Abstract
Background
For many years, lung cancer has been the most common and deadly cancer worldwide. Early diagnosis of non‐small cell lung cancer (NSCLC) in particular is very difficult because the symptoms are often ignored. The five‐year survival rate is very low despite great improvements to therapy. Thus, there is an urgent need to identify prognostic biomarkers and target molecules for the clinical diagnosis and individualized treatment of NSCLC.
Methods
We performed quantitative real‐time PCR to determine the expression levels of the long non‐coding RNA (lncRNA) linc01433 in NSCLC and normal matched lung tissue. Subsequently, we established cell lines with overexpression or knockdown of linc01433 to evaluate the effects on proliferation and metastasis in vitro. Epithelial‐to‐mesenchymal transition was examined using Western blot.
Results
Linc01433 was significantly overexpressed in NSCLC tissues compared to normal lung tissues. In addition, linc01433 levels were associated with smoking history. Linc01433 overexpression in lung cancer cells increased proliferation, migration, and invasion abilities, as well as epithelial‐to‐mesenchymal transition.
Conclusions
Linc01433 is a cancer‐related lncRNA that may have an oncogene‐like effect in NSCLC.
Keywords: lncRNA, metastasis, migration and invasion, NSCLC, prognostic marker
Introduction
For many years, lung cancer has been the most common and deadly cancer worldwide. In 2015, 4 292 000 new cancer cases and 2 814 000 cancer deaths were estimated to have occurred in China1 while 1 658 370 new cancer cases and 589 430 cancer deaths occurred in the United States.2 Non‐small cell lung cancer (NSCLC) is the predominant type of lung cancer and accounts for approximately 85% of cases.3, 4 Surgery, chemotherapy, radiotherapy, targeted therapy, and immunotherapy are used alone or in combination to treat NSCLC.5, 6, 7 The five‐year overall survival rate of NSCLC remains as low as 16% because most patients are already at a locally advanced or metastatic stage when diagnosed.8 This may be at least partially attributable to inadequate understanding of the pathogenesis of NSCLC and the lack of early diagnostic biomarkers and therapeutic targets.9, 10, 11, 12, 13 Thus, there is an urgent need to reveal the mechanisms behind the generation and progression of NSCLC.
Epithelial‐to‐mesenchymal transition (EMT) is an essential process as a crucial driver of organ pathological fibrosis and tumor progression.14, 15 However, the mechanisms involved in EMT during cancer progression intermediates with lncRNA remain poorly understood.
Genetic and epigenetic changes have been widely acknowledged as the leading events in cancer. According to the Encyclopedia of DNA Elements (ENCODE) project, nearly 98% of human genomes do not encode proteins.16 The mechanisms and functions of microRNAs in cancer have been thoroughly reviewed in recent decades.11, 17 Long non‐coding RNAs (lncRNAs) are a heterogeneous group of non‐coding transcripts of more than 200 nucleotides in length that have many molecular functions, such as modulating transcription patterns, regulating protein activities, playing structural or organizational roles, altering RNA processing events, and serving as precursors to small RNAs.18, 19
For example, the lncRNA MALAT‐1 acts as a regulator of gene expression governing the hallmarks of metastasis in lung cancer.20 MALAT‐1 is associated with poor prognosis in NSCLC and induces proliferation and migration.21 MALAT‐1 also promotes brain metastasis by inducing EMT in lung cancer.22 Moreover, higher expression of HOTAIR is thought to be related to metastasis and is associated with poor survival in patients with lung cancer.23, 24 Col‐1, a potent inducer of EMT, regulates the expression of HOTAIR in NSCLC cells,25 whereas HOTAIR interacts with a chromatin modifier, lymphoid‐specific helicase (LSH), to target genes in NSCLC.26 Although substantial research of lung‐cancer‐related lncRNAs has been conducted,25, 26, 27, 28, 29, 30, 31, 32 no lncRNA that can serve as a single molecular marker to diagnose lung cancer has been identified, therefore we need to explore other lncRNAs to obtain a better understanding of this disease. In our previous study, we focused on one of the genes with a change in expression between tumor tissues and matched normal lung tissues, determined by RNA‐sequencing.33 Specifically, we found that the linc01433 expression level was significantly higher in tumor tissues, suggesting that it may be a novel prognostic factor for human NSCLC.
Methods
Samples and clinical data
Under approval from the Ethics Committee of the Second Xiangya Hospital of Central South University (Hunan, China) and upon the provision of written informed consent from all patients, we collected 45 paired NSCLC and normal lung tissues from patients who had undergone surgery at this hospital during 2010–2012 and were diagnosed with NSCLC (stage I, II, or III) based on histopathological evaluation. None of the patients had received radiotherapy or chemotherapy prior to surgery. All tissue samples were flash‐frozen in liquid nitrogen immediately after collection and stored at −80°C until use.
Cell culture
A normal human bronchial epithelioid cell line (BEAS‐2B), human NSCLC cell lines (A549, H1299, H520, PC9), and 293T were obtained from the Cancer Research Institution of Central South University (Hunan, China). BEAS‐2B and 293T were cultured in Dulbecco’s modified Eagle medium supplemented with 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA, USA) and 1% penicillin/streptomycin/gentamicin. A549 was cultured in Dulbecco’s modified Eagle medium‐F12 supplemented with 10% bovine calf serum (Invitrogen) and 1% penicillin/streptomycin/gentamicin. H520 was cultured in RPMI 1640 (Invitrogen), supplemented with 10% FBS and 1% penicillin/streptomycin/gentamicin. H1299 and PC9 were cultured in RPMI 1640, supplemented with 10% bovine calf serum and 1% penicillin/streptomycin/gentamicin. All cells were cultured in a humidified incubator at 37°C with 5% CO2.
Stable overexpression and knockdown of linc01433 in cell lines
The linc01433 coding region was sub‐cloned into the retroviral vector pLVX‐EF1α‐IRES‐Puro (Clontech, Mountain View, CA, USA), purchased from Beijing Genomics Institute (Beijing, China). Linc01433 small hairpin RNA (shRNA) vectors (GV248, linc01433‐shRNA 1–3, and control shRNA) were purchased from GeneChem (Shanghai, China). Transfection of plasmids was performed using LipoMax (Sudgen Biotech, Bellevue, WA, USA), in accordance with the manufacturer’s protocol. Stable shRNA‐expressing colonies and overexpressing colonies were selected using puromycin. Knockdown and overexpression of linc01433 was confirmed by quantitative real‐time PCR (qRT‐PCR).
RNA isolation, reverse‐transcription PCR and quantitative real‐time PCR
Total RNA was extracted from NSCLC cells or tissues using TRIZOL Reagent (Invitrogen), following the manufacturer’s protocol. Cytoplasmic RNA and nuclear RNA were separated and purified using RNAiso Blood (Takara, Dalian, China), following the manufacturer’s instructions. RNA and DNA concentrations were determined using NanoDrop (Thermo Scientific, Waltham, MA, USA). Total RNA was reverse‐transcribed into complementary DNA using a Prime Script RT Reagent Kit with gDNA Eraser (Takara). qRT‐PCR was carried out on an Applied Biosystems 7500 Real‐Time PCR System (Thermo Scientific), using a Fast Start Universal SYBR Green Master (Roche, Basel, Switzerland), in accordance with the manufacturer’s instructions.
The following primers for qRT‐PCR were purchased from Sangon Biotech (Shanghai, China): linc01433: forward, 5′‐ACTTCCTCACCCATCGACAA‐3′, and reverse, 5′‐CTCACCAACAACCGCTAGTG‐3′; β‐actin (as an internal control in most qRT‐PCR): forward, 5′‐CACCATTGGCAATGAGCGGTTC‐3′, and reverse, 5′‐AGGTCTTTGCGGATGTCCACGT‐3′; GAPDH: forward, 5′‐ GTCTCCTCTGACTTCAACAGCG‐3′, and reverse, 5′‐ ACCACCCTGTTGCTGTAGCCAA‐3′; and U6: forward, 5′‐ CTCGCTTCGGCAGCACA‐3′, and reverse, 5′‐ AACGCTTCACGAATTTGCGT‐3′.
The relative fold changes in messenger RNA expression were calculated using the 2−ΔΔCt method. Calculation of The ΔCt value was calculated using the following formula: ΔCt(target) = Ct(target) − Ct(β‐actin).
Cell proliferation assays
Cells were detached using 0.25% trypsin‐ethylene‐diamine‐tetraacetic acid (Invitrogen) and seeded into 96‐well plates at a density of 5 × 102 per well in 200 μL. We used a Bio‐Rad TC10TM automated cell counter to count cells (Bio‐Rad, Hercules, CA, USA). For MTS assay, the Cell Titer 96 AQueous One Solution Cell Proliferation Assay kit (Promega, Madison, WI, USA) was used following the manufacturer’s instructions. Briefly, 30 minutes before each of the set time points (12, 24, 48, and 72 hours), 20 μL of MTS reagent diluted 10 times was added to each well and cells were incubated at 37°C for 30 minutes. The absorbance was detected at 490 nm using a microplate reader (BioTek, Winooski, Vermont, USA). All experiments were repeated three times.
Cell migration and invasion assays
Cells were collected 48 hours post‐transfection; 5 × 104 (for migration assays) or 1 × 105 (for invasion assays) cells in serum‐free medium were placed into the upper chamber of an insert (8 μm pore size; Falcon, San Jose, CA, USA). Matrigel Matrix (Falcon) diluted nine times was added to the upper chamber of an insert before the serum‐free medium. Medium containing 10% FBS was added to the lower chamber. After 24 hours of incubation the cells remaining on the upper membrane were removed with cotton wool; cells that had migrated or invaded through the membrane were stained with methanol and 0.1% crystal violet, imaged, and counted using Image J software (NIH, Bethesda, MD, USA). Experiments were independently repeated three times.
Western blot assay
Cells stably overexpressing linc01433 were lysed by RIPA buffer with a protease inhibitor cocktail (Roche). A protein assay kit (Bio‐Rad) was used to determine the concentration of protein. Protein lysates weighing 50 μg were electrophoresed on 12% sodium dodecyl sulfate‐polyacrylamide gel electrophoresis, transferred onto 0.45‐μm polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA), and then incubated with an Epithelial–Mesenchymal Transition Antibody Sampler Kit (Cell Signaling, Danvers, MA, USA). Pierce ECL Western Blotting Substrate (Thermo Scientific) was used to detect the bands and quantify the intensity. β‐Actin antibody was used as a control.
Statistical analysis
All statistical analyses in this study were performed using SPSS version 23.0 (IBM, Armonk, NY, USA), and P < 0.05 was considered to represent statistical significance. The experimental data are presented as the mean ± standard deviation. Student’s t and chi‐squared tests were used to compare differences between groups. Pearson’s correlation analysis was applied to analyze the correlation between linc01433 in tissues.
Results
Higher expression of linc01433 in lung cancer tissue
To explore whether linc01433 was upregulated in NSCLC, we first performed qRT‐PCR to determine the linc01433 expression levels in NSCLC and normal matched lung tissue. The linc01433 expression level in an independent panel of 45 primary lung tumors was significantly higher than in normal matched lung tissue (P < 0.0001) (Fig 1a). However, this difference was more significant in lung squamous cell carcinoma (SCC) (P < 0.001) (Fig 1b) than in adenocarcinoma (ADC) (P < 0.05) (Fig 1c).
Figure 1.
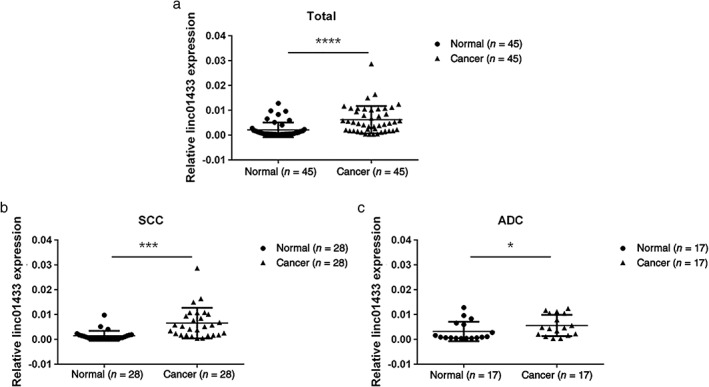
Linc01433 expression was significantly increased in human non‐small cell lung cancer (NSCLC) tissues compared to normal lung tissues. (a) Dot blot analysis based on quantitative reverse‐transcription PCR analysis of linc01433 expression in 45 paired lung cancer and corresponding normal lung tissues. Normal (n = 45), cancer (n = 45). Dot blot analysis of linc01433 expression in (b) squamous cell carcinoma (SCC) normal (n = 28), cancer (n = 28); and (c) adenocarcinoma (ADC) normal (n = 17), cancer (n = 17). *P < 0.05, ***P < 0.001, ****P < 0.0001.
Table 1.
Correlation between linc01433 expression and clinicopathological parameters of NSCLC (n = 45)
| Clinical parameters | Factors | n | Relative high | Relative low | P |
|---|---|---|---|---|---|
| Ages | ≤ 60 | 21 | 6 | 15 | 0.231 |
| >60 | 24 | 11 | 13 | ||
| Gender | Male | 34 | 14 | 20 | 0.401 |
| Female | 11 | 3 | 8 | ||
| Smoking history | Yes | 20 | 11 | 9 | 0.032* |
| No | 25 | 6 | 19 | ||
| Type | SCC | 28 | 11 | 17 | 0.788 |
| ADC | 17 | 6 | 11 | ||
| Differentiation | Poor | 25 | 9 | 16 | 0.783 |
| Well or moderate | 20 | 8 | 12 | ||
| T‐classification | T1–T2 | 27 | 10 | 17 | 0.900 |
| T3–T4 | 18 | 7 | 11 | ||
| N‐ classification | N0 | 21 | 8 | 13 | 0.967 |
| N1‐N3 | 24 | 9 | 15 | ||
| Clinical Stage | I–II | 26 | 8 | 18 | 0.257 |
| III–IV | 19 | 9 | 10 |
P < 0.05.
Chi‐square test. ADC, adenocarcinoma; NSCLC, non‐small cell lung cancer; SCC, squamous cell carcinoma.
Correlation between clinicopathological parameters and relative expression of linc01433
Subsequently, using the relative linc01433 expression level, we determined an average of 0.0062. NSCLC patients were divided into high (≥ 0.0062, n = 17) and low (< 0.0062, n = 28) groups based on the average linc01433 expression in 45 tissues. Moreover, to assess the clinical significance of linc01433, we evaluated the correlation between expression level and clinicopathological parameters. Linc01433 expression was closely associated with smoking history, but was not linked to other clinical risk factors such as age, gender, tumor type and differentiation, T‐classification, or lymphatic metastasis.
Expression level and localization of linc01433 in cell lines
We then explored linc01433 expression levels in five NSCLC cell lines (H520, H1299, PC9, H358, and A549). Our results revealed a higher level of linc01433 expression in three of the NSCLC cell lines (A549, H358, and PC9) than in BEAS‐2B, while no significant difference was identified in H520 or H1299 (P < 0.01) (Fig 2a). Furthermore, we determined the localization of linc01433 in cells; GAPDH and small nuclear RNA U6 were used as internal controls of the cytoplasm and nucleus, respectively.34, 35 The results of qRT‐PCR analysis of linc01433 expression levels in the nuclear/cytoplasmic fractions in A549 and H358 cells are presented in Figure 2b,c. We found that linc01433 localized in both the nucleus and the cytosol, but more so in the nucleus in A549 and H358 cell lines.
Figure 2.
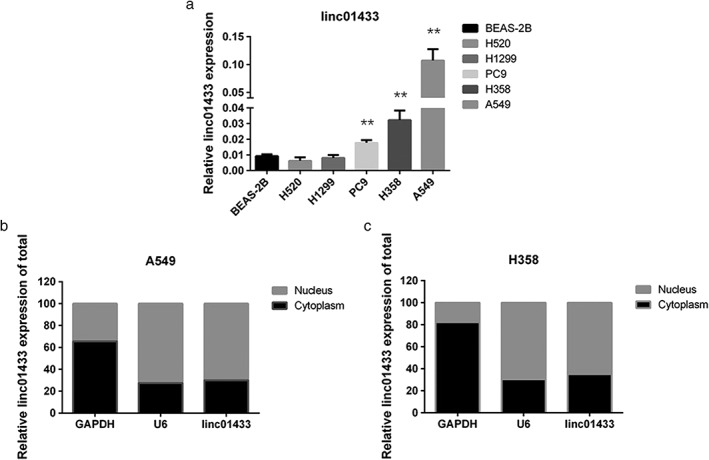
Expression and localization of linc01433 in cell lines. (a) Relative expression of linc01433 in five non‐small cell lung cancer (NSCLC) cell lines and normal human bronchial epithelial cells (BEAS‐2B). Linc01433 expression was higher in A549, H358, and PC9 than in the BEAS‐2B cell line. BEAS‐2B, H520, H1299, PC9, H358, A549. (b, c) Quantitative reverse‐transcription PCR analysis of linc01433 expression levels in different subcellular fractions in two NSCLC cell lines (A549 and H358). Nucleus, cytoplasm. Linc01433 localized in both the nucleus and the cytosol, but more so in the nucleus in the A549 and H358 cell lines. Nucleus, cytoplasm. **P < 0.01.
Linc01433 overexpression promoted cell proliferation, migration, invasion, and epithelial‐to‐mesenchymal transition (EMT)
We selected H520, H1299, and PC9 for further study given the lower levels of linc01433 in these cancer cells (Fig 3a). After observing stable ectopic expression of linc01433 in H520, H1299, and PC9 cells, we found that linc01433 promoted cell growth compared to the control group (Fig 3b,c). Moreover, stable expression of linc01433 was associated with increased migration and invasion activities (Fig 3d,e). E‐cadherin, claudin‐1, β‐catenin, vimentin, and ZEB1 protein levels were also examined by Western blotting. Linc01433 overexpression resulted in reductions of E‐cadherin and claudin‐1 and increases of vimentin, β‐catenin, and ZEB1 (Fig 3f).
Figure 3.
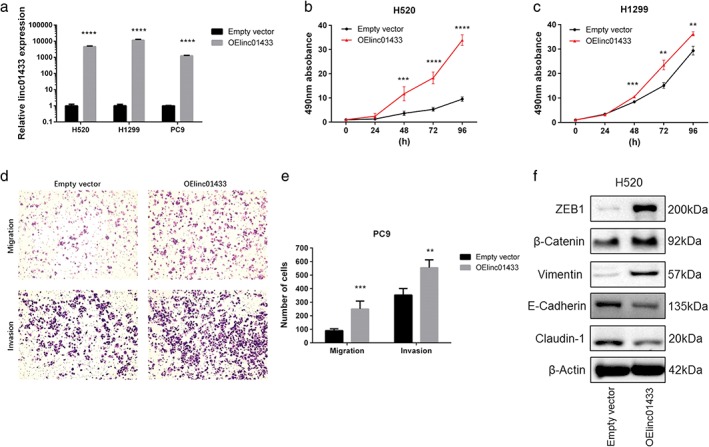
Overexpression of linc01433 promoted non‐small cell lung cancer (NSCLC) cell proliferation, migration, invasion, and epithelial‐to‐mesenchymal transition (EMT) in vitro. (a) Relative linc01433 expression in H520, H1299, and PC9 cells transfected with pLVX‐EF1α‐IRES‐Puro‐linc01433 vector. Empty vector, OElinc01433. (b, c) The effects of linc01433 overexpression on cell proliferation of H520 and H1299 in an in vitro MTS assay. Transfection of linc01433 significantly promoted the growth of OElinc01433 cells. Empty vector, OElinc01433. (d, e) The effects of linc01433 overexpression on cell migration and invasion. Significantly more H520‐OElinc01433 cells migrated and invaded through the basement membrane compared to empty vector cells. Empty vector, OElinc01433. (f) The effect of linc01433 overexpression on the expression of EMT‐related proteins in H520 cells. Claudin‐1, E‐cadherin, vimentin, β‐catenin, ZEB1, and β‐actin expression determined by Western blotting. Reductions of E‐cadherin and claudin‐1 and increases of vimentin, β‐catenin, and ZEB1 in H520‐OE linc01433 cells. **P < 0.01, ***P < 0.001, ****P < 0.0001.
Knockdown of linc01433 suppressed cell proliferation, migration, invasion, and EMT
We selected A549 for further study given that it had one of the highest levels of linc01433 expression (Fig 4a). After stable knockdown of linc01433 expression in A549 cells using three separate shRNA sequences, we found that linc01433 suppressed cell growth compared to the control group (Fig 4b). Stable knockdown of linc01433 was also associated with decreased migration and invasion activities (Fig 4c,d). Moreover, linc01433 knockdown resulted in increases of E‐cadherin and claudin‐1 and decreases of vimentin, β‐catenin, and ZEB1 (Fig 4e).
Figure 4.
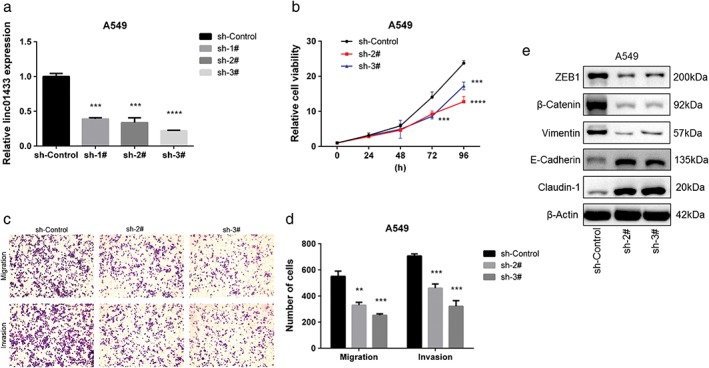
Knockdown of linc01433 suppressed non‐small cell lung cancer (NSCLC) cell proliferation, migration, invasion, and epithelial‐to‐mesenchymal transition (EMT) in vitro. (a) Relative linc01433 expression in A549 cells transfected with shRNAs. sh‐control, sh‐1#, sh‐2#, sh‐3#. (b) The effect of linc01433 knockdown on cell proliferation of A549 in an in vitro MTS assay. Knockdown of linc01433 significantly suppressed the growth of A549 cells. sh‐control, sh‐2#, sh‐3. (c,d) The effects of linc01433 knockdown on cell migration and invasion. Significantly fewer A549‐ShRNA cells migrated and invaded through the basement membrane compared to A549‐control cells. sh‐control, sh‐2#, sh‐3# (e) The effect of linc01433 knockdown on the expression of EMT‐related proteins in A549 cells. Claudin‐1, E‐cadherin, vimentin, β‐catenin, ZEB1, and β‐actin expression determined by Western blotting. Increases of E‐cadherin and claudin‐1 expression was increased, while vimentin, β‐catenin, and ZEB1 was reduced in A549‐shRNA cells. **P < 0.01, ***P < 0.001, ****P < 0.0001.
Discussion
Non‐small cell lung cancer is a heterogeneous group of diseases characterized by different biological behaviors. To improve treatment of patients with NSCLC, novel tumor‐specific biomarkers are needed for diagnosis, targeted therapy, and to predict prognosis36 LncRNAs are associated with pathological and physiological processes in numerous human cancers. The dysregulated expression of certain lncRNAs has been proven to be associated to proliferation and metastasis in NSCLC.25, 27, 28, 29, 30, 31 Linc01433 is located on chromosome 20p13 in humans and has a length of 682 bp. In our study, we identified that linc01433 was upregulated in NSCLC tissues, thus it could be a new clinicopathological biomarker and prognostic factor for this disease. Nevertheless, the biological roles and underlying molecular mechanisms of linc01433 in NSCLC tumorigenesis and progression are still unclear.
In the present study, we determined the expression levels of linc01433 in several NSCLC cell lines in order to select certain lines for use in subsequent experiments. As our results show, linc01433 exhibited lower expression in H520, H1299, and PC9 cells, thus we chose these three cell lines to achieve stable overexpression. Moreover, as linc01433 was more highly expressed in A549 cells, we selected A549 to establish a stable knockdown cell line. Most lncRNAs display strong nuclear localization36 however, we found that linc01433 was localized in both the nucleus and the cytoplasm, but more so in the nucleus in A549 and H358 cell lines.
Subsequently, we found that linc01433 overexpression in H520, H1299, and PC9 cells enhanced proliferation, migration, and invasion abilities, suggesting that this lncRNA is involved in tumor metastasis. Metastasis is a very complex biological process in carcinomas,15 the mechanisms of which remain largely unknown. However, many recent studies have shown that EMT activation is detrimental to the dissemination and invasion of certain cancer cells.37, 38, 39, 40 During EMT, epithelial tumor cells lose their epithelial traits, such as E‐cadherin expression,41 and instead display mesenchymal traits, such as vimentin expression.42 E‐cadherin is considered to be an active suppressor of the invasion and growth of many epithelial cancers,43 while higher expression of claudin‐1 is a good prognostic factor in NSCLC, but only in the SCC subgroup.44 Moreover, vimentin is an EMT biomarker associated with increased invasiveness of NSCLC cells.42 β‐catenin is a key downstream effector in the Wnt signaling pathway with a close relationship to EMT.45 Moreover, ZEB1 (TCF8), as a regulator of EMT, is associated with tumor grade and metastasis in lung cancer.46 In the present study, linc01433 overexpression was found to inhibit the expression of epithelial markers (E‐cadherin and claudin‐1) in H520 cells, while it increased the levels of mesenchymal markers (vimentin, β‐catenin, and ZEB1) in A549 cells. These results suggest that linc01433 could promote the transition from the epithelial to the mesenchymal stage.
Our data clarify that linc01433 is upregulated in NSCLC tumor tissues and some cell lines. In addition, linc01433 levels were associated with smoking history. Linc01433 overexpression promoted cell proliferation, migration, and invasion, and facilitated EMT in vitro. Moreover, linc01433 knockdown suppressed cell proliferation, migration, and invasion, as well as EMT, in vitro. Linc01433 is thus a cancer‐related lncRNA that may have an oncogene‐like effect on NSCLC. As chromatin modifiers and metabolism are involve in tumorigenicity,33, 47, 48, 49, 50 and the interplay of lncRNAs on metabolism is regarded as a hallmark of cancer, it will be interesting to determine whether linc01433 could regulate other possible metabolic targets and the potential mechanisms.
Disclosure
No authors report any conflict of interest.
Acknowledgments
We would like to thank all laboratory members for their critical discussion of this manuscript. The National Natural Science Foundation of China (81672787, 81370118, 81672308) supported the study.
Contributor Information
Yongguang Tao, Email: taoyong@csu.edu.cn.
Hongcan Shi, Email: shihongcan@hotmail.com.
References
- 1. Chen W, Zheng R, Baade PD et al Cancer statistics in China, 2015. CA Cancer J Clin 2016; 66: 115–32. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65: 5–29. [DOI] [PubMed] [Google Scholar]
- 3. Zhan Y, Zang H, Feng J, Lu J, Chen L, Fan S. Long non‐coding RNAs associated with non‐small cell lung cancer. Oncotarget 2017; 8: 69174–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gazdar AF, Bunn PA, Minna JD. Small‐cell lung cancer: What we know, what we need to know and the path forward. (Published erratum appears in Nat Rev Cancer 2017;17:765). Nat Rev Cancer 2017; 17: 725–37. [DOI] [PubMed] [Google Scholar]
- 5. Rosenzweig KE, Gomez JE. Concurrent chemotherapy and radiation therapy for inoperable locally advanced non‐small‐cell lung cancer. J Clin Oncol 2017; 35: 6–10. [DOI] [PubMed] [Google Scholar]
- 6. Kris MG, Gasper LE, Chaft JE et al Adjuvant systemic therapy and adjuvant radiation therapy for stage I to IIIA completely resected non‐small‐cell lung cancers: American Society of Clinical Oncology/Cancer Care Ontario Clinical Practice Guideline update. J Clin Oncol 2017; 35: 2960–74. [DOI] [PubMed] [Google Scholar]
- 7. Hiley CT, Le Quesne J, Santis G et al Challenges in molecular testing in non‐small‐cell lung cancer patients with advanced disease. Lancet 2016; 388: 1002–11. [DOI] [PubMed] [Google Scholar]
- 8. Wao H, Mhaskar R, Kumar A, Miladinovic B, Djulbegovic B. Survival of patients with non‐small cell lung cancer without treatment: A systematic review and meta‐analysis. Syst Rev 2013; 2: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reclusa P, Taverna S, Pucci M et al Exosomes as diagnostic and predictive biomarkers in lung cancer. J Thorac Dis 2017; 9 (Suppl 13): S1373–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Osmani L, Askin F, Gabrielson E, Li QK. Current WHO guidelines and the critical role of immunohistochemical markers in the subclassification of non‐small cell lung carcinoma (NSCLC): Moving from targeted therapy to immunotherapy. Semin Cancer Biol 2017. https://doi.org/10.1016/j.semcancer.2017.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moretti F, D'Antona P, Finardi E et al Systematic review and critique of circulating miRNAs as biomarkers of stage I–II non‐small cell lung cancer. Oncotarget 2017; 8: 94980–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mayo‐de‐Las‐Casas C, Garzón Ibáñez M, Jordana‐Ariza N et al An update on liquid biopsy analysis for diagnostic and monitoring applications in non‐small cell lung cancer. Expert Rev Mol Diagn 2018; 18: 35–45. [DOI] [PubMed] [Google Scholar]
- 13. Forest F, Stachowicz ML, Casteillo F et al EGFR, KRAS, BRAF and HER2 testing in metastatic lung adenocarcinoma: Value of testing on samples with poor specimen adequacy and analysis of discrepancies. Exp Mol Pathol 2017; 103: 306–10. [DOI] [PubMed] [Google Scholar]
- 14. Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial‐mesenchymal transition. Nat Rev Mol Cell Biol 2014; 15: 178–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nieto MA, RYJ H, Jackson RA, Theiry JP. EMT: 2016. Cell 2016; 166: 21–45. [DOI] [PubMed] [Google Scholar]
- 16. Encode Project Consortium . An integrated encyclopedia of DNA elements in the human genome. Nature 2012; 489: 57–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhou Q, Huang SX, Zhang F et al MicroRNAs: A novel potential biomarker for diagnosis and therapy in patients with non‐small cell lung cancer. Cell Prolif 2017; 50: e12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Esteller M. Non‐coding RNAs in human disease. Nat Rev Genet 2011; 12: 861–74. [DOI] [PubMed] [Google Scholar]
- 19. Mercer TR, Dinger ME, Mattickv JS. Long non‐coding RNAs: Insights into functions. Nat Rev Genet 2009; 10: 155–9. [DOI] [PubMed] [Google Scholar]
- 20. Gutschner T, Hämmerle M, Eissmann M et al The noncoding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cells. Cancer Res 2013; 73: 1180–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schmidt L, Spieker T, Koschmieder S et al The long noncoding MALAT‐1 RNA indicates a poor prognosis in non‐small cell lung cancer and induces migration and tumor growth. J Thorac Oncol 2011; 6: 1984–92. [DOI] [PubMed] [Google Scholar]
- 22. Shen L, Chen L, Wang Y, Jiang X, Xia H, Zhuang Z. Long noncoding RNA MALAT1 promotes brain metastasis by inducing epithelial‐mesenchymal transition in lung cancer. J Neurooncol 2015; 121: 101–8. [DOI] [PubMed] [Google Scholar]
- 23. Zhao W, An Y, Liang Y, Xie XW. Role of HOTAIR long noncoding RNA in metastatic progression of lung cancer. Eur Rev Med Pharmacol Sci 2014; 18: 1930–6. [PubMed] [Google Scholar]
- 24. Nakagawa T, Endo H, Yokoyama M et al Large noncoding RNA HOTAIR enhances aggressive biological behavior and is associated with short disease‐free survival in human non‐small cell lung cancer. Biochem Biophys Res Commun 2013; 436: 319–24. [DOI] [PubMed] [Google Scholar]
- 25. Zhuang Y, Wang X, Nguyen HT et al Induction of long intergenic non‐coding RNA HOTAIR in lung cancer cells by type I collagen. J Hematol Oncol 2013; 6: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang R, Shi Y, Chen L et al The ratio of FoxA1 to FoxA2 in lung adenocarcinoma is regulated by LncRNA HOTAIR and chromatin remodeling factor LSH. Sci Rep 2015; 5: 17826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shi X, Sun M, Liu H et al A critical role for the long non‐coding RNA GAS5 in proliferation and apoptosis in non‐small‐cell lung cancer. Mol. Carcinog 2015; 54(Suppl 1): E1–12. [DOI] [PubMed] [Google Scholar]
- 28. Sun M, Liu XH, Wang KM et al Downregulation of BRAF activated non‐coding RNA is associated with poor prognosis for non‐small cell lung cancer and promotes metastasis by affecting epithelial‐mesenchymal transition. Mol Cancer 2014; 13: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang M, Ma X, Zhu C et al The prognostic value of long non coding RNAs in non small cell lung cancer: A meta‐analysis. Oncotarget 2016; 7: 81292–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang EB, Yin DD, Sun M et al P53‐regulated long non‐coding RNA TUG1 affects cell proliferation in human non‐small cell lung cancer, partly through epigenetically regulating HOXB7 expression. Cell Death Dis 2014; 5: e1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gutschner T, Hämmerle M, Diederichs S. MALAT1 ‐‐ A paradigm for long noncoding RNA function in cancer. J Mol Med (Berl) 2013; 91: 791–801. [DOI] [PubMed] [Google Scholar]
- 32. Xu E, Yu X, Zeng Q et al Functional role of lncRNA DB327252 in lung cancer. J Thorac Dis 2016; 8: 2793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jiang Y, Mao C, Yang R et al EGLN1/c‐Myc induced lymphoid‐specific helicase inhibits ferroptosis through lipid metabolic gene expression changes. Theranostics 2017; 7: 3293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rodríguez R, Polston PM, Wu MJ, Wu J, Sobsey MD. An improved infectivity assay combining cell culture with real‐time PCR for rapid quantification of human adenoviruses 41 and semi‐quantification of human adenovirus in sewage. Water Res 2013; 47: 3183–91. [DOI] [PubMed] [Google Scholar]
- 35. Lou G, Ma N, Xu Y et al Differential distribution of U6 (RNU6‐1) expression in human carcinoma tissues demonstrates the requirement for caution in the internal control gene selection for microRNA quantification. Int J Mol Med 2015; 36: 1400–8. [DOI] [PubMed] [Google Scholar]
- 36. Ettinger DS, Wood DE, Aisner DL et al Non‐small Cell Lung Cancer, version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017; 15: 504–35. [DOI] [PubMed] [Google Scholar]
- 37. Zhang Q, Li X, Li X, Li X, Chen Z. LncRNA H19 promotes epithelial‐mesenchymal transition (EMT) by targeting miR‐484 in human lung cancer cells. J Cell Biochem 2017. https://doi.org/10.1002/jcb.26537 [DOI] [PubMed] [Google Scholar]
- 38. Imani S, Wei C, Cheng J et al MicroRNA‐34a targets epithelial to mesenchymal transition‐inducing transcription factors (EMT‐TFs) and inhibits breast cancer cell migration and invasion. Oncotarget 2017; 8: 21362–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu Z, Long J, Du R, Ge C, Guo K, Xu Y. miR‐204 regulates the EMT by targeting snai1 to suppress the invasion and migration of gastric cancer. Tumour Biol 2016; 37: 8327–35. [DOI] [PubMed] [Google Scholar]
- 40. Xiao D, He J. Epithelial mesenchymal transition and lung cancer. J Thorac Dis 2010; 2: 154–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jiang Y, Ren W, Wang W et al Inhibitor of β‐catenin and TCF (ICAT) promotes cervical cancer growth and metastasis by disrupting E‐cadherin/β‐catenin complex. Oncol Rep 2017; 38: 2597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Richardson A, Havel LS, Koyen AE et al Vimentin is required for lung adenocarcinoma metastasis via heterotypic tumor cell‐cancer‐associated fibroblast interactions during collective invasion. Clin Cancer Res 2018; 24: 420–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yang YL, Chen MW, Xian L. Prognostic and clinicopathological significance of downregulated E‐cadherin expression in patients with non‐small cell lung cancer (NSCLC): A meta‐analysis. PLoS ONE 2014; 9: e99763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Moldvay J, Fábián K, Jäckel M et al Claudin‐1 protein expression is a good prognostic factor in non‐small cell lung cancer, but only in squamous cell carcinoma cases. Pathol Oncol Res 2017; 23: 151–6. [DOI] [PubMed] [Google Scholar]
- 45. Yang S, Liu Y, Li MY et al FOXP3 promotes tumor growth and metastasis by activating Wnt/β‐catenin signaling pathway and EMT in non‐small cell lung cancer. Mol Cancer 2017; 16: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Larsen JE, Nathan V, Osborne JK et al ZEB1 drives epithelial‐to‐mesenchymal transition in lung cancer. J Clin Invest 2016; 126: 3219–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. He X, Yan B, Liu S et al Chromatin remodeling factor LSH drives cancer progression by suppressing the activity of fumarate hydratase. Cancer Res 2016; 76: 5743–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. He Y, Gao M, Cao Y, Tang H, Liu S, Tao Y. Nuclear localization of metabolic enzymes in immunity and metastasis. Biochim Biophys Acta 2017; 1868: 359–71. [DOI] [PubMed] [Google Scholar]
- 49. Jia J, Shi Y, Chen L et al Decrease in lymphoid specific helicase and 5‐hydroxymethylcytosine is associated with metastasis and genome instability. Theranostics 2017; 7: 3920–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Xiao D, Huang J, Pan Y et al Chromatin remodeling factor LSH is upregulated by the LRP6‐GSK3β‐E2F1 axis linking reversely with survival in gliomas. Theranostics 2017; 7: 132–43. [DOI] [PMC free article] [PubMed] [Google Scholar]


