Abstract
Interoception, i.e. the perception and appraisal of internal bodily signals, is related to the phenomenon of craving, and is reportedly disrupted in alcohol use disorders. The hormone oxytocin influences afferent transmission of bodily signals and, through its potential modulation of craving, is proposed as a possible treatment for alcohol use disorders. However, oxytocin’s impact on interoception in alcohol users remains unknown. Healthy alcohol users (n = 32) attended two laboratory sessions to perform tests of interoceptive ability (heartbeat tracking: attending to internal signals and, heartbeat discrimination: integrating internal and external signals) after intranasal administration of oxytocin or placebo. Effects of interoceptive accuracy, oxytocin administration and alcohol intake, were tested using mixed-effects models. On the tracking task, oxytocin reduced interoceptive accuracy, but did not interact with alcohol consumption. On the discrimination task, we found an interaction between oxytocin administration and alcohol intake: Oxytocin, compared with placebo, increased interoceptive accuracy in heavy drinkers, but not in light social drinkers. Our study does not suggest a pure interoceptive impairment in alcohol users but instead potentially highlights reduced flexibility of internal and external attentional resource allocation. Importantly, this impairment seems to be mitigated by oxytocin. This attentional hypothesis needs to be explicitly tested in future research.
Keywords: alcohol, addiction, alcohol use, oxytocin, interoception, attention
Introduction
Interoception classically refers to the signaling, representation, and perception of internal bodily sensations coming from the viscera, e.g. heartbeats, gastric distension or visceral pain (Cameron, 2001). Interoceptive processes are the safeguards of homeostatic control and contribute to motivational and affective behaviors (Tsakiris and Critchley, 2016). In the context of drug addiction, indirect evidence suggests that interoceptive processes underpin urges to take a drug, also known as drug craving (Naqvi and Bechara, 2010). For example, lesion studies implicate the insular cortex, a brain region supporting interoceptive states, in the phenomenon of craving (Gray and Critchley, 2007). Neuroimaging studies further reveal abnormalities in the morphometry, functional activity and connectivity of insular cortex in alcohol, cannabis and, also, tobacco users (Berk et al., 2015; Maria et al., 2015; Grodin et al., 2017). In addition to lesion and neuroimaging investigations, more direct evidence concerning the relationship between interoceptive ability and craving can be drawn from behavioral studies. Interoceptive ability (i.e. interoceptive accuracy) commonly measured using heartbeat tracking and heartbeat discrimination tasks (Garfinkel et al., 2015; Brener and Ring, 2016). In the heartbeat tracking task, participants silently count their own heartbeats, by focusing their attention on their internal cues. In the heartbeat discrimination task, participants attend to both internal and external cues, judging the synchrony between their own heartbeats and sequences of tones either presented in synch or out of synch with their own heartbeats. In this task, participants flexibly switch attention between external and internal cues to integrate the perceptions for the synchronicity judgment. Interestingly, the interoceptive accuracy of alcohol-dependent subjects and drug users has only been tested using the heartbeat tracking task. Diminished interoceptive abilities are observed in these populations and this deficit is positively associated with both subjective craving sensations and alexithymia (e.g. difficulties in identifying and describing emotions) (Ates Çöl et al., 2016; Sönmez et al., 2016). We recently reported that alexithymia (a possible outcome of aberrant processing of bodily sensations) may play a role in alcohol use (Betka et al., 2017). Indeed, the effective integration of interoceptive inputs is crucial for both subjective experience and social skills (Park and Tallon-Baudry, 2014; Shah et al., 2017); i.e. skills typically impaired in alcohol and drug use disorders (D’Hondt et al., 2014; Verdejo-Garcia, 2014).
Oxytocin (OT) is a neuropeptide hormone that is mostly synthesized in the hypothalamus, and released into the bloodstream via the posterior pituitary gland (Sokol and Valtin, 1967). OT receptors are present in central and peripheral tissues, including the brain, heart, gastrointestinal tract and uterus (Gimpl and Fahrenholz, 2001). Many human studies focus on the impact of OT administration on social cognition. However, underlying mechanisms remain unclear, since rodent ligands for OT receptors are not selective for human OT receptors (Paloyelis et al., 2014; Leng and Ludwig, 2015; Quintana et al., 2015; Valstad et al., 2016, 2017). Nevertheless, OT administration can increase trust and enhance the detection of emotional signals of others (Domes et al., 2007; Keri and Kiss, 2011; Schulze et al., 2011; Lischke et al., 2012; Van and Bakermans-Kranenburg, 2012; Perry et al., 2013; Kanat et al., 2015). Relatedly, OT administration can also improve capacity for ‘mind reading’ (mentalization) (Guastella et al., 2010), empathy (Hurlemann et al., 2010; Panksepp and Panksepp, 2013) and mimicry of angry faces (Korb et al., 2016). One proposed mechanisms by which OT impacts emotional regulation is via the modulation of attention resources. Indeed, intranasal OT will preferentially increase attention toward social cues, such as emotional faces, compared with neutral or non-social cues (Tollenaar et al., 2013; Clark-Elford et al., 2014; Dal Monte et al., 2014; Domes et al., 2016; Kanat et al., 2017; Pfundmair et al., 2017). There is emerging interest in whether the modulation of interoceptive processing underpins the impact of the OT system on social cognition. OT is hypothesized to modulate attention toward interoceptive signals (i.e. precision of central interoceptive representations), which can inform generative models of emotional and selfhood (Quattrocki and Friston, 2014). Intranasal OT administration may not markedly influence performance on heartbeat discrimination in healthy human participants (Yao et al., 2017), yet electrophysiological studies show that OT gates the transmission of viscerosensory afferent information (Peters et al., 2008). Correspondingly, OT has a direct impact on neurons within nucleus of the solitary tract, the main visceroceptive relay within the brainstem (Craig, 2002; Karelina and Norman, 2009).
Interestingly, a new wave of translational research suggests that OT administration can inhibit alcohol consumption. One clinical trial showed that intranasal OT attenuated alcohol withdrawal symptoms in alcoholic patients (Pedersen et al., 2013). In rodents, the overexpression of OT receptors in mice reduces the rewarding proprieties of ethanol (Bahi, 2015) and OT injections decrease ethanol consumption, ethanol preference and ethanol-triggered dopamine release within the accumbens nucleus (Peters et al., 2013, 2017; MacFadyen et al., 2016; King et al., 2017). Such observations further implicate the OT system in the development and maintenance of addiction (McGregor and Bowen, 2012; Buisman-Pijlman et al., 2014; Lee et al., 2016). Indeed, in adolescent animals, OT exposure will reduce the expression of anxiety and protect against development of adult alcohol and drug seeking behaviors (Bowen et al., 2011; Hicks et al., 2016). These effects are bidirectional: Alcohol injection is known to inhibit endogenous OT release (Fuchs et al., 1967). Again, Interoception appears to be an important mediator: The processing of bodily sensations is impaired in alcohol-dependent individuals and the degree of this impairment correlates with emotional impairments, including alexithymia. OT can improve the emotional skills of alexithymic individuals (Luminet et al., 2011), most likely through its impact on brain centers supporting both interoception and emotion (Crockford et al., 2014; Lancaster et al., 2015; Strauss et al., 2015).
In summary, interoception is a crucial facet of emotional regulation, which seems to be impaired in alcohol-dependent individuals. OT is a facilitator of empathic emotional feelings and enhances afferent viscerosensory transmission. Moreover, OT may reduce alcohol withdrawal symptoms and diminish alcohol intake. We, therefore, sought to characterize the impact of intranasal OT on interoceptive processing in alcohol users. We hypothesized that interoceptive skills are negatively correlated with alcohol use severity and that OT administration improves interoception and hence can reduce the impairments observed in heavy alcohol users.
Finally, higher level of alexithymia, anxiety, and depression are usually observed in alcohol use disorder (Evren et al., 2009). As these three conditions are associated with abnormal interoceptive profiles (Paulus and Stein, 2010; Barrett et al., 2016; Garfinkel et al., 2016; Betka et al., 2017), we thought it crucial to account for them in our analyses. However, we did not have any fresh hypotheses regarding the influence of these variables.
Materials and methods
Participants
Thirty-two male volunteers (mean age 25.1 years; range 18–36 years) took part in the experiment. Participants were recruited via advertisements placed around the University of Sussex and Brighton and Sussex Medical School. All participants were healthy individuals with no history of psychiatric or neurological diseases and were not taking medication. During the screening, participants were directly asked if they had any history or received any diagnostic of alcohol or drug use disorders. The average number of years of education was M = 16.9 (s.d. = 2.62). All participants gave their written informed consent and were compensated for their time. The study was reviewed and approved by the BSMS Research Governance and Ethics committee.
Procedure
The study was conducted at the Clinical Imaging Science Center in Brighton, UK. Participants were told that the goal of the study was to explore the impact of OT on emotional regulation. The study was composed of three sessions. Participants were asked to abstain drinking 24 h before each session. Prior to any session, participants were breathalyzed and a urinary sample was collected to test for drug use. The urinary drug test was undertaken to confirm the absence of drug use to exclude drug use disorder. The alcohol test was undertaken to ensure that participants abstained before the sessions. In the case of positive results, the participant would be excluded. During the baseline session and following the consent, demographic data (e.g. age, education level) were recorded, a blood sample was collected and psychometric questionnaires were administered. A within-subject design was used; a drug sequence was randomly allocated to each participant for the second and third session. On the second and third sessions, each participant self-administered 40 IU of OT nasal spray (Syntocinon; Novartis, Basel, Switzerland) or placebo (same composition as Syntocinon except for OT) in the presence of the experimenter, and subsequently performed the behavioral tasks 40 min after the administration (see Supplementary Material for details regarding the nasal spray administration). The second and third sessions were separated by 2–3 days. One participant specified he was hungover on one of the sessions; his data were discarded from the analyses. We failed to collect blood from two participants. Plasma OT measures are detailed in Supplementary Material.
Questionnaires
Alcohol use questionnaire
The Alcohol use questionnaire (Mehrabian and Russell, 1978) is a 15-item scale measuring in detailed way the quantity of alcohol consumption [alcohol units (8 g) drunk per week; Units per week]. Participants were asked to estimate the number of drinking days, the usual quantity consumed and their drinking pattern over the preceding 6 months. For the purpose of our study, we used only the drinking quantity (i.e. alcohol units per week). Following UK guidelines, alcohol consumption of 14 or less units of alcohol per week is considered as mild social drinking. Alcohol consumption of more than 14 units of alcohol per week is considered as harmful drinking or moderate-to-heavy drinking (https://www.nhs.uk).
Toronto alexithymia scale-20 items
The Toronto alexithymia scale-20 items (TAS-20) (Bagby et al., 1994) consists of 20 items rated on a five-point Likert scale (from 1 ‘strongly disagree’ to 5 ‘strongly agree’). The TAS-20 is composed of three factors. The first factor measures difficulties in identifying feelings, the second factor measures difficulties in describing feelings and the third factor measures the way the participant uses externally oriented thoughts. The total alexithymia score is the sum of responses across all 20 items. We only considered the total score in our analyses.
Spielberger state/trait anxiety inventory
Trait anxiety was assessed using the Trait version of the Spielberger State/Trait Anxiety Inventory (STAI; Spielberger et al., 1983). This questionnaire is composed of 20 questions, assessing trait anxiety with questions such as ‘I lack self-confidence’ and ‘I have disturbing thoughts’. Participants were asked to answer each statement using a response scale (which runs from 1 ‘Almost never’ to 4 ‘Almost always’) in order to capture a stable dispositional tendency (trait) for anxiety.
Beck depression index II
Symptoms and severity of depression were evaluated using the Beck depression index (BDI) (Beck et al., 1996). Participants responded to 21 questions designed to assess the individual’s level of depression (e.g. Sadness, pessimism, past failure etc.). The BDI items are scored on a scale from 0 to 3. All items were then summed for a BDI total score.
Interoceptive accuracy
Interoceptive accuracy was gauged by the participants’ ability to detect their own heartbeats using a heartbeat tracking task (Schandry, 1981) and a heartbeat discrimination task (Whitehead et al., 1977; Katkin et al., 1983). During each task, heartbeats were indexed and recorded using sensitive pulse oximetry (standard for our laboratory). A soft finger sensor was used to avoid pressure-induced pulsatile sensation at the fingertip and to gain accurate timing of pulse onsets from oximetric waveform output (Nonin4600 pulse oximeter, Nonin Medical Inc. Plymouth, MN, USA). Participants’ heartbeats were monitored and recorded with the pulse oximeter sensor mounting attached to their index finger. The task was composed of 20 trials.
For the heartbeat tracking task, participants were required to count their heartbeats during six randomized time windows of varying length (25, 30, 35, 40, 45 and 50 s) and, at the end of each time window, to report the number of heartbeats detected to the experimenter. To derive measures for interoceptive accuracy, heartbeat tracking scores were calculated on a trial-by-trial basis based upon the ratio of perceived to actual heartbeats 1 − (|nbeatsreal − nbeatsreported|)/((nbeatsreal + nbeatsreported)/2) (Hart et al., 2013; Garfinkel et al., 2015). This measure calculates interoceptive accuracy, independent of the number of heartbeats in the trial by normalizing the absolute error in perceived heartbeats as a function of the overall number of heartbeats. Mean interoceptive accuracy was computed by averaging accuracy for all trials. Moreover, a measure of heart rate was computed for each trial.
For the heartbeat discrimination task, each trial consisted of ten tones presented at 440 Hz and having 100 ms duration, which were triggered by the heartbeat. Under the synchronous condition, stimuli were presented at the start of the rise of the pulse oximetry signal (i.e. indicator of cardiac systole O’Rourke et al., 2001). Under the asynchronous condition, a delay of 300 ms was inserted (i.e. diastolic phase). At the end of each trial, participants signaled to the experimenter whether they believed the tones to be synchronous or asynchronous with their heartbeats. Therefore, the outcome of each trial was binary (1= Accurate, 0= Inaccurate). Mean interoceptive accuracy for the heartbeat discrimination task was calculated as a ratio of correct to incorrect synchronicity judgments, for each participant. Moreover, a measure of heart rate was computed for each trial.
Statistical analyses
Analysis of interoceptive tracking accuracy used linear mixed-effects models as the outcome was continuous. We analysed interoceptive discrimination accuracy using generalized linear mixed models as the outcome was binary (Inaccurate =0; Accurate =1; binomial family function), using the lme4 package (Bates et al., 2015) in the R environment (version 3.4.2; RCoreTeam, 2013). P values were computed using lmerTest package (Kuznetsova et al., 2014).
Both types of interoceptive accuracy were analysed with drug (two levels: Placebo = 0; OT = 1), units of alcohol per week (continuous predictor), their interaction and control variables (heart rate, anxiety, depression, alexithymia and drug sequence) as fixed factors. Participants were treated as a random factor. In order to specify the random effect structure, we used a data-driven approach for both tasks (Barr et al., 2013). Models including (i) random intercepts, (ii) random uncorrelated intercepts and slopes and (iii) random correlated intercepts and slopes were run. Models’ goodness of fits were compared using likelihood ratio tests. For the tracking task, the model including random correlated intercepts and slopes explained more variance than the two other models (Model 1: AIC = −293.91; Model 2: AIC = −354.91; Model 3: AIC = −385.46; Models 1–2 comparison: χ2(11) = 61.00; P < 0.001; Models 2–3 comparison: χ2(13) = 34.55; P < 0.001; Models 1–3 comparison: χ2(13) = 95.55; P < 0.001). For the discrimination task, the model including random uncorrelated intercepts and slopes explained more variance than the model including random intercept alone (Model 1: AIC = 1687.4; Model 2: AIC =1684; χ2(10) = 3.33; P < 0.001). The model including random correlated intercepts and slopes did not adequately converge, even after rescaling and optimization.
All continuous predictor variables were centered. Plasma OT levels were missing for two participants from whom we were unable to collect blood samples. Generalized and linear mixed models omit cases with missing data. Therefore, to check the effect of plasma OT levels on accuracy, we ran a first version of our final models, restricted to the participants with plasma OT information. Thereafter, we ran a second model, similar to the first one, but including plasma OT levels as predictor (fixed factor). For both tasks, we compared the two models using likelihood ratio tests.
Results
Sample description, psychometric measures and correlations
Thirteen participants were drinking between <14 alcohol units per week and were considered as mild social drinkers. Nineteen participants were drinking >14 alcohol units per week and were considered as moderate-to-heavy drinkers. Means, standard deviations and ranges of psychometric measures, basal plasma OT level as well as interoceptive accuracy and heart rate were computed for both tasks (Table 1). Correlations coefficients revealed no relationship between psychometric measures and basal plasma OT level (Table 2).
Table 1.
Mean and standard deviations of psychometric measures, basal plasma OT level as well as interoceptive accuracy and heart rate for both tasks
| Mean | s.d. | Range | |
|---|---|---|---|
| TAS-20 | 55.42 | 9.76 | 36–74 |
| Units per week | 24.82 | 18.09 | 4.8–69.50 |
| STAI | 48.19 | 12.21 | 22–66 |
| BDI | 12.84 | 9.98 | 0–48 |
| Basal plasma OT level (pg/ml) | 1.61 | 0.57 | 0.95–3.07 |
| Mean discrimination Accuracy (Placebo) | 0.53 | 0.13 | 0.30–0.85 |
| Mean discrimination Accuracy (OT) | 0.58 | 0.18 | 0.30–0.95 |
| Mean heart rate during the discrimination task (Placebo; bpm) | 64.07 | 9.97 | 47.20–88.80 |
| Mean heart rate during the discrimination task (OT; bpm) | 66.67 | 11.15 | 46–80–88.10 |
| Mean tracking Accuracy (Placebo) | 0.78 | 0.12 | 0.55–0.97 |
| Mean tracking Accuracy (OT) | 0.68 | 0.23 | 0.25–0.97 |
| Mean heart rate during the tracking task (Placebo; bpm) | 62.12 | 9.89 | 44.30–83.80 |
| Mean heart rate during the tracking task (OT; bpm) | 65.45 | 11.17 | 43.30–85.70 |
Table 2.
Correlations coefficients for psychometric measures and basal plasma OT level
| TAS-20 | Units per week | STAI | BDI | ||
|---|---|---|---|---|---|
| Units per week | Pearson Correlation | 0.192 | |||
| Sig. (two-tailed) | 0.301 | ||||
| STAI | Pearson Correlation | 0.308 | −0.179 | ||
| Sig. (two-tailed) | 0.091 | 0.337 | |||
| BDI | Pearson Correlation | 0.354 | −0.117 | 0.496** | |
| Sig. (two-tailed) | 0.051 | 0.531 | 0.005 | ||
| Basal plasma OT level | Pearson Correlation | −0.149 | 0.113 | −0.057 | −0.179 |
| Sig. (two-tailed) | 0.440 | 0.558 | 0.770 | 0.352 | |
**p < 0.01.
Tracking task
Results of the mixed-effects regression model, for this task, are presented in Table 3.
Table 3.
Mixed-effects regression model to explain accuracy on tracking task using predictors for OT/placebo (drug), alcohol intake (units per week) and their interaction, with heart rate (HR), anxiety (STAI), depression (BDI), alexithymia (TAS-20) and drug sequence as control variables, including all participants
| β | SE | z value | P value | |
|---|---|---|---|---|
| (Intercept) | 0.794 | 0.025 | 32.254 | 0.000*** |
| Drug | –0.078 | 0.034 | –2.287 | 0.030* |
| Units per week | –0.002 | 0.001 | –1.689 | 0.105 |
| HR | –0.005 | 0.002 | –3.301 | 0.002** |
| STAI | –0.005 | 0.002 | –2.687 | 0.013* |
| BDI | 0.007 | 0.002 | 3.148 | 0.004** |
| TAS-20 | –0.001 | 0.002 | –0.676 | 0.506 |
| Sequence | –0.050 | 0.030 | –1.689 | 0.102 |
| Drug*Units | 0.002 | 0.002 | 1.256 | 0.220 |
Formula: Accuracy ∼ Drug * Units per week + HR + STAI + BDI + TAS-20 + Sequence + (1 + Drug|ID); signif. codes: 0 ‘***’ 0.001 ‘**’ 0.01 ‘*’ 0.05.
We found a main effect of OT on accuracy, with reduced accuracy under OT compared with placebo (β = −0.08, SE = 0.03, P = 0.03). The main effect of heart rate was significant: participants with lower heart rate were more accurate than participants with higher heart rate (β = −0.01, SE = 0.01, P = 0.002). A main effect of anxiety as well as a main effect of depression were found; anxiety was associated with reduced accuracy whereas depression was associated with increased accuracy (anxiety: β = −0.01, SE = 0.01, P = 0.013; depression: β = 0.01, SE = 0.01, P =0.004). No main effect of units per week or interaction between OT/placebo and units per week was observed.
In order to check the effect of plasma OT level on accuracy, we compared a model restricted to the participants with plasma OT information and a second one similar to the first one but including plasma OT levels as predictor. Adding plasma OT levels did not significantly improve the model fit [χ2(14) = 2.33; P = 0.13]. Moreover, the main effect of plasma was not significant (β = 0.05, SE = 0.04, P = 0.16; see Supplementary Tables S1 and S2).
Discrimination task
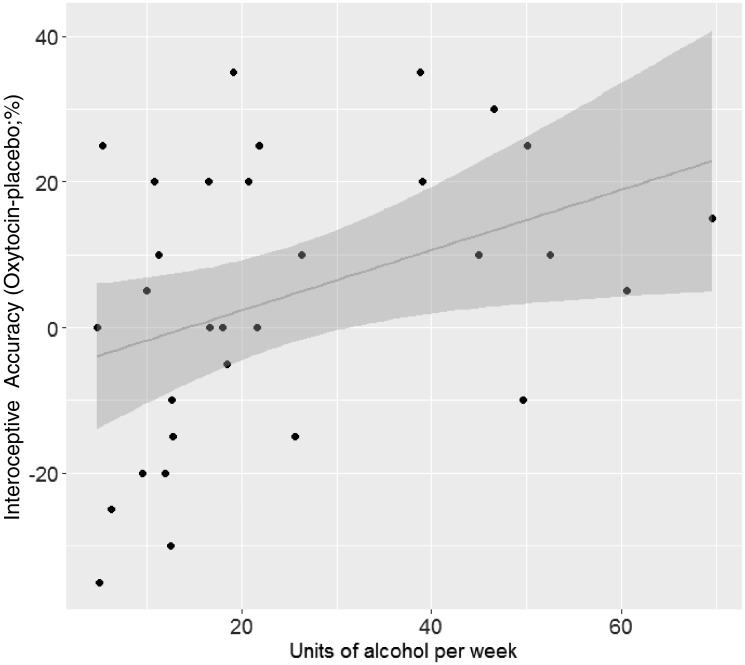
Results of the mixed-effects regression model, for the (interoceptive/exteroceptive, cross-modal) discrimination task, are presented in Table 4. Crucially, we found a significant interaction between drug and units of alcohol (β = 0.02, SE = 0.01, P = 0.025): The more alcohol drunk, the more OT increases interoceptive accuracy compared with placebo (Figure 1).
Table 4.
Mixed-effects regression model to explain accuracy on discrimination task using predictors for OT/placebo (drug), alcohol intake (units per week) and their interaction, with heart rate (HR), anxiety (STAI), depression (BDI), alexithymia (TAS-20) and drug sequence as control variables, including all participants
| Β | SE | z value | P value | |
|---|---|---|---|---|
| (Intercept) | 0.097 | 0.107 | 0.904 | 0.366 |
| Drug | 0.236 | 0.140 | 1.686 | 0.092. |
| Units per week | –0.002 | 0.005 | –0.346 | 0.730 |
| HR | –0.013 | 0.006 | –2.043 | 0.041* |
| STAI | –0.010 | 0.007 | –1.511 | 0.131 |
| BDI | –0.004 | 0.008 | –0.435 | 0.664 |
| TAS-20 | 0.001 | 0.008 | 0.170 | 0.865 |
| Sequence | 0.032 | 0.137 | 0.236 | 0.813 |
| Drug*Unit | 0.018 | 0.008 | 2.239 | 0.025* |
Formula: Accuracy ∼ Drug * Units per week + HR + STAI + BDI + TAS-20 + Sequence + (-1 + Drug|ID); signif. codes: 0.01 ‘*’ 0.05 ‘.’ 0.1.
Fig. 1.
Scatterplot illustrating the interaction between Drug (OT/Placebo) and alcohol units per week on interoceptive accuracy during the heartbeat discrimination task. The y-axis is displaying the difference between interoceptive accuracy under OT and under placebo, in percentage. The shaded area represents the SE.
We also found a trend of main effect of drug on accuracy, with greater accuracy under OT than under placebo (β = 0.23, SE = 0.14, P = 0.092). A significant main effect of heart rate was also observed: participants with lower heart rate were more accurate than participants with higher heart rate (β = −0.01, SE = 0.01, P = 0.041).
No main effect of anxiety, depression and alexithymia or OT/placebo sequence was observed.
In order to check the effect of plasma OT level on accuracy, we compared one model, restricted to the participants with plasma OT information, to a second model, similar to the first one but including plasma OT levels as predictor. Adding plasma OT levels did not significantly improve the model fit [χ2(11) = 2.74; P = 0.10]. Moreover, the main effect of plasma was not significant (β = −0.21, SE = 0.13, P = 0.10; see Supplementary Tables S3 and S4).
Discussion
In this study, we examined interoceptive processing in alcohol users and characterized the impact of intranasal OT on these processes.
Our main findings were that OT administration was associated with a reduction of interoceptive accuracy on the tracking task. However, it tended to be associated with an increase of interoceptive accuracy on the discrimination task. Moreover, we found a significant interaction between OT and alcohol intake on the discrimination task: when compared with placebo, OT administration was associated with improved interoceptive accuracy in heavy drinkers, but not in mild social drinkers. Interestingly, we did not find any main effect of units of alcohol on either interoceptive tasks; indicating that (non-clinical) alcohol consumption alone did not seem to be a strong predictor of interoceptive accuracy. Unlike alcohol-dependent patients, these alcohol users do not seem to be markedly impaired in interoceptive accuracy (Ates Çöl et al., 2016; Sönmez et al., 2016). However, interaction between alcohol intake and OT was observed independently of heart rate, alexithymia, anxiety or depression (as we controlled for these potential confounds). Moreover, the fact that this interaction was found only in the discrimination task (and not in the tracking task) suggests that OT does not have a general impact on interoceptive processing.
Indeed, while these two tasks are often used interchangeably in the objective measurement of interoception, heartbeat discrimination and heartbeat tracking tasks involve different cognitive mechanisms (Garfinkel et al., 2015, 2016). Heartbeat tracking requires the participant to focus only on his/her internal cardiac sensations, whereas performance of the heartbeat discrimination task requires the participant to attend flexibly to, switch perception between, and integrate external (e.g. sound) and internal (e.g. actual heartbeat) cues. Therefore, in the discrimination task, this integration of both internal and external sensorial information is crucial for making accurate judgments of synchronicity. Although specific studies of the integration of internal and external cues have yet to be undertaken in alcohol use disorders, there is evidence to suggest that the multimodal integration of emotional information is impaired in alcohol-dependent individuals (Maurage et al., 2009; Brion et al., 2017). For example, electroencephalography reveals a reduced amplitude and increased latency of event-related potentials during crossmodal emotional processing in alcohol-dependent patients. However, this deficit is not observed in binge drinking suggesting that sensory integration processes are more vulnerable to chronic alcohol use compared with more acute alcohol intoxication or other drinking patterns (Maurage et al., 2008; Brion et al., 2017).
The ability to switch between internally and externally focused attention is evoked by the discrimination task. This capacity is impaired in specific psychiatric disorders, including obsessive and compulsive disorders (Stern et al., 2017). A cognitive-physiological theory of ‘alcohol myopia’ suggests that alcohol allows drinkers to narrow their perceptual abilities in order to focus in more immediate and salient aspects of experience (e.g. externally oriented thoughts) (Steele and Josephs, 1990; Fairbairn and Sayette, 2013). This is supported by the recent demonstration that alcohol disrupts key nodes within the salience network, suggesting compromised internal monitoring (Padula et al., 2011; Gorka et al., 2017; Grodin et al., 2017). Interestingly, intranasal OT is proposed to direct attentional resources from internal to external cues, through concurrent modulation of functional connectivity within the ventral attention network and salience network (Abu-Akel et al., 2015; Shamay-Tsoory and Abu-Akel, 2016; Brodmann et al., 2017; Yao et al., 2017). These elements, plausibly account for the different directions of OT effects on the two interoceptive tasks, and inform understanding of the potential mechanism through which OT enhances interoceptive discrimination accuracy in heavy drinkers compared with mild social drinkers.
The alcohol myopia theory suggests that heavy drinkers are biased toward the processing of interoceptive cues and consequently experience difficulty in switching attention to exteroceptive one. Heavy drinkers, before marked alcohol-induced neurodegeneration, may not be overtly impaired and might employ compensatory mechanisms to manage deficits in attentional switching. However, we expect deficits become exacerbated and pathological with prolonged heavy drinking. The expression of such deficits may be mitigated by OT: As already mentioned, intranasal OT increases attentional resources toward exteroceptive stimulations. Correspondingly, we showed OT-induced reduction of accuracy during interoceptive tracking. Indeed, as the tracking task mainly involves internally focused attention (Garfinkel et al., 2015), OT may directly weaken attentional resource allocation toward bodily sensations, compromising interoceptive performance, and perhaps boost the capacity to switch from internal to external focused attention. Alternatively, by increasing externally focused attention, OT may potentiate the integration of internal–external inputs. These complementary mechanisms can also explain why OT tended to increase interoceptive accuracy on the discrimination task and why heavy drinkers, potentially less able to switch between internal and external cues, show a greater benefit from OT administration than mild social drinkers. Our findings are also consistent with a model arising from a predictive coding framework, in which OT is proposed to modulate the precision of interoceptive signals (i.e. narrowing the attribution of salience to internal bodily signals). This neuromodulation allows attentional deployment toward relevant external cues and, thereby favors associative learning between internal and external cues, a process fundamental to social cognition (Quattrocki and Friston, 2014).
Our results of this study should be considered in light of several constraints. First, low heart rate is associated with increased accuracy on cardiac interoceptive tasks, an effect that we also observed on both heartbeat tracking and discrimination tasks. Relatedly, beliefs about heart rate may further influence heartbeat counting performance (Ring and Brener, 1996; Ring et al., 2015). We managed this issue by including heart rate as a co-variable in analyses to account for its bias on cardioception. A second limitation of the study is the generalization of our findings to other dimensions of interoception. Even if it has been shown that cardioception aligns with sensitivity to gastric functions (Herbert et al., 2012), it would be interesting to investigate this relationship in alcohol use disorders. Finally, a last important constraint was that our sample was composed only of males to avoid taking menstrual cycle variability in our within-subject design. Further studies are needed to verify if our findings regarding OT modulation of attentional resources are generalizable to women. Finally, we measured basal plasma OT level to account for inter-individual differences. However, knowledge of the relationship between central and peripheral endogenous OT, and pharmacokinetic aspects of intranasal OT action remain rudimentary (Quintana et al., 2015; Valstad et al., 2016, 2017).
In conclusion, this study is, to date, the first study to examine the impact of OT on interoceptive performance accuracy in relation to non-clinical alcohol use. Our results do not suggest pure interoceptive impairment in alcohol users, but instead highlight a potential reduced flexibility of attentional resource allocation between internal and external cues; importantly, this impairment might be mitigated by acute intranasal OT intake. In this study, attention mechanisms are suggested to explain the relationship between OT and interoception in alcohol users. However, this hypothesis needs to be explicitly tested in future research. Nevertheless, our findings may usefully inform the development of new therapeutic approaches in alcohol and drug use disorders, potentially targeting mechanisms of attentional control and switching between interoceptive and exteroceptive sensory representations. Further studies involving neuroimaging will be helpful to build on this mechanistic understanding.
Supplementary data
Supplementary data are available at SCAN online.
Supplementary Material
Acknowledgements
We thank Clare Brown and Matthew Stephens for their assistance. S.B. is grateful to the Society for the Study of Addiction (SSA) for funding support under their PhD Studentship scheme, and states that the opinions expressed are those of the author(s) and do not necessarily reflect the views of the SSA itself.
Funding
This study was supported in part by Rotary Foundation, the Society for the Study of Addiction and the European Research Council (Advanced Grant CCFIB AG 234150 awarded to HDC).
Conflict of interest. None declared.
Competing interest: Authors have no conflicts or other disclosures beyond funding information provided.
References
- Abu-Akel A., Palgi S., Klein E., Decety J., Shamay-Tsoory S. (2015). Oxytocin increases empathy to pain when adopting the other- but not the self-perspective. Society for Neuroscience, 10(1), 7–15. [DOI] [PubMed] [Google Scholar]
- Ates Cöl I., Sonmez M.B., Vardar M.E. (2016). Evaluation of interoceptive awareness in alcohol-addicted patients. Archives of Neuropsychiatry, 53(1), 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagby R.M., Parker J.D., Taylor G.J. (1994). The twenty-item Toronto Alexithymia Scale–I. Item selection and cross-validation of the factor structure. Journal of Psychosomatic Research, 38(1), 23–32. [DOI] [PubMed] [Google Scholar]
- Bahi A. (2015). The oxytocin receptor impairs ethanol reward in mice. Physiology Behavior, 139, 321–7. [DOI] [PubMed] [Google Scholar]
- Barr D.J., Levy R., Scheepers C., Tily H.J. (2013). Random effects structure for confirmatory hypothesis testing: keep it maximal. Journal of Memory Language, 68(3), 255.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett L.F., Quigley K.S., Hamilton P. (2016). An active inference theory of allostasis and interoception in depression. Philosophical Transactions of the Royal Society of London. Series B Biological Science, 371(1708), 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D., Maechler M., Bolker B., Walker S. (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67, 1–48. [Google Scholar]
- Beck A.T., Steer R.A., Ball R., Ranieri W. (1996). Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. Journal of Personality Assessessment, 67(3), 588–97. [DOI] [PubMed] [Google Scholar]
- Berk L., Stewart J.L., May A.C., et al. (2015). Under pressure: adolescent substance users show exaggerated neural processing of aversive interoceptive stimuli. Addiction, 110(12), 2025–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betka S., Pfeifer G., Garfinkel S., et al. (2017). How do self-assessment of alexithymia and sensitivity to bodily sensations relate to alcohol consumption? Alcoholism, Clinical and Experimental Research. [DOI] [PubMed] [Google Scholar]
- Bowen M.T., Carson D.S., Spiro A., Arnold J.C., McGregor I.S. (2011). Adolescent oxytocin exposure causes persistent reductions in anxiety and alcohol consumption and enhances sociability in rats. PLoS One. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brener J., Ring C. (2016). Towards a psychophysics of interoceptive processes: the measurement of heartbeat detection. Philosophical Transactions of the Royal Society of London. Series B Biological Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brion M., D’Hondt F., Lannoy S., Pitel A.-L., Davidoff D.A., Maurage P. (2017). Crossmodal processing of emotions in alcohol-dependence and Korsakoff syndrome. Cognitive Neuropsychiatry, 22(5), 436–51. [DOI] [PubMed] [Google Scholar]
- Brodmann K., Gruber O., Goya-Maldonado R. (2017). Intranasal oxytocin selectively modulates large-scale brain networks in humans. Brain Connectivity, 7(7), 454–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisman-Pijlman F.T., Sumracki N.M., Gordon J.J., Hull P.R., Carter C.S., Tops M. (2014). Individual differences underlying susceptibility to addiction: role for the endogenous oxytocin system. Pharmacology Biochemistry Behavior, 119, 22–38. [DOI] [PubMed] [Google Scholar]
- Cameron O.G. (2001). Interoception: the inside story–a model for psychosomatic processes. Psychosomtic Medicine, 63(5), 697–710. [DOI] [PubMed] [Google Scholar]
- Clark-Elford R., Nathan P.J., Auyeung B., et al. (2015). Effects of oxytocin on attention to emotional faces in healthy volunteers and highly socially anxious males. International Journal of Neuropsychopharmacology, 18(2), 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig A.D. (2002). How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience 3(8), 655–66. [DOI] [PubMed] [Google Scholar]
- Crockford C., Deschner T., Ziegler T.E., Wittig R.M. (2014). Endogenous peripheral oxytocin measures can give insight into the dynamics of social relationships: a review. Frontiers in Behavioral Neuroscience, 8, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Monte O., Noble P.L., Costa V.D., Averbeck B.B. (2014). Oxytocin enhances attention to the eye region in rhesus monkeys. Frontiers in Neuroscience, 8, 41.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes G., Heinrichs M., Gläscher J., Büchel C., Braus D.F., Herpertz S.C. (2007). Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biological Psychiatry, 62(10), 1187–90. [DOI] [PubMed] [Google Scholar]
- Domes G., Normann C., Heinrichs M. (2016). The effect of oxytocin on attention to angry and happy faces in chronic depression. BMC Psychiatry, 16(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Hondt F., Campanella S., Kornreich C., Philippot P., Maurage P. (2014). Below and beyond the recognition of emotional facial expressions in alcohol dependence: from basic perception to social cognition. Neuropsychiatric Disease and Treatment, 10, 2177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evren C., Evren B., Dalbudak E. (2009). Alexithymia and personality dimensions in relation to depression and anxiety in male alcohol-dependent inpatients. International Journal of Psychiatry in Clinical Practice, 13(1), 3–10. [DOI] [PubMed] [Google Scholar]
- Fairbairn C.E., Sayette M.A. (2013). The effect of alcohol on emotional inertia: a test of alcohol myopia. Journal of Abnormal Psychology, 122(3), 770–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs F., Fuchs A.-R., Poblete V.F., Risk A. (1967). Effect of alcohol on threatened premature labor. American Journal of Obstetrics and Gynecology, 99(5), 627–37. [DOI] [PubMed] [Google Scholar]
- Garfinkel S.N., Seth A.K., Barrett A.B., Suzuki K., Critchley H.D. (2015). Knowing your own heart: distinguishing interoceptive accuracy from interoceptive awareness. Biological Psychology, 104, 65–74. [DOI] [PubMed] [Google Scholar]
- Garfinkel S.N., Tiley C., O’Keeffe S., Harrison N.A., Seth A.K., Critchley H.D. (2016). Discrepancies between dimensions of interoception in autism: implications for emotion and anxiety. Biological Psychology, 114, 117–26. [DOI] [PubMed] [Google Scholar]
- Gimpl G., Fahrenholz F. (2001). The oxytocin receptor system: structure, function, and regulation. Physiological Review, 81(2), 629–83. [DOI] [PubMed] [Google Scholar]
- Gorka S.M., Phan K.L., Childs E. (2017). Acute calming effects of alcohol are associated with disruption of the salience network. Addiction Biology. [DOI] [PubMed] [Google Scholar]
- Gray M.A., Critchley H.D. (2007). Interoceptive basis to craving. Neuron, 54(2), 183–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodin E.N., Cortes C.R., Spagnolo P.A., Momenan R. (2017). Structural deficits in salience network regions are associated with increased impulsivity and compulsivity in alcohol dependence. Drug and Alcohol Dependence, 179, 100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella A.J., Einfeld S.L., Gray K.M., et al. (2010). Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biological Psychiatry, 67(7), 692–4. [DOI] [PubMed] [Google Scholar]
- Hart N., McGowan J., Minati L., Critchley H.D. (2013). Emotional regulation and bodily sensation: interoceptive awareness is intact in borderline personality disorder. Journal of Personality Disorders, 27(4), 506–18. [DOI] [PubMed] [Google Scholar]
- Herbert B.M., Muth E.R., Pollatos O., Herbert C. (2012). Interoception across modalities: on the relationship between cardiac awareness and the sensitivity for gastric functions. PLoS One [Database]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks C., Cornish J.L., Baracz S.J., Suraev A., McGregor I.S. (2016). Adolescent pre-treatment with oxytocin protects against adult methamphetamine-seeking behavior in female rats. Addiction Biology, 21(2), 304–15. [DOI] [PubMed] [Google Scholar]
- Hurlemann R., Patin A., Onur O.A., et al. (2010). Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. Journal Neuroscience, 30(14), 4999–5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanat M., Heinrichs M., Mader I., van Elst L.T., Domes G. (2015). Oxytocin modulates amygdala reactivity to masked fearful eyes. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanat M., Spenthof I., Riedel A., van Elst L.T., Heinrichs M., Domes G. (2017). Restoring effects of oxytocin on the attentional preference for faces in autism. Transl Psychiatry, 7(4), e1097.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karelina K., Norman G.J. (2009). Oxytocin influence on NTS: beyond homeostatic regulation. Journal of Neuroscience, 29(15), 4687–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katkin E., Reed S.D., Deroo C. (1983). A methodological analysis of 3 techniques for the assessment of individual-differences in heartbeat detection. Psychophysiology, 20(4), 452. [Google Scholar]
- Keri S., Kiss I. (2011). Oxytocin response in a trust game and habituation of arousal. Physiology & Behavior, 102(2), 221–4. [DOI] [PubMed] [Google Scholar]
- King C.E., Griffin W.C., Luderman L.N., Kates M.M., McGinty J.F., Becker H.C. (2017). Oxytocin reduces ethanol self-administration in mice. Alcoholism, Clinical and Experiamental Research, 41(5), 955–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korb S., Malsert J., Strathearn L., Vuilleumier P., Niedenthal P. (2016). Sniff and mimic - Intranasal oxytocin increases facial mimicry in a sample of men. Hormones and Behavior, 84, 64–74. [DOI] [PubMed] [Google Scholar]
- Kuznetsova A., Brockhoff P.B., Christensen R.H.B. (2014) lmerTest: tests for random and fixed effects for linear mixed effect models (Lmer Objects of Lme4 Package). R Package Version 2.0-6 Available: http://cran.r-project.org/web/packages/lmerTest/index.html.
- Lancaster K., Carter C.S., Pournajafi-Nazarloo H., et al. (2015). Plasma oxytocin explains individual differences in neural substrates of social perception. Frontiers in Human Neuroscience, 9, 132.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.R., Rohn M.C., Tanda G., Leggio L. (2016). Targeting the oxytocin system to treat addictive disorders: rationale and progress to date. CNS Drugs, 30 (2), 109–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng G., Ludwig M. (2015). Intranasal oxytocin: myths and delusions. Biological Psychiatry. [DOI] [PubMed] [Google Scholar]
- Lischke A., Berger C., Prehn K., Heinrichs M., Herpertz S.C., Domes G. (2012). Intranasal oxytocin enhances emotion recognition from dynamic facial expressions and leaves eye-gaze unaffected. Psychoneuroendocrinology, 37 (4), 475–81. [DOI] [PubMed] [Google Scholar]
- Luminet O., Grynberg D., Ruzette N., Mikolajczak M. (2011). Personality-dependent effects of oxytocin: greater social benefits for high alexithymia scorers. Biological Psychology, 87(3), 401–6. [DOI] [PubMed] [Google Scholar]
- MacFadyen K., Loveless R., DeLucca B., et al. (2016). Peripheral oxytocin administration reduces ethanol consumption in rats. Pharmacology and Biochemistry Behavior, 140, 27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maria M., Hartwell K.J., Hanlon C.A., et al. (2015). Right anterior insula connectivity is important for cue-induced craving in nicotine-dependent smokers. Addiction Biology, 20(2), 407–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurage P., Campanella S., Philippot P., Charest I., Martin S., de Timary P. (2009). Impaired emotional facial expression decoding in alcoholism is also present for emotional prosody and body postures. Alcohol and Alcoholism, 44(5), 476–85. [DOI] [PubMed] [Google Scholar]
- Maurage P., Philippot P., Joassin F., et al. (2008). The auditory-visual integration of anger is impaired in alcoholism: an event-related potentials study. Journal of Psychiatry & Neuroscience, 33(2), 111–22. [PMC free article] [PubMed] [Google Scholar]
- McGregor I.S., Bowen M.T. (2012). Breaking the loop: oxytocin as a potential treatment for drug addiction. Hormones Behavior, 61(3), 331–9. [DOI] [PubMed] [Google Scholar]
- Mehrabian A., Russell J.A. (1978). A questionnaire measure of habitual alcohol use. Psychological Reports, 43(3 Pt 1), 803–6. [DOI] [PubMed] [Google Scholar]
- Naqvi N.H., Bechara A. (2010). The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Structure and Function, 214(5–6), 435–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rourke M.F., Pauca A., Jiang X.J. (2001). Pulse wave analysis. British Journal of Clinical Pharmacology, 51(6), 507–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padula C.B., Simmons A.N., Matthews S.C., et al. (2011). Alcohol attenuates activation in the bilateral anterior insula during an emotional processing task: a pilot study. Alcohol and Alcoholism, 46(5), 547–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paloyelis Y., Doyle O.M., Zelaya F.O., et al. (2014). A spatiotemporal profile of in vivo cerebral blood flow changes following intranasal oxytocin in humans. Biological Psychiatry. [DOI] [PubMed] [Google Scholar]
- Panksepp J., Panksepp J.B. (2013). Toward a cross-species understanding of empathy. Trends in Neurosci, 36(8), 489.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.D., Tallon-Baudry C. (2014). The neural subjective frame: from bodily signals to perceptual consciousness. Philosophical Transactions of the Royal Society of London. Series B Biological Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus M.P., Stein M.B. (2010). Interoception in anxiety and depression. Brain Structure and Function, 214(5–6), 451–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne R.A., Symeonides C.N., Webb D.J., Maxwell S.R. (2006). Pulse transit time measured from the ECG: an unreliable marker of beat-to-beat blood pressure. Journal of Applied Physiology (1985), 100(1), 136–41. [DOI] [PubMed] [Google Scholar]
- Pedersen C.A., Smedley K.L., Leserman J., et al. (2013). Intranasal oxytocin blocks alcohol withdrawal in human subjects. Alcoholism, Clinical and Experimental Research, 37(3), 484–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry A., Aviezer H., Goldstein P., Palgi S., Klein E., Shamay-Tsoory S.G. (2013). Face or body? Oxytocin improves perception of emotions from facial expressions in incongruent emotional body context. Psychoneuroendocrinology, 38 (11), 2820–5. [DOI] [PubMed] [Google Scholar]
- Peters J.H., McDougall S.J., Kellett D.O., Jordan D., Llewellyn-Smith I.J., Andresen M.C. (2008). Oxytocin enhances cranial visceral afferent synaptic transmission to the solitary tract nucleus. Journal of Neuroscience, 28(45), 11731–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S., Slattery D.A., Flor P.J., Neumann I.D., Reber S.O. (2013). Differential effects of baclofen and oxytocin on the increased ethanol consumption following chronic psychosocial stress in mice. Addiction Biology, 18(1), 66–77. [DOI] [PubMed] [Google Scholar]
- Peters S.T., Bowen M.T., Bohrer K., McGregor I.S., Neumann I.D. (2017). Oxytocin inhibits ethanol consumption and ethanol-induced dopamine release in the nucleus accumbens. Addiction Biology, 22(3), 702–11. [DOI] [PubMed] [Google Scholar]
- Pfundmair M., Zwarg C., Paulus M., Rimpel A. (2017). Oxytocin promotes attention to social cues regardless of group membership. Hormones and Behavior, 90, 136–40. [DOI] [PubMed] [Google Scholar]
- Quattrocki E., Friston K. (2014). Autism, oxytocin and interoception. Neuroscience & Biobehavioral Reviews, 47, 410–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana D.S., Alvares G.A., Hickie I.B., Guastella A.J. (2015). Do delivery routes of intranasally administered oxytocin account for observed effects on social cognition and behavior? A two-level model. Neuroscience & Biobehavioral Reviews, 49, 182–92. [DOI] [PubMed] [Google Scholar]
- RCoreTeam. (2013). R: A language and Environment for Statistical Computing Vienna: R Foundation for Statistical Computing. Available: http://www.R-project.org.
- Ring C., Brener J. (1996). Influence of beliefs about heart rate and actual heart rate on heartbeat counting. Psychophysiology, 33(5), 541–6. [DOI] [PubMed] [Google Scholar]
- Ring C., Brener J., Knapp K., Mailloux J. (2015). Effects of heartbeat feedback on beliefs about heart rate and heartbeat counting: a cautionary tale about interoceptive awareness. Biological Psychology, 104, 193–8. [DOI] [PubMed] [Google Scholar]
- Schandry R. (1981). Heart beat perception and emotional experience. Psychophysiology, 18(4), 483–8. [DOI] [PubMed] [Google Scholar]
- Schulze L., Lischke A., Greif J., Herpertz S.C., Heinrichs M., Domes G. (2011). Oxytocin increases recognition of masked emotional faces. Psychoneuroendocrinology, 36(9), 1378–82. [DOI] [PubMed] [Google Scholar]
- Shah P., Catmur C., Bird G. (2017). From heart to mind: linking interoception, emotion, and theory of mind. Cortex, 93, 220–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory S.G., Abu-Akel A. (2016). The social salience hypothesis of oxytocin. Biological Psychiatry, 79(3), 194–202. [DOI] [PubMed] [Google Scholar]
- Sokol H.W., Valtin H. (1967). Evidence for the synthesis of oxytocin and vasopressin in separate neurons. Nature, 214(5085), 314–6. [DOI] [PubMed] [Google Scholar]
- Spielberger C.D., Gorsuch L.,R., Lushene R., Vagg R.,P., Jacobs A.,G. (1983). Manual for the State-Trait Anxiety Inventory. Palo Alto: CA: Consulting Psychologists Press. [Google Scholar]
- Steele C.M., Josephs R.A. (1990). Alcohol myopia. Its prized and dangerous effects. American Psychology, 45(8), 921–33. [DOI] [PubMed] [Google Scholar]
- Stern E.R., Muratore A.F., Taylor S.F., Abelson J.L., Hof P.R., Goodman W.K. (2017). Switching between internally and externally focused attention in obsessive-compulsive disorder: abnormal visual cortex activation and connectivity. Psychiatry Research, 265, 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss G.P., Keller W.R., Koenig J.I., Sullivan S.K., Gold J.M., Buchanan R.W. (2015). Endogenous oxytocin levels are associated with the perception of emotion in dynamic body expressions in schizophrenia. Schizophrenia Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sönmez M.B., Kahyacı Kılıç E., Ateş Çöl I., Görgülü Y., Köse Çınar R. (2016). Decreased interoceptive awareness in patients with substance use disorders. Journal of Substance Use, 22(1), 1–6. [Google Scholar]
- Tollenaar M.S., Chatzimanoli M., van der Wee N.J., Putman P. (2013). Enhanced orienting of attention in response to emotional gaze cues after oxytocin administration in healthy young men. Psychoneuroendocrinology, 38(9), 1797–802. [DOI] [PubMed] [Google Scholar]
- Tsakiris M., Critchley H. (2016). Interoception beyond homeostasis: affect, cognition and mental health. Philosophical Transactions of the Royal Society of London. Series B Biological Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valstad M., Alvares G.A., Andreassen O.A., Westlye L.T., Quintana D.S. (2016). The relationship between central and peripheral oxytocin concentrations: a systematic review and meta-analysis protocol. Systematic Review, 5(1), 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valstad M., Alvares G.A., Egknud M., et al. (2017). The correlation between central and peripheral oxytocin concentrations: a systematic review and meta-analysis. Neuroscience & Biobehavioral Review, 78, 117–24. [DOI] [PubMed] [Google Scholar]
- Van I.M.H., Bakermans-Kranenburg M.J. (2012). A sniff of trust: meta-analysis of the effects of intranasal oxytocin administration on face recognition, trust to in-group, and trust to out-group. Psychoneuroendocrinology, 37(3), 438–43. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A. (2014). Social cognition in cocaine addiction. Proceedings of the National Academy of Sciences of the United States of America, 111(7), 2406–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead W.E., Drescher V.M., Heiman P., Blackwell B. (1977). Realtion of heart rate control to heartbeat perception. Biofeedback and Self Regulation, 2(4), 371–92. [PubMed] [Google Scholar]
- Yao S., Becker B., Zhao W., et al. (2017). Oxytocin modulates attention switching between interoceptive signals and external social cues. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.