Abstract
Background
Circulating tumor cell (CTC) counts at baseline and follow‐up are an independent prognostic factor in patients receiving standard chemotherapy for non‐small cell lung cancer (NSCLC). This study further explored the role of CTCs in EGFR‐mutated and ALK‐rearranged NSCLC patients administered targeted therapies as first‐line treatment.
Methods
CTCs were enumerated with a novel high‐efficiency detection method from the blood of 43 patients with EGFR‐mutated or ALK‐rearranged NSCLC at baseline and at disease‐progression. Patients were stratified into favorable and unfavorable groups with baseline CTC counts of < 8 or ≥ 8 CTCs/3.2 mL, respectively.
Results
A total of 76.7% of the patients were positive for ≥ 2 CTCs /3.2 ml blood at baseline. The median progression‐free survival (PFS) and overall survival (OS) rates of the favorable compared to the unfavorable group were longer (11.6 vs. 8.5 months, P = 0.004 for PFS; 21.00 vs. 17.7 months, P = 0.013 for OS). Multivariate analysis demonstrated that baseline CTC count was a strong predictor of PFS (hazard ratio 2.835; 95% confidence interval 1.240–6.483; P = 0.014) and OS (hazard ratio 3.317; 95% confidence interval 1.360–8.092; P = 0.008).
Conclusion
Baseline CTC count could be a predictive biomarker for EGFR‐mutated and ALK‐rearranged NSCLCs, which allows for better guidance and monitoring of patients over the course of molecular targeted therapies.
Keywords: ALK, circulating tumor cells, EGFR, molecular targeted therapy, non‐small cell lung cancer
Introduction
Non‐small cell lung cancer (NSCLC) is one of the most common cancers in the world, with increasing incidence in recent decades.1 Recent advances in molecular therapies targeted to specific oncogenic driver alterations, such as EGFR mutations and ALK rearrangements, have greatly improved the quality of life and long‐term prognosis of patients with advanced NSCLC.2, 3 However, more precise regimens involve greater complexity in terms of decision‐making. A biomarker for better personalization and monitoring of patient treatment would thus be of great potential clinical utility.
It is widely recognized that circulating tumor cells (CTCs) are of great prognostic value as a tumor biomarker for metastatic breast, colorectal, and prostate cancers.4, 5, 6 We recently demonstrated that CTC counts at baseline and CTC count changes during treatment could also serve as independent predictive and prognostic markers for NSCLC patients receiving first‐line chemotherapy.7, 8 However, previous analyses were limited to patient subgroups without oncogenic alterations, or patients with positive EGFR mutations/ALK rearrangements administered chemotherapy as first‐line therapy. Little is known about the prevalence and clinical significance of CTCs in EGFR‐mutant and ALK‐rearranged NSCLC.
The aims of this study were to further explore the clinical value of CTC in patients with EGFR mutations or ALK alterations receiving targeted therapy as first‐line treatment, and to evaluate the relevance of CTC‐related characteristics in this oncogene‐addicted group of NSCLC patients.
Methods
Study design
Patients with histologically proven advanced (stage IIIb or IV) NSCLC who attended the Peking Union Medical College Hospital (PUMCH) between October 2013 and September 2015 were included in this prospective study. Adult males and females with a sensitizing EGFR mutation or ALK rearrangement in primary tumor tissue and no prior history of treatment with EGFR/ALK‐targeted agents were eligible for the study. Previous surgical and adjuvant chemotherapy was permitted one year after treatment for recurrent NSCLC. All patients received EGFR or ALK targeted therapy as first‐line treatment. Peripheral blood samples (3.2 mL) were collected for CTC analysis at baseline and at disease progression. All of the samples were collected under protocols approved by the PUMCH Ethics Committee and written informed consent from the patients.
Tumor assessments were performed using a computed tomography scan and/or magnetic resonance imaging every six or eight weeks, and disease status was evaluated using Response Evaluation Criteria in Solid Tumors (RECIST). Progression‐free survival (PFS) and overall survival (OS) were measured from the date of informed consent to the date of disease progression or death. Patients who were progression‐free and alive at the time of final analysis were censored.
Circulating tumor cell (CTC) collection and analysis
Circulating tumor cells were enumerated and identified using a novel technique that combines subtraction enrichment, leukocyte common antigen (CD45) immunostaining, and fluorescence in situ hybridization. The technical details, including sensitivity, accuracy, linearity, and reproducibility, are described in our previous reports.7, 8
Herein, ≥ 2 CTCs per 3.2 mL of blood was classified as CTC‐positive, and a cut‐off threshold of 8 CTCs per 3.2 mL of blood was selected to stratify patients into favorable (< 8 CTCs per 3.2 mL of blood) and unfavorable (≥ 8 CTCs per 3.2 mL of blood) prognostic groups, based on preliminary studies.7, 8
Statistical analysis
Differences between groups were tested using a chi‐square test or with a Mann–Whitney U test in the case of continuous variables. Survival curves were plotted using the Kaplan–Meier method and compared by log‐rank test. Cox proportional hazards regression analysis was used to estimate univariate and multivariate hazard ratios for PFS and OS. Statistical analysis was carried out using SPSS version 19.0 (IBM Corp., Armonk, NY, USA) and a two‐tailed P value of < 0.05 was considered statistically significant.
Results
Patient characteristics
A total of 43 patients were enrolled in this study. The clinicopathological characteristics of the patients at the time of study entry are presented in Table 1. The histologic type in all 43 patients was lung adenocarcinoma. EGFR mutations were detected in 36 patients (L858R point mutations [n = 11] and exon 19 deletions [n = 25]) using an amplification‐refractory mutation system (AmoyDx, Xiamen, China), while seven patients were identified as harboring ALK rearrangements using the Ventana immunohistochemistry platform (Roche, Basel, Switzerland). All patients received molecular targeted agents as first‐line therapy: 28 cases received gefitinib, 8 icotinib, and 7 crizotinib.
Table 1.
Patient demographics and clinical characteristics (n = 43)
| Characteristics | n | Proportion (%) |
|---|---|---|
| Age | ||
| < 60 | 26 | 60.5 |
| ≥ 60 | 17 | 39.5 |
| Gender | ||
| Male | 15 | 34.9 |
| Female | 28 | 65.1 |
| Smoking history | ||
| Yes | 14 | 32.6 |
| No | 29 | 67.4 |
| ECOG | ||
| 0 | 33 | 76.7 |
| 1 | 10 | 23.3 |
| TNM status | ||
| IIIb | 6 | 14.0 |
| IV | 37 | 86.0 |
| Tumor size | ||
| > 3 cm | 23 | 53.5 |
| ≤ 3 cm | 20 | 46.5 |
| Mutation status | ||
| EGFR | 36 | 83.7 |
| ALK | 7 | 16.3 |
ECOG, Eastern Cooperative Oncology Group; TNM, tumor node metastasis.
CTC count at baseline
Overall, 33 patients (76.7%) had positive CTC counts at baseline, with ≥ 2 CTCs per 3.2 mL of blood (range 0–50). In total, 21% of patients had an unfavorable CTC count (≥ 8 CTCs) at baseline, while 79% had a favorable count (< 8 CTCs): 48.8%, ≤ 3; 58.1%, ≤ 4; 62.8%, ≤ 5; and 69.8%, ≤ 6. No significant associations were observed between baseline CTC counts and clinicopathological characteristics, including age, gender, smoking history, Eastern Cooperative Oncology Group performance status, tumor stage, tumor size, mutant status (EGFR or ALK), the number of metastases, and metastatic organs (i.e. the bone, liver, and adrenal gland), as shown in Table 2.
Table 2.
Relationship between circulating tumor cell count and clinicopathological characteristics
| Variable | n | P | Z Statistics |
|---|---|---|---|
| Age | 0.726 | −0.350 | |
| < 60 | 26 | ||
| ≥ 60 | 17 | ||
| Gender | 1.000 | 0.000 | |
| Male | 15 | ||
| Female | 28 | ||
| Smoking history | 0.167 | −1.383 | |
| Yes | 14 | ||
| No | 29 | ||
| ECOG | 0.750 | −0.318 | |
| 0 | 33 | ||
| 1 | 10 | ||
| TNM status | 0.069 | −1.817 | |
| IIIb | 6 | ||
| IV | 37 | ||
| Tumor size | 0.059 | −1.887 | |
| > 3 cm | 23 | ||
| ≤ 3 cm | 20 | ||
| Mutation status | 0.246 | −1.159 | |
| EGFR | 36 | ||
| ALK | 7 | ||
| Liver metastases | 0.784 | −0.274 | |
| Yes | 4 | ||
| No | 39 | ||
| Bone metastases | 0.990 | −0.012 | |
| Yes | 22 | ||
| No | 21 | ||
| Adrenal metastases | 0.950 | −0.063 | |
| Yes | 4 | ||
| No | 39 | ||
| No. of metastatic sites | 0.149 | −1442 | |
| < 2 | 27 | ||
| ≥ 2 | 16 |
ECOG, Eastern Cooperative Oncology Group; TNM, tumor node metastasis.
Prognostic significance of CTC count at baseline
At the final analysis on 30 March 2017, 34 patients had experienced disease progression, 25 patients had died, and 5 patients were lost to follow‐up. The median OS and PFS rates of all patients were 20.2 and 10.0 months, respectively.
In CTC univariate analysis, the median OS rate for patients with a favorable CTC count (< 8 CTCs) at baseline was 21.0 months compared to 17.7 months for patients with unfavorable counts (≥ 8 CTCs): log‐rank test P = 0.013 (Fig 1); Cox proportional hazards regression: hazard ratio (HR) 2.739, 95% confidence interval (CI) 1.194–6.283; P = 0.017 (Table 3). Consistent with the results of our previous studies, the median PFS rate of patients in the favorable group was also significantly longer than the rate in the unfavorable group: 11.6 vs. 8.5 months, log‐rank test P = 0.004 (Fig 2); Cox proportional hazards regression: HR 3.084; 95% CI 1.357–7.011; P = 0.007 (Table 3).7, 8
Figure 1.
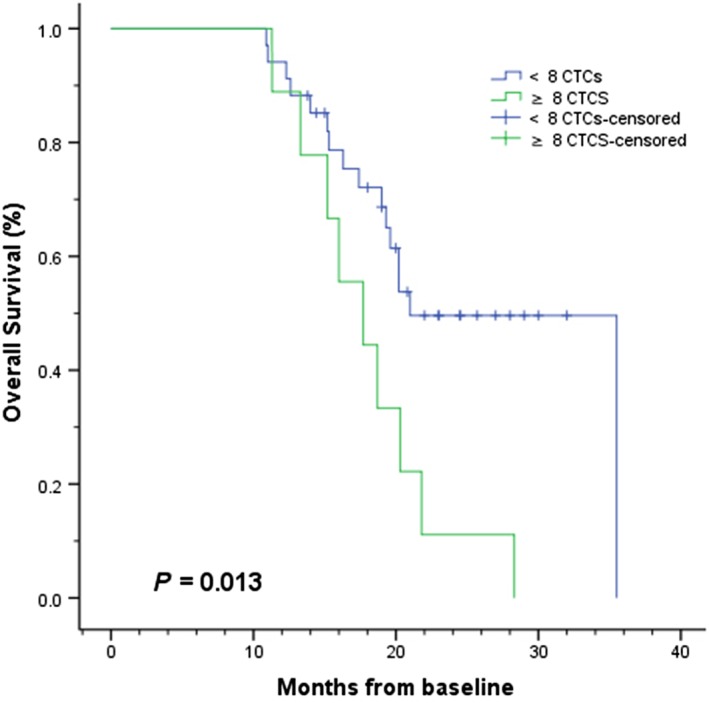
Kaplan–Meier curves of overall survival according to baseline circulating tumor cell (CTC) counts in patients with EGFR‐mutated or ALK‐rearranged non‐small cell lung cancer (n = 43). < 8 CTCs, ≥ 8 CTCS, < 8 CTCs‐censored, ≥ 8 CTCS‐censored.
Table 3.
Univariate and multivariate Cox proportional hazards regression analysis for prediction of OS and PFS
| Variable | OS HR (95.0% CI) | P | PFS HR (95.0% CI) | P |
|---|---|---|---|---|
| Univariate Cox proportional hazards regression analysis | ||||
| Age: < 60 vs. ≥ 60 | 0.479 (0.189–1.210) | 0.120 | 0.435 (0.205–0.922) | 0.030 |
| Gender: female vs. male | 1.437 (0.623–3.312) | 0.395 | 1.620 (0.805–3.261) | 0.176 |
| Smoking history: yes vs. no | 0.344 (0.146–0.808) | 0.014 | 0.314 (0.143–0.689) | 0.004 |
| ECOG PS: 0 vs. 1 | 0.294 (0.087–0.994) | 0.049 | 0.478 (0.206–1.107) | 0.085 |
| Tumor stage: IIIb vs. IV | 1.274 (0.378–4.294) | 0.696 | 1.342 (0.470–3.829) | 0.582 |
| Tumor size: < 3 vs. ≥ 3 cm | 1.523 (0.674–3.440) | 0.312 | 2.065 (1.000–4.267) | 0.050 |
| Mutation status: EGFR vs. ALK | 1.547 (0.519–4.609) | 0.434 | 1.930 (0.780–4.777) | 0.155 |
| Liver metastasis: yes vs. no | 0.491 (0.144–1.669) | 0.254 | 1.222 (0.371–4.022) | 0.742 |
| Bone metastasis: yes vs. no | 0.737 (0.330–1.646) | 0.456 | 0.761 (0.387–1.497) | 0.429 |
| Adrenal metastasis: yes vs. no | 3.121 (0.419–23.258) | 0.267 | 1.300 (0.397–4.260) | 0.665 |
| Baseline CTCs: < 8 vs. ≥ 8 | 2.739 (1.194–6.283) | 0.017 | 3.084 (1.357–7.011) | 0.007 |
| Stepwise multivariate Cox proportional hazards regression analysis | ||||
| Smoking history: yes vs. no | 0.227 (0.087–0.593) | 0.002 | 0.301 (0.135–0.669) | 0.003 |
| ECOG PS: 0 vs. 1 | 0.216 (0.058–0.805) | 0.022 | — | — |
| Baseline CTCs: < 8 vs. ≥ 8 | 3.317 (1.360–8.092) | 0.008 | 2.835(1.240–6.483) | 0.014 |
Bold value indicates P<0.05 are statistically significant. CI, confidence interval; CTCs, circulating tumor cells; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; OS, overall survival; PFS, progression‐free survival.
Figure 2.
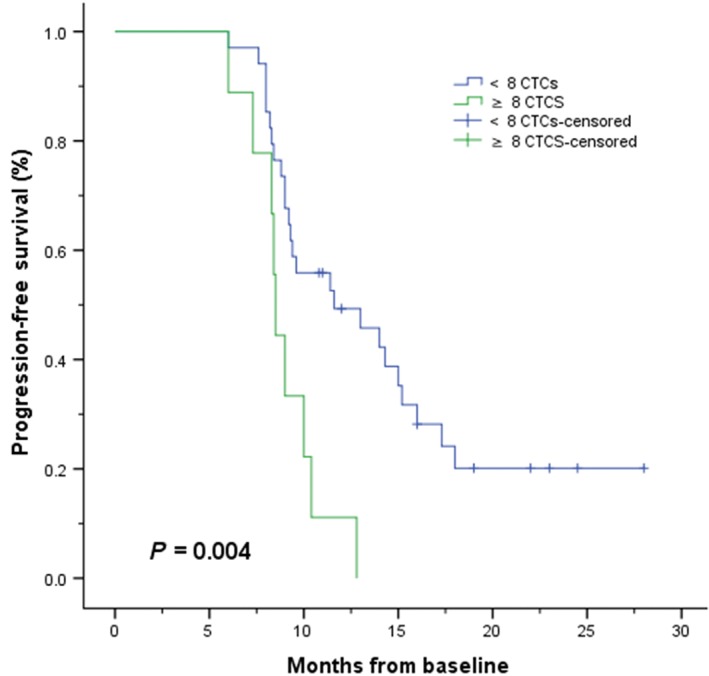
Kaplan–Meier curves of progression‐free survival according to baseline circulating tumor cell (CTC) counts in patients with EGFR‐mutated or ALK‐rearranged non‐small cell lung cancer (n = 43). < 8 CTCs, ≥ 8 CTCS, < 8 CTCs‐censored, ≥ 8 CTCS‐censored.
In univariate analyses, age and a smoking history were significantly correlated to OS, while a smoking history and Eastern Cooperative Oncology Group performance status were significantly associated with PFS (Table 3). These clinical factors were then included in multivariate forward stepwise Cox proportional hazards regression analyses. The baseline CTC count remained an independent prognostic factor for OS and PFS (HR 3.317, 95% CI 1.360–8.092, P = 0.008 for OS; HR 2.835, 95% CI 1.24 0–6.483, P = 0.014 for PFS) (Table 3, Table S1).
Changes in CTC count during follow‐up
In the current study, the CTC counts of 29 patients were collected and analyzed at the time of disease progression, and changes in the CTC count from baseline in these patients are shown in Figure 3. The CTC count was increased in 14 patients, decreased in 13, and stable in 2. The median CTC counts were 6 (range 0–50) and 7 (range 0–38) CTCs/3.2 mL of blood at baseline and at disease progression, respectively. Further analysis showed no significant difference between CTC counts at these two time‐points (P = 0.932).
Figure 3.
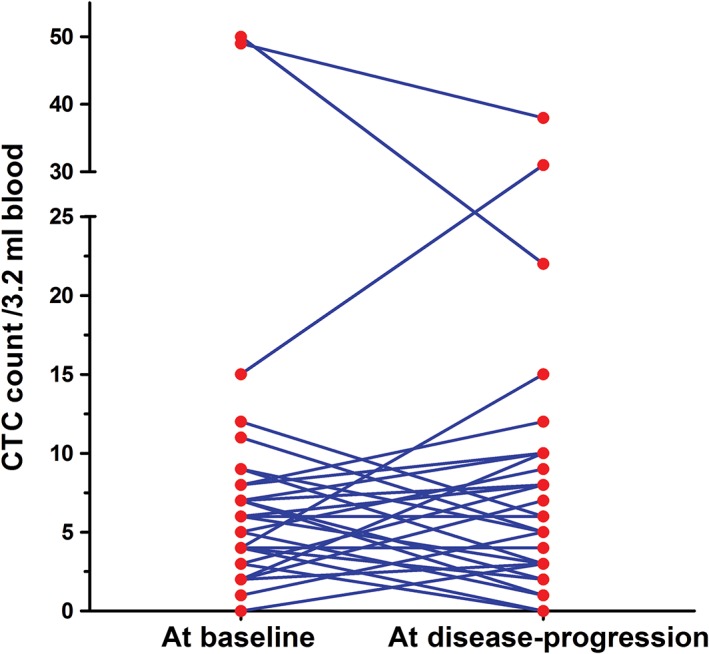
Dynamic changes in circulating tumor cell (CTC) count from baseline to disease progression (n = 29).
Discussion
In recent years, CTCs have emerged as an important tumor biomarker for a wide range of human cancers.4, 5, 6, 7, 8, 9, 10, 11 Our previous reports of CTC analyses in advanced NSCLC mainly focusing on patients without oncogenic mutations receiving chemotherapy as first‐line treatment demonstrated that CTC counts at baseline and during follow‐up are a strong independent predictor of survival outcomes.7, 8 The current study extends our previous analyses to patients harboring EGFR mutations or ALK rearrangements, and yielded remarkably consistent results showing poorer PFS and OS rates in patients with unfavorable compared to favorable baseline CTC counts.
To our knowledge, this study provides the first complete evidence of the prognostic value of baseline CTC counts for predicting PFS and OS rates in patients with positive EGFR/ALK. To date few reports have investigated the prognostic significance of CTCs in EGFR‐mutated or ALK‐rearranged NSCLCs.12, 13, 14, 15 Yang et al. performed similar CTC analyses using the CellSearch system (Johnson & Johnson, Brunswick, NJ, USA) and reported PFS values of 11.1 versus 6.8 months (P = 0.009) for EGFR‐mutated NSCLC patients with < 5 CTCs compared to those with ≥ 5 CTCs at baseline.12 Their multivariate analysis indicated that baseline CTC number was the most significant prognostic factor of PFS (HR 8.635, 95% CI 2.341–15.613; P < 0 0.001). He et al. reported that a higher baseline CTC count was significantly associated with inferior PFS (5.6 vs. 11.5 months; P = 0.005) and OS (18.3 vs. 22.8 months; P = 0.010) rates in advanced NSCLC patients with EGFR mutations.13 These findings in EGFR‐mutated NSCLC patients correspond to the results of our study.
Furthermore, we enrolled patients with ALK‐rearranged NSCLC, and no significant difference was observed between EGFR‐mutated and ALK‐rearranged NSCLC patients. However, another study of ALK‐rearranged NSCLC patients did not confirm the prognostic role of baseline CTC count. Pailler et al. enrolled 41 ALK‐rearranged NSCLC patients treated with crizotinib, and evaluated CTC levels with aberrant ALK‐fluorescence in situ hybridization patterns at baseline and after two months of crizotinib treatment.14 They found no significant relationship between baseline CTC levels and PFS (data not shown). Discrepancies may have arisen as a result of targeting different CTC populations and NSCLC oncogenic mutations. We examined 34 cases with EGFR mutations, but only seven cases with ALK rearrangement in the final analysis of prognostic significance. Larger samples are needed to elucidate more fully the association between baseline CTC counts and ALK‐rearranged NSCLC. Nonetheless, with respect to the remarkable difference in survival outcomes between unfavorable and favorable CTC groups of advanced NSCLC patients, it is critical that this novel biomarker be carefully balanced when evaluating patient prognosis in future clinical trials.
In this prospective study, we examined CTC levels at two time‐points: baseline and disease progression. No significant increase in CTC level at disease progression was observed (increase/stable, n = 16; decrease, n = 13). Similarly, Pailler et al. also reported no significant difference between CTC levels at these two time points.14 However, they did report a significant association between a decrease in CTC level with ALK‐copy number gain after crizotinib treatment and longer PFS (HR 4.485, 95% CI 1.543–13.030; P = 0 0.006). Punnoose et al. conducted a study of 41 patients treated with erlotinib and pertuzumab and reported that increasing CTC levels during treatment were closely associated with shorter PFS (P = 0.006) and a better RECIST response (P = 0.019).15 Previous studies have demonstrated that a dynamic change in CTC count during treatment may serve as a predictive biomarker of chemotherapy response.16, 17 Unfortunately, in the present study we did not monitor changes in CTC levels over the course of treatment as it was difficult to keep patients in the study. Further analysis of changes in CTC levels can be expected from future large‐scale prospective studies.
We performed CTC analysis using the Cyttel method (Cyttel, Beijing, China), which is independent to the epithelial cell adhesion molecule (EpCAM) method and thus obtains higher efficiency than the CellSearch system.18 A threshold of eight CTCs was chosen to define unfavorable and favorable prognostic groups, and was validated as a suitable CTC threshold for EpCAM‐independent CTC detection methods. The CTC detection rates in this study were consistent with those previously reported by studies of NSCLC patients without mutations. No correlations were found between baseline CTC counts and clinicopathological characteristics in EGFR‐mutated or ALK‐rearranged NSCLC patients.7, 19
The main limitations of this study were the inadequate sample size of ALK‐rearranged cases and a lack of analysis of other oncogenes, such as KRAS, BRAF, and c‐MET. CTC detection during treatment would have yielded further valuable information. Nonetheless, our data provides a basis for future studies.
In conclusion, our data demonstrated the prognostic significance of the presence and characterization of CTCs in EGFR‐mutated and ALK‐rearranged NSCLC patients treated with targeted therapies. The baseline CTC count remains an important prognostic factor contributing to risk stratification and individualized treatment for advanced NSCLC patients. Further studies are warranted to validate these results.
Acknowledgment
We thank colleagues at the Lung Cancer Center of Peking Union Medical Hospital for their contribution to sample collection.
Disclosure
No authors report any conflict of interest.
Supporting information
Table S1 Stepwise multivariate Cox analysis of overall survival (OS) and progression‐free survival (PFS).
References
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 2. Shea M, Costa DB, Rangachari D. Management of advanced non‐small cell lung cancers with known mutations or rearrangements: Latest evidence and treatment approaches. Ther Adv Respir Dis 2016; 10: 113–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sgambato A, Casaluce F, Maione P, Gridelli C. Targeted therapies in non‐small cell lung cancer: A focus on ALK/ROS1 tyrosine kinase inhibitors. Expert Rev Anticancer Ther 2018; 18: 71–80. [DOI] [PubMed] [Google Scholar]
- 4. Lv Q, Gong L, Zhang T et al. Prognostic value of circulating tumor cells in metastatic breast cancer: A systemic review and meta‐analysis. Clin Transl Oncol 2016; 18: 322–30. [DOI] [PubMed] [Google Scholar]
- 5. Groot Koerkamp B, Rahbari NN, Büchler MW, Koch M, Weitz J. Circulating tumor cells and prognosis of patients with resectable colorectal liver metastases or widespread metastatic colorectal cancer: A meta‐analysis. Ann Surg Oncol 2013; 20: 2156–65. [DOI] [PubMed] [Google Scholar]
- 6. Onstenk W, de Klaver W, de Wit R, Lolkema M, Foekens J, Sleijfer S. The use of circulating tumor cells in guiding treatment decisions for patients with metastatic castration‐resistant prostate cancer. Cancer Treat Rev 2016; 46: 42–50. [DOI] [PubMed] [Google Scholar]
- 7. Zhang Z, Xiao Y, Zhao J et al Relationship between circulating tumour cell count and prognosis following chemotherapy in patients with advanced non‐small‐cell lung cancer. Respirology 2016; 21: 519–25. [DOI] [PubMed] [Google Scholar]
- 8. Tong B, Xu Y, Zhao J et al Prognostic significance of circulating tumor cells in non‐small cell lung cancer patients undergoing chemotherapy. Oncotarget 2017; 8: 86615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu Y, Ling Y, Qi Q et al Prognostic value of circulating tumor cells in advanced gastric cancer patients receiving chemotherapy. Mol Clin Oncol 2017; 6: 235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Okubo K, Uenosono Y, Arigami T et al Clinical impact of circulating tumor cells and therapy response in pancreatic cancer. Eur J Surg Oncol 2017; 43: 1050–5. [DOI] [PubMed] [Google Scholar]
- 11. Aggarwal C, Wang X, Ranganathan A et al Circulating tumor cells as a predictive biomarker in patients with small cell lung cancer undergoing chemotherapy. Lung Cancer 2017; 112: 118–25. [DOI] [PubMed] [Google Scholar]
- 12. Yang B, Qin A, Zhang K et al Circulating tumor cells predict prognosis following tyrosine kinase inhibitor treatment in EGFR‐mutant non‐small cell lung cancer patients. Oncol Res 2017; 25: 1601–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. He W, Li W, Jiang B et al Correlation between epidermal growth factor receptor tyrosine kinase inhibitor efficacy and circulating tumor cell levels in patients with advanced non‐small cell lung cancer. Onco Targets Ther 2016; 9: 7515–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pailler E, Oulhen M, Borget I et al. Circulating tumor cells with aberrant ALK copy number predict progression‐free survival during crizotinib treatment in ALK‐rearranged non‐small cell lung cancer patients. Cancer Res 2017; 77: 2222–30. [DOI] [PubMed] [Google Scholar]
- 15. Punnoose EA, Atwal S, Liu W et al. Evaluation of circulating tumor cells and circulating tumor DNA in non‐small cell lung cancer: Association with clinical endpoints in a phase II clinical trial of pertuzumab and erlotinib. Clin Cancer Res 2012; 18: 2391–401. [DOI] [PubMed] [Google Scholar]
- 16. Hirose T, Murata Y, Oki Y et al Relationship of circulating tumor cells to the effectiveness of cytotoxic chemotherapy in patients with metastatic non‐small‐cell lung cancer. Oncol Res 2012; 20: 131–7. [DOI] [PubMed] [Google Scholar]
- 17. Krebs MG, Sloane R, Priest L et al Evaluation and prognostic significance of circulating tumor cells in patients with non‐small‐cell lung cancer. J Clin Oncol 2011; 29: 1556–63. [DOI] [PubMed] [Google Scholar]
- 18. Krebs MG, Hou JM, Sloane R et al Analysis of circulating tumor cells in patients with non‐small cell lung cancer using epithelial marker‐dependent and ‐independent approaches. J Thorac Oncol 2012; 7: 306–15. [DOI] [PubMed] [Google Scholar]
- 19. Chen YY, Xu GB. Effect of circulating tumor cells combined with negative enrichment and CD45‐FISH identification in diagnosis, therapy monitoring and prognosis of primary lung cancer. (Published erratum appears in Med Oncol 2015;32:190) Med Oncol 2014; 31: 240. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Stepwise multivariate Cox analysis of overall survival (OS) and progression‐free survival (PFS).


