Abstract
Background
The study was conducted to retrospectively evaluate the safety and effectiveness of computed tomography (CT)‐guided percutaneous microwave ablation (MWA) for peripheral non‐small cell lung cancer (NSCLC) in 11 patients with a single lung after pneumonectomy.
Methods
From May 2011 to March 2015, 11 single‐lung patients (8 men and 3 women; mean age 60.3 years, range 46–71) with peripheral NSCLC underwent 12 sessions of MWA. Eleven tumors measuring 13–52 mm (mean 30.2 mm) were treated. Follow‐up was performed via CT scan at 1, 3, 6, 12, 18, and 24 months after the procedure and annually thereafter. Clinical outcomes were evaluated and complications after MWA were summarized.
Results
At a median follow‐up period of 20 months (range 6–38), four patients showed evidence of local recurrence at a rate of 36.4% (4/11). Median overall survival was 20 months. The overall survival rates at one, two, and three years after MWA were 88.7%, 63.6%, and 42.3%, respectively. Complications after MWA included pneumothorax (33.3%), hemoptysis (33.3%), intrapulmonary bleeding (25%), pleural effusion (16.7%), and pulmonary infection (8.3%). None of the patients died during the procedure or in the 30 days after MWA.
Conclusion
CT‐guided percutaneous MWA is safe and effective for the treatment of peripheral NSCLC in patients with a single lung after prior pneumonectomy.
Keywords: Microwave ablation, NSCLC, pneumonectomy, single lung
Introduction
Primary lung cancer is the leading cause of death among all types of cancer worldwide. Non‐small cell lung cancer (NSCLC) accounts for approximately 80% of all lung cancer cases.1 Surgery remains the gold standard to treat patients with lung malignancies and some patients require pneumonectomy. In such patients, metastatic lung malignancies might occur in the contralateral single lung; however, in consideration of the special medical condition of patients with a single lung, surgery and traditional radiotherapy are not the preferred local treatment. In the last decade, many new local treatment methods, including thermal ablation and stereotactic body radiotherapy, have been developed to treat lung tumor patients who receive limited benefit from traditional chemotherapy or radiotherapy.2, 3 Primary and metastatic lung tumors can be successfully treated with microwave ablation (MWA) therapies.4, 5, 6, 7, 8, 9 Several investigations have been conducted on radiofrequency (RFA) for the treatment of primary or metastatic lung tumors in patients with a single lung.10, 11, 12, 13 The purpose of this multi‐center study was to retrospectively evaluate the safety and clinical outcomes of percutaneous MWA in patients who underwent contralateral pneumonectomy for metastatic malignancies in a single lung.
Methods
This retrospective multi‐center study was approved by five hospitals: Shandong Provincial Hospital Affiliated to Shandong University, the Hospital Affiliated to Binzhou Medical College, the 88th Hospital of Chinese People's Liberation Army, the Second People's Hospital of Dezhou, and Weifang People's Hospital Affiliated to Weifang Medical College. From May 2011 to March 2015, 11 single‐lung patients (8 men, 3 women; mean age 60.3 years, range 46–71) with peripheral malignancies underwent computed tomography (CT)‐guided percutaneous MWA in 12 sessions (one patient underwent two sessions as a result of incomplete ablation). A CT‐guided biopsy was conducted before MWA to obtain a pathological diagnosis. An interdisciplinary group consisting of a radiation oncologist, a thoracic surgeon, a radiologist, interventional radiologists, and a medical oncologist evaluated all patients. In this study, patients were deemed unable to tolerate curative lung resection because of insufficient respiratory reserve on the basis of clinical evaluation, results of respiratory test (forced expiratory volume in one second [FEV1] < 1L, FEV1% < 50%, maximum voluntary ventilation < 50%), or medically inoperable with heart dysfunction and other comorbidities (such as severe diabetes). All patients had previously undergone pneumonectomy 9 to 63 months before MWA (median 27.5 months) for the treatment of primary lung tumors. Of the 11 primary lung tumors, six were adenocarcinoma, four squamous, and one sarcoma. The largest diameter of the tumors ranged from 13 to 52 mm (mean 30.2 mm). Patient and tumor characteristics are listed in Table 1. All patients were informed in detail about the risks and benefits associated with MWA and provided written informed consent for the procedure. Ethical approval to conduct this study was obtained by the institutional review boards of the participating hospitals.
Table 1.
Patient and tumor characteristics
| No | Age/ Gender | Stage | Pathology | Interval (months) | Location | Size (mm) | Power/Time (W/min) | Local recurrence (months) | Comorbidity | Follow‐up (months/ status) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 61/M | IIa | ADC | 18 | Right inferior | 27 | 60/6 | None | None | 38/survived |
| 2 | 70/F | Ib | SQ | 63 | Left superior | 42 | 70/8 | 13 | Chronic bronchitis | 17/died |
| 3 | 46/F | Ib | SQ | 23 | Left superior | 28 | 60/7 | None | Diabetes (type I) | 16/survived |
| 4 | 55/M | IIa | ADC | 15 | Right middle | 25 | 60/6 | 26 | None | 32/died |
| 5 | 62/F | Ib | ADC | 12 | Right superior | 33 | 70/8 | None | Hypertension | 26/survived |
| 6 | 54/M | IIa | SQ | 18 | Right inferior | 18 | 60/5 | None | None | 37/survived |
| 7 | 64/M | Ib | ADC | 9 | Left superior | 44 | 70/8 | None | Chronic bronchitis | 23/survived |
| 8 | 59/M | IIIa | ADC | 11 | Left inferior | 30 | 60/8 | 9 | Diabetes (type II) | 11/died |
| 9 | 71/M | Ia | SQ | 37 | Right inferior | 20 | 60/6 | None | Ischemic heart disease | 10/survived |
| 10 | 68/M | IIIa | ADC | 14 | Left inferior | 13 | 60/4 | None | Ischemic heart disease | 6/survived |
| 11 | 53/M | Ia | Sarcoma | 46 | Left superior | 52 | 70/8, 60/6 & | 16 | Diabetes (type II) | 20/ died |
Stage: initial diagnosis. Interval: period between the date of the initial procedure and before microwave ablation (MWA). ADC, adenocarcinoma; SQ, squamous cell carcinoma.
Anesthesia
Local anesthetic and preemptive analgesia were used during the procedure.14 The patients abstained from solid food for 12 h prior to the procedure. For preemptive analgesia, injections of morphine (10 mg, subcutaneous), diazepam (10 mg, intramuscular), and flurbiprofen axetil (50 mg, intravenous) were administered before the procedure. An intravenous injection of flurbiprofen axetil (50 mg) was also administered after the procedure. Local anesthetic was applied at the selected puncture points using 2% lidocaine.
Instrumentation and procedure
A GE Light Speed 16 or VCT 64 spiral CT system (GE Healthcare, Atlanta, GA, USA) was used for imaging guidance and monitoring. ECO‐2450B MWA (ECO Microwave Electronic Institute, Nanjing, China; registration standard: YZB/country 1475–2013; China: SFDA certified No.[III] 20112251456), or KY‐2450B MWA (Kangyou Microwave Institute, Nanjing, China; registration standard: YZB/country 0247‐2011; China: SFDA [III] 2011 No:3251059) systems were used. The main frequency was 2450 GHz, and the output power was 0–100W (continually adjustable). The microwave antenna had an effective length of 100–180 mm and an outside diameter of 15–18 G, with a 15 mm active tip, and with a water circulation cooling system to reduce surface temperature. In general, the ablation power selected was 60–70 W and the ablative duration ranged from four to 8 minutes. This procedure has been described previously.6, 7 For tumors larger than 3.5 cm, ablation was performed with two antennae.6, 7, 15, 16, 17 Immediately after the procedure, CT scanning was again performed to evaluate the tumor size, tumor morphology, status of the adjacent organs, and to determine whether any complications, such as intrapulmonary bleeding and pneumothorax, were present. During the procedure, the patient's heart rate, blood pressure, electrocardiogram, and peripheral blood oxygen saturation level were carefully monitored. Vital signs, clinical symptoms, and urine volume were also closely observed during the post‐MWA hospital stay.
Follow‐up imaging and outcome
All patients underwent a non‐contrast chest CT scan 24–48 hours after the procedure to detect early‐onset, asymptomatic complications and ground glass opacities surrounding the lesions. Patients were requested to have serial repeat contrast‐enhanced CT (CECT) scans at 1, 3, 6, 9, 12, 18, and 24 months after the procedure and annually thereafter. Local efficacy was assessed according to the standards drafted by Xin et al.9 Complete ablation is defined as lesion disappearance, complete cavernous formation, fibrotic progression or scar, solid nodule involution or no change, without contrast‐enhanced signs on the CT scan, and/or atelectasis. Incomplete ablation is defined as incomplete cavernous formation, with some solid or liquid components remaining and irregular peripheral or internal enhancement signs on CT scans; partial fibrosis, with solid residues in the fibrotic lesion, which presents as irregular peripheral or internal enhancement signs on CT scans; and/or solid nodules with unchanged or increased size, which also present as irregular peripheral or internal enhancement signs on CT scans. Focal enhancement at the ablation site or enlargement of the ablated tumor after a series of shrinkage was considered local recurrence if technical success had been confirmed. Local recurrence is defined as a recurrence within or at the margin of the ablation site. Distant metastatic disease is defined as newly identified lung cancer metastases other than within the same lobe and in nodes other than mediastinal or hilar. Overall survival duration is defined as the interval between the date of the initial procedure and death or final follow‐up.
Complication assessment
Complications were assessed according to the standards drafted by the International Working Group on Image‐Guided Tumor Ablation in 2005 and 2014.18, 19 Major complications were defined as clinical symptoms experienced during or after ablation that may be life threatening, resulting in substantial damage and dysfunction and requiring hospitalization or prolonged hospitalization. Minor complications were defined as self‐limiting complications without sequelae that only required a short hospital stay for observation or treatment. Side effects referred to pain, post‐ablation syndrome, and asymptomatic minor bleeding or fluid accumulation on CT.
Statistical analysis
The data were processed using SPSS version 19.0 (IBM Corp., Armonk, NY, USA). Survival curves were constructed using the Kaplan–Meier method. Confidence intervals (CIs) were calculated using the Rothmann method.
Results
The biopsy results of the ablated lesions in the 11 patients were the same as the original pathological diagnoses after surgical resection. Successful ablation was achieved for all lesions in a single session. Double antennae were used for three lesions (No. 2, No. 7, No. 11). None of the procedures were interrupted as a result of complications, such as severe pneumothorax. The technical success rate of a single session was 100%. Hospital stay ranged from two to eight days (average 3.4 days).
The follow‐up CT scan one month after MWA revealed that complete ablation was achieved for 10 of the 11 lesions (complete ablation rate 90.9%; Figs 1,2); incomplete ablation occurred in one patient (No. 11, tumor 52 mm), however, complete ablation was achieved after the second MWA.
Figure 1.
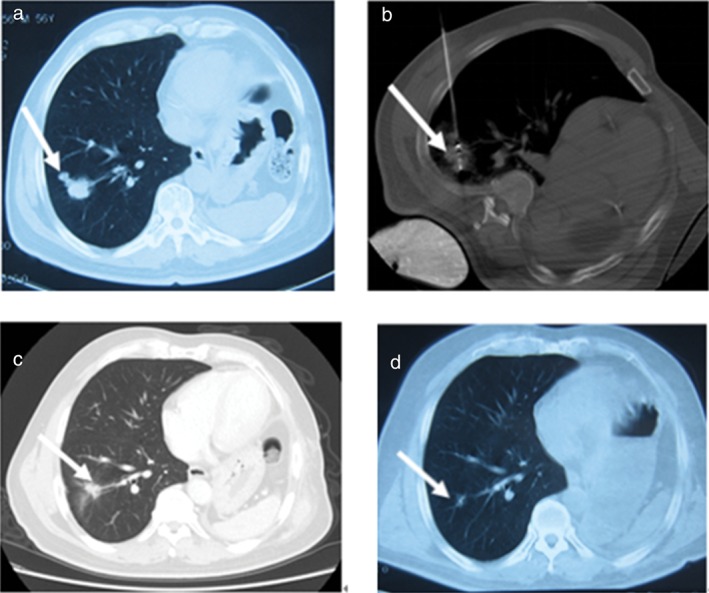
No 1. Male 61‐year‐old patient with 2.7 × 2.5 cm right inferior lung cancer (adenocarcinoma) completely ablated by microwave ablation (MWA). (a) Tumor lesion (arrow) on computed tomography prior to MWA; (b) the microwave antenna punctured the lesion (arrow); (c) the lesion significantly shrank 24 months after ablation (arrow); and (d) became a fiber scar 36 months after ablation (arrow).
Figure 2.
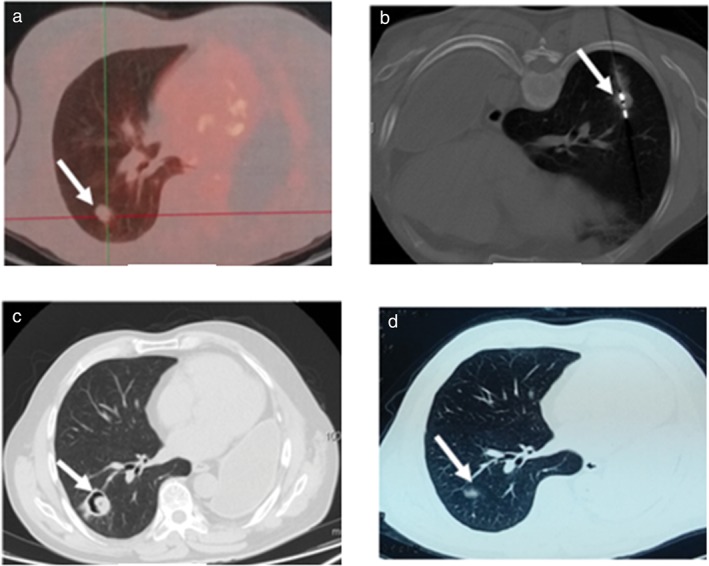
No 6. Male 54‐year‐old patient with 1.7 × 1.8 cm right inferior lung cancer (squamous) completely ablated by microwave ablation (MWA). (a) Tumor lesion (arrow) on positron emission tomography‐computed tomography prior to MWA (standardized uptake value 4.3); (b) the microwave antenna punctured the lesion (arrow); (c) three months after MWA a cavitating structure developed at the site of the ablated lesion (arrow); (d) 32 months after MWA, the lesion became a fiber scar (arrow).
The median follow‐up post‐ablation was 20 months (range 6–38) with CECT (Figs 1,2). No patient was lost to follow‐up. During the follow‐up period, local lesion recurrence was detected in four patients (No. 2, No. 4, No. 8, No. 11) and 11 lesions (recurrence rate 36.4%). Three patients received systematic chemotherapy as adjuvant treatment, one patient received radiation therapy for brain metastases, and the remaining patients received no anticancer therapy. Four patients developed distant metastatic disease and four patients died. The median overall survival was 20 months. The overall one, two, and three‐year survival rates were 88.7%, 63.6%, and 42.3%%, respectively (Fig 3).
Figure 3.
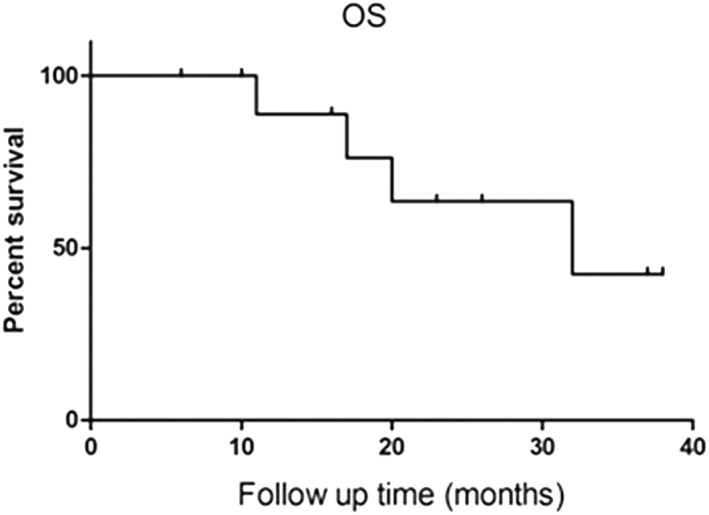
Graph shows overall survival (OS).
Side effects and complications
Pain was the common side effect experienced under local anesthesia during the procedure. In 12 sessions of 11 patients treated with MWA, the patients in four sessions experienced moderate pain, but this did not affect the procedure. Five patients suffered mild to moderate pain after MWA, but no severe post‐ablation pain occurred. Four patients exhibited post‐ablation syndrome, with symptoms of fever (under 38.5°C), fatigue, general malaise, nausea, and vomiting. Four patients developed pneumothorax: one was managed with chest tube placement, while the remaining three patients were self‐limited. Four patients had hemoptysis during the procedure: one (moderate hemoptysis) instance was resolved quickly with ablation, and the remaining three patients were self‐limited. Three patients had mild intrapulmonary bleeding during the procedure, two patients had mild pleural effusion, and one patient developed pneumonia and was treated with sputum culture‐specific antibiotics. The side effects and complications are listed in Table 2. None of the patients died during the procedure or in the 30 days following MWA.
Table 2.
Grade of complications during and following MWA
| Grade | Complications | Number |
|---|---|---|
| Major complications | Pneumothorax | 1 (8.3%) |
| Pneumonia | 1 (8.3%) | |
| Minor complications | Pneumothorax | 3 (25%) |
| Pleural effusion | 2 (16.7%) | |
| Hemoptysis | 4 (33.3%) | |
| Mild intrapulmonary bleeding | 3 (25%) | |
| Side effects | Pain | 4 (33.3%) |
| Pain post‐ablation | 5 (41.7%) | |
| Post‐ablation syndrome | 4 (33.3%) |
MWA, microwave ablation.
Discussion
Surgical resection is the preferred treatment for patients who develop a new lung lesion; however, further resection is not always feasible. Resection of recurrent or metastatic tumors on the residual lung after pneumonectomy is unusual.20 These patients, with limited respiratory function and poor medical conditions (such as cardiopulmonary dysfunctions), may not tolerate another lung parenchyma resection. The postoperative mortality rate is high, particularly if pulmonary resection is extensive, with the removal of a large amount of parenchyma.21 Dupuy et al. first demonstrated RFA of a pulmonary tumor in 2000.22 Since then, many publications have confirmed the feasibility, safety, and efficacy of RFA of lung malignancy. MWA has several advantages over RFA: larger volumes of necrosis are ablated in a shorter procedural time; less “heat sink” effect occurs, for the better treatment of perivascular tissue; and the ablation zone is maximized by positioning multiple MWA antennae into a larger lesion simultaneously.15, 16, 23, 24, 25 Based on the substantial advantages of MWA, more patients with pulmonary malignancies have been administered MWA treatment as an alternative option.
In our series, the median overall survival duration was 20 months. The one, two, and three‐year overall survival rates were 88.7%, 63.6%, and 42.3%%, respectively. Our data are similar to the results of previous studies of lung RFA.12, 13, 26 These results suggest that MWA is effective in improving the survival of peripheral lung malignancies in patients with a single lung after pneumonectomy.
Four patients (36.7%) in our study experienced local recurrence. A meta‐analysis of 17 series of RFA of lung tumors reported a 3–38.1% local tumor recurrence rate.27 There is evidence that tumor size is an independent predictive factor of local tumor recurrence, with the most common threshold for significance reported to be 3 cm or 3.5 cm. Our data are similar to the results of previous studies.10, 28 Two cases of local tumor recurrence were observed in three tumors measuring more than 3.5 cm.
Despite the clinical benefits, MWA can lead to complications. Pneumothorax, the most common complication of lung MWA, is reported at wide rates of 8–63%, with 3.8–15.7% requiring chest tube placement.9, 29, 30, 31, 32, 33 In our series, the incidence of pneumothorax was 33.3%, similar to the rate reported in patients with two lungs, and only one case required chest tube placement. The other major complication was pulmonary infection (8.3%). Minor complications included hemoptysis (33.3%), intrapulmonary bleeding (25%), and pleural effusion (16.7%). The side effects were mild to moderate pain and post‐ablation syndrome (33.3%). These side effects and complications could be well controlled through observation or proper treatment. None of the patients died during the procedure or in the 30 days following MWA. Our results suggest that MWA is feasible and safe for the treatment of peripheral lung malignancies in patients with a single lung; however, major risks of single‐lung MWA do exist. Several investigators have reported mortality rates associated with lung RFA, and half of the periprocedural deaths occurred in single‐lung patients.
Our study has several limitations. First, our series was a retrospective study involving a small sample population. Investigation with a larger sample should be conducted. Second, the follow‐up period was relatively short and does not allow definitive conclusions about survival rate. Third, without the aid of positron emission tomography‐CT or histopathologic proof, CECT was employed to evaluate efficacy (such as complete ablation, local recurrence), which might have caused inaccuracy. Finally, certain patients undergoing MWA were subsequently treated with systemic chemotherapy and/or external beam radiation therapy, which might have affected clinical efficacy.
Computed tomography‐guided percutaneous MWA is safe and effective for the treatment of peripheral NSCLC in patients with a single lung.
Disclosure
No authors report any conflict of interest.
References
- 1. Ferlay J, Soerjomataram I, Ervik M et al GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11. International Agency for Research on Cancer, Lyon: 2013. [Cited February 2018.] Available from URL: http://globocan.iarc.fr. [Google Scholar]
- 2. Vogl TJ, Naguib NN, Lehnert T, Nour‐Eldin NE. Radiofrequency, microwave and laser ablation of pulmonary neoplasms: Clinical studies and technical considerations‐‐Review article. Eur J Radiol 2011; 77: 346–57. [DOI] [PubMed] [Google Scholar]
- 3. Davis JN, Medbery C III, Sharma S et al Stereotactic body radiotherapy for early‐stage non‐small cell lung cancer: Clinical outcomes from a National Patient Registry. J Radiat Oncol 2015; 4: 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dupuy DE. Image‐guided thermal ablation of lung malignancies. Radiology 2011; 260: 633–55. [DOI] [PubMed] [Google Scholar]
- 5. Yang X, Ye X, Huang G et al Repeated percutaneous microwave ablation for local recurrence of inoperable stage I non‐small cell lung cancer. J Cancer Res Ther 2017; 13: 683–8. [DOI] [PubMed] [Google Scholar]
- 6. Yang X, Ye X, Zheng A et al Percutaneous microwave ablation of stage I medically inoperable non‐small cell lung cancer: Clinical evaluation of 47 cases. J Surg Oncol 2014; 110: 758–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen J, Lin ZY, Wu ZB, Chen ZW, Chen YP. Magnetic resonance imaging evaluation after radiofrequency ablation for malignant lung tumors. J Cancer Res Ther 2017; 13: 669–75. [DOI] [PubMed] [Google Scholar]
- 8. Ye X, Fan WJ, Chen JH et al Chinese expert consensus workshop report: Guidelines for thermal ablation of primary and metastatic lung tumors. Thorac Cancer 2015; 6: 112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Simon CJ, Dupuy DE, DiPetrillo TA et al Pulmonary radiofrequency ablation: Long‐term safety and efficacy in 153 patients. Radiology 2007; 243: 268–75. [DOI] [PubMed] [Google Scholar]
- 10. Ambrogi MC, Fanucchi O, Lencioni R, Cioni R, Mussi A. Pulmonary radiofrequency ablation in a single lung patient. Thorax 2006; 61: 828–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Modesto A, Giron J, Massabeau C, Sans N, Berjaud J, Mazieres J. Radiofrequency ablation for non‐small‐cell lung cancer in a single‐lung patient: Case report and review of the literature. Lung Cancer 2013; 80: 341–3. [DOI] [PubMed] [Google Scholar]
- 12. Hess A, Palussière J, Goyers JF, Guth A, Aupérin A, de Baère T. Pulmonary radiofrequency ablation in patients with a single lung: Feasibility, efficacy, and tolerance. Radiology 2011; 258: 635–42. [DOI] [PubMed] [Google Scholar]
- 13. Ong CK, Lirk P, Seymour RA, Jenkins BJ. The efficacy of preemptive analgesia for acute postoperative pain management: A meta‐analysis. Anesth Analg 2005; 100: 757–73. [DOI] [PubMed] [Google Scholar]
- 14. Crocetti L, Bozzi E, Faviana P et al Thermal ablation of lung tissue: in vivo experimental comparison of microwave and radiofrequency. Cardiovasc Intervent Radiol 2010; 33: 818–27. [DOI] [PubMed] [Google Scholar]
- 15. Dupuy DE. Microwave ablation compared with radiofrequency ablation in lung tissue‐is microwave not just for popcorn anymore? Radiology 2009; 251: 617–8. [DOI] [PubMed] [Google Scholar]
- 16. Song Z, Qi H, Zhang H et al Microwave ablation: Results with three different diameters of antennas in ex vivo bovine and in vivo porcine liver. J Cancer Res Ther 2017; 13: 737–41. [DOI] [PubMed] [Google Scholar]
- 17. Goldberg SN, Grassi CJ, Cardella JF et al Image‐guided tumor ablation: Standardization of terminology and reporting criteria. Radiology 2005; 235: 728–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ahmed M, Solbiati L, Brace CL et al Image‐guided tumor ablation: Standardization of terminology and reporting criteria‐‐a 10‐year update. Radiology 2014; 273: 241–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Terzi A, Lonardoni A, Scanagatta P, Pergher S, Bonadiman C, Calabrò F. Lung resection for bronchogenic carcinoma after pneumonectomy: A safe and worthwhile procedure. Eur J Cardiothorac Surg 2004; 2: 456–9. [DOI] [PubMed] [Google Scholar]
- 20. Donington JS, Miller DL, Rowland CC et al Subsequent pulmonary resection for bronchogenic carcinoma after pneumonectomy. Ann Thorac Surg 2002; 74: 154–8. [DOI] [PubMed] [Google Scholar]
- 21. Dupuy DE, Zagoria RJ, Akerley W, Mayo‐Smith WW, Kavanagh PV, Safran H. Percutaneous radiofrequency ablation of malignancies in the lung. AJR Am J Roentgenol 2000; 174: 57–9. [DOI] [PubMed] [Google Scholar]
- 22. Poulou LS, Botsa E, Thanou I, Ziakas PD, Thanos L. Percutaneous microwave ablation vs radiofrequency ablation in the treatment of hepatocellular carcinoma. World J Hepatol 2015; 7: 1054–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Di Vece F, Tombesi P, Ermili F, Maraldi C, Sartori S. Coagulation areas produced by cool‐tip radiofrequency ablation and microwave ablation using a device to decrease back‐heating effects: A prospective pilot study. Cardiovasc Intervent Radiol 2014; 37: 723–9. [DOI] [PubMed] [Google Scholar]
- 24. Planché O, Teriitehau C, Boudabous S et al In vivo evaluation of lung microwave ablation in a porcine tumor mimic model. Cardiovasc Intervent Radiol 2013; 36: 221–8. [DOI] [PubMed] [Google Scholar]
- 25. Carrafiello G, Mangini M, Fontana F et al Radiofrequency ablation for single lung tumours not suitable for surgery: Seven years’ experience. Radiol Med 2012; 117: 1320–32. [DOI] [PubMed] [Google Scholar]
- 26. Zhu JC, Yan TD, Morris DL. A systematic review of radiofrequency ablation for lung tumors. Ann Surg Oncol 2008; 15: 1765–74. [DOI] [PubMed] [Google Scholar]
- 27. Hiraki T, Sakurai J, Tsuda T et al Risk factors for local progression after percutaneous radiofrequency ablation of lung tumors: Evaluation based on a preliminary review of 342 tumors. Cancer 2006; 107: 2873–80. [DOI] [PubMed] [Google Scholar]
- 28. Vogl TJ, Naguib NN, Gruber‐Rouh T, Koitka K, Lehnert T, Nour‐Eldin NE. Microwave ablation therapy: Clinical utility in treatment of pulmonary metastases. Radiology 2011; 261: 643–51 (Published erratum appears in Radiology 2013;266:1000). [DOI] [PubMed] [Google Scholar]
- 29. Carrafiello G, Mangini M, Fontana F et al Complications of microwave and radiofrequency lung ablation: Personal experience and review of the literature. Radiol Med 2012; 117: 201–13. [DOI] [PubMed] [Google Scholar]
- 30. Nakamura T, Matsumine A, Yamakado K, Takao M, Uchida A, Sudo A. Clinical significance of radiofrequency ablation and metastasectomy in elderly patients with lung metastases from musculoskeletal sarcomas. J Cancer Res Ther 2013; 9: 219–23. [DOI] [PubMed] [Google Scholar]
- 31. Belfiore G, Ronza F, Belfiore MP et al Patients’ survival in lung malignancies treated by microwave ablation: Our experience on 56 patients. Eur J Radiol 2013; 82: 177–81. [DOI] [PubMed] [Google Scholar]
- 32. Zheng A, Wang X, Yang X et al Major complications after lung microwave ablation: A single‐center experience on 204 sessions. Ann Thorac Surg 2014; 98: 243–8. [DOI] [PubMed] [Google Scholar]
- 33. Splatt AM, Steinke K. Major complications of high‐energy microwave ablation for percutaneous CT‐guided treatment of lung malignancies: Single‐centre experience after 4 years. J Med Imaging Radiat Oncol 2015; 59: 609–16. [DOI] [PubMed] [Google Scholar]


