. DPLC incidence was lowest among all types of lung cancers (20 cases, 0.07%), and was most commonly diagnosed in elderly male patients (15/19) (Fig 4).
Figure 4.
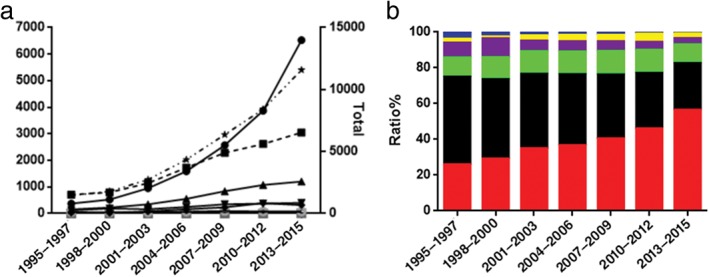
Changing trends in histological types of lung cancer by (a) number ( ) Adenocarcinoma (ADC), (
) Adenocarcinoma (ADC), ( ) squamous cell carcinoma (SQCC), (
) squamous cell carcinoma (SQCC), ( ) small cell lung cancer (SCLC), (
) small cell lung cancer (SCLC), ( ) others, (
) others, ( ) uncertain, (
) uncertain, ( ) poorly differentiated carcinoma, and (
) poorly differentiated carcinoma, and ( ) double primary lung cancer (DPLC) and (b) proportion. (
) double primary lung cancer (DPLC) and (b) proportion. ( ) Adenocarcinoma (ADC), (
) Adenocarcinoma (ADC), ( ) squamous cell carcinoma (SQCC), (
) squamous cell carcinoma (SQCC), ( ) small cell lung cancer (SCLC), (
) small cell lung cancer (SCLC), ( ) others, (
) others, ( ) uncertain, (
) uncertain, ( ) poorly differentiated carcinoma, and (
) poorly differentiated carcinoma, and ( ) double primary lung cancer (DPLC).
) double primary lung cancer (DPLC).
Relationship between histological type and age
Figure 5 presents the relationship between histological type and age. Over the study period, the number of ADC cases in different age groups increased to varying degrees. The proportion of ADC in the young and middle‐aged groups was slightly higher than in the elderly group in 1995–1997 (28.35% vs. 27.15% vs. 24.11%, respectively). In 2013–2015, the proportion of ADC increased significantly to 66.53% (P < 0.001) in the young, 58.43% (P < 0.001) in the middle‐aged, and 53.4% (P < 0.001) in the elderly group.
Figure 5.
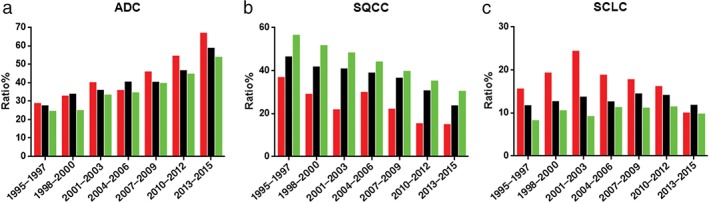
Trends in proportions in different age groups of (a) adenocarcinoma (ADC), (b) squamous cell carcinoma (SQCC), and (c) small cell lung cancer (SCLC). ( ) Young, (
) Young, ( ) middle‐aged, and (
) middle‐aged, and ( ) old.
) old.
The number of cases of SQCC in different age groups increased during the study period; however, the proportion of SQCC in all groups showed a decreasing trend. The proportion of SQCC in young, middle‐aged, and elderly patients was 36.6%, 46.19%, and 56.16% in 1995–1997, decreasing to 14.67%, 23.43%, and 30.08%, respectively, (all P < 0.001) in 2013–2015.
The proportion of SCLC in young patients increased from 15.46% (1995–1997) to a peak of 24.24% (2001–2003; P = 0.019), then decreased to 9.85% (2013–2015) (P < 0.001). The difference between 1995–1997 and 2013–2015 was significant (P = 0.03). The proportion of SCLC in middle‐aged patients increased from 11.59% (1995–1997) to 14.33%, and then decreased to 11.70% (2013–2015), but this was not significant (P = 1). The proportion of SCLC in elderly patients increased from 8.09% to 11.30%, then decreased to 9.62% in 2013–2015; the difference between 1995–1997 and 2013–2015 was not significant (P = 0.242). Our results indicate a downward trend in the proportion of SCLC in young patients.
In 1995–1997, SQCC was the most common histological type in young, middle‐aged, and elderly patients (36.6%, 46.19%, and 56.16%); followed by ADC (28.35%, 27.15%, and. 24.11%); and SCLC (15.46%, 11.59%, and 8.09%), respectively. In 2013–2015, ADC became the most common histological type in young, middle‐aged, and elderly patients (66.53%, 58.43%, and 53.4%); followed by SQCC (14.67%, 23.43%, and 30.08%); and SCLC (9.85%, 11.70, and 9.62%), respectively.
Histological trends in men and women
Figure 6 shows the trends of different histological types of lung cancer in men and women. The proportion of men with ADC decreased significantly from 61.87% (1995–1997) to 51.79% (2013–2015), whereas the number of women with ADC during the same period increased significantly from 38.13% to 48.21% (P < 0.001) in comparison to all ADC patients. The proportion of women with ADC among all women with lung cancer increased significantly from 49.14% (1995–1997) to 79.35% (2013–2015) (P < 0.001). A similar increase was observed in the proportion of men with ADC compared to all men with lung cancer, from 20.09% (1995–1997) to 44.39% (2013–2015) (P < 0.001). In addition, the proportion of men and women with ADC compared to all cases of lung cancer increased significantly from 16.04% and 9.89% (1995–1997) to 29.19% and 27.18% (2013–2015), respectively (P < 0.001). The male‐to‐female ratio decreased from 1.622 (16.04%/9.89%) to 1.07 (29.19%/27.18%). ADC incidence increased in both men and women, but more significantly in women. At present, more men are diagnosed with lung ADC, but the ratio between men and women was lowest during 2013–2015.
Figure 6.
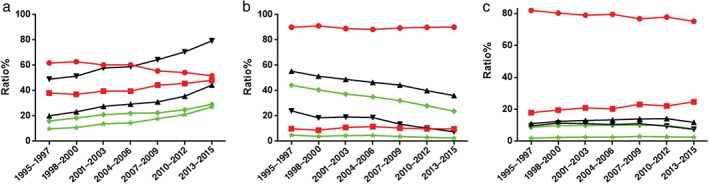
Trends in adenocarcinoma by gender in (a) adenocarcinoma (ADC), (b) squamous cell carcinoma (SQCC), and (c) small cell lung cancer (SCLC). KEY: (a) ( ) women with ADC compared to all lung cancer cases; (
) women with ADC compared to all lung cancer cases; ( ) men with ADC compared to all lung cancer cases; (
) men with ADC compared to all lung cancer cases; ( ) women with ADC compared to all women with lung cancer; (
) women with ADC compared to all women with lung cancer; ( ) men with ADC compared to all men with lung cancer; (
) men with ADC compared to all men with lung cancer; ( ) women with ADC as a proportion of all patients with ADC; (
) women with ADC as a proportion of all patients with ADC; ( ) men with ADC as a proportion of all patients with ADC; (b) (
) men with ADC as a proportion of all patients with ADC; (b) ( ) women with SQCC compared to all lung cancer cases; (
) women with SQCC compared to all lung cancer cases; ( ) men with SQCC compared to all lung cancer cases; (
) men with SQCC compared to all lung cancer cases; ( ) women with SQCC compared to all women with lung cancer; (
) women with SQCC compared to all women with lung cancer; ( ) men with SQCC compared to all men with lung cancer; (
) men with SQCC compared to all men with lung cancer; ( ) women with SQCC as a proportion of all patients with SQCC; (
) women with SQCC as a proportion of all patients with SQCC; ( ) men with SQCC as a proportion of all patients with SQCC; (c) (
) men with SQCC as a proportion of all patients with SQCC; (c) ( ) women with SCLC compared to all lung cancer cases; (
) women with SCLC compared to all lung cancer cases; ( ) men with SCLC compared to all lung cancer cases; (
) men with SCLC compared to all lung cancer cases; ( ) women with SCLC compared to all women with lung cancer; (
) women with SCLC compared to all women with lung cancer; ( ) men with SCLC compared to all men with lung cancer; (
) men with SCLC compared to all men with lung cancer; ( ) women with SCLC as a proportion of all patients with SCLC; (
) women with SCLC as a proportion of all patients with SCLC; ( ) men with SCLC as a proportion of all patients with SCLC.
) men with SCLC as a proportion of all patients with SCLC.
The majority of SQCC patients were male. The proportion of male SQCC patients decreased slightly from 90.14% (1995–1997) to 88.35% (P = 0.228), then rose again to 90.23% (2013–2015), whereas that of female SQCC patients first increased from 9.86% to 11.65%, then decreased to 9.77%, compared to all patients with SQCC. The difference between 1995–1997 and 2013–2015 was not significant (P = 0.944), indicating that although the proportion of men and women with SQCC fluctuated slightly, the overall trend was stable. The proportion of men with SQCC among all men with lung cancer decreased significantly from 24.05% (1995–1997) to 7.51% (2013–2015) (P < 0.001). A similar decrease was observed in women with SQCC compared to all women with lung cancer, from 55.41% (1995–1997) to 36.14% (2013–2015) (P < 0.001). In addition, the proportion of male and female SQCC cases compared to all lung cancer cases decreased significantly from 44.26% and 4.84% (1995–1997) to 23.77% and 2.57% (2013–2015) (P < 0.001). The male‐to‐female ratio increased from 9.14 (44.26%/4.84%) to 9.24 (23.77%/2.57%). In conclusion, men were at higher risk of developing SQCC. The proportion of SQCC in both male and female lung cancer cases tended to decrease; however, the decrease in women was more evident. At present, ADC is the main histological type of lung cancer in both men and women.
SCLC was predominant in men throughout the study. The proportion of men with SCLC decreased from 82.05% (1995–1997) to 75.25% (2013–2015), whereas the proportion of women with SCLC increased from 17.95% to 24.75% (P = 0.072), compared to all patients with SCLC, suggesting that although the proportion of men and women with SCLC fluctuated, it was stable overall. In addition, the proportion of men with SCLC compared to men with all lung cancers increased from 8.85% and (1995–1997) to 10.13% (P = 0.168), then decreased to 7.82% (2013–2015) (P < 0.001). Similarly, the proportion of women with SCLC compared to women with all lung cancers increased from 1.94% (1995–1997) to 3.05% (P = 0.022), then decreased to 2.57% (2013–2015) (P = 0.069). The proportion of SCLC in men and women compared to all cases of lung cancer increased from 9.62% and 11.08% (1995–1997) to 14.21% (P = 0.005) and 11.41% (P = 0.527), then decreased to 7.51% (P = 0.012) and 11.90% (2013–2015) (P < 0.001); the difference between 1995–1997 and 2013–2015 was insignificant (P = 0.208 and P = 0.462 in women and men, respectively). Our results suggest that the proportion of SCLC in men tended to decrease, and in women showed a downward trend after an upward trend.
Discussion
Cancer statistics show that incidence and mortality rates of lung cancer were highest in China during the period studied.3 Lung cancer incidence in men was basically stable, while it rose slightly in women during 2000–2011 in China. Similar to other studies, our results showed that the total number of lung cancer diagnoses increased during 1995–2015.5 This increase is possibly related to the aging population, intensified industrialization, the promotion of medical care, and improved diagnosis and treatment of lung cancer in China.
Our study used the 1981/1999/2004/2015 editions of the WHO Classifications of Lung Tumors. There are some overlapping classifications in the 1999 edition; for example, SCLC could be classified as both SQCC and an independent type. In this study, we classified SCLC separately, and this did not affect the proportion of different histological types. ADC, SQCC, and large cell carcinoma subtypes were classified more carefully. The main features of the 2004 edition are: (i) no major changes to the basic classification; (ii) the classification of several types of carcinosarcomas (giant cell, spindle cell, and pleomorphic carcinomas, carcinosarcoma and pulmonary blastoma) as a major class named “sarcomatoid carcinoma;” and (iii) increased consideration of molecular genetics. In the 2015 edition, SCLC, large cell neuroendocrine carcinoma, atypical carcinoid, and carcinoid are classified as lung neuroendocrine tumors, but we classified SCLC separately for statistical analysis. The 2015 edition adjusts the morphological classification of SQCC and includes a more detailed classification of ADC. However, we did perform more detailed morphological classification of ADC and SQCC, and these changes did not affect the proportion of histological types. Currently, the most common histological type of lung cancer in our hospital is ADC in both genders, followed by SQCC and SCLC, similar to worldwide distribution.6 Data from other countries showed that in the first half of the 20th century, SQCC was the predominant lung cancer caused by smoking, followed by SCLC. However, Vincent et al. reported that the number of ADC cases exceeded that of SQCC in 1977.7 Since then, many clinical studies have confirmed this result.8, 9 In some countries, such as Serbia and Iran, however, this trend has not been observed noted, and SQCC predominates in both sexes.10, 11, 12 Although global data confirms the increased incidence of ADC, only a few studies analyzing a short time period have been conducted in China.13, 14, 15, 16, 17
The proportion of ADC compared to all lung cancer types increased yearly to 56.36%, and the proportion of SQCC decreased to 26.34% in 2015 in our study. Since 2007–2009, the proportion of ADC exceeded that of SQCC, and ADC became the predominant histological type. The proportion of ADC increased in both sexes, and the increase in women was more evident than in men. More men are diagnosed with SQCC than women, and although the proportion of SQCC decreased in both sexes, the decrease in men was more evident. This differs somewhat from the results of a study of Tianjin, China, which showed that the proportion of women diagnosed with ADC increased from 42.3% to 55.6% during 1981–2005.18 According to a sample survey, the smoking rate was 45.8% in men and 12% in women in Tianjin in 2006, and the smoking rate in women was higher than the smoking rate worldwide.19 The significantly higher proportion of SQCC in women in Tianjin compared to our results for Sichuan may be related to the higher female smoking rate in Tianjin.
In recent years, an increasing number of epidemiological investigations have noted the growing incidence of lung cancer, which is related to air pollution, smoking, genetics, and other factors. Among these factors, the primary cause of lung cancer is smoking.20, 21 The histological types of lung cancer most closely associated with smoking are SQCC and SCLC, whereas the association between ADC and smoking is weak.22, 23, 24 A study of the Sichuan area showed that the smoking rate in Chengdu decreased from 37.57% in 1996 to 30.62% in 2002, with rates of male and female smoking decreasing from 70.47% to 62.82% and 8.85% to 5.22%, respectively.25 The reduction in smoking rates in the national and Chengdu populations may in part explain the decreasing trend in the proportion of SQCC in our study.
Exposure to a pathogenic environment may play a major role in the pathogenesis of lung cancer among non‐smokers. Many studies have suggested that there are certain types of pathogenic environments, including indoor (e.g. indoor cigarette smoking environments) and outdoor air pollution. Epidemiological studies suggest that the increased incidence of ADC in women in China may be related to exposure to indoor nitrogen oxides.26, 27, 28 In 2010, the International Agency for Research on Cancer reported that the environmental pollution caused by combustion of biomass energy is a human carcinogen.29 In addition, the study found that there was a dose‐response relationship between the indoor environment of cooking smoke and lung cancer in non‐smoking women.30, 31 Sichuan is a large agricultural province. In the past 10 years, although more natural gas is now used as fuel in urban areas, coal, biomass, and other fuels remain the main energy sources in vast rural areas. Tobacco smoke in the environment is also a high risk factor for ADC in non‐smoking women.32, 33, 34 A 2011 report on smoking control in China showed that 74% of non‐smokers in China were exposed to secondhand smoke. In 2006, a local study showed that passive smoking in non‐smoking women was 82.5%.35 China's secondhand smoke exposure rate has been increasing since 1996, and in Sichuan it is slightly higher than the national rate. Indoor air pollution caused by local energy utilization may be associated with the increasing incidence of lung ADC in our study, and may also explain in part why the proportion of ADC in women is higher in our study than in Tianjin.
Some studies have also suggested that long‐term outdoor air pollution may increase the risk of ADC in developed countries and is associated with increased mortality.36, 37, 38, 39, 40 A Sichuan air quality report showed that between 2000 and 2013, the concentration of SO2 decreased; the concentration of NO2 began to rise in 2008; and the concentration of respirable particulate matter (PM10) increased in 2000–2002, decreased slightly, and finally increased.41 Although air quality has improved in recent years, air pollution remains a serious issue. The PM10 level in China is significantly higher than global standards.42 A prospective cohort study of the effects of air pollution in Europe reported that the risk ratios of lung cancer are 1.22/10 μg/m3 and 1.18/5 μg/m3 increase in PM10. For ADC, under the same conditions, the risk ratios are 1.51 and 1, respectively.43 The high concentration of PM10 in the Sichuan area may be an important factor related to the increased incidence of lung ADC observed in our study. Liaw et al. showed a dose‐response relationship of carcinogens in the atmosphere, especially NO, with the incidence of lung ADC.44 In addition to the regularly monitored pollutants, such as SO2, NO2, and PM10, NO and PM2.5 should also be considered air pollution factors associated with lung cancer. The development of precise monitoring and epidemiological studies of PM2.5 and NO in China is urgently required.
In recent years, the incidence of lung cancer in women has gradually increased worldwide, especially in North America, Europe, and other developed countries, and the male‐to‐female ratio is gradually declining.2 The lung cancer trend in China is similar.3 Our results showed a trend of increasing lung cancer incidence in women, consistent with global trends. Although the incidence of female smoking was low, the incidence of lung cancer in women was higher than in some European countries. This may be related to indoor air pollution caused by poorly ventilated coal fuel stoves and cooking fumes.45 Different explanations have been offered to account for the increasing incidence of lung cancer in women, including active smoking, indoor environmental pollution, air pollution, estrogen levels, genetic susceptibility, and viral infection. In addition, passive smoking is a major issue in Sichuan province, with women being the largest population affected.46 This may explain the increased proportion of women with lung cancer in our study. A retrospective study by Kligerman and White showed that as a result of factors such as high expression of CYPlA1 and P53 gene mutations, genetic susceptibility to carcinogens in tobacco smoke and air pollution is greater in women than in men.47 In addition, estrogen has been shown to promote the proliferation of lung cancer cells and the interaction between the ER and EGFR signaling pathways in lung cancer. Estrogen and its signaling pathway have even been suggested as target therapy sites for lung cancer.48
In our study, the number of young patients with lung cancer obviously increased, but the proportion of young patients, compared to all lung cancer cases, declined. This may be related to the aging population and the increase in elderly patients with lung cancer. A report in Shanghai found that lung cancer incidence in young male and female patients increased in 2002–2005.49 The differences between our results and the data from the Shanghai study may be related to economic patterns, the proportion of the migratory population, and population composition. Currently, there is no indication that the incidence of lung cancer in young people has increased or decreased, thus an epidemiological investigation of the overall population is needed.
Many studies have shown a downward trend in the age of lung cancer diagnosis in developed countries. A University of California, Los Angeles study showed that over the past 30 years in developed countries, the diagnostic rate of lung cancer rose in patients < 50 and > 80, as a result of a two‐fold increase in the adolescent smoking rate and the aging population.50 However, national data reported for China in 1989–2008 indicated the average age of onset of lung cancer had increased significantly, by 2.55 years in men and 2.91 years in women.51 The data in our study showed a decreased proportion of lung cancer in youth and an increased proportion in elderly people, with yearly increases in the median and average ages and variability in the age of highest occurrence, but little change in those aged 60–64 years. This is consistent with national data trends. After stratification of the elderly lung cancer population, the main growth group was the 70‐year‐old group, similar to results of the University of California, Los Angeles study. This result may be related to the aging population, and subsequent increases in the elderly population and life expectancy. In 2015, the average life expectancy in Sichuan ranked sixth among all provinces in China, reaching 75.07 years. This may partly explain the increase in the proportion of elderly lung cancer patients and the decline in the proportion of lung cancer in young people.
This study has potential limitations. First, the data originated from our Department of Pathology, which cares for a wide range of patients covering China's northwest, southwest, and central regions, and includes some minority nationalities. Therefore, bias related to differences in air pollution, smoking, genetic specificity, and other possible pathogenic factors in different ethnicities and areas may be present. Second, the data showed a trend of instability because of the small amount of histological data in some age groups. Additionally, our study data cannot fully reflect the specific characteristics of changes in lung cancer incidence. Large‐scale epidemiological investigations are needed to identify trends in pathological types and characteristics. However, despite these limitations, our results provide information of trends in pathological types and characteristics of lung cancer in southwest China.
Disclosure
No authors report any conflict of interest.
Acknowledgments
The Department of Thoracic Oncology, West China Hospital of Sichuan University supported this study.
Contributor Information
Yongsheng Wang, Email: wangys75@gmail.com.
Zongguo Pang, Email: pzgxy@hotmail.com.
References
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016; 66: 7. [DOI] [PubMed] [Google Scholar]
- 3. Chen WQ, Zheng RS, Baade et al Cancer statistics in China, 2015. CA Cancer J Clin 2016; 66: 115–32. [DOI] [PubMed] [Google Scholar]
- 4. Toh CK. The changing epidemiology of lung cancer. Methods Mol Biol 2009; 472: 397–411. [DOI] [PubMed] [Google Scholar]
- 5. Chen WQ, Zhang SW, Zou XN. Estimation and projection of lung cancer incidence and mortality in China. Chin J Lung Cancer 2010; 13: 488–92 (In Chinese.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gabrielson E. Worldwide trends in lung cancer pathology. Respirology 2006; 11: 533–8. [DOI] [PubMed] [Google Scholar]
- 7. Vincent RG, Pickren JW, Lane WW et al The changing histopathology of lung cancer: A review of 1682 cases. Cancer 1977; 39: 1647–55. [DOI] [PubMed] [Google Scholar]
- 8. Ishchenko BI, Krivets NP. Differential x‐ray diagnosis of delimited pneumosclerosis with centra1 lung cancer. Vopr Onkol 1982; 28: 70–5 (In Russian.) [PubMed] [Google Scholar]
- 9. Yesner R, Carter D. Pathology of carcinoma of the lung. Changing patterns. Clin Chest Med 1982; 3: 257–89. [PubMed] [Google Scholar]
- 10. Stojsic J, Radojicic J, Markovic J et al Gender and age trends of histological types of lung cancer in a 20‐year period: Pathological perspective. J BUON 2010; 15: 136–40. [PubMed] [Google Scholar]
- 11. Hajmanoochehri F, Mohammadi N, Zohal MA, Sodagar A, Ebtehaj M. Epidemiological and clinicopathological characteristics of lung cancer in a teaching hospital in Iran. Asian Pac J Cancer Prev 2014; 15: 2495–500. [DOI] [PubMed] [Google Scholar]
- 12. Al‐Alao BS, O'Callaghan DS, Gately K et al Surgical resection for non‐small cell lung cancer: Clinical features and outcomes for a consecutive series at an Irish tertiary referral centre. Ir J Med Sci 2013; 182: 217–25. [DOI] [PubMed] [Google Scholar]
- 13. Zou XN, Lin DM, Wan X et al Histological subtypes of lung cancer in Chinese males from 2000 to 2012. Biomed Environ Sci 2014; 27: 3–9. [DOI] [PubMed] [Google Scholar]
- 14. Yang P, Xie L, Yang X, Li JH, Li S, Cun DZ. Characteristics of 793 cases of lung cancer in Yunnan Province. J Kunming Med Univ 2013; 34: 65–7. [Google Scholar]
- 15. Gong SN, Sang CL, Xu ZY et al Clinicopathological characteristics of 130 cases of lung cancer in the youth. Chin J Lung Cancer 2014; 17: 465–8 (In Chinese.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang SX, Zhou W, Tan H, Yin M. Clinical study of lung cancer with different types of pathology diagnosed through bronchoscope. China J Endosc 2007; 13: 966–8 (In Chinese.) [Google Scholar]
- 17. Lin PS, Chen CX, Lin SQ, Yin JF, Zhang B. Analysis of cases characteristics of lung cancer from 2004 to 2013. Chin Med Rec 2014; 15 (6): 60–2 (In Chinese.) [Google Scholar]
- 18. Kong JY, Xu FX, He M, Chen K, Qian B. The incidence of lung cancer by histological type: A population‐based study in Tianjin, China during 1981–2005. Respirology 2014; 19: 1222–8. [DOI] [PubMed] [Google Scholar]
- 19. Zheng WL, Li W, Xie J. A cross‐sectional study on the smoking status of residents aged above 15 years old in Tianjin. Chin J Prev Control Chron Non‐Commun Dis 2011; 19: 150–1 (In Chinese.) [Google Scholar]
- 20. Haussmann HJ. Smoking and lung cancer: Future research directions. Int J Toxicol 2007; 26: 353–64. [DOI] [PubMed] [Google Scholar]
- 21. Thun MJ, Henley SJ, Calle EE. Tobacco use and cancer: An epidemiologic perspective for geneticists. Oncogene 2002; 21: 7307–25. [DOI] [PubMed] [Google Scholar]
- 22. Barbone F, Bovenzi M, Cavallieri F, Stanta G. Cigarette smoking and histologic type of lung cancer in men. Chest 1997; 112: 1474–9. [DOI] [PubMed] [Google Scholar]
- 23. Khuder SA, Mutgi AB. Effect of smoking cessation on major histologic types of lung cancer. Chest 2001; 120: 1577–83. [DOI] [PubMed] [Google Scholar]
- 24. Devesa SS, Bray F, Vizcaino AP, Parkin DM. International lung cancer trends by histologic type: Male:female differences diminishing and adenocarcinoma rates rising. Int J Cancer 2005; 117: 294–9. [DOI] [PubMed] [Google Scholar]
- 25. Centers for Disease Control and Prevention (CDC) . School‐based tobacco‐use prevention – People's Republic of China, May 1989–January 1990. MMWR Morb Mortal Wkly Rep 1993; 42: 370–1. [PubMed] [Google Scholar]
- 26. Xiao Y, Shao Y, Yu X, Zhou G. The epidemic status and risk factors of lung cancer in Xuanwei City, Yunnan Province, China. Front Med 2012; 6: 388–94. [DOI] [PubMed] [Google Scholar]
- 27. Du YX, Cha Q, Chen XW et al An epidemiological study of risk factors for lung cancer in Guangzhou, China. Lung Cancer 1996; 14 (Suppl 1): S9–37. [DOI] [PubMed] [Google Scholar]
- 28. Ko YC, Cheng LS, Lee CH et al Chinese food cooking and lung cancer in women nonsmokers. Am J Epidemiol 2000; 151: 140–7. [DOI] [PubMed] [Google Scholar]
- 29. Reid BC, Ghazarian AA, DeMarini DM. Research opportunities for cancer associated with indoor air pollution from solid‐fuel combustion. Environ Health Perspect 2012; 120: 1495–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhou BS, Wang TJ, Guan P, Wu JM. Indoor air pollution and pulmonary adenocarcinoma among females: A case‐control study in Shenyang, China. Oncol Rep 2000; 7: 1253–9. [DOI] [PubMed] [Google Scholar]
- 31. Yu IT, Chiu YL, Au JS, Wong TW, Tang JL. Dose‐response relationship between cooking fumes exposures and lung cancer among Chinese nonsmoking women. Cancer Res 2006; 66: 4961–7. [DOI] [PubMed] [Google Scholar]
- 32. Brownson RC, Reif JS, Keefe TJ, Ferguson SW, Pritzl JA. Risk factors for adenocarcinoma of the lung. Am J Epidemiol 1987; 125: 25–34 (Published erratum appears in Am J Epidemiol 1987;126: 363). [DOI] [PubMed] [Google Scholar]
- 33. Wang XZ, Qin YK, Gu JD et al Systematic review of studies of workplace exposure to environmental tobacco smoke and lung cancer risk. Chin J Lung Cancer 2011; 14: 345–50 (In Chinese.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fu X, Feng T, Wu M, Zhang L, Jiang C. [Relationship between environmental tobacco smoke and lung cancer risk among nonsmokers in China: A meta‐analysis.] Chin J Prev Med 2015;49:644–8. (In Chinese.) [PubMed] [Google Scholar]
- 35. Han JX, Ma L, Zhang HW et al A cross sectional study of passive smoking of non‐smoking women and analysis of influence factors on women passive smoking. J Hyg Res 2006; 35: 609–11 (In Chinese.) [PubMed] [Google Scholar]
- 36. Vineis P, Hoek G, Krzyzanowski M et al Air pollution and risk of lung cancer in a prospective study in Europe. Int J Cancer 2006; 119: 169–74. [DOI] [PubMed] [Google Scholar]
- 37. Chen F, Jackson H, Bina WF. Lung adenocarcinoma incidence rates and their relation to motor vehicle density. Cancer Epidemiol Biomarkers Prev 2009; 18: 760–4. [DOI] [PubMed] [Google Scholar]
- 38. Chen F, Cole P, Bina WF. Time trend and geographic patterns of lung adenocarcinoma in the United States, 1973–2002. Cancer Epidemiol Biomarkers Prev 2007; 16: 2724–9. [DOI] [PubMed] [Google Scholar]
- 39. Katanoda K, Sobue T, Satoh H et al An association between long‐term exposure to ambient air pollution and mortality from lung cancer and respiratory diseases in Japan. J Epidemiol 2011; 21: 132–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang WS, Zhao H, Wang X, Deng Q, Fan WY, Wang L. An evidence‐based assessment for the association between long‐term exposure to outdoor air pollution and the risk of lung cancer. Eur J Cancer Prev 2016; 25: 163–72. [DOI] [PubMed] [Google Scholar]
- 41. Zou J, Yang L. The ambient air quality and trends in cities of Sichuan Province. Sichuan Environ 2010(4); 29: 50–3 (In Chinese.) [Google Scholar]
- 42. Kan HD, Chen RJ, Tong SL. Ambient air pollution, climate change, and population health in China. Environ Int 2012; 42:10–9. [DOI] [PubMed] [Google Scholar]
- 43. Raaschou‐Nielsen O, Andersen ZJ, Beelen R et al Air pollution and lung cancer incidence in 17 European cohorts: Prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE). Lancet Oncol 2013; 14: 813–22. [DOI] [PubMed] [Google Scholar]
- 44. Liaw YP, Ting TF, Ho CC, Chiou ZY. Cell type specificity of lung cancer associated with nitric oxide. Sci Total Environ 2010; 408: 4931–4. [DOI] [PubMed] [Google Scholar]
- 45. International Agency for Research on Cancer . Personal habits and indoor combustions In: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 100E IARC Press, Lyon: 2012. [PMC free article] [PubMed] [Google Scholar]
- 46. He J, Chen XF, Deng Y et al Investigation on smoking among residents over age of 18 in Sichuan. J Occup Health Damage 2012; 27: 274–7 (In Chinese.) [Google Scholar]
- 47. Kligerman S, White C. Epidemiology of lung cancer in women: Risk factors, survival, and screening. AJR Am J Roentgenol 2011; 196: 287–95. [DOI] [PubMed] [Google Scholar]
- 48. Márquez‐Garbán DC, Chen HW, Fishbein MC, Goodglick L, Pietras RJ. Estrogen receptor signaling pathways in human non‐small cell lung cancer. Steroids 2007; 72: 135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang R, Wu CX, Zhang J, Bao PP, Chen HQ. Lung cancer in young patients aged from 15 to 44 years: Incidence trend, current status and survival analysis from 2002 to 2005. Tumor 2009; 29: 1146–52. [Google Scholar]
- 50. Wang J, Xu F, Zhou QH et al Advances in epidemiology of lung cancer. Chin J Lung Cancer 2005; 8: 395–400 (In Chinese.) [DOI] [PubMed] [Google Scholar]
- 51. Han RQ, Zheng RS, Zhang SW, Wu M, Chen W. Trend analyses on the differences of lung cancer incidence between gender, area and average age in China during 1989–2008. Chin J Lung Cancer 2013; 16: 445–51 (In Chinese.) [DOI] [PMC free article] [PubMed] [Google Scholar]


