Abstract
Background
Exopolysaccharides with structural diversity have shown wide applications in biomaterial, food, and pharmaceutical industries. Herein, we isolated an endophytic strain, 14‐DS‐1, from the traditional medicinal plant Codonopsis pilosula to elucidate the characteristics and anti‐cancer activities of purified exopolysaccharides.
Methods
HPLC and GC‐MS were conducted to purify and characterize the exopolysaccharides isolated from 14‐DS‐1. Quantitative RT‐PCR, cell migration assays, immunofluorescence staining, and flow cytometry analysis were conducted to investighate the biological activity of DSPS.
Results
We demonstrated that exopolysaccharides isolated from 14‐DS‐1 (DSPS), which were predominately composed of six monosaccharides, showed anti‐cancer activities. Biological activity analysis revealed that exposure to DSPS induced macrophage activation and polarization by promoting the production of TNF‐α and nitric oxide. Further analysis revealed that DSPS treatment promoted macrophage infiltration, whereas cancer cell migration was suppressed. In addition, DSPS exposure led to S‐phase arrest and apoptosis in cancer cells. Immunofluorescence staining revealed that treatment with DSPS resulted in defects in spindle orientation and positioning.
Conclusion
These findings thus suggest that DSPS may have promising potential in cancer therapy.
Keywords: Cancer, Codonopsis pilosula, endophyte, exopolysaccharide, macrophage
Introduction
Endophytes are endosymbionts; these organisms, including bacteria and fungi, are ubiquitously found in plants and evolve along with the host plant.1, 2, 3, 4 Endophytes provide the host with many benefits: they can promote growth, contribute to nutrient acquisition, or improve resistance to biotic and abiotic stresses. Endophytes isolated from medicinal plants are reported to be capable of producing the same or similar secondary metabolites as their host plants.5, 6, 7, 8, 9, 10 For example, the endophytic fungus isolated from the yew tree, Taxusbrevifolia, can produce the anti‐cancer drug, paclitaxel.11 Codonopsispilosula is an herbaceous perennial plant mainly grown in China, Japan, and Korea that is widely used in traditional medicine. C. pilosula extracts are very complex, consisting of polysaccharides, saponins, sesquiterpenes, polyphenolic glycosides, alkaloids, polyacetylenes, and phytosteroids.12, 13, 14 Polysaccharides isolated from C. pilosula are one of the plant's important active constituents, with multiple biological activities, including antioxidant, anti‐cancer, and immunomodulatory properties.15, 16, 17, 18, 19, 20, 21 Given that C. pilosula endophytes are able to produce similar bioactive molecules as the plant itself, isolation and identification of C. pilosula endophytes could lead to the discovery of novel bioactive molecules.
Microbial polysaccharides, including intracellular polysaccharides, capsular polysaccharides, and exopolysaccharides (EPSs), are natural macromolecules abundant in microorganisms that are important for the maintenance of cell wall integrity and for the regulation of host‐pathogen interactions.22, 23 EPSs secreted by microorganisms into the extracellular environment are structurally diverse, allowing for a variety of potential applications in the biomaterial and pharmaceutical industries. A growing body of evidence has revealed that microbial EPSs are beneficial to human health; they show promising activities, including immunomodulation and cytotoxic effects against cancer cells.24, 25, 26, 27, 28, 29 Importantly, emerging studies have revealed that the structural units of the EPS not only determine its function, but the ecological niches of the host microorganism also contribute to EPS activity.30, 31, 32, 33, 34, 35, 36, 37 For example, EPS produced by Paenibacilluspolymyxa, a bacterium isolated from Stemona japonica (Blume) Miquel, exhibited robust scavenging activities for superoxide and hydroxyl radicals.5 Thus, isolating and characterizing novel EPSs from medicinal plants may lead to the identification of promising biological macromolecules. In this study, we sought to isolate endophytes from the root of C. pilosula and to elucidate the characteristics and anti‐cancer activities of purified EPSs.
Methods
Chemicals and antibodies
All chemicals were purchased from Sigma‐Aldrich (St. Louis, MO, USA). Fluorescein isothiocyanate (FITC)‐conjugated phalloidin was obtained from ThermoFisher Scientific (Waltham, MA, USA). Antibodies against α‐tubulin and γ‐tubulin were obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Rhodamine or fluorescein‐conjugated secondary antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA, USA). The annexin V‐FITC/PI apoptosis detection kit used was obtained from Sigma‐Aldrich. Transwell inserts were obtained from BD Biosciences (San Jose, CA, USA).
Cell culture and treatment
RAW264.7 macrophages, BT549 and MDA‐MB‐231 breast cancer cells, and HeLa cervical cancer cells were obtained from American Type Culture Collection (Rockville, MD, USA) and cultured in medium as described previously.38 Cells were maintained at 37°C in a humidified incubator containing 5% CO2. Various concentrations of exopolysaccharides isolated from 14‐DS‐1 (DSPS) were added to cell culture medium for the indicated times.
Isolation of endophytes from C. pilosula
Fresh C. pilosula roots were washed several times under running tap water and sterilized with 75% ethanol for 20 seconds and then with 2.5% sodium hypochlorite for five minutes. C. pilosula roots were cut vertically into small segments to expose the inner surface and then inoculated on agar plates. Plates were incubated at 37°C for 48 hours to promote endophyte growth.39 Each endophytic culture was checked for purity and transferred to freshly prepared agar plates. Appropriate controls were also set up in which no plant tissues were inoculated.
Physiological characteristics and phylogenetic relationship determination
The physiological characteristics of the isolated strain 14‐DS‐1 were characterized according to the procedures outlined in Bergey's Manual of Systematic Bacteriology.40 In brief, 16S rDNA from the 14‐DS‐1strain was sequenced, followed by analysis with the BLASTn program (https://blast.ncbi.nlm.nih.gov/Blast.cgi). To analyze the phylogenetic relationship, a neighbor‐joining phylogenetic tree was constructed using the CLUSTAL program (http://www.genome.jp/tools-bin/clustalw), as previously described.41, 42, 43 The defined strain 14‐DS‐1 was deposited in the China General Microbiological Culture Collection Center.
Extraction and purification of exopolysaccharides (EPSs) from C. pilosula endophytes
The 14‐DS‐1 strain was cultured in Luria‐Bertani liquid medium and agitated at 37°C for 48 hours. The biomass of the bacteria was removed by centrifugation at 5000 rpm for 10 minutes, and then the cell‐free supernatants were treated once with Sevage reagent (Sinopharm, Shanghai, China) to remove free protein at 4°C overnight. The volume ratio of the supernatants and Sevage reagent was 4:1, while chloroform and butanol was 5:1. Samples were clarified by centrifugation at 8000 rpm for 15 minutes, and the supernatants were treated with 10% ethanol (v/v) at 4°C overnight. The following day, the samples underwent centrifugation at 10000 rpm at 4°C for 30 minutes. A crude EPS sample was then dissolved in deionized water. The EPS solution was loaded onto a Sephadex G‐50 flow column (16 × 100 cm; Sigma‐Aldrich) and eluted with deionized water. Only one major fraction was detected, and the collected fraction was termed DSPS.
Molecular weight determination
The molecular weight of DSPS was determined by high performance liquid chromatography. Size‐exclusion chromatographic (SEC) separation was carried out using a Shodex 803 column connected to a Waters model 515 HPLC system (Waters, Rydalmere NSW, Australia) coupled to a refractive index detector (Wyatt‐OptilabrEX) and a Wyatt‐DAWN HELEOS‐II laser light scattering spectrometer (Wyatt Technology Corporation, Santa Barbara, CA, USA).
Monosaccharide composition analysis
The purified polysaccharide sample (2 mg) was hydrolyzed with 2 mL of 2 M trifluoroacetic acid at 110°C for 1.5 hours. After hydrolysis, the solution was evaporated to dryness at 50°C, and then a stream of methanol (3 mL) was used to remove the excess acid. This procedure was repeated five times. Sodium borohydride (60 mg) was then added to obtain the reducing reaction at room temperature for eight hours. Several drops of glacial acetic acid were added to stop the reaction. The solution was then evaporated to dryness at 50°C. Methanol (3 mL) was used to remove the reducing agent five times, and then the residue was dried at 110°C for 15 minutes. Acetylation was performed using acetic anhydride (3 mL) and pyridine (1 mL) at 100°C for five hours. The mixture was then evaporated to dryness and trichloromethane (5 mL) was added to dissolve the residue. The organic phase consisted of washing with distilled water (2 mL) four times to remove impurities. Finally, the water was removed with anhydrous sodium sulfate and the mixture was transferred into a vial for gas chromatography‐mass spectrometer (GC‐MS) (Agilent Technologies, Santa Clara, CA, USA) analysis. The GC‐MS was used to separate the monosaccharides. A capillary column Hp‐5 (Agilent 19091J‐413) (30 m × 0.25 mm × 320 μm) was used, with 30.0 mL/min for hydrogen, 400.0 mL/min for air, and with helium as carrier gas at a constant flow of 1 mL/min. The temperature program was set as: initial temperature 120°C, 3°C/min ramp to 250°C, and held for five minutes. The total duration of analysis was 35 minutes. The temperature of the injection port was 250°C and a 1 μL volume was injected in splitless mode.
Quantitative reverse transcriptase‐PCR
Quantitative reverse transcriptase‐PCR (RT‐PCR) assay was performed as previously described.44, 45 Briefly, total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. RNA was reverse transcribed to complementary DNA using a reverse transcription PCR Purification kit (Promega, Madison, WI, USA). TNF‐α messenger RNA expression was evaluated by quantitative RT‐PCR (TNF‐α primers: 5′‐CCCAAAGGGATGAGAAGTTCCCAAAT‐3′ and 5′‐CCACTTGGTGGTTTGCTACGACG‐3′; β‐actin primers: 5′‐CAGAAGGAGATTACTGCTCTGGCT‐3′, and 5′‐TACTCCTGCTTGCTGATCCACATC‐3′).
Quantification of nitric oxide production
Nitric oxide (NO) production was examined as previously described.46 In brief, RAW264.7 cells were treated with various concentrations of DSPS for 24 hours. NO production was estimated by measuring nitrite levels in cell supernatants with Greiss reagent (1% sulfanilamide, 0.1% napthyl ethyl diamine dihydrochloride, 2.5% phosphoric acid; Sigma‐Aldrich). Absorbance was read at 540 nm, and NaNO2 was used as a standard to quantify NO production.
Cell migration assays
For transwell migration assay (BD Biosciences), RAW264.7 cells resuspended in serum‐free medium were pretreated with various concentrations of DSPS. Cells were then added to insert chambers and incubated for 16 hours. Cells on the upper surface of the inserts were completely wiped off with a cotton swab, while cells that migrated to the underside of the inserts were fixed with 4% paraformaldehyde for 15 minutes at room temperature. Cells were then stained with 0.5% crystal violet in 20% methanol for 30 minutes, washed three times with distilled water, and visualized with a Leica stereomicroscope (Leica Microsystems, Wetzlar, Germany).47, 48 For wound healing assay, cells were grown to 90% confluence in a 12‐well plate, and a scratch wound was created in the monolayer using a pipette tip. Cell debris was removed by washing three times with phosphate‐buffered saline (PBS). Cells were incubated in complete culture medium for the indicated times to allow wound healing. Phase‐contrast images of the wound were captured at the indicated time points.
Fluorescence microscopy
Cells were fixed with 4% paraformaldehyde for 20 minutes, permeabilized with 0.05% Triton X‐100 (Sigma‐Aldrich) in PBS for 30 minutes, and blocked with 2.5% bovine serum albumin for one hour at room temperature. Cells were then sequentially incubated with the indicated primary and secondary antibodies, followed by staining with 4′,6‐diamidino‐2‐phenylindole. Coverslips were mounted with 90% glycerol and visualized with a Leica TCS SP8 confocal microscope (Leica Microsystems). The spindle angle and positioning were determined as previously described.49, 50, 51
Fluorescence‐activated cell sorting analysis
Cell cycle progression and apoptosis analysis were performed as previously described.52 In brief, cells were collected and washed twice with ice cold PBS, and then fixed with 70% ethanol. Cell suspensions were clarified by centrifugation at 1000 × g for 10 minutes, and the pellets were resuspended in phosphate/citrate buffer (pH 7.5) for 30 minutes. To analyze cell cycle progression, cells were washed with PBS and stained with propidium iodide (PI) for 30 minutes. To analyze apoptosis, cells were stained with Annexin V‐FITC/PI for 15 minutes using the Annexin V‐FITC/PI Apoptosis Detection Kit (Sigma‐Aldrich). Samples were analyzed using a flow cytometer (BD Accuri C6 Plus, BD Biosciences).
Results
Strain isolation and biochemical and molecular characterization of 14‐DS‐1
To isolate and identify endophytes capable of producing bioactive molecules, we collected fresh C. pilosula root tissue and successfully isolated a strain, 14‐DS‐1, which was capable of producing EPSs. Characterization of this strain revealed that 14‐DS‐1 is an aerobic, gram‐positive bacterium that utilizes sucrose, glucose, and maltose as carbon sources (Fig 1a,b). We then analyzed the phylogeny of this strain by 16S rDNA sequencing. The 16S rDNA gene sequences of 14‐DS‐1 (GenBank accession number: KY658460) were amplified by PCR, sequenced, and compared to all sequences in GenBank. The 14‐DS‐1 strain belonged to a sub‐branch of the genus Bacillus, Bacillusaryabhattai; based on sequence alignment, 14‐DS‐1 is 99% similar to this strain. To elucidate the evolutionary relationship, we constructed a phylogenetic tree using the neighbor‐joining method. We found that 14‐DS‐1 is the closest match to Bacillus sp., a genus that includes the Bacillus sp. SG1‐2, Bacillus megaterium, and Bacillus aryabhattai strains, with 98% similarity (Fig 1c).
Figure 1.
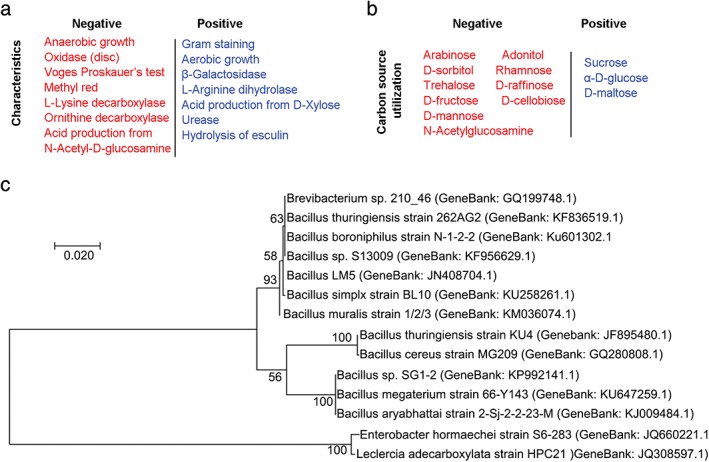
Determination of the physiological characteristics and phylogenetic relationships of the isolated strain. (a) Characteristics of the endophytic strain 14‐DS‐1 isolated from Codonopsis pilosula. (b) Carbon‐source utilization analysis of the strain 14‐DS‐1. (c) Neighbor‐joining phylogenetic relationship analysis based on 16S rDNA sequencing. Genbank accession numbers are annotated in parentheses. Scale bars, 0.02 substitutions per nucleotide position.
Characterization of EPSs purified from 14‐DS‐1
As EPSs constitute the major active biomolecule produced by endophytic bacteria, we next isolated and purified EPSs from 14‐DS‐1. The deproteinized and decolorized constituents secreted by 14‐DS‐1 contained ~32% EPSs. The crude EPSs were further purified by Sephadex G‐50 flow column chromatography (Sigma‐Aldrich). Only one fraction was obtained, which was named DSPS (Fig 2a). The molecular weight of DSPS was determined using a Wyatt‐DAWN HELEOS‐II laser light scattering spectrometer (Wyatt Technology Corporation), and the molecular weight was found to be 1.68 × 104 Da (data not shown). The monosaccharide composition of DSPS was analyzed by GC‐MS, revealing that it is mainly composed of galactose and glucose, as well as rhamnose, fucose, arabinose, and mannose (Fig 2b). Additionally, we obtained the infrared spectrum of DSPS (Fig 2c).
Figure 2.
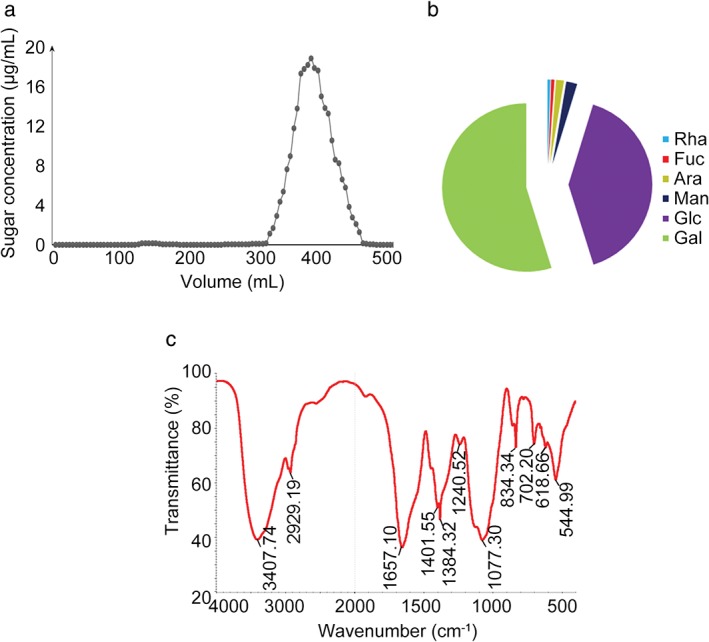
Characterization of DSPS purified from the strain 14‐DS‐1. (a) Exopolysaccharides were isolated using a Sephadex G‐50 flow column. (b) Analysis of the monosaccharide composition of DSPS. Rha, Fuc, Ara, Man, Glc, Gal. (c) The infrared spectrum of DSPS.
DSPS promotes macrophage polarization toward the classically activated macrophage (M1) phenotype
As the primary phagocytic cell type, macrophages are activated to phagocytose foreign cells and cellular debris.53 We investigated the effect of DSPS on macrophage activation in RAW264.7 cells. Activated macrophages undergo morphological transformation, changing from round‐shaped cells to flattened spreading cells with pseudopodium‐like protrusions. We found that in the control cells and those treated with low concentrations of DSPS, cells remained round; however, exposure to a higher concentration of DSPS (5 mg/mL) led to macrophage activation, as evidenced by an elongated morphology in appropriately 25% of cells (Fig 3a,b). Macrophages can be polarized toward an anti‐cancer, M1, or alternatively, pro‐tumor, M2, phenotype, depending on the stimuli. To determine the role of DSPS in macrophage activation, we analyzed the production of inflammatory mediators, TNF‐α and NO, both of which are secreted by the M1 macrophage subset.54 We found that higher concentrations of DSPS significantly elevated the production of TNF‐α and NO, indicating a critical role of DSPS in promoting polarization of macrophages to the M1 phenotype (Fig 3c,d). This result may provide valuable information for the development of macrophage‐mediated immune therapy for cancer treatment.
Figure 3.
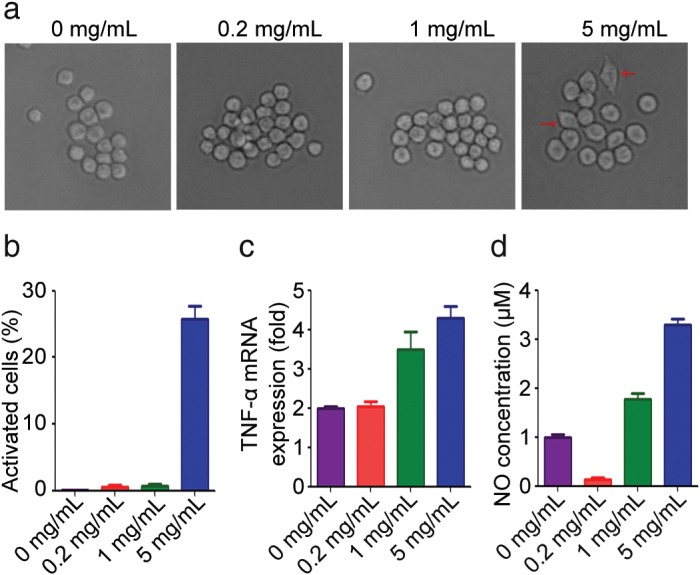
DSPS activates macrophages. (a) RAW264.7 cells were treated with various concentrations of DSPS for 24 hours, and the cellular morphology was analyzed with a phase‐contrast microscope. (b) Quantification of activated macrophages for experiments performed as described in (a). (c) Quantitative reverse transcriptase‐PCR analysis of the relative expression of TNF‐α messenger RNA (mRNA). (d) Quantification of nitric oxide (NO) production by RAW264.7 cells.
DSPS plays roles in the differentiation and migration of macrophages and in cancer cell migration
As macrophage infiltration to the site of tumors is a prerequisite for macrophages to inhibit tumor progression, we explored the regulatory role of DSPS in macrophage migration. RAW264.7 cells were treated with different concentrations of DSPS, and cell migration was analyzed using a transwell assay. We found that high concentration of DSPS (1 mg/mL and 5 mg/mL) significantly promoted RAW264.7 cell migration across the permeable membrane (Fig 4a,b).
Figure 4.
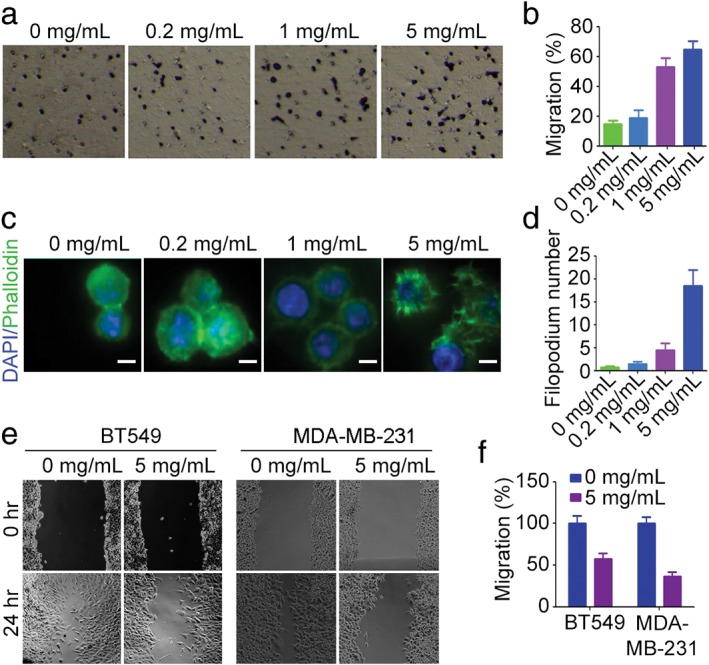
DSPS affects cell migration. (a) Transwell migration assay showing the effect of DSPS treatment on RAW264.7 cell migration. (b) Quantification of cell migration; cells were treated as in panel (a). (c) Immunofluorescence staining of actin (fluorescein isothiocyanate‐conjugated phalloidin) and nuclei (4′, 6‐diamidino‐2‐phenylindole) in RAW264.7 cells. Scale bars, 5 μm. (d) Quantification of filopodia presented in panel (c). (e) Representative wound healing assay analyzing the effects of DSPS on the migration of BT549 and MDA‐MB‐231 breast cancer cells. (f) Quantification of migrated cells treated as in panel (e).
To investigate the underlying mechanism of the DSPS mediated enhancement of macrophage migration, we analyzed the effect of DSPS on the formation of filopodia: actin‐rich membrane protrusions critical for cell adhesion and migration. Immunostaining of F‐actin revealed that high concentrations of DSPS led to an increase in filopodia formation (Fig 4c,d), suggesting a role of DSPS in filopodium‐mediated microphage migration. Additionally, we investigated the effect of DSPS on cancer cell migration. In contrast to the results observed in macrophages, DSPS remarkably repressed the migration of BT549 and MDA‐MB‐231 breast cancer cells (Fig 4e,f), suggesting its potential as an inhibitor of tumor metastasis.
DSPS affects cell cycle progression and promotes apoptosis of cancer cells
To further investigate the anti‐cancer properties of DSPS, we analyzed its effect on cell cycle progression. MDA‐MB‐231 cells treated with various concentrations of DSPS were stained with 4′,6‐diamidino‐2‐phenylindole and analyzed by flow cytometry. Treatment with DSPS (5 mg/mL) resulted in an increase in S phase cells and a decline in G2/M cells, indicating that DSPS inhibits the DNA replication process (Fig 5a,b).
Figure 5.
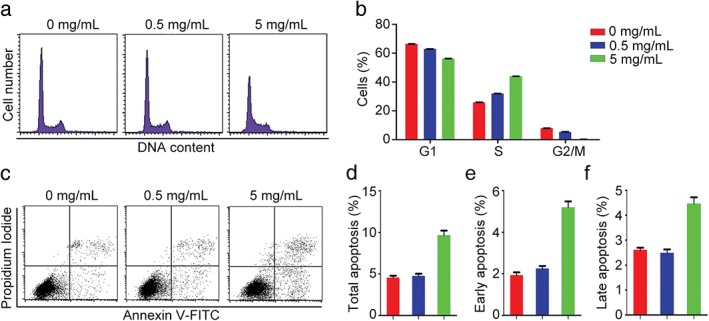
DSPS alters cell cycle progression and promotes apoptosis. (a) MDA‐MB‐231 cells treated with various concentrations of DSPS were stained with PI, and the cells were analyzed by flow cytometry to determine cell cycle progression. (b) Quantification of the percentage of cells in G1, S, and G2/M phases of cells treated as in panel A. 0 mg/mL, 0.5 mg/mL, 5 mg/mL. (c–f) MDA‐MB‐231 cells treated with various concentrations of DSPS and stained with Annexin V fluorescein isothiocyanate (FITC)/propidium iodide (c); the percentage of total apoptotic cells (d), cells in early apoptosis (e), and cells in late apoptosis (f) were quantified.
Next, we sought to determine whether DSPS treatment could induce cell death. Using Annexin V‐FITC/PI staining, we found that treatment with DSPS (5 mg/mL) remarkably increased the percentage of apoptotic cells, including cells in both early and late stages of apoptosis (Fig 5c–f). Collectively, these data suggest a promising anti‐proliferative role of DSPS in the treatment of cancer cells.
DSPS affects spindle orientation and positioning in cancer cells
As spindle orientation is essential for proper cell division and cell fate determination, we investigated the effect of DSPS on this process. Immunofluorescence microscopy revealed that DSPS treatment increased the z‐value between the bipolar spindles without affecting the overall morphology or spindle length (Fig 6a,b); this change resulted in an increase in the spindle angle (Fig 6a–c). Next, we measured the distance between the cell center and the spindle center to determine the effect of DSPS on spindle positioning (Fig 6d). Exposure to DSPS (5 mg/mL) significantly increased the distance between the cell center and the spindle center, suggesting a spindle‐positioning defect (Fig 6e,f). Taken together, these data reveal a critical role of DSPS in the regulation of spindle orientation and positioning.
Figure 6.
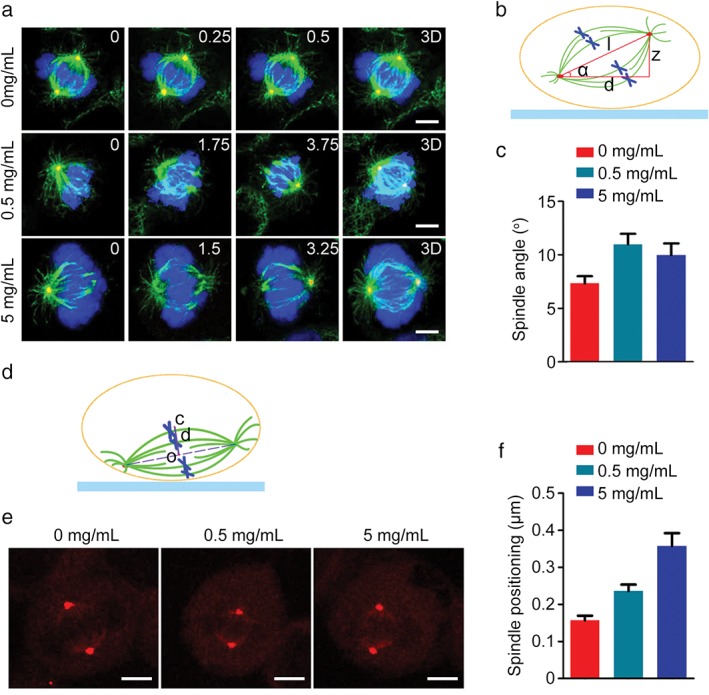
DSPS impairs spindle orientation and positioning. (a) HeLa cells treated with various concentrations of DSPS were stained with α‐tubulin (green) and γ‐tubulin (red) antibodies and 4′, 6‐diamidino‐2‐phenylindole (blue). The position of the z stage is indicated in micrometers; 3D, xy projection. Scale bars, 5 μm. (b) A diagram illustrating the spindle angle (α) measurement. (c) Determination of average spindle angle of cells treated as in panel (a). 0 mg/mL, 0.5 mg/mL, 5 mg/mL. (d) A diagram illustrating the distance (d) between the cell center (c) and the spindle center (o). (e) HeLa cells treated with DSPS were immunostained with γ‐tubulin to visualize spindle poles. Scale bars, 5 μm. (f) Quantification of the distance between the cell center and the spindle center. 0 mg/mL, 0.5 mg/mL, 5 mg/mL.
Discussion
C. pilosula is a traditional medicinal plant with promising anti‐cancer activity. However, little progress has been made in isolating and identifying endophytes from this plant capable of producing active secondary metabolites.45, 55, 56, 57 In this study, we isolated an endophyte strain, 14‐DS‐1, characterized its phylogenetic relationships, and analyzed its physiological properties. Importantly, our study revealed that EPS is the predominant component of 14‐DS‐1 extracts, and DSPS, as its active ingredient, exhibits promising immunoregulatory and anti‐cancer properties. EPSs, which are composed of repeating units of sugars or sugar derivatives, can be grouped into homopolysaccharides or heteropolysaccharides, depending on their monosaccharide compositions.45, 58, 59, 60, 61 Our analysis revealed that DSPS is a heteropolysaccharide comprising six monosaccharides. EPSs produced by endophytes are strain‐dependent, and their structural and compositional diversity conveys unique and varied biological activities.31, 44, 62, 63, 64 Given that endophytes can be grown in large‐scale cultures, have a short growth period, and are easily isolated, the 14‐DS‐1 strain isolated in this study may have great potential for future applications.
Because of their role in phagocytosis and antigen presentation, macrophages are critical for cancer immunosurveillance.65, 66, 67, 68 Macrophage activation and infiltration in response to inflammation is important for macrophage‐related immunosurveillance. Our data demonstrate that DSPS promotes macrophage activation and migration, suggesting an immunomodulatory function for this EPS. While macrophages are important to inhibit tumor development, a growing body of evidence demonstrates that cancer cells can foster macrophages present in the tumor microenvironment to promote tumor growth.69, 70, 71 Specifically, macrophages play distinct roles in cancer progression depending on their polarized phenotype.72 Classically activated macrophages (M1) suppress tumor growth, metastasis, and angiogenesis. However, in response to the tumor microenvironment, M1 cells can be induced to become alternatively polarized M2 cells, a change that facilitates the escape of cancer cells from immune surveillance. M1 and M2 subtypes can be shifted reversibly in response to different environmental stimuli.67, 72, 73 Therefore, identification of biological molecules that can repolarize the tumor‐promoting M2 cells into anti‐cancer M1 subsets would be of therapeutic value for the treatment of cancer. Importantly, we revealed that DSPS isolated from 14‐DS‐1 enhances the production of TNF‐α and NO, inflammatory mediators secreted by anti‐cancer M1 subsets,74, 75, 76 suggesting a potential application for this molecule in cancer treatment.
Further analysis revealed that treatment with DSPS also increased macrophage migration; in contrast, cancer cell migration was inhibited by this polysaccharide. However, the molecular mechanism underlying the role of DSPS in cell migration remains to be elucidated. Additionally, we found that exposure to DSPS resulted in synthesis phase (S phase) cell cycle arrest and apoptosis of cancer cells, demonstrating its anti‐cancer activity. Furthermore, our data demonstrate that treatment with DSPS resulted in impaired spindle orientation and positioning, events critical for proper cell division and cell fate determination.50, 51 Given that inappropriate cell division can affect cell cycle progression and initiate apoptotic cell death, DSPS‐induced defects in spindle orientation and positioning suggest a mechanism for the anti‐cancer effect of this molecule, underscoring its potential for use in cancer therapy.
Disclosure
No authors report any conflict of interest.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (31741039, 31701216, and 31770997), the Natural Science Foundation of Shandong Province (ZR2017MC008), and the China Postdoctoral Science Foundation (2016M600553).
Contributor Information
Songbo Xie, Email: xiesongbo@sdnu.edu.cn.
Min Liu, Email: minliu@sdnu.edu.cn.
References
- 1. Abdalla MA, Matasyoh JC. Endophytes as producers of peptides: An overview about the recently discovered peptides from endophytic microbes. Nat Prod Bioprospect 2014; 4: 257–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nair DN, Padmavathy S. Impact of endophytic microorganisms on plants, environment and humans. Sci World J 2014; 2014: 250693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang CM, An LG, Huang Y. Two new species of Terschellingia (Nematoda: Monhysterida: Linhomoeidae) from the East China Sea. Cah Biol Mar 2017; 58: 33–41. [Google Scholar]
- 4. Yang HT, Zou SS, Zhai LJ et al Pathogen invasion changes the intestinal microbiota composition and induces innate immune responses in the zebrafish intestine. Fish Shellfish Immunol 2017; 71: 35–42. [DOI] [PubMed] [Google Scholar]
- 5. Liu J, Luo JG, Ye H, Sun Y, Lu ZX, Zeng XX. Production, characterization and antioxidant activities in vitro of exopolysaccharides from endophytic bacterium Paenibacillus polymyxa EJS‐3. Carbohydr Polym 2009; 78: 275–81. [Google Scholar]
- 6. Zheng LP, Zou T, Ma YJ, Wang JW, Zhang YQ. Antioxidant and DNA damage protecting activity of exopolysaccharides from the endophytic bacterium Bacillus cereus SZ1. Molecules 2016; 21: pii: E174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lü L, Zhao ZT. Lecanora shangrilaensis sp nov., on pinecones from China. Mycotaxon 2017; 132: 441–4. [Google Scholar]
- 8. Wang WC, Zhao ZT, Zhang LL. Four new records of Rhizocarpon from China. Mycotaxon 2015; 130: 739–47. [Google Scholar]
- 9. Zhao X, Zhang LL, Sun LY, Hu L, Zhao ZT. Four new records of Lecanoraceae in China. Mycotaxon 2015; 130: 707–15. [Google Scholar]
- 10. Zhao XX, Zhao ZT, Miao CC, Ren ZJ, Zhang LL. Five Lecidea lichens new to China. Mycotaxon 2017; 132: 317–26. [Google Scholar]
- 11. Xie S, Zhou J. Harnessing plant biodiversity for the discovery of novel anticancer drugs targeting microtubules. Front Plant Sci 2017; 8: 720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mizutani K, Yuda M, Tanaka O et al Chemical studies on Chinese traditional medicine, dangshen. I. Isolation of (Z)‐3‐ and (E)‐2‐hexenyl beta‐D‐glucosides. Chem Pharm Bull (Tokyo) 1988; 36: 2689–90. [DOI] [PubMed] [Google Scholar]
- 13. Nörr H, Wagner H. New constituents from Codonopsis pilosula . Planta Med 1994; 60: 494–5. [DOI] [PubMed] [Google Scholar]
- 14. Wong MP, Chiang TC, Chang HM. Chemical studies on Dangshen, the root of Codonopsis pilosula . Planta Med 1983; 49: 60. [DOI] [PubMed] [Google Scholar]
- 15. Chu X, Liu XJ, Qiu JM, Zeng XL, Bao HR, Shu J. Effects of Astragalus and Codonopsis pilosula polysaccharides on alveolar macrophage phagocytosis and inflammation in chronic obstructive pulmonary disease mice exposed to PM2.5. Environ Toxicol Pharmacol 2016; 48: 76–84. [DOI] [PubMed] [Google Scholar]
- 16. He JY, Ma N, Zhu S, Komatsu K, Li ZY, Fu WM. The genus Codonopsis (Campanulaceae): A review of phytochemistry, bioactivity and quality control. J Nat Med 2015; 69: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li Z, Zhu L, Zhang H et al Protective effect of a polysaccharide from stem of Codonopsis pilosula against renal ischemia/reperfusion injury in rats. Carbohydr Polym 2012; 90: 1739–43. [DOI] [PubMed] [Google Scholar]
- 18. Qin T, Ren Z, Lin D et al Effects of selenizing Codonopsis pilosula polysaccharide on macrophage modulatory activities. J Microbiol Biotechnol 2016; 26: 1358–66. [DOI] [PubMed] [Google Scholar]
- 19. Wang ZT, Ng TB, Yeung HW, Xu GJ. Immunomodulatory effect of a polysaccharide‐enriched preparation of Codonopsis pilosula roots. Gen Pharmacol 1996; 27: 1347–50. [DOI] [PubMed] [Google Scholar]
- 20. Xu C, Liu Y, Yuan G, Guan M. The contribution of side chains to antitumor activity of a polysaccharide from Codonopsis pilosula . Int J Biol Macromol 2012; 50 (4): 891. [DOI] [PubMed] [Google Scholar]
- 21. Yang C, Gou Y, Chen J, An J, Chen W, Hu F. Structural characterization and antitumor activity of a pectic polysaccharide from Codonopsis pilosula . Carbohydr Polym 2013; 98: 886–95. [DOI] [PubMed] [Google Scholar]
- 22. Aslam SN, Newman MA, Erbs G et al Bacterial polysaccharides suppress induced innate immunity by calcium chelation. Curr Biol 2008; 18: 1078–83. [DOI] [PubMed] [Google Scholar]
- 23. Bates JM, Akerlund J, Mittge E, Guillemin K. Intestinal alkaline phosphatase detoxifies lipopolysaccharide and prevents inflammation in zebrafish in response to the gut microbiota. Cell Host Microbe 2007; 2: 371–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nwodo UU, Green E, Okoh AI. Bacterial exopolysaccharides: Functionality and prospects. Int J Mol Sci 2012; 13: 14002–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roca C, Alves VD, Freitas F, Reis MA. Exopolysaccharides enriched in rare sugars: Bacterial sources, production, and applications. Front Microbiol 2015; 6: 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang K, Li W, Rui X, Chen X, Jiang M, Dong M. Characterization of a novel exopolysaccharide with antitumor activity from Lactobacillus plantarum 70810. Int J Biol Macromol 2014; 63: 133–9. [DOI] [PubMed] [Google Scholar]
- 27. Wu Z, Wang G, Pan D et al Inflammation‐related pro‐apoptotic activity of exopolysaccharides isolated from Lactococcus lactis subsp. lactis . Benef Microbes 2016; 7: 761–8. [DOI] [PubMed] [Google Scholar]
- 28. Matsuzaki C, Hayakawa A, Matsumoto K, Katoh T, Yamamoto K, Hisa K. Exopolysaccharides produced by Leuconostoc mesenteroides strain NTM048 as an Immunostimulant to enhance the mucosal barrier and influence the systemic immune response. J Agric Food Chem 2015; 63: 7009–15. [DOI] [PubMed] [Google Scholar]
- 29. Li W, Xia X, Tang W et al Structural characterization and anticancer activity of cell‐bound exopolysaccharide from Lactobacillus helveticus MB2‐1. J Agric Food Chem 2015; 63: 3454–63. [DOI] [PubMed] [Google Scholar]
- 30. Liu SB, Chen XL, He HL et al Structure and ecological roles of a novel exopolysaccharide from the arctic sea ice bacterium Pseudoalteromonas sp. strain SM20310. Appl Environ Microbiol 2013; 79: 224–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sungur T, Aslim B, Karaaslan C, Aktas B. Impact of exopolysaccharides (EPSs) of Lactobacillus gasseri strains isolated from human vagina on cervical tumor cells (HeLa). Anaerobe 2017; 47: 137–44. [DOI] [PubMed] [Google Scholar]
- 32. Wang JS, Zhang Q, Cui F et al Genome‐wide analysis of gene expression provides new insights into cold responses in Thellungiella salsuginea . Front Plant Sci 2017; 8: 713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang PF, Song H, Li CS et al Genome‐wide dissection of the heat shock transcription factor family genes in Arachis. Front Plant Sci 2017; 8: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhao X, Leavitt SD, Zhao ZT et al Towards a revised generic classification of lecanoroid lichens (Lecanoraceae, Ascomycota) based on molecular, morphological and chemical evidence. Fungal Divers 2016; 78: 293–304. [Google Scholar]
- 35. Zhu QL, Sun L, Lian JJ et al The phospholipase C (FgPLC1) is involved in regulation of development, pathogenicity, and stress responses in Fusarium graminearum . Fungal Genet Biol 2016; 97: 1–9. [DOI] [PubMed] [Google Scholar]
- 36. Feng ZT, Deng YQ, Zhang SC et al K+ accumulation in the cytoplasm and nucleus of the salt gland cells of Limonium bicolor accompanies increased rates of salt secretion under NaCl treatment using NanoSIMS. Plant Sci 2015; 238: 286–96. [DOI] [PubMed] [Google Scholar]
- 37. Cao S, Du XH, Li LH et al Overexpression of Populus tomentosa cytosolic ascorbate peroxidase enhances abiotic stress tolerance in tobacco plants. Russ J Plant Physiol 2017; 64: 224–34. [Google Scholar]
- 38. An J, Hou L, Li C et al Cloning and expression analysis of four DELLA genes in peanut. Russ J Plant Physiol 2015; 62: 116–26. [Google Scholar]
- 39. Han G, Wang M, Yuan F, Sui N, Song J, Wang B. The CCCH zinc finger protein gene AtZFP1 improves salt resistance in Arabidopsis thaliana . Plant Mol Biol 2014; 86: 237–53. [DOI] [PubMed] [Google Scholar]
- 40. Guerrero R. Bergey's manuals and the classification of prokaryotes. Int Microbiol 2001; 4: 103–9. [DOI] [PubMed] [Google Scholar]
- 41. Mailund T, Brodal GS, Fagerberg R, Pedersen CN, Phillips D. Recrafting the neighbor‐joining method. BMC Bioinformatics 2006; 7: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. He CQ, Liu YX, Wang HM, Hou PL, He HB, Ding NZ. New genetic mechanism, origin and population dynamic of bovine ephemeral fever virus. Vet Microbiol 2016; 182: 50–6. [DOI] [PubMed] [Google Scholar]
- 43. Ji W, Niu DD, Si HL, Ding NZ, He CQ. Vaccination influences the evolution of classical swine fever virus. Infect Genet Evol 2014; 25: 69–77. [DOI] [PubMed] [Google Scholar]
- 44. Zhu YY, Xing WX, Shan SJ et al Characterization and immune response expression of the Rig‐I‐like receptor mda5 in common carp Cyprinus carpio. J Fish Biol 2016; 88: 2188–202. [DOI] [PubMed] [Google Scholar]
- 45. Bai B, Zhao J, Li Y et al OsBBX14 delays heading date by repressing florigen gene expression under long and short‐day conditions in rice. Plant Sci 2016; 247: 25–34. [DOI] [PubMed] [Google Scholar]
- 46. Kaur G, Tirkey N, Bharrhan S, Chanana V, Rishi P, Chopra K. Inhibition of oxidative stress and cytokine activity by curcumin in amelioration of endotoxin‐induced experimental hepatoxicity in rodents. Clin Exp Immunol 2006; 145: 313–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yang Y, Mu T, Li T et al Effects of FSTL1 on the proliferation and motility of breast cancer cells and vascular endothelial cells. Thorac Cancer 2017; 8: 606–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Qin H, Zhou J, Zhou P et al Prognostic significance of RelB overexpression in non‐small cell lung cancer patients. Thorac Cancer 2016; 7: 415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Xie W, Yang Y, Gao S et al The tumor suppressor CYLD controls epithelial morphogenesis and homeostasis by regulating mitotic spindle behavior and adherens junction assembly. J Genet Genomics 2017; 44: 343–53. [DOI] [PubMed] [Google Scholar]
- 50. Luo Y, Ran J, Xie S et al ASK1 controls spindle orientation and positioning by phosphorylating EB1 and stabilizing astral microtubules. Cell Discov 2016; 2: 16033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yang Y, Liu M, Li D et al CYLD regulates spindle orientation by stabilizing astral microtubules and promoting dishevelled‐NuMA‐dynein/dynactin complex formation. Proc Natl Acad Sci U S A 2014; 111: 2158–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Li D, Sun X, Zhang L et al Histone deacetylase 6 and cytoplasmic linker protein 170 function together to regulate the motility of pancreatic cancer cells. Protein Cell 2014; 5: 214–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Henneke P, Golenbock DT. Phagocytosis, innate immunity, and host‐pathogen specificity. J Exp Med 2004; 199: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schepetkin IA, Quinn MT. Botanical polysaccharides: Macrophage immunomodulation and therapeutic potential. Int Immunopharmacol 2006; 6: 317–33. [DOI] [PubMed] [Google Scholar]
- 55. Meng X, Yang DY, Li XD, Zhao SY, Sui N, Meng QW. Physiological changes in fruit ripening caused by overexpression of tomato SlAN2, an R2R3‐MYB factor. Plant Physiol Biochem 2015; 89: 24–30. [DOI] [PubMed] [Google Scholar]
- 56. Song J, Shi WW, Liu RR et al The role of the seed coat in adaptation of dimorphic seeds of the euhalophyte Suaeda salsa to salinity. Plant Spec Biol 2017; 32: 107–14. [Google Scholar]
- 57. Song J, Zhou JC, Zhao WW et al Effects of salinity and nitrate on production and germination of dimorphic seeds applied both through the mother plant and exogenously during germination in Suaeda salsa . Plant Spec Biol 2016; 31: 19–28. [Google Scholar]
- 58. Liu SS, Wang WQ, Li M, Wan SB, Sui N. Antioxidants and unsaturated fatty acids are involved in salt tolerance in peanut. Acta Physiol Plant 2017; 39: 207. [Google Scholar]
- 59. Zhao BT, Zhu XF, Jung JH, Xuan YH. Effect of brassinosteroids on ammonium uptake via regulation of ammonium transporter and N‐metabolism genes in Arabidopsis . Biol Plantarum 2016; 60: 563–71. [Google Scholar]
- 60. Liu M, Xie S, Zhou J. Use of animal models for the imaging and quantification of angiogenesis. Exp Anim 2018; 67: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Li H, Yang GW, Ma F et al Molecular characterization of a fish‐specific toll‐like receptor 22 (TLR22) gene from common carp (Cyprinus carpio L.): Evolutionary relationship and induced expression upon immune stimulants. Fish Shellfish Immunol 2017; 63: 74–86. [DOI] [PubMed] [Google Scholar]
- 62. Zhao BT, Dai AH, Wei HC et al Arabidopsis KLU homologue GmCYP78A72 regulates seed size in soybean. Plant Mol Biol 2016; 90: 33–47. [DOI] [PubMed] [Google Scholar]
- 63. Li T, Li H, Peng SQ, Zhang FM, An LG, Yang GW. Molecular characterization and expression pattern of X box‐binding protein‐1 (XBP1) in common carp (Cyprinus carpio L.): Indications for a role of XBP1 in antibacterial and antiviral immunity. Fish Shellfish Immunol 2017; 67: 667–74. [DOI] [PubMed] [Google Scholar]
- 64. Liu TT, Yan JH. Review of the Palearctic Atemelia Herrich‐Schaffer (Lepidoptera, Yponomeutoidea, Praydidae), with description of a new leafmining species. Zootaxa 2017; 4250: 327–36. [DOI] [PubMed] [Google Scholar]
- 65. Guo X, Zhao Y, Yan H et al Single tumor‐initiating cells evade immune clearance by recruiting type II macrophages. Genes Dev 2017; 31: 247–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhou J, Qu Z, Sun F et al Myeloid STAT3 promotes lung tumorigenesis by transforming tumor immunosurveillance into tumor‐promoting inflammation. Cancer Immunol Res 2017; 5: 257–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sun S, Zhou J. Molecular mechanisms underlying stress response and adaptation. Thorac Cancer 2018; 9: 218–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. He X, Liu Z, He Q et al Identification of novel microtubule‐binding proteins by taxol‐mediated microtubule stabilization and mass spectrometry analysis. Thorac Cancer 2015; 6: 649–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Dehne N, Mora J, Namgaladze D, Weigert A, Brüne B. Cancer cell and macrophage cross‐talk in the tumor microenvironment. Curr Opin Pharmacol 2017; 35: 12–9. [DOI] [PubMed] [Google Scholar]
- 70. Zheng X, Turkowski K, Mora J et al Redirecting tumor‐associated macrophages to become tumoricidal effectors as a novel strategy for cancer therapy. Oncotarget 2017; 8: 48436–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhu B, Chen S, Wang H et al The protective role of DOT1L in UV‐induced melanomagenesis. Nat Commun 2018; 9: 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wang H, Zhang L, Yang L, Liu C, Zhang Q, Zhang L. Targeting macrophage anti‐tumor activity to suppress melanoma progression. Oncotarget 2017; 8: 18486–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Murray PJ, Allen JE, Biswas SK et al Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014; 41: 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Dentan C, Lesnik P, Chapman MJ, Ninio E. Phagocytic activation induces formation of platelet‐activating factor in human monocyte‐derived macrophages and in macrophage‐derived foam cells. Relevance to the inflammatory reaction in atherogenesis. Eur J Biochem 1996; 236: 48–55. [DOI] [PubMed] [Google Scholar]
- 75. Grimes GR, Moodie S, Beattie JS et al GPX‐macrophage expression atlas: A database for expression profiles of macrophages challenged with a variety of pro‐inflammatory, anti‐inflammatory, benign and pathogen insults. BMC Genomics 2005; 6: 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Yoon WJ, Kim SS, Oh TH, Lee N, Hyun CG. Abies koreana essential oil inhibits drug‐resistant skin pathogen growth and LPS‐induced inflammatory effects of murine macrophage. Lipids 2009; 44: 471–6. [DOI] [PubMed] [Google Scholar]


