Abstract
The success of our political institutions, environmental stewardship and evolutionary fitness all hinge on our ability to prioritize collective-interest over self-interest. Despite considerable interest in the neuro-cognitive processes that underlie group cooperation, the evidence to date is inconsistent. Several papers support models of prosocial restraint, while more recent work supports models of prosocial intuition. We evaluate these competing models using a sample of lesion patients with damage to brain regions previously implicated in intuition and deliberation. Compared to matched control participants (brain damaged and healthy controls), we found that patients with dorsolateral prefrontal cortex (dlPFC) damage were less likely to cooperate in a modified public goods game, whereas patients with ventromedial prefrontal cortex (vmPFC) damage were more likely to cooperate. In contrast, we observed no association between cooperation and amygdala damage relative to controls. These findings suggest that the dlPFC, rather than the vmPFC or amygdala, plays a necessary role in group-based cooperation. These findings suggest cooperation does not solely rely on intuitive processes. Implications for models of group cooperation are discussed.
Keywords: prosocial behavior, lesions, cooperation, public goods game
Introduction
Prioritizing collective-interest over self-interest is essential for adaptive group living and is critical for resolving numerous social problems, including voter turnout (Ostrom, 1990), climate change (Jacquet et al., 2013), scientific integrity (Everett and Earp, 2015) and evolutionary fitness (Axelrod and Hamilton, 1981). For centuries, philosophers have debated whether prosocial tendencies are rooted in institutions that regulate our selfish impulses (Hobbes, 1650) or emerge through natural intuitions, only to be suppressed by civilized and calculated self-interest (Rousseau, 1754). This debate persists to this day.
This debate is often framed through the lens of dual-process theories that carve the mind into two core systems (Kahneman, 2011): intuition—immediate, reflexive mental processes—and deliberation—delayed, reflective processing. Some argue, for instance, that humans’ unique capacity for self-reflection (i.e. compared to other primates) provides a critical avenue to promote prosocial behavior (Stevens and Hauser, 2004). There is additional evidence that depleting cognitive resources impairs helping behavior (DeWall et al., 2008; Capraro and Cococcioni, 2016) and amplifies dishonesty (Mead et al., 2009), supporting models of prosocial restraint, whereby cooperation stems from deliberate restraint of our selfish impulses (Martinsson et al., 2012; Achtziger et al., 2016; Lohse, 2016).
These models have been recently challenged by opposing models of prosocial intuition (Zaki and Mitchell, 2013), whereby intuitive cooperation is compromised by calculated self-interest (Rand et al., 2014). Research supporting these models often involves experimental economic games that use real money to pit self-interest against collective-interest. In the public goods game (PGG), for instance, the group benefits when everyone contributes their money, even though it is in each individual’s self-interest to keep their money (i.e. free-riding). Observations that people are relatively slower to free-ride in these games was initially interpreted as evidence for intuitive cooperation (Rand et al., 2012). Follow-up investigations however suggest these slower reaction times more likely index decision conflict, rather than deliberation, per se (Evans et al., 2015; Krajbich et al., 2015). In addition, some recent meta-analyses and preregistered studies indicate that experimentally inducing intuition (e.g. manipulating time-pressure) tends to boost group contributions in these games (Rand, 2016; Everett et al., 2017), whereas others have reported inconclusive or null results (Tinghög et al., 2013; Bouwmeester et al., 2017). Given this mixed evidence with behavioral measurements and manipulations, we leverage neuropsychological samples to help evaluate these conflicting models of prosocial restraint and prosocial intuition.
Despite extensive research devoted to promoting group-based cooperation (Van Lange, 1999), we still know relatively little about the neuro-cognitive processes that guide this adaptive behavior. Early work in social neuroscience attempted to map intuition and deliberation onto specific brain systems (Lieberman, 2007; see also Cunningham et al., 2007). Evidence from functional magnetic resonance imaging (fMRI) suggests the ventromedial prefrontal cortex (vmPFC) is implicated in immediate, automatic evaluations (Satpute and Lieberman, 2006; Lebreton et al., 2009), whereas the dorsolateral prefrontal cortex (dlPFC) is associated with delayed, controlled deliberation and self-restraint (Hare et al., 2009; Hutcherson et al., 2012). In addition, patients with vmPFC damage show blunted affective processing (Bechara et al., 1996; but see Dunn et al., 2006 for a review of alternate conclusions), while dlPFC damage appears to impair deliberative processes, such as working memory, reasoning and self-regulation (Barbey et al., 2013; Zhu et al., 2014). Evidence from fMRI, brain lesions and single-cell recordings further implicates subcortical structures like the amygdala in rapid, affective processing that precedes evaluations in the vmPFC (Phelps and LeDoux, 2005).
Thus, examining cooperative behavior among patients with damage to their dlPFC, vmPFC or amygdala can help arbitrate between prosocial restraint vs intuition models. Unlike the inherent correlational nature of fMRI, lesion approaches can effectively probe the causal contribution of these regions (Fellows et al., 2005). Prior studies leveraging this method have suggested causal links between dlPFC damage and honesty (Zhu et al., 2014), vmPFC damage and fairness (Koenigs and Tranel, 2007), as well as amygdala damage and trust (Koscik and Tranel, 2011). While these studies provide valuable insight into dyadic social decision-making, no study (to our knowledge) has examined how damage to these regions impacts cooperation in group contexts. We measure group cooperation using a PGG, pitting the prosocial intuition hypothesis—whereby patients with vmPFC or amygdala damage should contribute to the public goods less frequently than controls, against the prosocial restraint hypothesis—whereby patients with dlPFC damage should contribute to the public good less frequently. PGGs measure group cooperation by allowing players to make fiscal contributions to their group that are then multiplied and distributed equally (i.e. even to players who keep all their money for themselves). Even though it is in the group’s collective-interest if all players contribute, it is in each individual’s self-interest to contribute nothing and reap the benefits of other’s generosity (i.e. free-riding).
Materials and methods
Participants
We report how we determined our sample size, all data exclusions, all manipulations and all measures in the study. Thirty-seven patients (MAge = 40.7 years, s.d.Age = 11.5 years) with brain lesions due to stroke, tumor, head injury and surgical resection of tumor, epileptogenic tissue, abscesses and cortical malformations were recruited from the New York University Patient Registry for the Study of Perception, Emotion and Cognition. Using lesion masks created by a clinical neuropsychologist prior to data collection (Supplementary Material Section S1), patients were classified into different groups reflecting the primary location of resection or tissue damage. This procedure resulted in 10 patients with frontal lobe damage extending into vmPFC or dlPFC, 16 patients with anterior temporal lobe (ATL) resections (involving the amygdala and hippocampus), and 11 brain-damaged comparison (BDC) patients with mixed lobar (frontal, parietal or temporal) lesions different than those resected/damaged in the frontal and ATL groups.
Among the 10 frontal patients, 2 had primarily vmPFC damage and 2 had primarily dlPFC damage. The remaining six frontal patients had substantive damage to both vmPFC and dlPFC and could not be cleanly categorized into either group (see Supplementary Table S1). Twenty-nine age-matched (M = 36.1 years, s.d. = 10.9 years) healthy comparison participants (HC) with no brain damage were recruited from the NYC community. Our target sample size for patients was to recruit all eligible volunteers within the registry. Given the limited ability to recruit eligible frontal patients (n = 10), we intentionally oversampled healthy controls by a factor of three (n ∼ 30). All participants gave written informed consent. Across all groups, seven participants failed to meet our inclusion criteria (see Supplementary Material Section S2), leaving a final sample of 26 HC participants, 8 frontal patients (1 vmPFC, 1 dlPFC, 6 mixed), 14 Amygdala patients and 11 BDC patients (see Figure 1). Since most frontal patients had a mixture of vmPFC and dlPFC damage, our primary analysis treated degree of damage to each sub-region as a continuous regressor (see Supplementary Material Section S4).
Fig. 1.
Lesion overlap of patients. (A) Patients with vmPFC damage (n = 7) are shown in axial slices Z = [−24, −19, −14, −9, −4, 1, 6]. (B) Patients with dlPFC damage (n = 7) are shown in sagittal slices X = [64, 59, 54, 49, 44, 39, 34]. Both images include patients with combined vmPFC and dlPFC damage (n = 6). Patients with right hemisphere damage (n = 2) were flipped to generate the overlap lesion reconstruction. (C) ATL patients with amygdala damage (n = 14) are shown in sagittal slices X = [44, 39, 34, 29, 24, 19, 14]. For symmetric visualization purposes, lateralized damage has been reflected onto both hemispheres.
Neuroanatomical analysis
Each patient’s lesion was manually reconstructed by a clinical neuropsychologist using MRIcron and FSL View software (Supplementary Material Section S1). ROIs were constructed with MarsBar toolbox by combining corresponding structures from the Harvard-Oxford Maximum Probability Atlases (Supplementary Material Section S1).
Stimuli and task
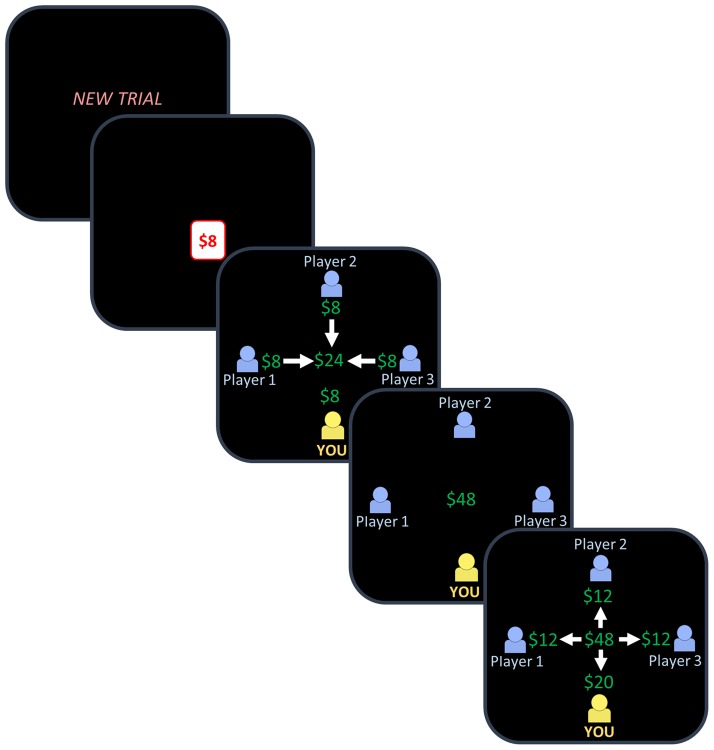
Participants played 20 one-shot PGGs. In each PGG, participants were given $8.00 that they could either keep for themselves or contribute to benefit the group (see Figure 2). Players interacted in groups of four; all contributions were doubled and split equally amongst all group members. There was no time limit for making decisions and reaction times were recorded. After each choice, participants were shown feedback about the other players’ decisions and resulting payouts. To ensure all payouts were based on real economic interactions with other humans without deception, other players’ decisions were based on real responses from publicly available data. These decisions were predetermined and presented in randomized order. On average, the other players gave 60% of the time with the following distribution: 0 givers (1 trial), 1 giver (6 trials), 2 givers (9 trials) and 3 givers (4 trials). Participants were informed that their decisions were anonymously recorded and could be used to influence the payment of future participants.
Fig. 2.
The PGG. Each game began with a prompt indicating that a new round was about to begin. Participants decided whether to keep or give $8.00. Participants then saw three stages of guided feedback: (1) each player’s contribution to the public good, (2) the total contribution multiplied by two, then (3) each player’s resulting payout. Each feedback stage was presented for 5 s. The next trial began immediately afterwards, for a total of 20 games. Each game consisted of a separate one-shot interaction with three new players. In the example above, the participant decides to keep and then learns, in the feedback stage, that the other three players chose to give. Decisions to keep and give were depicted in yellow and blue, respectively. Not shown to scale.
After reading through instructions with visualized examples of gameplay, participants completed a brief comprehension quiz. Participants were unable to begin the task until they correctly answered all quiz questions. The experimenter then walked through two example rounds illustrating how, no matter what the other players decided, it was always in the participant’s self-interest to keep their money and always in the group’s collective-interest to give their money. During debriefing, participants were again assessed as to (1) whether they understood the task and (2) whether they believed their decisions could actually influence others. Participants who misunderstood instructions or expressed suspicion about the prosocial nature of the task were excluded (see Supplementary Material Section S2). This intensive comprehension assessment helped alleviate concerns that cooperative behavior stemmed from misunderstanding or confusion (Burton-Chellew et al., 2016). Participants received $10 for participating, and could earn up to $20 more based on one randomly selected trial. The procedure typically lasted 15 min but was nested within a longer, 90-min protocol with other measures which are being analyzed for a separate report (see Supplementary Section S3 for full protocol).
Data availability
All materials, analysis scripts and summary data will be made available to other scientists upon publication on the Open Science Framework (https://osf.io/r8gwx/).
Analysis strategy
Our primary analysis aimed to test the relationship between group cooperation and damage to vmPFC, dlPFC and amygdala. Cooperation was operationalized as a trial-by-trial binary outcome (0 = keep, 1 = give). Given the multilevel structure of the data, we used generalized estimating equations (GEE) to fit a multiple logistic regression with exchangeable correlation matrices clustered on each patient.1 Six (out of the eight) frontal patients had diffuse damage overlapping both vmPFC and dlPFC (r(6) = 0.26, 95% bootstrapped CI [−0.23, 0.87], P = 0.536). To help assess the independent effects of each brain region, we simultaneously included vmPFC, dlPFC and amygdala damage as predictors of each participant’s decision to give. This ROI analysis converged with the traditional group analysis.
Results
Behavioral results
Participants chose to cooperate on 38.5% of trials (s.d. = 48.6%), indicating they were more inclined to free-ride than give their money to the group. Participants spent an average of 1.85 s deciding (s.d. = 1.87). Although they were faster to keep (M = 1.75 s, s.d. = 1.48) than to give (M = 2.02 s, s.d. = 2.35), this difference was not significant (P > 0.13). Finally, although cooperation tended to decrease over the course of the 20 trials (see Supplementary Figure S1), this trend was not significant,2χ2(1) = 2.05, P = 0.153. Thus, we find insufficient evidence that decisions in our task change over time or differ in response time.
Primary analysis
The prosocial intuition hypothesis predicts reduced cooperation among vmPFC or amygdala patients, whereas the prosocial restraint hypothesis predicts reduced cooperation among dlPFC patients. In contrast to the prosocial intuition hypothesis, vmPFC damage was associated with heightened cooperation [odds ratio (OR) = 1.55, 95% CI [1.06, 2.26], χ2(1) = 5.09, P = 0.024], such that the odds of cooperating were 1.55 times larger for each additional 10% of vmPFC damage. Consistent with the prosocial restraint hypothesis, dlPFC damage was associated with reduced cooperation (OR = 0.49, 95% CI [0.27, 0.90], χ2(1) = 5.31, P = 0.021), such that the odds of free-riding were 2.04 times larger for each additional 10% of dlPFC damage (Figure 3; see Supplementary Material Section S3 for analysis details). The degree of amygdala damage was not significantly associated with cooperative behavior (OR = 0.82, 95% CI [0.62, 1.06], P = 0.133). The overall results were consistent with models of prosocial restraint.
Fig. 3.
Cooperation as a function of ROI damage. (A) vmPFC (red) and dlPFC (blue) ROIs are pictured at the following slices: Sagittal X = 4 (top), Coronal Y = 40 (middle), Sagittal X = 44 (bottom). (B) Predicted probabilities of giving are plotted against damage within each ROI, after adjusting for external ROI damage. Lines are interpolated through the range of the observed levels of predictors. Bands indicate 95% confidence intervals.
Secondary analyses
In addition to the ROI analysis using continuous regressors, we also tested whether overall cooperation rates differed between lesion groups (Figure 4; see Supplementary Material Section S5 for analysis details). The amygdala group (29.6% overall cooperation) cooperated at similar rates compared to the HC (41.0%) and BDC groups (43.8%), with no statistical difference between the HC and BDC participants (all Ps > 0.15). In contrast to the prosocial intuition hypothesis, the vmPFC patient cooperated more often (90%) than both the HC groups [OR = 13, χ2(1) = 104.07, P < 0.001] and BDC groups [OR = 10, χ2(1) = 38.57, P < 0.001]. Moreover, a bootstrapped 95% confidence interval indicated a lower bound of 15.36% cooperation among the amygdala group, suggesting neither amygdala nor vmPFC appear necessary for cooperation. Consistent with the prosocial restraint hypothesis, the patient with isolated dlPFC damage did not cooperate (0%) across the entire task, indicating the dlPFC may be necessary for cooperation. Although we are reluctant to draw general conclusions about task-specific effects of damage from single patients, this overall pattern of results (along with the primary ROI analysis) is consistent with the prosocial restraint hypothesis. A secondary analysis using voxel-based lesion symptom mapping produced convergent results consistent with models of prosocial restraint (Supplementary Material Section S6 and Supplementary Figure S4).
Fig. 4.
Frequency of giving between isolated lesion groups. Proportions of ‘give’ decisions are shown for each participant group. Across all twenty trials, no differences were observed between healthy comparison (light grey), BDC (dark grey), and amygdala patients (green; all Ps >0.26). However, all three comparison groups differed significantly from the vmPFC and dlPFC patients. Results show that dlPFC damage is associated with reduced cooperation, and vmPFC damage is associated with greater cooperation. Violin plot width represents the density of the distribution for each frequency of giving (e.g. healthy comparison participants give 41% of the time more often than 100% of the time). Plot height is constrained to the range of the data for each group (e.g. amygdala patients never gave more than 90% of the time). Circles reflect mean for each lesion group. Vertical lines indicate ±1 SEM. Since the isolated vmPFC and dlPFC groups each consist of one patient, horizontal lines at the mean are used instead of error bars.
Several follow-up analyses were conducted in order to rule out alternative explanations. There is extensive evidence, for instance, that cooperation decays once participants learn that others are exploiting their generosity by free-riding (Frey and Meier, 2004). However, vmPFC patients may be particularly insensitive to trial-by-trial feedback, given fMRI work implicating this region in social norm learning (Behrens et al., 2008). To address this possibility, we used the number of ‘givers’ (i.e. other players who cooperated on each trial) to compute two additional measures for each participant: (1) the number of givers on the trial preceding each decision and (2) the running average of givers across all trials that preceded each decision. Moreover, given evidence linking increased self-interest with slower decisions (Rand et al., 2012) and experience (Rand et al., 2014), we also entered reaction time and trial number into the model. Hierarchically entering these covariates into the analysis did not predict cooperation, nor did it qualitatively change the prior associations between ROI damage and cooperation (see Supplementary Table S2). Moreover, constraining the analysis to each participant’s first decision (trial 1) revealed results consistent with the full data (Supplementary Figures S5 and S6). Prior PGG studies have occasionally revealed higher contributions from women and younger participants (Rand et al., 2012). However, our results did not significantly change when adjusting for participant age and gender (Supplementary Table S4). Overall, these follow-up analyses suggest the associations between ROI damage and cooperation are robust, even when adjusting for learning (see Supplementary Figures S10 and S11 for raw temporal data) and individual differences (see Supplementary Material Section S4 for further analyses).
Discussion
We investigated the necessity of several brain regions for group-based cooperation. A sample of lesion patients provided no evidence that the vmPFC or amygdala are necessary for cooperation in a PGG. This is particularly striking given prior fMRI investigations linking vmPFC (Rilling et al., 2002) and amygdala (Fermin et al., 2016) with cooperative decisions in two-player games. In contrast, the extent of dlPFC damage associated with reduced cooperation, and the patient with the greatest dlPFC damage did not cooperate on a single trial. Considering recent associations between dlPFC damage and greater dishonesty (Zhu et al., 2014), this study adds to a growing body of literature showing that the lateral prefrontal cortex may be necessary for a broad range of prosocial behaviors.
Our findings have implications for competing models of prosocial behavior. The prosocial intuition hypothesis predicts that (1) impaired intuition (e.g. vmPFC or amygdala damage) will decrease cooperation, and (2) impaired deliberation (e.g. dlPFC damage) should increase (or at minimum preserve) cooperation. None of these predicted outcomes was observed, suggesting intuition did not guide cooperation in our sample. Instead, our behavioral and neuropsychological data were more consistent with prosocial restraint models.
We believe that there may still be contexts in which intuitive processes guide cooperative behavior (Rand, 2016). For instance, recent findings suggest that concerns over equity (i.e. fair allocations of resources) are intuitive whereas concerns over efficiency (i.e. maximizing collective resources, a likely component of our PGG) are deliberative (Capraro et al., 2017). In addition, incentivizing cooperation (by manipulating the payoff contingencies) results in relatively faster and more frequent decisions to cooperate, whereas more costly cooperation produces the opposite pattern (Krajbich et al., 2015). In our task, free-riding was the most common response among healthy controls. As a result, the neuropsychological associations we observed may reverse under conditions when cooperation is the modal response or involves efficiency tradeoffs.
The dual-process models of cooperation we tested have admittedly been largely silent to neuroscience. We hope that this investigation helps trigger the development of multi-level theories of cooperation and encourages future work leveraging the methods and samples from neuroscience to develop a more comprehensive understanding of human cooperation. For instance, there is growing evidence that models pitting intuition against deliberation may not capture the dynamic and widely distributed nature of evaluation and moral decision-making (Cunningham et al., 2007; Van Bavel et al., 2015). Thus, future investigations should examine broader neural substrates implicated in prosocial behavior, such as the ventral striatum (Fareri et al., 2015), insular cortex (Lamm et al., 2011), subgenual anterior cingulate cortex (FeldmanHall et al., 2015) and temporal parietal junction (Hutcherson et al., 2015). We also believe this approach will move theoretical debates beyond broad constructs, like intuition and deliberation, and focus on the underlying neural computations of value that guide cooperation.
One limitation of our primary analysis is that it assumes the ROIs we selected are fungible homogenous substrates (e.g. 10% damage to anterior vmPFC is equivalent to 10% damage to posterior vmPFC). Given evidence suggesting anterior vmPFC encodes more abstract, secondary reinforcers (Klein-Flügge et al., 2013), future investigations should leverage larger samples that can fully capitalize on more spatially granular analyses, such as voxel-based lesion-symptom mapping (Rorden et al., 2007). Nevertheless, while our sample size is smaller than typically seen in psychology papers, it is quite normal for lesion studies (e.g. Bechara et al., 1996; Koenigs and Tranel, 2007; Spunt et al., 2015). Indeed, lesion samples can provide critical insights into underlying cognitive processes. For instance, our models of human memory were revolutionized by a single participant (H.M.; Scoville and Milner, 1957).
Conclusion
The success of our political institutions, environmental stewardship, scientific progress and evolutionary fitness all hinge on our ability to prioritize collective-interest over self-interest. The present research illustrates how combining behavioral and neurological measures can help advance ancient philosophical debates about human benevolence. Understanding the neural systems involved helps clarify the underlying neuro-cognitive processes guiding cooperation and may ultimately inform interventions that push the limits of our cooperative potential.
Prior to this paper, JW presented these findings at various meetings and conferences. In addition, an earlier draft of the paper was posted as a preprint on PsyArXiv (https://osf.io/juhbx/).
Supplementary Material
Acknowledgements
The authors would like to thank Augustus Baker for extensive assistance with data collection. The authors would also like to thank Elizabeth Phelps, Ph.D. for providing lab equipment and helpful comments on the manuscript as well as Oliver Vikbladh for technical assistance with the region of interest analysis and overlay maps. Furthermore, the authors would like to thank members of the NYU Social Perception and Evaluation Lab for insightful comments on this research and earlier drafts of this manuscript.
Supplementary data
Supplementary data are available at SCAN online.
Funding
The authors gratefully acknowledge the financial support of the National Science Foundation grant #1349089 awarded to Jay Van Bavel and the National Science Foundation Graduate Research Fellowship awarded to Julian Wills.
Conflict of interest. None declared. Competing financial interests: The authors declare no competing financial interests.
Appendix
NYU PROSPEC Collaboration: The New York University Patient Registry for the Study of Perception, Emotion, and Cognition (NYU PROSPEC) includes the following group of clinical researchers and their affiliations.
Karen Blackmon1,2, Orrin Devinsky1,3,4, Werner K. Doyle1, Daniel J. Luciano1, Ruben I. Kuzniecky1,5, Siddhartha S. Nadkarni1,4, Blanca Vazquez1, Soul Najjar6,7, Eric Geller8, John G. Golfinos3,9, Dimitris G. Placantonakis3, Daniel Friedman1, Jeffrey H. Wisoff3,10, Uzma Samadani3,4,11,12
1Department of Neurology, New York University School of Medicine, New York, NY 10016, USA
2Department of Physiology, Neuroscience, and Behavioral Sciences, St. George's University School of Medicine, St. George, Grenada, West Indies
3 Department of Neurosurgery, New York University School of Medicine, New York, NY 10016, USA
4Department of Psychiatry, New York University School of Medicine, New York, NY 10016, USA
5 Department of Neurology, Hofstra Northwell Health School of Medicine, New York, NY 10075, USA
6 Lenox Hill Hospital and Staten Island University Hospital, Northwell Health, New York, NY, 10075, USA
7 Department fo Neurology, Hofstra North Shore Long Island Jewish School of Medicine, Hempstead, NY 11549, USA
8 St. Barnabas Medical Center, Livingston, NJ 07039, USA
9 Department of Otolaryngology-Head and Neck Surgery, New York University School of Medicine, New York, NY 10016, USA
10 Department of Pediatrics, New York University School of Medicine, New York, NY 10016, USA
11 Veterans Affairs New York Harbor Health Care System, New York, NY 10010, USA
12 Department of Physiology & Neuroscience, New York University School of Medicine, New York, NY 10016, USA
Footnotes
We used the geepack R package (https://cran.r-project.org/web/packages/geepack/index.html) to analyze this.
We conducted a logistic regression and report the test statistic of trial number predicting cooperation.
Contributor Information
NYU PROSPEC Collaboration:
Karen Blackmon, Orrin Devinsky, Werner K Doyle, Daniel J Luciano, Ruben I Kuzniecky, Siddhartha S Nadkarni, Blanca Vazquez, Soul Najjar, Eric Geller, John G Golfinos, Dimitris G Placantonakis, Daniel Friedman, Jeffrey H Wisoff, and Uzma Samadani
References
- Achtziger A., Alós-Ferrer C., Wagner A.K. (2016). The impact of self-control depletion on social preferences in the ultimatum game. Journal of Economic Psychology, 53, 1–16. [Google Scholar]
- Axelrod R., Hamilton W. (1981). The evolution of cooperation. Science, 211(4489), 1390–6. http://dx.doi.org/10.1126/science.7466396. [DOI] [PubMed] [Google Scholar]
- Barbey A.K., Koenigs M., Grafman J. (2013). Dorsolateral prefrontal contributions to human working memory. Cortex, 49(5), 1195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A., Tranel D., Damasio H., Damasio A.R. (1996). Failure to respond autonomically to anticipated future outcomes following damage to prefrontal cortex. Cerebral Cortex, 6(2), 215–25. [DOI] [PubMed] [Google Scholar]
- Behrens T.E., Hunt L.T., Woolrich M.W., Rushworth M.F. (2008). Associative learning of social value. Nature, 456(7219), 245–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwmeester S., Verkoeijen P.P.J.L., Aczel B., et al. (2017). Registered replication report: Rand, Greene, and Nowak (2012). Perspectives on Psychological Science, 12(3), 527.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton-Chellew M.N., El Mouden C., West S.A. (2016). Conditional cooperation and confusion in public-goods experiments. Proceedings of the National Academy of Sciences, 113(5), 1291–6. https://doi.org/10.1073/pnas.1509740113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capraro V., Cococcioni G. (2016). Rethinking spontaneous giving: extreme time pressure and ego-depletion favor self-regarding reactions. Scientific Reports, 6(1), 27219.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capraro V., Corgnet B., Espín A.M., Hernán-González R. (2017). Deliberation favours social efficiency by making people disregard their relative shares: evidence from USA and India. Royal Society Open Science, 4(2), 160605.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham W.A., Zelazo P.D., Packer D.J., Van Bavel J.J. (2007). The iterative reprocessing model: a multilevel framework for attitudes and evaluation. Social Cognition, 25(5), 736. [Google Scholar]
- DeWall C.N., Baumeister R.F., Gailliot M.T., Maner J.K. (2008). Depletion makes the heart grow less helpful: helping as a function of self-regulatory energy and genetic relatedness. Personality and Social Psychology Bulletin, 34(12), 1653–62. [DOI] [PubMed] [Google Scholar]
- Dunn B.D., Dalgleish T., Lawrence A.D. (2006). The somatic marker hypothesis: a critical evaluation. Neuroscience & Biobehavioral Reviews, 30(2), 239–71. [DOI] [PubMed] [Google Scholar]
- Evans A.M., Dillon K.D., Rand D.G. (2015). Fast but not intuitive, slow but not reflective: decision conflict drives reaction times in social dilemmas. Journal of Experimental Psychology: General, 144(5), 951–66. [DOI] [PubMed] [Google Scholar]
- Everett J.A., Earp B.D. (2015). A tragedy of the (academic) commons: interpreting the replication crisis in psychology as a social dilemma for early-career researchers. Frontiers in Psychology, 6, 1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett J., Ingbretsen Z., Cushman F., Cikara M. (2017). Deliberation erodes cooperative behaviour – even towards competitive outgroups, even when using a control condition, and even when controlling for sample bias. Journal of Experimental Social Psychology, 73, 76–81. [Google Scholar]
- Fareri D.S., Chang L.J., Delgado M.R. (2015). Computational substrates of social value in interpersonal collaboration. Journal of Neuroscience, 35(21), 8170–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FeldmanHall O., Dalgleish T., Evans D., Mobbs D. (2015). Empathic concern drives costly altruism. Neuroimage, 105, 347–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows L.K., Heberlein A.S., Morales D.A., Shivde G., Waller S., Wu D.H. (2005). Method matters: an empirical study of impact in cognitive neuroscience. Journal of Cognitive Neuroscience, 17(6), 850–8. [DOI] [PubMed] [Google Scholar]
- Fermin A.S., Sakagami M., Kiyonari T., Li Y., Matsumoto Y., Yamagishi T. (2016). Representation of economic preferences in the structure and function of the amygdala and prefrontal cortex. Scientific Reports, 6(1), 20982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey B.S., Meier S. (2004). Social comparisons and pro-social behavior: testing “conditional cooperation” in a field experiment. American Economic Review, 94(5), 1717–22. [Google Scholar]
- Hare T.A., Camerer C.F., Rangel A. (2009). Self-control in decision-making involves modulation of the vmPFC valuation system. Science, 324(5927), 646–8. [DOI] [PubMed] [Google Scholar]
- Hobbes T. (1650). Human Nature (1650). Leviathan (Engl. 1651).
- Hutcherson C.A., Plassmann H., Gross J.J., Rangel A. (2012). Cognitive regulation during decision making shifts behavioral control between ventromedial and dorsolateral prefrontal value systems. Journal of Neuroscience, 32(39), 13543–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcherson C.A., Bushong B., Rangel A. (2015). A neurocomputational model of altruistic choice and its implications. Neuron, 87(2), 451–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquet J., Hagel K., Hauert C., Marotzke J., Röhl T., Milinski M. (2013). Intra- and intergenerational discounting in the climate game. Nature Climate Change, 3(12), 1025–8. [Google Scholar]
- Kahneman D. (2011). Thinking, Fast and Slow. Macmillan. [Google Scholar]
- Klein-Flügge M.C., Barron H.C., Brodersen K.H., Dolan R.J., Behrens T.E.J. (2013). Segregated encoding of reward–identity and stimulus–reward associations in human orbitofrontal cortex. Journal of Neuroscience, 33(7), 3202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M., Tranel D. (2007). Irrational economic decision-making after ventromedial prefrontal damage: evidence from the Ultimatum Game. The Journal of Neuroscience, 27(4), 951–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koscik T.R., Tranel D. (2011). The human amygdala is necessary for developing and expressing normal interpersonal trust. Neuropsychologia, 49(4), 602–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajbich I., Bartling B., Hare T., Fehr E. (2015). Rethinking fast and slow based on a critique of. reaction-time reverse inference. Nature Communications, 6(1), 7455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C., Decety J., Singer T. (2011). Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage, 54(3), 2492–502. [DOI] [PubMed] [Google Scholar]
- Lebreton M., Jorge S., Michel V., Thirion B., Pessiglione M. (2009). An automatic valuation system in the human brain: evidence from functional neuroimaging. Neuron, 64(3), 431–9. [DOI] [PubMed] [Google Scholar]
- Lieberman M.D. (2007). Social cognitive neuroscience: a review of core processes. Annual Review of the Psychology, 58(1), 259–89. [DOI] [PubMed] [Google Scholar]
- Lohse J. (2016). Smart or selfish—when smart guys finish nice. Journal of Behavioral and Experimental Economics, 64, 28–40. [Google Scholar]
- Martinsson P., Myrseth K.O.R., Wollbrant C. (2012). Reconciling pro-social vs. selfish behavior: on the role of self-control. Judgment and Decision Making, 7(3), 304. [Google Scholar]
- Mead N.L., Baumeister R.F., Gino F., Schweitzer M.E., Ariely D. (2009). Too tired to tell the truth: self-control resource depletion and dishonesty. Journal of Experimental Social Psychology, 45(3), 594–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrom E. (1990). Governing the Commons: The Evolution of Institutions for Collective Action. Cambridge University Press. [Google Scholar]
- Phelps E.A., LeDoux J.E. (2005). Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron, 48(2), 175–87. [DOI] [PubMed] [Google Scholar]
- Rand D.G. (2016). Cooperation, fast and slow: meta-analytic evidence for a theory of social heuristics and self-interested deliberation. Psychological Science, 27, 1192–206. [DOI] [PubMed] [Google Scholar]
- Rand D.G., Greene J.D., Nowak M.A. (2012). Spontaneous giving and calculated greed. Nature, 489(7416), 427–30. [DOI] [PubMed] [Google Scholar]
- Rand D.G., Peysakhovich A., Kraft-Todd G.T., et al. (2014). Social heuristics shape intuitive cooperation. Nature Communications, 5, 3677. [DOI] [PubMed] [Google Scholar]
- Rilling J.K., Gutman D.A., Zeh T.R., Pagnoni G., Berns G.S., Kilts C.D. (2002). A neural basis for social cooperation. Neuron, 35(2), 395–405. [DOI] [PubMed] [Google Scholar]
- Rorden C., Karnath H.-O., Bonilha L. (2007). Improving lesion-symptom mapping. Journal of Cognitive Neuroscience, 19(7), 1081–8. [DOI] [PubMed] [Google Scholar]
- Rousseau J.J. (1754). A discourse on a subject proposed by the academy of Dijon: what is the origin of inequality among men, and is it authorised by natural law? Retrieved 23 January 2009, from the Constitution Society in the Rousseau site http://www.constitution.org/jjr/ineq.htm.
- Satpute A.B., Lieberman M.D. (2006). Integrating automatic and controlled processes into neurocognitive models of social cognition. Brain Research, 1079(1), 86–97. [DOI] [PubMed] [Google Scholar]
- Scoville W.B., Milner B. (1957). Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology, Neurosurgery, and Psychiatry, 20(1), 11.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spunt R.P., Elison J.T., Dufour N., Hurlemann R., Saxe R., Adolphs R. (2015). Amygdala lesions do not compromise the cortical network for false-belief reasoning. Proceedings of the National Academy of Sciences, 112(15), 4827–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J.R., Hauser M.D. (2004). Why be nice? Psychological constraints on the evolution of cooperation. Trends in Cognitive Sciences, 8(2), 60–5. [DOI] [PubMed] [Google Scholar]
- Tinghög G., Andersson D., Bonn C., et al. (2013). Intuition and cooperation reconsidered. Nature, 498(7452), E1–2. [DOI] [PubMed] [Google Scholar]
- Van Bavel J.J., FeldmanHall O., Mende-Siedlecki P. (2015). The neuroscience of moral cognition: from dual processes to dynamic systems. Current Opinion in Psychology, 6, 167. [Google Scholar]
- Van Lange P.A. (1999). The pursuit of joint outcomes and equality in outcomes: an integrative model of social value orientation. Journal of Personality and Social Psychology, 77(2), 337– 49. [Google Scholar]
- Zaki J., Mitchell J.P. (2013). Intuitive prosociality. Current Directions in Psychological Science, 22(6), 466–70. [Google Scholar]
- Zhu L., Jenkins A.C., Set E., et al. (2014). Damage to dorsolateral prefrontal cortex affects tradeoffs between honesty and self-interest. Nature Neuroscience, 17(10), 1319–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All materials, analysis scripts and summary data will be made available to other scientists upon publication on the Open Science Framework (https://osf.io/r8gwx/).