Abstract
Desiccation tolerance is initiated in wheat (Triticum aestivum L.) embryos in planta at 22 to 24 d after anthesis, at the time that the embryo water content has decreased from about 73% fresh weight (2.7 g water/g dry weight) to about 65% fresh weight (1.8 g water/g dry weight). To determine if desiccation tolerance is fully induced by the loss of a relatively small amount of water, detached wheat grains were treated to reduce the embryo water content by just a small amount to approximately 69% (2.2 g water/g dry weight). After 24 h of such incipient water loss, subsequently excised embryos were able to withstand severe desiccation, whereas those embryos that had not previously lost water could not. Therefore, a relatively small decrease in water content for only 24 h acts as the signal for the development of desiccation tolerance. Embryos that were induced into tolerance by a 24-h water loss had no detectable raffinose. The oligosaccharide accumulated at later times even in embryos of detached grains that had not become desiccation tolerant, although tolerant embryos (i.e. those that previously had lost some water) contained larger amounts of the carbohydrate. It is concluded that desiccation tolerance and the occurrence of raffinose are not correlated. Immunodetected dehydrins accumulated in embryos in planta as desiccation tolerance developed. Detachment of grains induced the appearance of dehydrins at an earlier age, even in embryos that had not been made desiccation tolerant by incipient drying. It is concluded that a small reduction in water content induces desiccation tolerance by initiating changes in which dehydrins might participate but not by their interaction with raffinose.
Desiccation tolerance in seeds is initiated approximately when mass maturity is established, approximately when maturation drying occurs (Bartels et al., 1988; Fischer et al., 1988; Ellis and Hong, 1994; Hay and Probert, 1995; Black et al., 1996; Sanhewe and Ellis, 1996). However, the precise time during maturation when tolerance is acquired depends on the species, the rate of water loss, and the final water content after drying (Hong and Ellis, 1992; Ellis and Hong, 1994; Wechsberg et al., 1994; Hay and Probert, 1995). Physiological regulation of the induction of desiccation tolerance is poorly understood, but one suggestion is that vascular isolation of the developing seeds (“ovular abscission”) is of major importance (Galau et al., 1991), followed by incipient water loss. The action of ABA might also be involved, as first indicated by Bartels et al. (1988). Tolerance can be induced experimentally in isolated seeds or embryos by slow drying to the final dehydration state (Adams et al., 1983; Blackman et al., 1991; for review, see Vertucci and Farrant, 1995; Bochicchio et al., 1997), implying that cellular changes conferring resistance are initiated as water is lost and can be consolidated as long as the removal of water is not too rapid. However, to our knowledge, the amount of water loss that is required to initiate tolerance has never been defined. This paper provides novel data relating to this point.
To withstand desiccation cells must be protected against potentially lethal changes that could follow dehydration. Mechanisms by which this might be achieved include the participation of certain soluble carbohydrates and/or LEA (late-embryogenesis abundant) proteins (for review, see Vertucci and Farrant, 1995). Important among the soluble sugars are Suc and the raffinose-family oligosaccharides (e.g. raffinose and stachyose), which are thought to be involved in glass formation or to interact protectively with membrane phospholipids, or both (for review, see Vertucci and Farrant, 1995). Accumulation of these carbohydrates has been correlated with the development of desiccation tolerance in several species, such as maize (Chen and Burris, 1990), soybean (Blackman et al., 1992), Brassica campestris (Leprince et al., 1990), and wheat (Black et al., 1996), and in germinated seeds of maize, pea, and soybean (Koster and Leopold, 1988). Raffinose-to-Suc ratios above certain critical values are considered important (Horbowicz and Obendorf, 1994). On the other hand, it has been suggested that desiccation tolerance can occur in the absence of oligosaccharide, e.g. raffinose, in maize (Bochicchio et al., 1997), and intolerance can occur even in its abundance, as in wild rice (Still et al., 1994); therefore, there is some uncertainty that requires a resolution.
There is good evidence that the LEA proteins play important parts in responses to various stresses, including water stress (Close, 1996). Whereas several LEAs, including dehydrins, accumulate in seeds during maturation drying (Galau et al., 1987; Blackman et al., 1991, 1992; Dure, 1993; Gee et al., 1994; Wechsberg et al., 1994; Vertucci and Farrant, 1995; Han et al., 1997; Kermode, 1997), their role is still unclear. It has been suggested, however, that the LEAs alone do not confer desiccation tolerance but that they interact with other protectants such as oligosaccharides (Blackman et al., 1992). According to this concept, tolerance should be induced only when LEAs are accompanied by, for example, the appropriate oligosaccharide. As in the case of the oligosaccharides that appear during maturation drying, little is known about the physiological regulation of LEA appearance in seeds. We show that accumulation of dehydrins can be provoked by grain detachment, irrespective of subsequent desiccation tolerance.
Our previous work (Black et al., 1996) showed that desiccation tolerance in wheat (Triticum aestivum) embryos is induced in planta early during maturation drying and is complete by the time the water content has decreased from approximately 74% to approximately 62% fresh weight (from 2.8 to 1.6 g water/g dry weight). Whether this reflects a causal relationship between water loss and the initiation of desiccation tolerance requires clarification. If it is causal, information is needed concerning the degree of water loss that is effective. Raffinose, the only detectable oligosaccharide in wheat, begins to accumulate at about this time, but the precise association between raffinose content and the acquisition of desiccation tolerance has not been determined and the possible participation of dehydrins has not been examined. Therefore, we have continued our investigations to answer the following questions concerning the physiological, biochemical, and molecular aspects of desiccation tolerance: (a) How much loss of water initiates the acquisition of desiccation tolerance? (b) Does tolerance depend on the enhancement of raffinose accumulation? (c) Does the appearance of dehydrin and ABA participate in the induction of tolerance? (d) Is the accumulation of raffinose and dehydrin regulated in the same physiological manner?
MATERIALS AND METHODS
Plant Material
A spring wheat (Triticum aestivum cv Sappo) was cultivated in a growth room as described by Garcia-Maya et al. (1990). Ears were tagged at anthesis, subsequently yielding grains of known age (daa). Mid-ear grains were detached as required, and where necessary embryos were excised immediately for testing of water content, desiccation tolerance, and analysis of soluble carbohydrate and dehydrin (see below).
Manipulation of Embryo Water Content
Detached grains were held in open Petri dishes on wire-net trays in desiccators in which the bottom compartment was filled with water (giving 100% RH) or saturated Na2CO3 (giving 90% RH); the grains at 100% RH were placed on filter paper moistened with water. The desiccators were kept in the growth room under the same light and temperature conditions as for wheat plant cultivation (16 h light/8 h dark, 18°C/14°C). This was done to hold conditions as comparable as possible with those experienced during in planta drying under our growing conditions. Grains were removed at intervals, and the embryos were excised immediately to provide samples for the determination of water content, desiccation tolerance, soluble carbohydrate content, and dehydrin occurrence. Those embryos for desiccation tolerance and determination of water content were used immediately after excision, whereas the others were divided into weighed batches and stored at −80°C for later extraction.
Water Content
Embryos were dried at 98°C to 100°C until constant dry weight was achieved (40–48 h) for determination of water content, generally expressed on a percentage fresh weight basis.
Desiccation Tolerance
Immediately after their excision, embryos were desiccated by stepwise treatment in the growth room (see above) for 24 h at 90% RH (saturated Na2CO3) (to approximately 25% water content) and for 24 h at 73% RH (saturated NaCl) (to a final water content of approximately 7% fresh weight ) (Black et al., 1996).
Germination Test
After desiccation (generally immediately but occasionally after 24 h of storage at 4°C), embryos were tested for germination by incubation for 7 d (maximum germination) at 20°C in darkness on filter paper wetted with a solution composed of one-half-strength Murashige and Skoog medium (minus growth regulators) containing 2% (w/v) Suc, 1.5 mm l-Ala, and 2.5 mm l-Gln, pH 5.5. All embryos that showed radicle and/or coleoptile elongation were scored as germinated. Failure of embryos to produce a “germinated” primary radicle, i.e. only an elongated coleoptile, was dependent on embryo age and was not imposed by desiccation. Younger embryos (e.g. 16–24 daa) showed a higher percentage (approximately 30%–40%) of coleoptile-only germination than fully mature (34 daa) embryos (100% radicles and coleoptile). Each germination test consisted of duplicates of 25 embryos: All experiments were performed at least three times, and percentages are therefore means of at least six values.
Determination of Soluble Sugars
Duplicate samples of weighed embryos or embryo parts (10–15 per sample) were extracted as described by Black et al. (1996) in 80% aqueous ethanol containing 250 μg/mL melezitose as an internal standard. Soluble sugars (duplicates of each sample extract) were determined as described by Black et al. (1996) and are usually calculated on a per-embryo basis. Most experiments were done at least twice and in many cases three times. Final values, therefore, are means of 8 to 12 determinations.
Dehydrins
Embryos in batches of 50 were placed in liquid N2, ground to a powder, and homogenized in Trizol reagent (GIBCO-BRL) (5 mL/g embryo, i.e. about 250 μL) and then centrifuged after the addition of 0.1 volume of chloroform. Following the manufacturer's procedure the proteins were precipitated from the eventual phenol:ethanol supernatant by the addition of 0.8 volume of propan-2-ol and were then purified according to the manufacturer's instructions. Protein concentration in the final supernatant was estimated by the method of Bradford (1976). Equal quantities of protein were fractionated by discontinuous SDS-PAGE using 12% polyacrylamide gels and electroblotted onto a nitrocellulose membrane. The membrane was probed with rabbit antiserum (diluted 1:500) raised against the oligopeptide corresponding to the consensus carboxy terminus of dehydrin proteins (Close et al., 1989) (courtesy of Dr. Peter Chandler, Commonwealth Scientific and Industrial Research Organization, Canberra, Australia) and then with donkey anti-rabbit horseradish peroxidase-linked whole antibody (Amersham). The resulting protein-antibody complex was detected using the chemiluminescence western-blotting detection system (ECL, Amersham).
Extraction and Measurement of ABA
ABA was extracted and detected by HPLC-ELISA methodology, as described by Julliard et al. (1994). Freeze-dried embryos (10–15 per batch) were extracted with 80% aqueous methanol containing 40 mg/L butylhydroxytoluene for 60 h at 4°C in darkness. The extracts were filtered through cellulose and 0.2-μm pore size filters (Millipore) and prepurified through a C18 Sep-Pak minicolumn (Millipore), reduced under a vacuum to a small volume of aqueous phase, and acidified with formic acid before HPLC purification. The collected fractions were reduced to dryness, taken up in distilled water, and tested by ELISA according to the procedure described by Julliard et al. (1994). The results are presented as means of values obtained in five replications.
RESULTS
Desiccation Tolerance and Soluble Carbohydrates in Planta
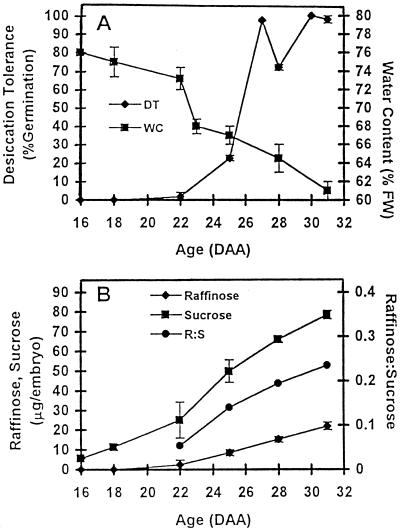
The water content and desiccation tolerance of embryos maturing in planta are shown in Figure 1A. Embryos younger than 22 daa had no tolerance, but this developed fully in the period 22 to 27 daa, when the water content decreased from approximately 73% to approximately 65% fresh weight (from 2.2 to 1.8 g water/g dry weight). Raffinose was absent from embryos that had no desiccation tolerance (16–18 daa), was barely detectable at 22 daa, and accumulated thereafter to reach approximately 20 μg/embryo (about 10 μg/mg fresh weight, 25 μg/mg dry weight) at 31 daa. Suc was found in 16-daa embryos at about 5.5 μg/embryo (approximately 11 μg/mg fresh weight, 55 μg/mg dry weight) and increased to approximately 78 μg/embryo (39 μg/mg fresh weight, 100 μg/mg dry weight) at 31 daa. The raffinose-to-Suc mass ratio increased more than 4-fold, from about 0.05 at 22 daa to more than 0.2 at 31 daa (Fig. 1B). Amounts of Glc and Fru were extremely low throughout seed development and showed no increase during maturation drying (data not shown).
Figure 1.
Desiccation tolerance, water, Suc, and raffinose contents of wheat embryos of different ages in planta. A, Desiccation tolerance (DT; percent germination after desiccation to approximately 7% water content) (♦) and water content (WC; fresh weight [FW] basis) (▪). B, Sugar contents (raffinose, ♦; Suc, ▪) and raffinose-to-Suc mass ratio (R:S, •). All values are means ± se of four to six samples.
Water Content and Desiccation Tolerance ex Planta
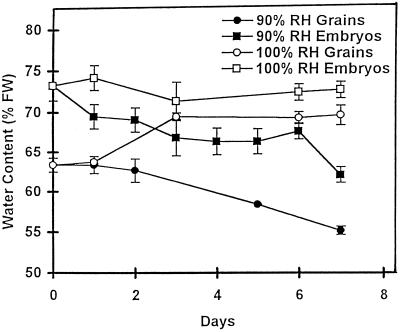
The results shown in Figure 1 raise the possibility that a relatively small loss of water may be responsible for provoking the onset of desiccation tolerance. This was tested by imposing treatments on detached whole grains that caused the embryos to lose a relatively small amount of water or, in the control, none at all (Fig. 2). Isolated wheat grains (22 daa) held at 90% RH lost water slowly over 7 d, decreasing from about 64% water content to approximately 55%. The water content of the embryos in the grains decreased from about 74% to 69% fresh weight (from about 2.8 to 2.2 g water/g dry weight) in the first 24 h but remained unchanged for the next 5 d, until it decreased further to about 63% (1.7 g water/g dry weight) after 7 d at 90% RH. The water content of grains at 100% RH increased from approximately 64% to about 70%. Embryos of these grains retained their water content at about 74% (Fig. 2).
Figure 2.
Water contents of detached whole grains (22 daa) and their embryos at different relative humidities. Grains were kept at 100% RH or 90% RH for up to 7 d. Water contents (fresh weight [FW] basis) of whole grains and excised embryos were determined at intervals. All values are means ± se of five to six samples.
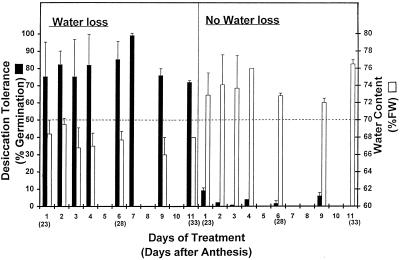
Embryos of grains kept at 100% RH for up to 11 d were highly germinable (90%–95% germination; detailed data not shown), but they had almost no survival after desiccation. Those embryos whose water content had previously decreased to about 69% or 63% survived subsequent rapid desiccation, i.e. they had become desiccation tolerant (Fig. 3). A striking effect is that a relatively small decrease in water content during the first 24 h was sufficient to induce maximum tolerance to further extreme dehydration; inductive loss of water for a longer period was not necessary. Desiccation tolerance was also provoked in younger embryos (starting at 16 and 18 daa) by a similar water loss experienced for 6 and 4 d, respectively (Table I).
Figure 3.
Effect of water loss on subsequent desiccation tolerance. Detached grains (22 daa) were held at 100% RH (no water loss) or at 90% RH (water loss) for up to 11 d. Chronological ages after detachment (daa) are shown in parentheses. At intervals, embryos were removed and their water content (open columns) and desiccation tolerance (percentage of germination after desiccation to approximately 7% water content; closed columns) were determined. The dashed line indicates the 70% water content. All values are means ± se of at least six samples. FW, Fresh weight.
Table I.
Induction of desiccation tolerance in young embryos
| Age | Treatment | WC | DT | Raffinose | R:S | |
|---|---|---|---|---|---|---|
| daa | % fresh wt | % germn | μg/embryo | μg/mg fresh wt | ||
| 16 | Freshly harvested | 76.4 ± 1.6 | 0 | 0 | 0 | –a |
| 6 d at 90% RH | 68.3 ± 1.5 | 85.5 ± 9.3 | 9.3 ± 2.9 | 8.3 ± 2.6 | 0.33 | |
| 6 d at 100% RH | 77.1 ± 0.6 | 12.2 ± 1.6 | 5.0 ± 0.4 | 4.5 ± 0.7 | 0.29 | |
| 18 | Freshly harvested | 76.0 ± 0.9 | 0 | 0 | 0 | – |
| 4 d at 90% RH | 69.5 ± 0.5 | 92.0 ± 3.1 | 11.4 ± 4.4 | 8.7 ± 3.7 | 0.35 | |
| 4 d at 100% RH | 74.5 ± 1.3 | 11.2 ± 4.3 | 1.2 ± 0.9 | 0.9 ± 0.7 | 0.06 | |
Detached grains aged 16 and 18 daa (approximately 20% and 33%, respectively, of the fresh weight of mature embryos at 32 daa) were kept at 90% or 100% RH for the times indicated. Embryos were removed for the determination of water content (WC), desiccation tolerance (DT) (percentage of germination [germn] after drying to about 7% water content), and raffinose (R) and Suc (S) contents. R:S is expressed as the mass ratio. Values are means ± se of five to six samples (water content and desiccation tolerance) or eight determinations (R and S).
–, Not applicable.
Water Content and Accumulation of Suc and Raffinose
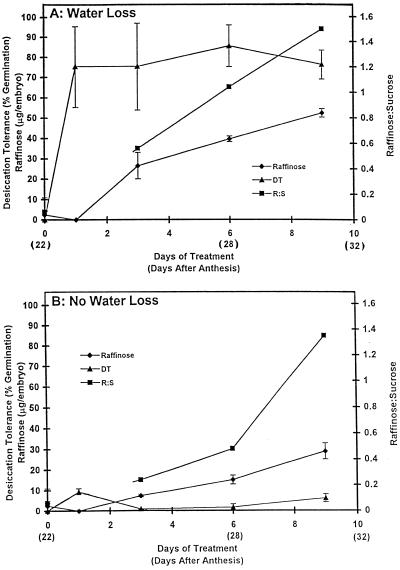
The amounts of these two carbohydrates were measured in embryos in which desiccation tolerance had or had not been induced by manipulation of the water content. Grains aged 22 daa were held at 90% and 100% RH for 9 d, and at intervals embryos were isolated and tested for desiccation tolerance and carbohydrate content (Fig. 4). As expected, embryos that had lost water showed a high degree of desiccation tolerance (about 80% were able to germinate after rapid drying), whereas those that previously had lost no water remained virtually desiccation intolerant. Before and after 1 d of incipient water loss raffinose was undetectable in the embryos (even though after 1 d the embryos were highly desiccation tolerant), but subsequently the content gradually increased to reach 51 ± 2 μg/embryo (42.5 μg/mg fresh weight, 113 μg/mg dry weight) at 9 d. In the 1-d-treated tolerant embryos the raffinose-to-Suc ratio was incalculably small, but at 3 d its value was about 0.6, increasing to approximately 1.5 at d 9. In those embryos that had not lost water (desiccation intolerant), raffinose was again virtually absent for the 1st d, increasing gradually to approximately 30 μg/embryo (20.7 μg/mg fresh weight, 82 μg/mg dry weight) at d 9, with an accompanying increase in the raffinose-to-Suc ratio to about 1.4.
Figure 4.
Raffinose content and the raffinose-to-Suc ratio in desiccation-tolerant and -intolerant embryos. Grains (22 daa) were held at 90% RH to cause incipient water loss of embryos and induction of desiccation tolerance (A) and at 100% RH when no water loss occurred and no desiccation tolerance was induced (B). At intervals, desiccation tolerance of isolated embryos was determined (DT; ▴) and raffinose (♦) and Suc contents were measured. Raffinose-to-Suc mass ratios (R:S; ▪) were calculated. All values for desiccation tolerance are means ± se of 6 readings, and those for raffinose are means ± se of 8 to 12 readings. On a dry-matter basis, the raffinose content increased from 65 μg/mg dry weight at 3 d to 113 μg/mg dry weight at 9 d (A) and from 23 μg/mg dry weight at 3 d to 82 μg/mg dry weight at 9 d (B).
The data in Table I show that embryos as young as 16 and 18 daa (starting age) could also be induced into the tolerant state by the incipient loss of water. This was also accompanied by an increase in raffinose content (determined after 4 and 6 d) and the raffinose-to-Suc ratio. Smaller increases in raffinose content and the raffinose-to-Suc ratio also occurred in the absence of water loss, accompanying the extremely low level of desiccation tolerance (Table I).
Intraembryo Distribution of Raffinose
To obtain information concerning the location of raffinose, embryos (22 daa) in which desiccation tolerance was induced over 6 d at 90% RH were divided into radicles, coleoptiles, and scutella, and batches of each part were extracted and assayed (Table II). No raffinose was detected after 1 d, but it accumulated at d 3 and 6 at 90% RH in coleoptiles and scutella, respectively. Raffinose did not appear in the radicles until 6 d of reduced water content. At d 6, on a per-organ basis, amounts of raffinose occurred increasingly in the order radicle, coleoptile, and scutellum, but on a concentration basis differences among the parts were relatively small. For unknown reasons, the sums of the parts were less than the total amounts shown in Figure 4.
Table II.
Intraembryo distribution of raffinose
| Days at 90% RH | Raffinose
|
R:S
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Coleoptile | Radicle | Scutellum | Coleoptile | Radicle | Scutellum | ||||
| μg/organ | μg/mg fresh wt | μg/organ | μg/mg fresh wt | μg/organ | μg/mg fresh wt | ||||
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | –a | – | – |
| 3 | 2.0 ± 1.5 | 8.7 ± 2.1 | 0 | 0 | 5.7 ± 1.9 | 7.9 ± 1.9 | 0.53 | – | 0.42 |
| 6 | 7.5 ± 1.0 | 19.9 ± 1.8 | 4.1 ± 0.2 | 13.1 ± 1.5 | 26.3 ± 4.9 | 20.1 ± 3.8 | 0.75 | 0.56 | 0.89 |
Grains aged 22 daa were detached and held at 90% RH for 1, 3, and 6 d, during which time the embryo water content decreased from 73% to 68% and desiccation tolerance was induced. Embryos were divided into separate organs for extraction. Values are means ± se of six determinations. R:S is expressed as the mass ratio.
–, Not applicable.
Accumulation of Dehydrin
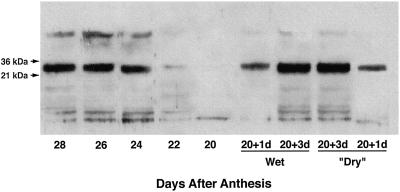
The occurrence of dehydrin was followed in embryos as they became desiccation tolerant in planta, in embryos inwhich tolerance was induced by manipulation of water content, and in embryos in which acquisition of desiccation tolerance was suppressed. The immunoblots (Fig. 5) show a major, strong dehydrin band (molecular mass approximately 25 kD) in in planta embryos at 24 to 28 daa (as tolerance is developing) but little or none at 20 to 22 daa (before the onset of tolerance). Within 1 d after detachment from the plant (21 daa), a strong dehydrin signal was found, similar to that in 24-daa in planta embryos, which increased at 3 d after detachment (23 daa). These increases in dehydrin occurred in embryos of detached grains irrespective of whether they had been primed into desiccation tolerance by adjustment of their water content.
Figure 5.
Dehydrins in embryos in planta and in desiccation-tolerant and -intolerant embryos of detached grains. Embryos were removed from grains at 20 to 28 daa (desiccation tolerance, 0%–92% germinability). Grains 20 daa were detached and kept for 1 and 3 d at 90% RH (“Dry”) when desiccation tolerance reached 65% and 89% germinability, respectively, and at 100% RH (Wet) when no tolerance developed. Embryos were extracted, and 10 μg of protein per lane was loaded on the gel. Dehydrins were detected by western blotting with an antiserum raised against an oligopeptide corresponding to the consensus carboxy terminus sequence of dehydrins. The experiment was carried out twice with similar results. There was insufficient preimmune serum to use on the blot shown, but in a previous experiment using a restricted range of samples preimmune serum was inactive.
ABA
The concentrations of free ABA and its glucosylpyranosyl ester in embryos induced into desiccation tolerance were compared with those in embryos that remained desiccation intolerant. ABA and its ester were found in all of the treatments. No significant differences in free-ABA content occurred among the samples, but small differences were found in the concentration of the ABA ester (Table III).
Table III.
Free and esterified ABA in embryos with or without water loss
| Treatment | Free ABA | ABA-GE |
|---|---|---|
| pmol/mg fresh wt | ||
| Freshly harvested | 10.2 ± 0.7 | 3.5 ± 0.2 |
| 6 d at 90% RH | 11.8 ± 1.1 | 5.3 ± 0.5 |
| 6 d at 100% RH | 8.7 ± 0.8 | 2.6 ± 0.3 |
Detached grains aged 22 daa were kept at 90% or 100% RH for 6 d, during which time the water content of the embryos decreased to about 68% or remained at about 74%, respectively. Free ABA and its glucopyranosyl ester (ABA-GE) in the embryos were determined by HPLC-ELISA method. All values are means ± se of five replicates.
DISCUSSION
With respect to the induction and mechanism of desiccation tolerance in wheat embryos the experimental findings point to the following conclusions. (a) Desiccation tolerance is induced by incipient loss of water for as little as 1 d. The small reduction in water content is effective not only at an age when embryos are on the verge of becoming desiccation tolerant (e.g. 22 daa) but also in younger embryos. (b) Incipient water loss does not cause an increase in ABA. (c) The presence of raffinose and therefore a critical raffinose-to-Suc ratio are not essential for the development of tolerance, as can be seen in embryos that have experienced water loss for just 1 d. Similarly, the accumulation of raffinose does not necessarily lead to the establishment of desiccation tolerance, as shown by embryos in detached grains held wet for several days, which acquired substantial amounts of raffinose but did not become tolerant. (d) Although water loss is not an essential initiator of raffinose biosynthesis, it does enhance raffinose accumulation in those embryos capable of producing the oligosaccharide. (e) Dehydrin accumulation is not physiologically regulated in the same manner as the induction of desiccation tolerance, i.e. experimentally by incipient water loss, but rather by an apparent detachment effect. We will now examine these conclusions in more detail.
Desiccation tolerance in wheat embryos was fully induced by a decrease in water content from about 73% fresh weight (2.7 g water/g dry weight) to approximately 69% fresh weight (2.2 g water/g dry weight), i.e. about 15% on a dry-matter basis, for only 1 d. After the “drying” induction a much higher proportion of embryos became desiccation tolerant than their in planta counterparts of the same age. Surprisingly, however, in previous studies emphasis has always been placed on the drying “rate” and not on the absolute amount of water loss required for the initiation of tolerance. That desiccation tolerance in seeds can be induced by slow drying is well documented (for review, see Vertucci and Farrant, 1995). Examination of those cases in which induced tolerance has been measured at intervals during the slow-drying treatment suggests that tolerance can be initiated when a relatively small decrease in water content has been achieved, e.g. in soybean (Blackman et al., 1992), upon a decrease from approximately 2.1 to approximately 1.7 g water/g dry weight (i.e. 68%–63% fresh weight). Surprisingly, this point has been ignored, possibly because continued drying has always been used. Clearly, wheat embryos become desiccation tolerant as a result of an initial, relatively small decrease in water content, and further slow drying is not necessary. The effects of a small perturbation in water content on events such as promotion of germinability and subsequent seedling growth have been recorded for other species (Rosenberg and Rinne, 1986), but the present finding with respect to desiccation tolerance is novel.
The clear requirement for a relatively slight water loss for the induction of desiccation tolerance indicates that induction is not “programmed” to occur only at a certain age (see, for example, the effects on 16-daa embryos), nor does it necessarily follow physical (and therefore, physiological) detachment from the parent plant (ovular abscission), because embryos in wet grains did not become tolerant even at 11 d after detachment. In principle, these findings agree with those for soybean (Blackman et al., 1992). The unexpected finding that the loss of water for only 1 d is enough to initiate tolerance in wheat shows that inductive events must be completed within this time. These events presumably include the perception of water loss, the transduction of this signal, and the final establishment of tolerance.
The initial loss of water from the embryos will lead to a reduction in turgor and an alteration in osmotic balance. Presumably, these are the signals that set in motion the events by which desiccation tolerance is established, although how they operate is at present obscure. The mechanism(s) of osmosensing in plants is unknown. Osmosensors such as those in yeast (Posas et al., 1996) may be responsible, but to our knowledge, none has been identified. The subsequent signal transduction chain in wheat embryos does not appear to be initiated specifically by ABA, as it can be in plant responses to water stress, because the signal (water loss) does not cause an increase in the ABA content. Nonetheless, ABA may participate, as the evidence suggests for other species such as barley and Arabidopsis (Bartels et al., 1988; Koornneef et al., 1989; Ooms et al., 1993; Tetteroo et al., 1994; Kermode, 1997). For now, other components of the transduction chain must remain the subject of speculation, but it is likely that they include factors that are being characterized with respect to the responses of vegetative tissues to drought and other stresses (Bohnert and Sheveleva, 1998).
The acquisition of desiccation tolerance has been suggested to depend on various carbohydrates such as Suc, raffinose-family oligosaccharides, and the Gal cyclitols (Horbowicz and Obendorf, 1994; Vertucci and Farrant, 1995; Obendorf, 1997). Most of the evidence for this comes from the observation that the carbohydrates accumulate during seed maturation, accompanying (apart from Suc, in which accumulation starts earlier) the development of tolerance (e.g. Chen and Burris, 1990; Leprince et al., 1990; Horbowicz and Obendorf, 1994; Black et al., 1996; Obendorf, 1997). Also, raffinose and stachyose are reported to appear in soybean axes only when detached seeds are held under conditions that provoke desiccation tolerance (Blackman et al., 1992). There are also several indications that in the case of raffinose and Suc tolerance is established as the raffinose-to-Suc ratio increases above a specific value (Chen and Burris, 1990). The evidence in wheat embryos stands against the idea that tolerance is achieved via an effect of raffinose. The most important argument is that there is no or barely detectable raffinose production under the water loss (1–2 d) that can bring about desiccation tolerance. Additionally, under certain conditions (“wet” embryos) the raffinose concentration and the raffinose-to-Suc ratio both increase to values similar to those in tolerant embryos, but no desiccation tolerance sets in. The latter point contrasts with the situation in soybean as reported by Blackman et al. (1992), in which embryonic axes of seeds held wet fail to produce the raffinose-family oligosaccharides. It appears, therefore, that in wheat embryos raffinose biosynthesis develops relatively slowly and is initiated by grain detachment or when a certain age is reached, or both. Over the long term (6 d) raffinose accumulates in all parts of the embryo in detached wheat grains under conditions in which desiccation tolerance develops. Suc contents also increase (data not shown), presumably at the expense of endosperm carbohydrates), and the raffinose-to-Suc ratio increases.
Although raffinose is not essential for the development of tolerance, conditions that induce tolerance (water loss) do, however, enhance the buildup of raffinose, even though water loss is not necessary for the latter to begin. After 6 and 9 d, for example, there was about 4 and 2 times, respectively, the amount of raffinose per embryo in embryos that experienced water loss than in those that did not (Fig. 4). In young embryos (16 and 18 daa) subjected to water loss for times during which they reach 22 daa, raffinose contents increased substantially, whereas in those embryos that remained in planta for 22 daa raffinose was almost undetectable. These findings suggest that raffinose biosynthesis can be regulated at three levels: (a) by a time-dependent program, (b) by detachment from the parent plant (ovular abscission), and (c) by incipient drying. The fact that the latter possibility presents the most favorable conditions indicates that metabolic processes involved in raffinose formation can progress adequately even at water potentials more negative than normal.
The mechanism by which incipient drying enhances raffinose accumulation is a matter for speculation. Several metabolic processes in seeds are known to be affected by dehydration, which in some cases regulates proteins at the level of gene expression (Cornford et al., 1986; Jiang and Kermode, 1994). Stages in raffinose biosynthesis might be similarly affected, although Blackman et al. (1992) concluded from their studies in soybean that galactinol synthase and raffinose synthetase are not likely to be points at which regulation occurs.
Our findings, like those of Bochicchio et al. (1997) in maize, cast doubt on a specific requirement for raffinose in the establishment of desiccation tolerance. Nonetheless, raffinose begins to accumulate at about the time when tolerance is initiated; therefore, the question remains what part does the oligosaccharide (and related ones) play. A positive correlation between seed longevity and oligosaccharide content and oligosaccharide:Suc has been found in several species (Bernal-Lugo and Leopold, 1992; Horbowicz and Obendorf, 1994; Liu and Huang, 1994; Steadman et al., 1996), and we have evidence that this is also the case in wheat (M. Black, H. Gee, and C. Cornford, unpublished data). The enhanced production of raffinose in response to the dehydration signal might be a preparation to maximize future longevity of the dried embryo rather than to initiate tolerance to desiccation. Thus, the assumption, which is often made or implied, that raffinose and other oligosaccharides play equivalent roles in the acquisition of desiccation tolerance, in storability, and in longevity should be reexamined.
The LEA proteins are thought to participate in desiccation tolerance (Dure, 1993; Close 1996; Han et al., 1997; Kermode, 1997). In orthodox seeds they have been studied generically as heat-stable proteins (e.g. Blackman et al., 1991; Kermode, 1997) or more specifically as the class II LEAs, the dehydrins (Gee et al., 1994; Wechsberg et al., 1994). Western blots show that a dehydrin-like protein of approximately 25 kD is just detectable in 22-daa wheat embryos in planta but appears strongly at 24 daa, i.e. at the onset of desiccation tolerance. Detachment of 20-daa grains induces a very strong dehydrin signal after 1 d (i.e. at 21 daa) and an even stronger signal after 3 d. Because there are no discernible differences in signal strength between embryos that will (incipient drying) or will not (no water loss) become desiccation tolerant, we must conclude that dehydrin accumulation is not regulated by factors that specifically control the induction of tolerance. Instead, it would appear that the dehydrin-like protein is produced in response to grain detachment (ovular abscission), even when this is not followed by dehydration, as was concluded for soybean (Blackman et al., 1991). Our findings in wheat on the beneficial effects of detachment are therefore consistent with the possibility that in planta there is a maternal suppression of dehydrin accumulation by the embryos that is relieved by ovular abscission (Galau et al., 1987).
If dehydrins participate in the initiation of desiccation tolerance by incipient drying, they are unlikely to do so, in wheat embryos, by an interaction with raffinose, as suggested for soybean (Blackman et al., 1992), because this oligosaccharide is not consistently present when embryos are induced into tolerance. The effect of decreased water potential in inducing desiccation tolerance, therefore, must operate through some other mechanism that is now obscure.
ACKNOWLEDGMENTS
We thank Regis Maldiney of Professor E. Miginiac's laboratory (Université Pierre et Marie Curie, Paris) for carrying out the determinations of ABA and Marie-Ange Picard for technical assistance in the sugar determinations. M.B. is thankful to l'Université Pierre et Marie Curie for support.
Abbreviation:
- daa
days after anthesis
LITERATURE CITED
- Adams CA, Fjerstad MC, Rinne RW. Characteristics of soybean maturation: necessity for slow dehydration. Crop Sci. 1983;23:265–267. [Google Scholar]
- Bartels D, Singh M, Salamini F. Onset of desiccation tolerance during development of the barley embryo. Planta. 1988;175:485–492. doi: 10.1007/BF00393069. [DOI] [PubMed] [Google Scholar]
- Bernal-Lugo I, Leopold AC. Changes in soluble carbohydrates during seed storage. Plant Physiol. 1992;98:1207–1210. doi: 10.1104/pp.98.3.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black M, Corbineau F, Grzesik M, Guy P, Côme D. Carbohydrate metabolism in the developing and maturing wheat embryo in relation to its desiccation tolerance. J Exp Bot. 1996;47:161–169. [Google Scholar]
- Blackman SA, Obendorf RL, Leopold AC. Maturation proteins and sugars in desiccation tolerance of developing soybean seeds. Plant Physiol. 1992;100:225–230. doi: 10.1104/pp.100.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman SA, Wettlaufer SH, Obendorf RL, Leopold AC. Maturation proteins associated with desiccation tolerance in soybean. Plant Physiol. 1991;96:868–874. doi: 10.1104/pp.96.3.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochicchio A, Vernieri P, Puliga S, Murelli C, Vazzana C. Desiccation tolerance in immature embryos of maize: sucrose, raffinose and the ABA-sucrose relation. In: Ellis RH, Black M, Murdoch AJ, Hong TD, editors. Basic and Applied Aspects of Seed Biology. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1997. pp. 13–22. [Google Scholar]
- Bohnert HJ, Sheveleva E. Plant stress adaptations: making metabolism move. Curr Opin Plant Biol. 1998;1:267–274. doi: 10.1016/s1369-5266(98)80115-5. [DOI] [PubMed] [Google Scholar]
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chen Y, Burris JS. Role of carbohydrates in desiccation tolerance and membrane behavior in maturing maize seed. Crop Sci. 1990;30:971–975. [Google Scholar]
- Close TJ. Dehydrins: emergence of a biochemical role of a family of plant dehydration proteins. Physiol Plant. 1996;97:795–803. [Google Scholar]
- Close TJ, Kortt AA, Chandler PM. A cDNA-based comparison of dehydration-induced proteins (dehydrins) in barley and corn. Plant Mol Biol. 1989;13:95–108. doi: 10.1007/BF00027338. [DOI] [PubMed] [Google Scholar]
- Cornford CA, Black M, Chapman JM, Baulcombe DC. Expression of α-amylase and other gibberellin-regulated genes in aleurone tissue of developing wheat grains. Planta. 1986;169:420–428. doi: 10.1007/BF00392140. [DOI] [PubMed] [Google Scholar]
- Dure LS., III . The Lea proteins of higher plants. In: Verma DPS, editor. Control of Plant Gene Expression. Boca Raton, FL: CRC Press; 1993. pp. 325–335. [Google Scholar]
- Ellis R, Hong TD. Desiccation tolerance and potential longevity of developing seeds of rice (Oryza sativa L.) Ann Bot. 1994;73:501–506. [Google Scholar]
- Fischer W, Bergfeld R, Plachy C, Schäfer R, Schopfer P. Accumulation of storage materials, precocious gemination and development of desiccation tolerance during seed maturation in mustard (Sinapis alba L.) Bot Acta. 1988;101:344–354. [Google Scholar]
- Galau GA, Bijaisorodat N, Hughes DW. Accumulation kinetics of cotton late embryogenesis-abundant (Lea) mRNAs and storage protein mRNAs: coordinate regulation during embryogenesis and the role of abscisic acid. Dev Biol. 1987;123:198–212. doi: 10.1016/0012-1606(87)90442-8. [DOI] [PubMed] [Google Scholar]
- Galau GA, Jakobsen KS, Hughes DW. The controls of late dicot embryogenesis and early germination. Physiol Plant. 1991;81:280–288. [Google Scholar]
- Garcia-Maya M, Chapman JM, Black M. Regulation of α-amylase formation and gene expression in the developing wheat embryo: role of abscisic acid, the osmotic environment and gibberellin. Planta. 1990;181:296–303. doi: 10.1007/BF00195879. [DOI] [PubMed] [Google Scholar]
- Gee OH, Probert RJ, Coomber SA. ‘Dehydrin-like’ proteins and desiccation tolerance in seeds. Seed Sci Res. 1994;4:135–141. [Google Scholar]
- Han B, Hughes DW, Galau GA, Bewley JD, Kermode AR. Changes in late-embryogenesis-abundant (LEA) messenger RNAs and dehydrins during maturation and premature drying of Ricinus communis L. seeds. Planta. 1997;201:27–35. doi: 10.1007/BF01258677. [DOI] [PubMed] [Google Scholar]
- Hay FR, Probert RJ. Seed maturity and the effects of different drying conditions on desiccation tolerance and seed longevity in foxglove (Digitalis purpurea L.) Ann Bot. 1995;76:639–647. [Google Scholar]
- Hong TD, Ellis RH. Development of desiccation tolerance in Norway maple (Acer platanoides L.) seeds during maturation drying. Seed Sci Res. 1992;2:169–172. [Google Scholar]
- Horbowicz M, Obendorf RL. Seed desiccation tolerance and storability: dependence on flatulence-producing oligosaccharides and cyclitols: review and survey. Seed Sci Res. 1994;4:385–405. [Google Scholar]
- Jiang L, Kermode AR. Role of desiccation in the termination of expression of genes for storage proteins. Seed Sci Res. 1994;4:149–174. [Google Scholar]
- Julliard J, Maldiney R, Sotta B, Miginiac E, Kerhoas L, Einhorn J. HPLC-ELISA and MS as complementary techniques to study plant hormone metabolites. Analysis. 1994;22:483–489. [Google Scholar]
- Kermode AR. Approaches to elucidate the basis of desiccation-tolerance in seeds. Seed Sci Res. 1997;7:75–95. [Google Scholar]
- Koornneef M, Hanhart CJ, Hilhorst HWM, Karssen CM. In vivo inhibition of seed development and protein accumulation in recombinants of abscisic acid biosynthesis and responsiveness mutants in Arabidopsis thaliana. Plant Physiol. 1989;90:463–469. doi: 10.1104/pp.90.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster KL, Leopold AC. Sugars and desiccation tolerance in seeds. Plant Physiol. 1988;88:829–832. doi: 10.1104/pp.88.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leprince O, Bonchart R, Deltour R. Changes in starch and soluble sugars in relation to the acquisition of desiccation tolerance during maturation of Brassica campestris seed. Plant Cell Environ. 1990;13:539–546. [Google Scholar]
- Liu T-P, Huang N-H. The relationship between carbohydrate composition of some tree seeds and their longevity. J Exp Bot. 1994;45:1289–1294. [Google Scholar]
- Obendorf R. Oligosaccharides and galactosyl cyclitols in seed desiccation tolerance. Seed Sci Res. 1997;7:63–74. [Google Scholar]
- Ooms JJJ, Leon-Kloosterziel KM, Bartels D, Koornneef M, Karssen CM. Acquisition of desiccation tolerance and longevity in seeds of Arabidopsis thaliana. Plant Physiol. 1993;102:1185–1191. doi: 10.1104/pp.102.4.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posas F, Wurgler-Murphy SM, Maeda T, Witten EA, Thai TC, Saito H. Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN-1-YPD1-SSK1 2-component osmosensor. Cell. 1996;86:865–875. doi: 10.1016/s0092-8674(00)80162-2. [DOI] [PubMed] [Google Scholar]
- Rosenberg LA, Rinne RW. Moisture loss as a prerequisite for seedling growth in soybean seeds (Glycine max L. Merr.) J Exp Bot. 1986;37:1663–1674. [Google Scholar]
- Sanhewe AJ, Ellis RH. Seed development and maturation in Phaseolus vulgaris. 1. Ability to germinate and to tolerate desiccation. J Exp Bot. 1996;47:949–958. [Google Scholar]
- Steadman KJ, Pritchard HW, Dey PM. Tissue-specific soluble sugars in seeds as indicators of storage category. Ann Bot. 1996;77:667–674. [Google Scholar]
- Still DW, Kovach DA, Bradford KJ. Development of desiccation tolerance during embryogenesis in rice (Oryza sativa) and wild rice (Zizania palustris) Plant Physiol. 1994;104:431–438. doi: 10.1104/pp.104.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetteroo FAA, Bomal C, Hoekstra FA, Karssen CM. Effect of abscisic acid and slow drying on soluble carbohydrate content in developing embryoids of carrot (Daucus carota L.) and alfalfa (Medicago sativa L.) Seed Sci Res. 1994;4:203–210. [Google Scholar]
- Vertucci CW, Farrant JM (1995) Acquisition and loss of desiccation tolerance. In J Kigel, G Galil, eds, Seed Development and Germination. Marcel Dekker, New York, pp 237–272
- Wechsberg GE, Bray CM, Probert RJ. Expression of ‘dehydrin-like’ proteins in orthodox seeds of Ranunculus scleratus during development and water stress. Seed Sci Res. 1994;4:241–246. [Google Scholar]